Celiac Disease Monocytes Induce a Barrier Defect in Intestinal Epithelial Cells
Abstract
1. Introduction
2. Results
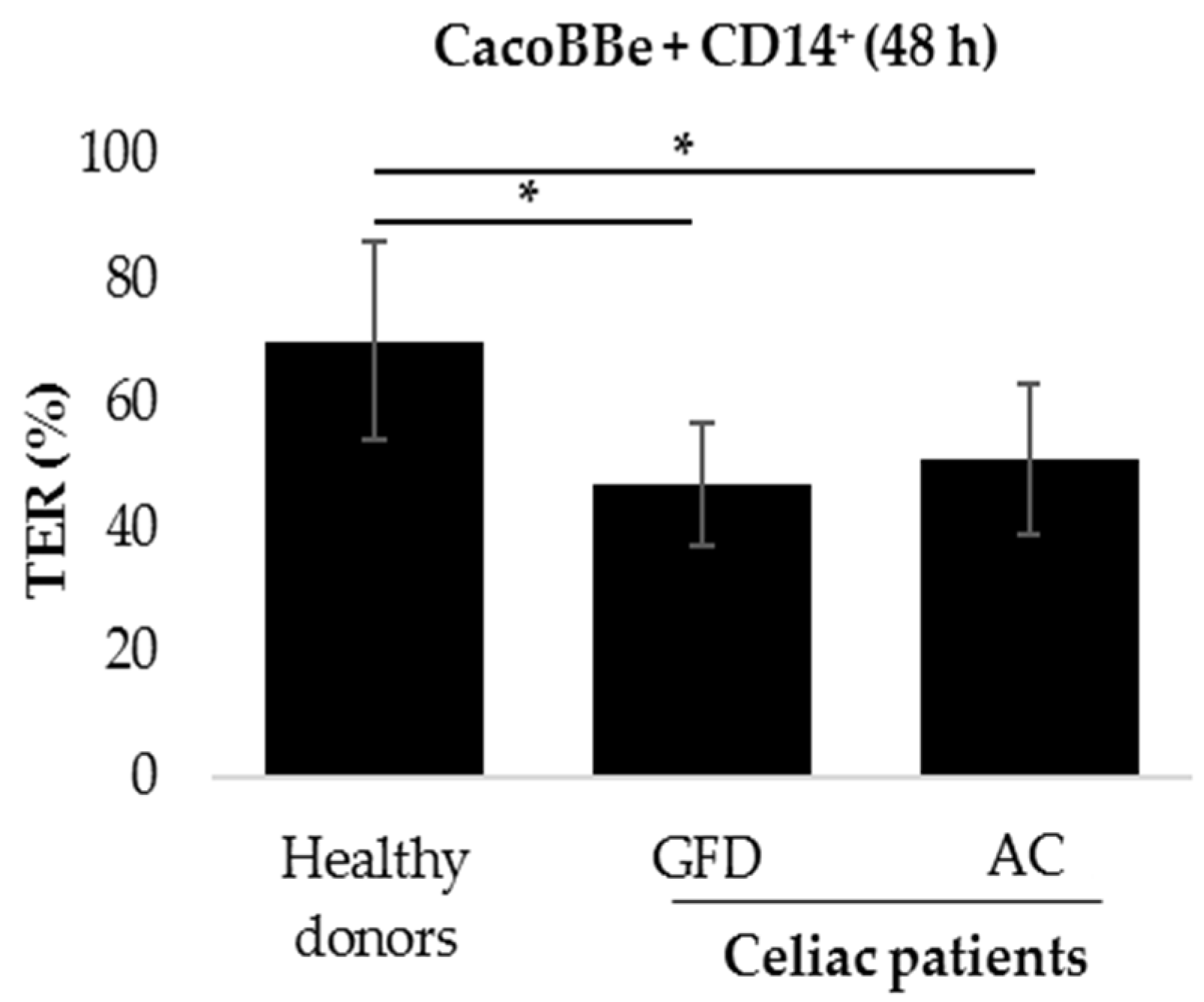
2.1. Monocytes Derived from Celiac Patients Induce a Barrier Defect in Intestinal Epithelial Cells
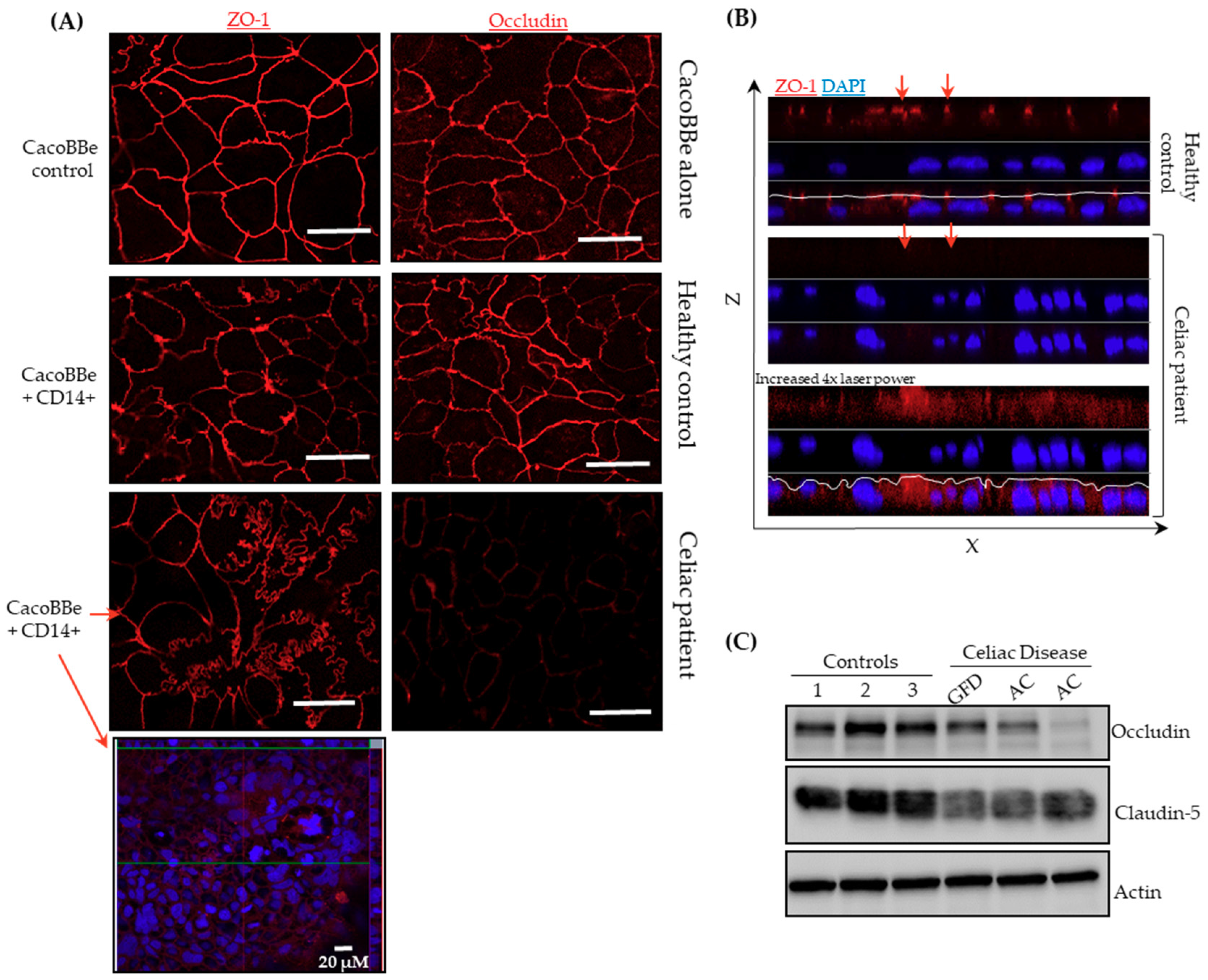
2.2. Celiac Monocytes Alter IEC-TJ Structure
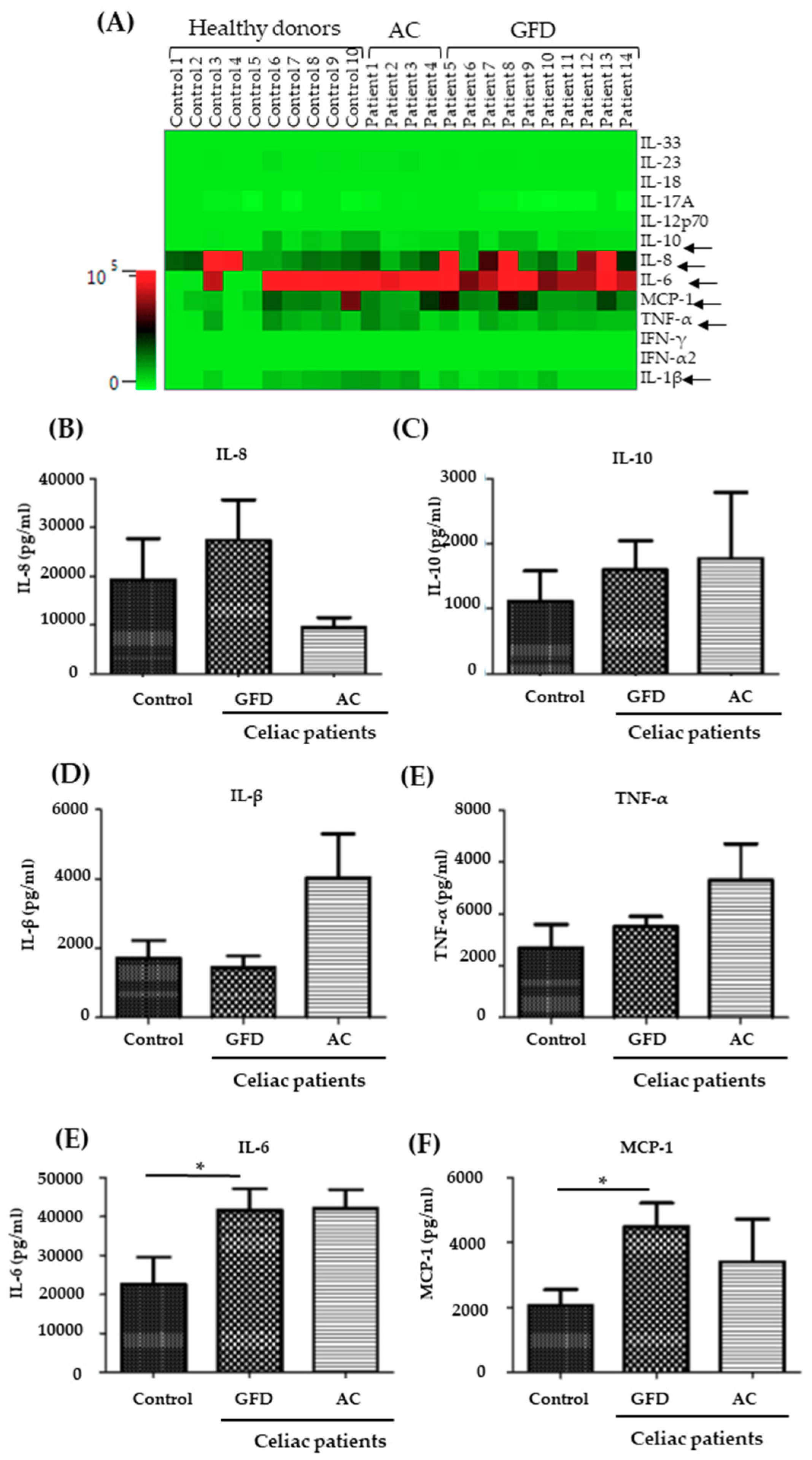
2.3. Monocytes Derived from Celiac Disease Patients Present Higher Levels of Proinflammatory Cytokine Production
3. Discussion
4. Materials and Methods
4.1. Human Material
4.2. Cell Line
4.3. PBMCs Isolation and CD14+ Sorting
4.4. Co-Culture and TER Measurement
4.5. Immunofluorescence
4.6. Western Blotting
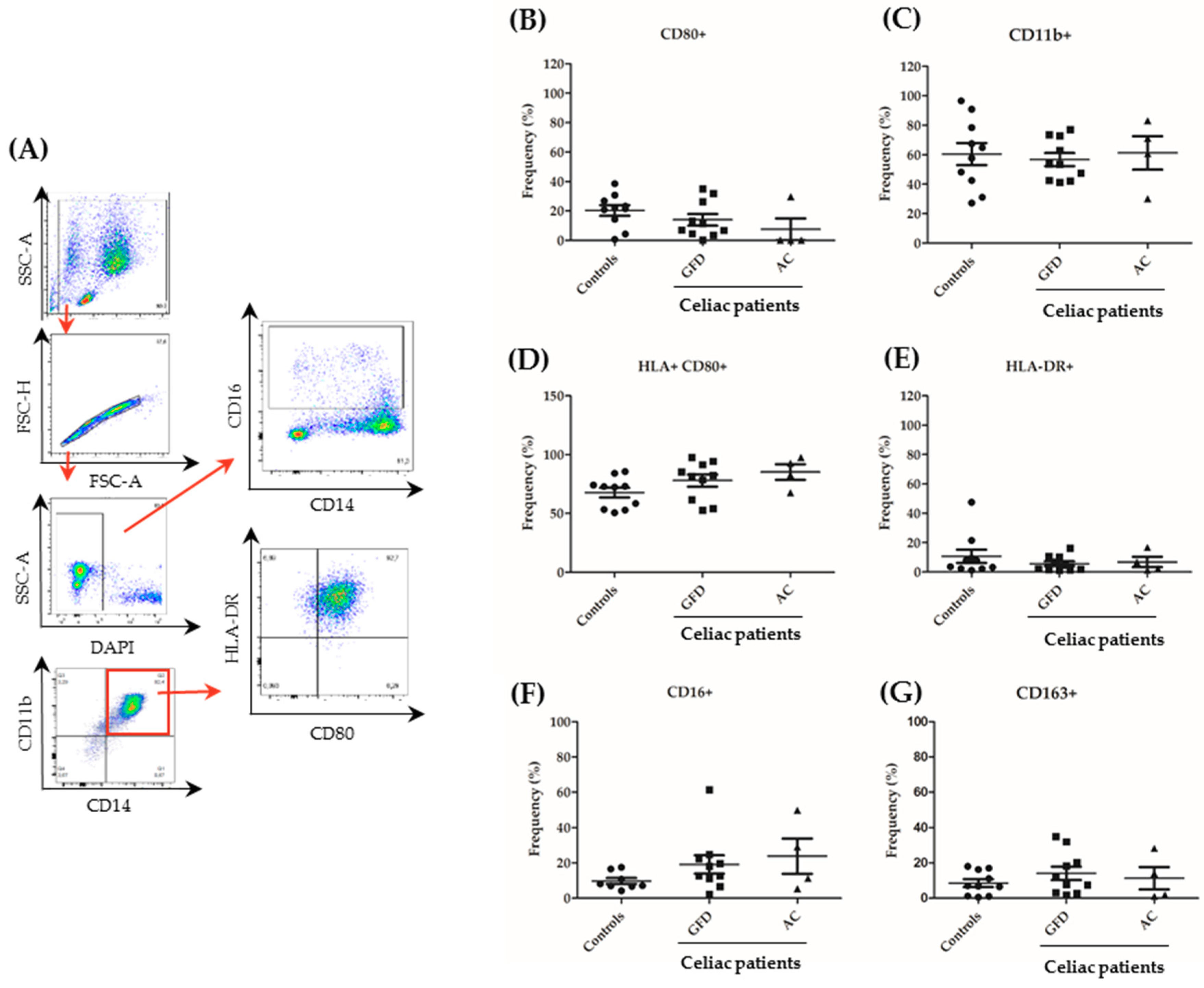
4.7. Flow Cytometric Assessment—Surface Markers and Cytokine Expression
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AC | Active celiac |
| CeD | Celiac disease |
| CCL2 | CC-chemokine ligand-2 |
| GFD | Gluten-free diet |
| GM-CSF | Granulocyte macrophage colony stimulating factor |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-10 | Interleukin-10 |
| IL-12p70 | Interleukin-12p70 |
| IL-15 | Interleukin-15 |
| IL-17A | Interleukin-17A |
| IL-18 | Interleukin-18 |
| IL-23 | Interleukin-23 |
| IL-33 | Interleukin-33 |
| IFN-γ | Interferon-γ |
| IFNα2 | Interferon-α2 |
| MACS | Magnetic cell sorting |
| MCP-1 | Monocyte chemotactic protein-1 |
| PBMCs | Peripheral blood mononuclear colony stimulating factor |
