The Role of Electron Transfer in the Fragmentation of Phenyl and Cyclohexyl Boronic Acids
Abstract
1. Introduction
2. Results and Discussion
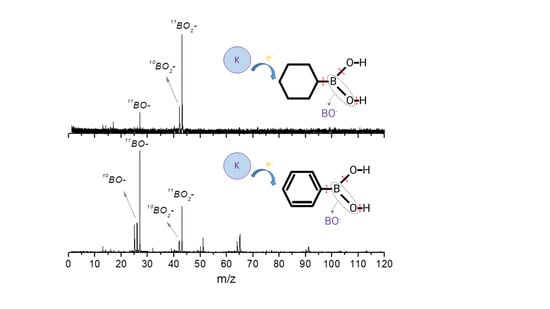
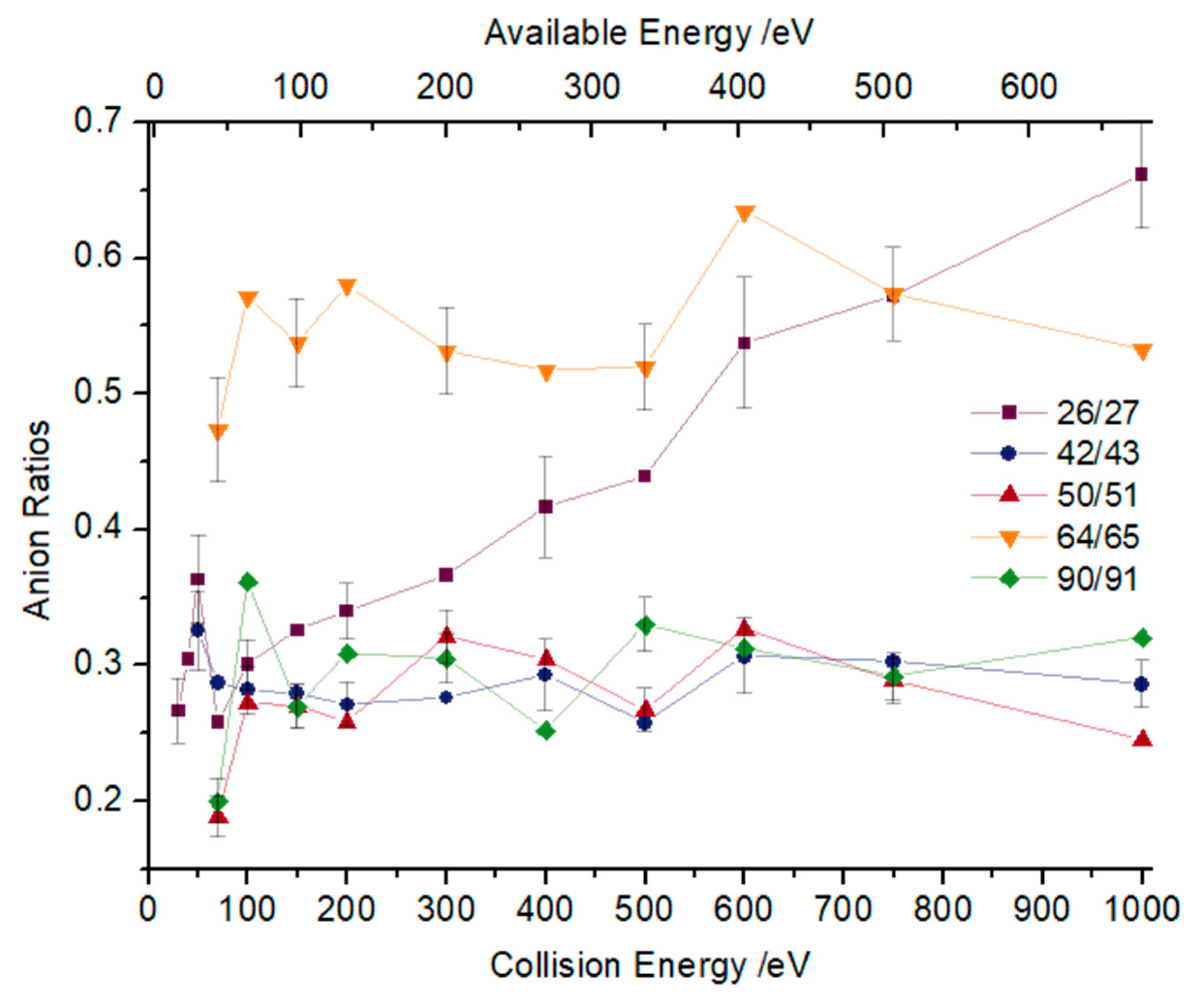
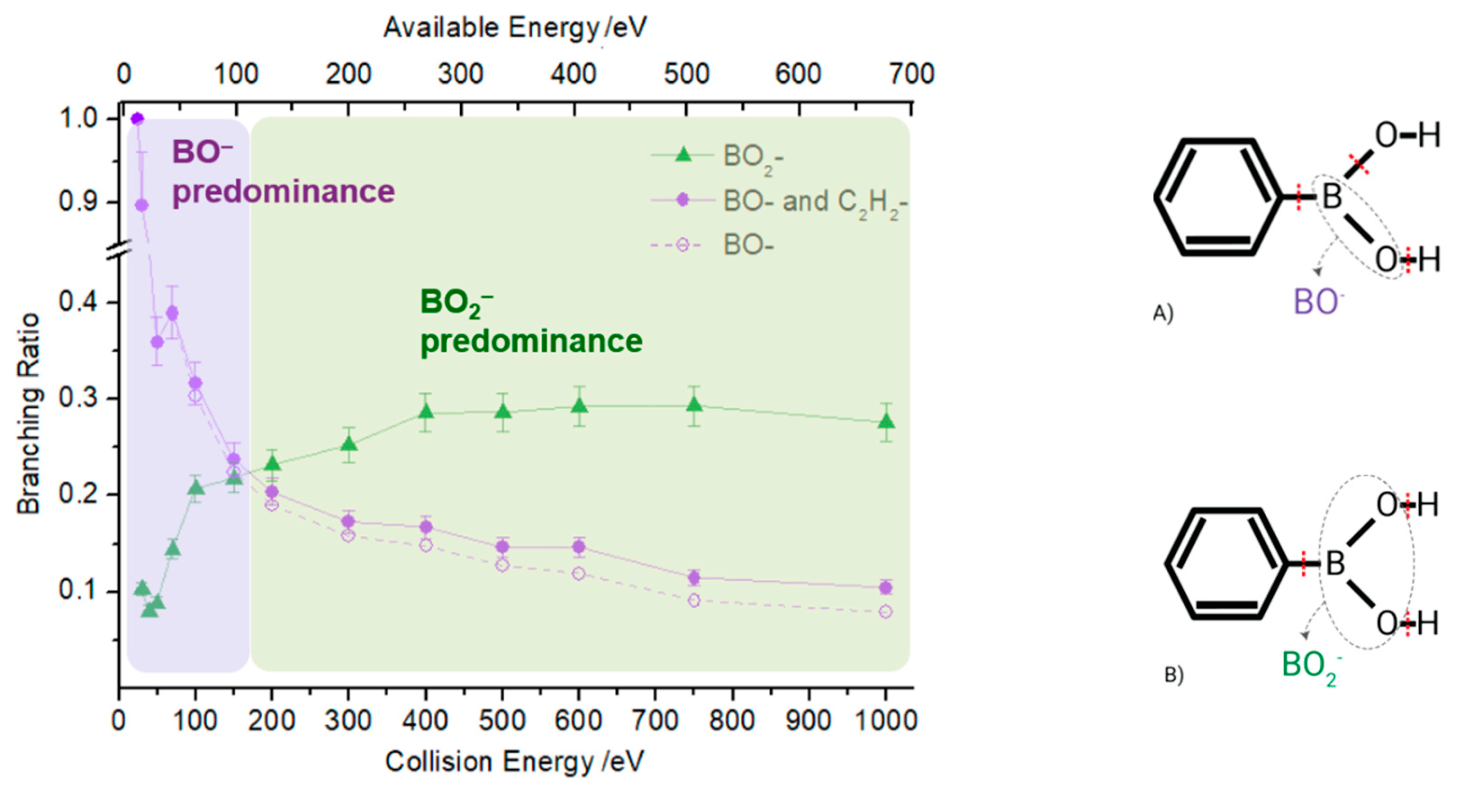
2.1. BO− and BO2−
2.2. Other Relevant Fragments
3. Experimental and Theoretical Methods
3.1. Experimental Methods
3.2. Theoretical Method
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PBA | Phenyl boronic acid |
| CHBA | Cyclohexyl boronic acid |
| HIV | Human immunodeficiency virus |
| LEE | Low-energy electrons |
| DNA | Deoxyribonucleic acid |
| RNA | Ribonucleic acid |
| DEA | Dissociative electron attachment |
| TNI | Temporary negative ion |
| TOF a. m. u. | Time-of-flight Atomic mass unit |
References
- Ban, H.S.; Nakamura, H. Boron-based drug design. Chem. Rec. 2015, 15, 616–635. [Google Scholar] [CrossRef] [PubMed]
- Santos, F.M.F.; Rosa, J.N.; Candeias, N.R.; Carvalho, C.P.; Matos, A.I.; Ventura, A.E.; Florindo, H.F.; Silva, L.C.; Pischel, U.; Gois, P.M.P. A Three-Component Assembly Promoted by Boronic Acids Delivers a Modular Fluorophore Platform (BASHY Dyes). Chem. A Eur. J. 2016, 22, 1631–1637. [Google Scholar] [CrossRef] [PubMed]
- Mader, H.S.; Wolfbeis, O.S. Boronic acid based probes for microdetermination of saccharides and glycosylated biomolecules. Microchim. Acta 2008, 162, 1–34. [Google Scholar] [CrossRef]
- Fu, H.; Fang, H.; Sun, J.; Wang, H.; Liu, A.; Sun, J.; Wu, Z. Boronic Acid-based Enzyme Inhibitors: A Review of Recent Progress. Curr. Med. Chem. 2014, 21, 3271–3280. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Gao, X.; Wang, B. Boronic acid compounds as potential pharmaceutical agents. Med. Res. Rev. 2003, 23, 346–368. [Google Scholar] [CrossRef]