| TER | Transepithelial resistance |
| Tglia | Trypsinized gliadin |
| TJ | Tight junctions |
References
- Mustalahti, K.; Catassi, C.; Reunanen, A.; Fabiani, E.; Heier, M.; McMillan, S.; Murray, L.; Metzger, M.H.; Gasparin, M.; Bravi, E.; et al. The prevalence of celiac disease in Europe: results of a centralized, international mass screening project. Ann. Med. 2010, 42, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Jabri, B.; Sollid, L.M. T Cells in Celiac Disease. J. Immunol. 2017, 198, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52. [Google Scholar] [CrossRef]
- Schumann, M.; Siegmund, B.; Schulzke, J.D.; Fromm, M. Celiac Disease: Role of the Epithelial Barrier. Cell Mol. Gastroenterol. Hepatol. 2017, 3, 150–162. [Google Scholar] [CrossRef]
- van Elburg, R.M.; Uil, J.J.; Mulder, C.J.; Heymans, H.S. Intestinal permeability in patients with coeliac disease and relatives of patients with coeliac disease. Gut 1993, 34, 354–357. [Google Scholar] [CrossRef]
- Cardoso-Silva, D.; Delbue, D.; Itzlinger, A.; Moerkens, R.; Withoff, S.; Branchi, F.; Schumann, M. Intestinal Barrier Function in Gluten-Related Disorders. Nutrients 2019, 11, 2325. [Google Scholar] [CrossRef] [PubMed]
- Matysiak-Budnik, T.; Moura, I.C.; Arcos-Fajardo, M.; Lebreton, C.; Menard, S.; Candalh, C.; Ben-Khalifa, K.; Dugave, C.; Tamouza, H.; van Niel, G.; et al. Secretory IgA mediates retrotranscytosis of intact gliadin peptides via the transferrin receptor in celiac disease. J. Exp. Med. 2008, 205, 143–154. [Google Scholar] [CrossRef]
- Menard, S.; Lebreton, C.; Schumann, M.; Matysiak-Budnik, T.; Dugave, C.; Bouhnik, Y.; Malamut, G.; Cellier, C.; Allez, M.; Crenn, P.; et al. Paracellular versus transcellular intestinal permeability to gliadin peptides in active celiac disease. Am. J. Pathol. 2012, 180, 608–615. [Google Scholar] [CrossRef]
- Schumann, M.; Gunzel, D.; Buergel, N.; Richter, J.F.; Troeger, H.; May, C.; Fromm, A.; Sorgenfrei, D.; Daum, S.; Bojarski, C.; et al. Cell polarity-determining proteins Par-3 and PP-1 are involved in epithelial tight junction defects in coeliac disease. Gut 2012, 61, 220–228. [Google Scholar] [CrossRef]
- Schumann, M.; Richter, J.F.; Wedell, I.; Moos, V.; Zimmermann-Kordmann, M.; Schneider, T.; Daum, S.; Zeitz, M.; Fromm, M.; Schulzke, J.D. Mechanisms of epithelial translocation of the alpha(2)-gliadin-33mer in coeliac sprue. Gut 2008, 57, 747–754. [Google Scholar] [CrossRef]
- Lissner, D.; Schumann, M.; Batra, A.; Kredel, L.I.; Kuhl, A.A.; Erben, U.; May, C.; Schulzke, J.D.; Siegmund, B. Monocyte and M1 Macrophage-induced Barrier Defect Contributes to Chronic Intestinal Inflammation in IBD. Inflamm. Bowel. Dis. 2015, 21, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Managlia, E.; Liu, S.X.L.; Yan, X.; Tan, X.D.; Chou, P.M.; Barrett, T.A.; De Plaen, I.G. Blocking NF-kappaB Activation in Ly6c(+) Monocytes Attenuates Necrotizing Enterocolitis. Am. J. Pathol. 2019, 189, 604–618. [Google Scholar] [CrossRef]