- Boudaïffa, B.; Cloutier, P.; Hunting, D.; Huels, M.A.; Sanche, L. Resonant formation of DNA strand breaks by low-energy (3 to 20 eV) electrons. Science 2000, 287, 1658–1660. [Google Scholar]
- Martin, F.; Burrow, P.D.; Cai, Z.; Cloutier, P.; Hunting, D.; Sanche, L. DNA strand breaks induced by 0–4 eV electrons: The role of shape resonances. Phys. Rev. Lett. 2004, 93, 6–9. [Google Scholar] [CrossRef]
- Sanche, L. Low energy electron-driven damage in biomolecules. Eur. Phys. J. D 2005, 35, 367–390. [Google Scholar] [CrossRef]
- Wang, C.R.; Nguyen, J.; Lu, Q. Bin Bond breaks of nucleotides by dissociative electron transfer of nonequilibrium prehydrated electrons: A new molecular mechanism for reductive DNA damage. J. Am. Chem. Soc. 2009, 131, 11320–11322. [Google Scholar] [CrossRef]
- Ferreira Da Silva, F.; Almeida, D.; Antunes, R.; Martins, G.; Nunes, Y.; Eden, S.; Garcia, G.; Limão-Vieira, P. Electron transfer processes in potassium collisions with 5-fluorouracil and 5-chlorouracil. Phys. Chem. Chem. Phys. 2011, 13, 21621–21629. [Google Scholar] [CrossRef]
- Mendes, M.; Probst, M.; Maihom, T.; García, G.; Limão-Vieira, P. Selective Bond Excision in Nitroimidazoles by Electron Transfer Experiments. J. Phys. Chem. A 2019, 123, 4068–4073. [Google Scholar] [CrossRef] [PubMed]
- Mendes, M.; Pamplona, B.; Kumar, S.; da Silva, F.F.; Aguilar, A.; García, G.; Bacchus-Montabonel, M.C.; Limao-Vieira, P. Ion-pair formation in neutral potassium-neutral pyrimidine collisions: Electron transfer experiments. Front. Chem. 2019, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Ferreira Da Silva, F.; García, G.; Limão-Vieira, P. Selective bond cleavage in potassium collisions with pyrimidine bases of DNA. Phys. Rev. Lett. 2013, 110, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Manura, J.; Manura, D. Isotope Distribution Calculator and Mass Spec Plotter. Sci. Instrum. Serv. 2009, 1996–2009. [Google Scholar]