- Morhardt, T.L.; Hayashi, A.; Ochi, T.; Quiros, M.; Kitamoto, S.; Nagao-Kitamoto, H.; Kuffa, P.; Atarashi, K.; Honda, K.; Kao, J.Y.; et al. IL-10 produced by macrophages regulates epithelial integrity in the small intestine. Sci. Rep. 2019, 9, 1223. [Google Scholar] [CrossRef] [PubMed]
- Bain, C.C.; Schridde, A. Origin, Differentiation, and Function of Intestinal Macrophages. Front Immunol. 2018, 9, 2733. [Google Scholar] [CrossRef] [PubMed]
- Cinova, J.; Palova-Jelinkova, L.; Smythies, L.E.; Cerna, M.; Pecharova, B.; Dvorak, M.; Fruhauf, P.; Tlaskalova-Hogenova, H.; Smith, P.D.; Tuckova, L. Gliadin peptides activate blood monocytes from patients with celiac disease. J. Clin Immunol. 2007, 27, 201–209. [Google Scholar] [CrossRef] [PubMed]
- Barilli, A.; Rotoli, B.M.; Visigalli, R.; Ingoglia, F.; Cirlini, M.; Prandi, B.; Dall'Asta, V. Gliadin-mediated production of polyamines by RAW264.7 macrophages modulates intestinal epithelial permeability in vitro. Biochim. Biophys. Acta 2015, 1852, 1779–1786. [Google Scholar] [CrossRef]
- Harris, K.M.; Fasano, A.; Mann, D.L. Monocytes differentiated with IL-15 support Th17 and Th1 responses to wheat gliadin: implications for celiac disease. Clin Immunol. 2010, 135, 430–439. [Google Scholar] [CrossRef]