- Zhai, H.J.; Wang, L.M.; Li, S.D.; Wang, L.S. Vibrationally resolved photoelectron spectroscopy of BO- and BO2-: A joint experimental and theoretical study. J. Phys. Chem. A 2007, 111, 1030–1035. [Google Scholar] [CrossRef]
- Alizadeh, E.; Orlando, T.M.; Sanche, L. Biomolecular Damage Induced by Ionizing Radiation: The Direct and Indirect Effects of Low-Energy Electrons on DNA. Annu. Rev. Phys. Chem. 2015, 66, 379–398. [Google Scholar] [CrossRef]
- Alizadeh, E.; Sanz, A.G.; García, G.; Sanche, L. Radiation Damage to DNA: The Indirect Effect of Low Energy Electrons. J. Phys. Chem. Lett. 2013, 4, 820–825. [Google Scholar] [CrossRef]
- Cunha, T.; Mendes, M.; Ferreira Da Silva, F.; Eden, S.; García, G.; Bacchus-Montabonel, M.C.; Limão-Vieira, P. Electron transfer driven decomposition of adenine and selected analogs as probed by experimental and theoretical methods. J. Chem. Phys. 2018, 148. [Google Scholar] [CrossRef]
- Available online: https://webbook.nist.gov (accessed on 6 November 2019).
- Luo, Y.-R. Bond dissociation energies. Q. Rev. Chem. Soc. 2009, 9, 65–98. [Google Scholar]
- Ervin, K.M.; Anusiewicz, I.; Skurski, P.; Simons, J.; Lineberger, W.C. The only stable state of O2- is the X 2∏g ground state and it (still!) has an adiabatic electron detachment energy of 0.45 eV. J. Phys. Chem. A 2003, 107, 8521–8529. [Google Scholar] [CrossRef]
- Moloney, J.N.; Cotter, T.G. ROS signalling in the biology of cancer. Semin. Cell Dev. Biol. 2018, 80, 50–64. [Google Scholar] [CrossRef] [PubMed]
- Antunes, R.; Almeida, D.; Martins, G.; Mason, N.J.; Garcia, G. Negative ion formation in potassium—Nitromethane collisions. Phys. Chem. Chem. Phys. 2010, 12, 12513–12519. [Google Scholar] [CrossRef] [PubMed]
- Bacchus-Montabonel, M.C.; Łabuda, M.; Tergiman, Y.S.; Sienkiewicz, J.E. Theoretical treatment of charge-transfer processes induced by collision of Cq+ ions with uracil. Phys. Rev. A At. Mol. Opt. Phys. 2005, 72, 1–9. [Google Scholar] [CrossRef]
- Erdmann, E.; Bacchus-Montabonel, M.C.; Łabuda, M. Modelling charge transfer processes in C2+-tetrahydrofuran collision for ion-induced radiation damage in DNA building blocks. Phys. Chem. Chem. Phys. 2017, 19, 19722–19732. [Google Scholar] [CrossRef]
- Bacchus-Montabonel, M.C.; Tergiman, Y.S. An ab initio study of ion induced charge transfer dynamics in collision of carbon ions with thymine. Phys. Chem. Chem. Phys. 2011, 13, 9761–9767. [Google Scholar] [CrossRef]
- Bacchus-Montabonel, M.C. Proton-induced damage on 2-aminooxazole, a potential prebiotic compound. J. Phys. Chem. A 2015, 119, 728–734. [Google Scholar] [CrossRef]
- Bacchus-Montabonel, M.C. Ab initio treatment of ion-induced charge transfer dynamics of isolated 2-deoxy-d-ribose. J. Phys. Chem. A 2014, 118, 6326–6332. [Google Scholar] [CrossRef]
- Almeida, D.; Bacchus-Montabonel, M.C.; Da Silva, F.F.; García, G.; Limão-Vieira, P. Potassium-uracil/thymine ring cleavage enhancement as studied in electron transfer experiments and theoretical calculations. J. Phys. Chem. A 2014, 118, 6547–6552. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Schäfer, A.; Huber, C.; Ahlrichs, R. Fully optimized contracted Gaussian basis sets of triple zeta valence quality for atoms Li to Kr. J. Chem. Phys. 1994, 100, 5829–5835. [Google Scholar] [CrossRef]
- Neese, F. The ORCA program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Werner, H.J.; Knowles, P.J.; Knizia, G.; Manby, F.R.; Schütz, M. Molpro: A general-purpose quantum chemistry program package. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 242–253. [Google Scholar] [CrossRef]
- Available online: http://wild.life.nctu.edu.tw/~jsyu/compchem/molpro-2010.1-manual.pdf (accessed on 7 November 2019).