- Kumar, V.; Gutierrez-Achury, J.; Kanduri, K.; Almeida, R.; Hrdlickova, B.; Zhernakova, D.V.; Westra, H.J.; Karjalainen, J.; Ricano-Ponce, I.; Li, Y.; et al. Systematic annotation of celiac disease loci refines pathological pathways and suggests a genetic explanation for increased interferon-gamma levels. Hum Mol. Genet 2015, 24, 397–409. [Google Scholar] [CrossRef]
- Grimm, M.C.; Pullman, W.E.; Bennett, G.M.; Sullivan, P.J.; Pavli, P.; Doe, W.F. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J. Gastroenterol. Hepatol. 1995, 10, 387–395. [Google Scholar] [CrossRef]
- Kamada, N.; Hisamatsu, T.; Okamoto, S.; Chinen, H.; Kobayashi, T.; Sato, T.; Sakuraba, A.; Kitazume, M.T.; Sugita, A.; Koganei, K.; et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J. Clin Invest. 2008, 118, 2269–2280. [Google Scholar]
- Lampinen, M.; Waddell, A.; Ahrens, R.; Carlson, M.; Hogan, S.P. CD14+CD33+ myeloid cell-CCL11-eosinophil signature in ulcerative colitis. J. Leukoc Biol. 2013, 94, 1061–1070. [Google Scholar] [CrossRef]
- Smith, P.D.; Smythies, L.E.; Shen, R.; Greenwell-Wild, T.; Gliozzi, M.; Wahl, S.M. Intestinal macrophages and response to microbial encroachment. Mucosal Immunol. 2011, 4, 31–42. [Google Scholar] [CrossRef]
- Vincentini, O.; Maialetti, F.; Gonnelli, E.; Silano, M. Gliadin-dependent cytokine production in a bidimensional cellular model of celiac intestinal mucosa. Clin Exp. Med. 2015, 15, 447–454. [Google Scholar] [CrossRef]
- Suzuki, T.; Yoshinaga, N.; Tanabe, S. Interleukin-6 (IL-6) regulates claudin-2 expression and tight junction permeability in intestinal epithelium. J. Biol. Chem. 2011, 286, 31263–31271. [Google Scholar] [CrossRef]