- Sergentu, D.C.; Amaouch, M.; Pilmé, J.; Galland, N.; Maurice, R. Electronic structures and geometries of the XF3 (X = Cl, Br, I, At) fluorides. J. Chem. Phys. 2015, 143. [Google Scholar] [CrossRef] [PubMed]
- Lawley, K.P.; Roos, B.O. AB Initio Methods in Quantum Chemistry II. Adv. Chem. Phys. 1987, 69, 399–466. [Google Scholar]

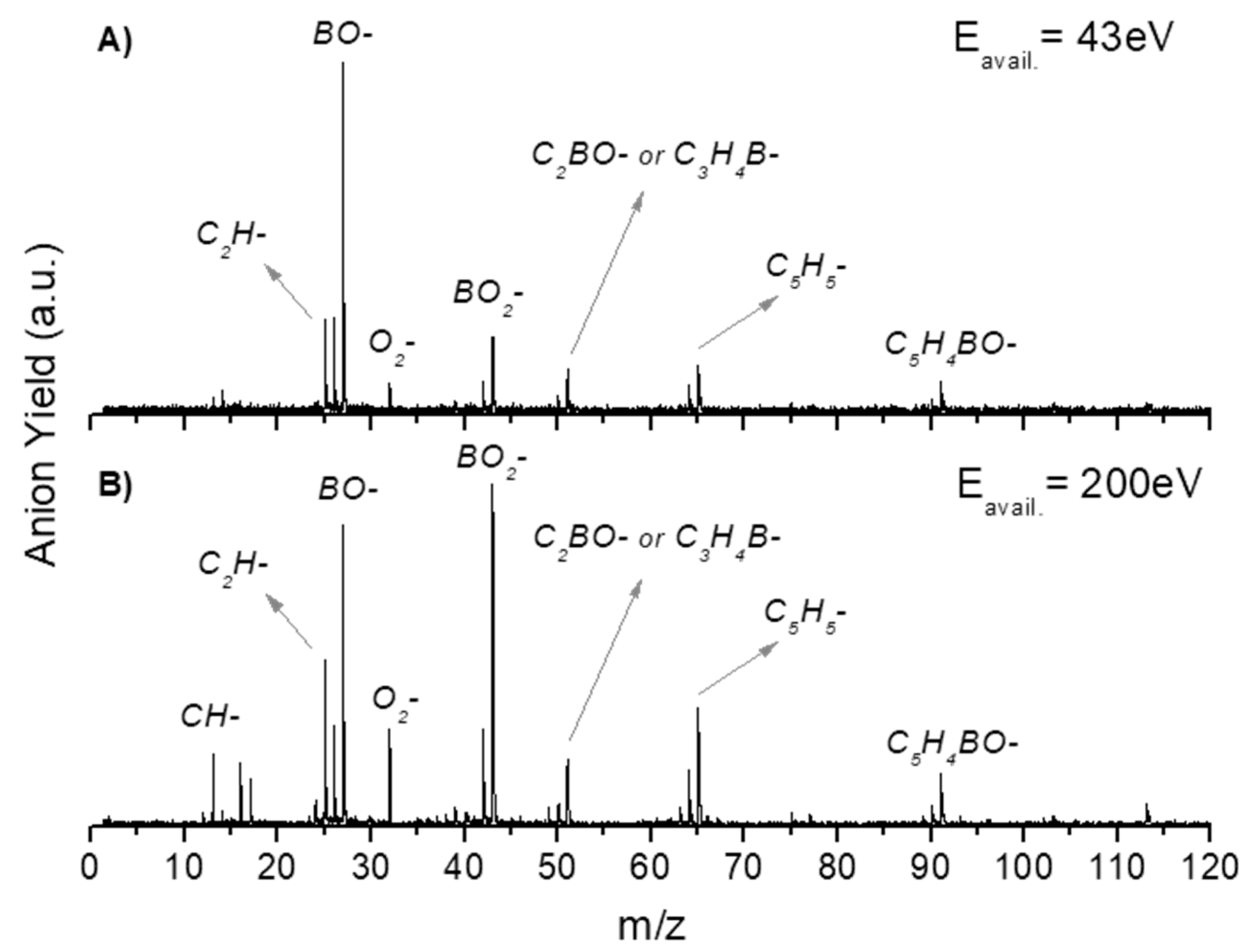