- Du Plessis, J.; Vanheel, H.; Janssen, C.E.; Roos, L.; Slavik, T.; Stivaktas, P.I.; Nieuwoudt, M.; van Wyk, S.G.; Vieira, W.; Pretorius, E.; et al. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. J. Hepatol. 2013, 58, 1125–1132. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Finamore, A.; Ara, C.; Di Sabatino, A.; Mengheri, E.; Corazza, G.R. Altered expression, localization, and phosphorylation of epithelial junctional proteins in celiac disease. Am. J. Clin. Pathol. 2006, 125, 502–511. [Google Scholar] [CrossRef]
- Bojarski, C.; Gitter, A.H.; Bendfeldt, K.; Mankertz, J.; Schmitz, H.; Wagner, S.; Fromm, M.; Schulzke, J.D. Permeability of human HT-29/B6 colonic epithelium as a function of apoptosis. J. Physiol. 2001, 535 Pt 2, 541–552. [Google Scholar] [CrossRef]
- Kredel, L.I.; Batra, A.; Stroh, T.; Kuhl, A.A.; Zeitz, M.; Erben, U.; Siegmund, B. Adipokines from local fat cells shape the macrophage compartment of the creeping fat in Crohn’s disease. Gut 2013, 62, 852–862. [Google Scholar] [CrossRef]
| Number of Subjects | 17 |
|---|---|
| Female/Male | 14/3 |
| Age at enrolment, median (range) | 46 (23-83) |
| Age at CeD diagnosis, median (range) | 32 (6-73) |
| Marsh Grade at enrolment, n (%) | |
| 0 | 5 (29) |
| 1 | 2 (12) |
| 2 | 0 (0) |
| 3a | 1 (6) |
| 3b | 3 (18) |
| 3c | 0 (0) |
| not available | 6 (35) |
| tTG at enrolment, n (%) | |
| positive | 6 (35) |
| negative | 2 (12) |
| not available | 9 (53) |
| HLA-DQ status, n (%) | |
| DQ2+ | 11 (65) |
| DQ8+ | 0 (0) |
| not available | 6 (35) |
| GFD status, n (%) | |
| Active CeD | 6 (35) |
| New CeD diagnosis | 4 |
| CeD, non-compliant to GFD | 2 |
| CeD on GFD | 11 (65) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delbue, D.; Cardoso-Silva, D.; Branchi, F.; Itzlinger, A.; Letizia, M.; Siegmund, B.; Schumann, M. Celiac Disease Monocytes Induce a Barrier Defect in Intestinal Epithelial Cells. Int. J. Mol. Sci. 2019, 20, 5597. https://doi.org/10.3390/ijms20225597
Delbue D, Cardoso-Silva D, Branchi F, Itzlinger A, Letizia M, Siegmund B, Schumann M. Celiac Disease Monocytes Induce a Barrier Defect in Intestinal Epithelial Cells. International Journal of Molecular Sciences. 2019; 20(22):5597. https://doi.org/10.3390/ijms20225597
Chicago/Turabian StyleDelbue, Deborah, Danielle Cardoso-Silva, Federica Branchi, Alice Itzlinger, Marilena Letizia, Britta Siegmund, and Michael Schumann. 2019. "Celiac Disease Monocytes Induce a Barrier Defect in Intestinal Epithelial Cells" International Journal of Molecular Sciences 20, no. 22: 5597. https://doi.org/10.3390/ijms20225597
APA StyleDelbue, D., Cardoso-Silva, D., Branchi, F., Itzlinger, A., Letizia, M., Siegmund, B., & Schumann, M. (2019). Celiac Disease Monocytes Induce a Barrier Defect in Intestinal Epithelial Cells. International Journal of Molecular Sciences, 20(22), 5597. https://doi.org/10.3390/ijms20225597