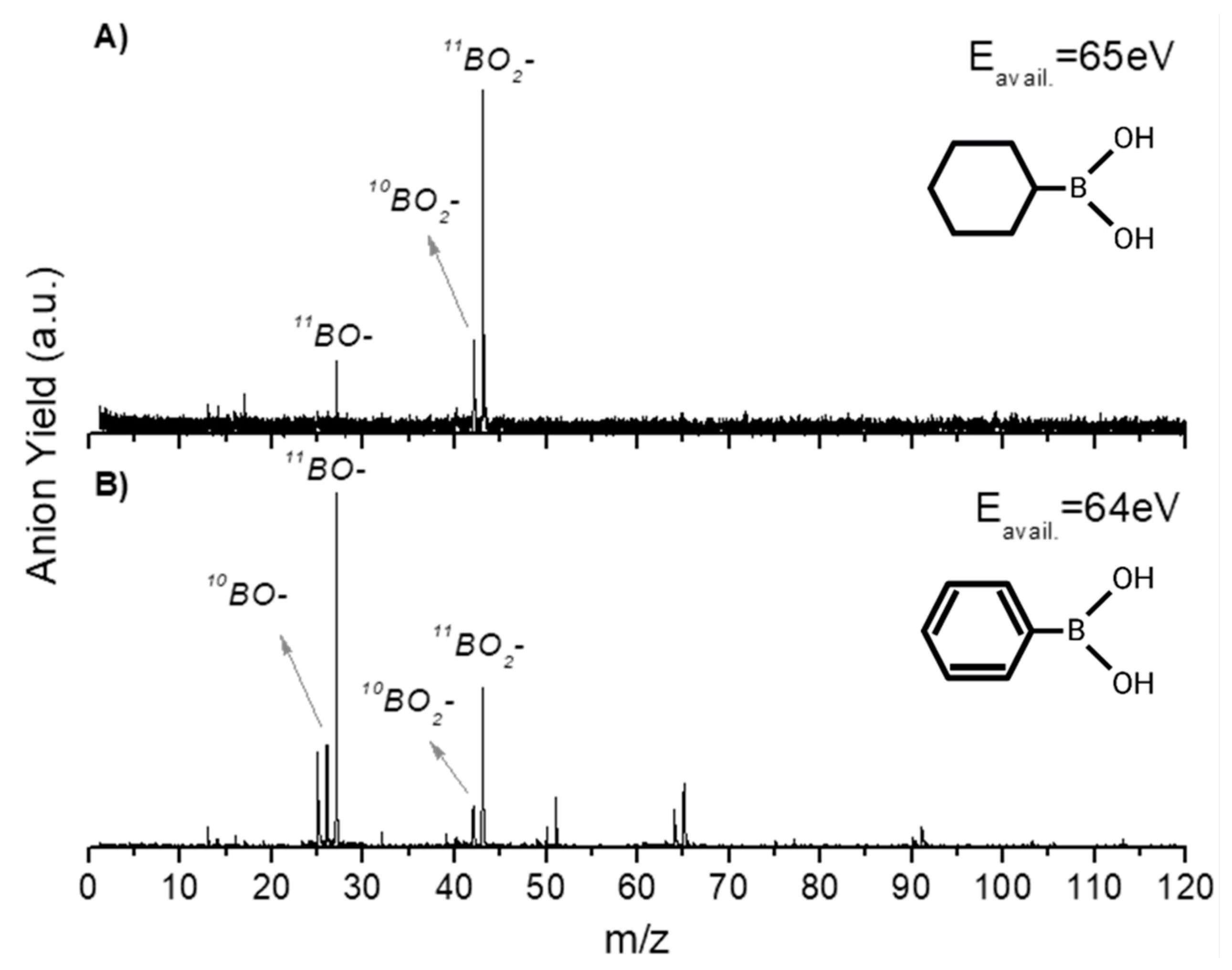
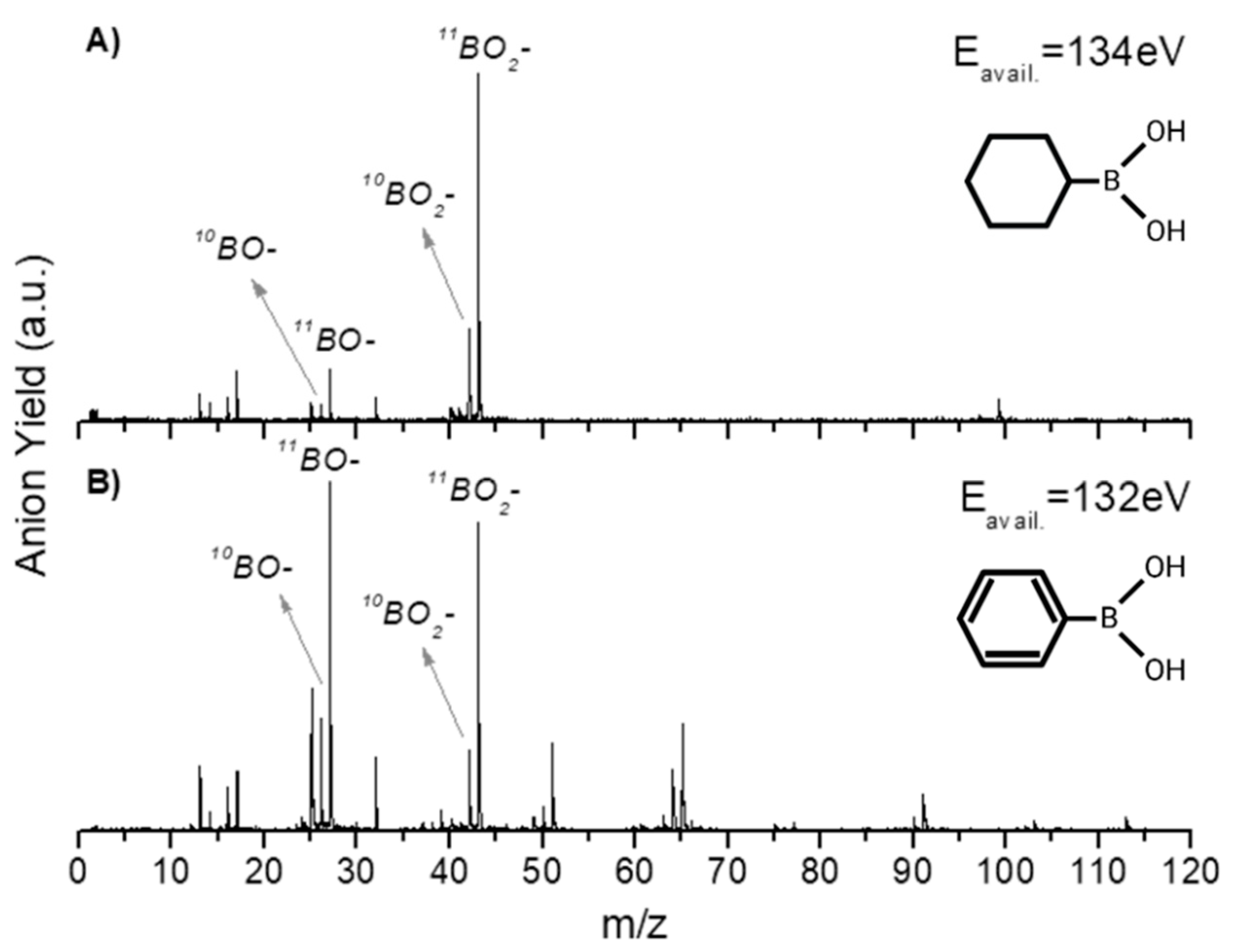
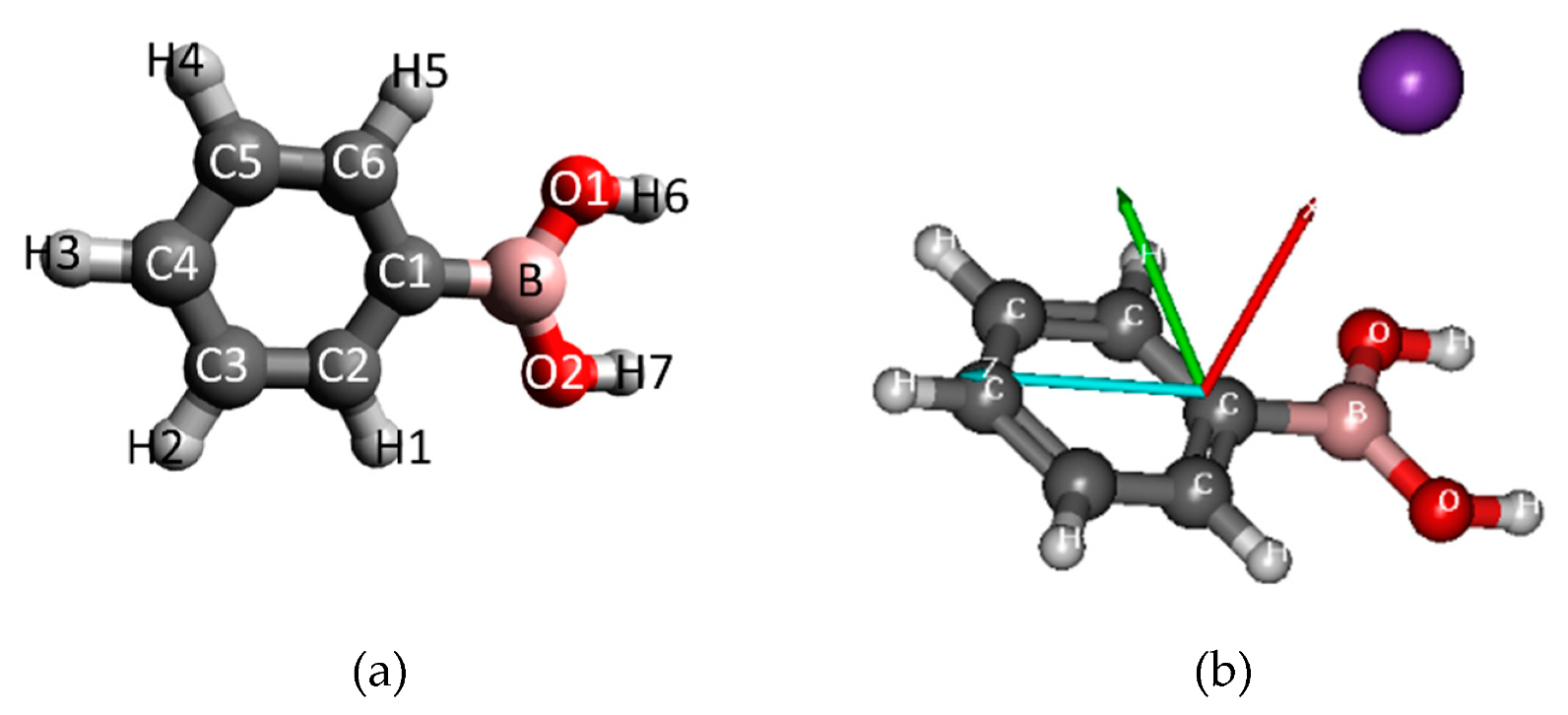
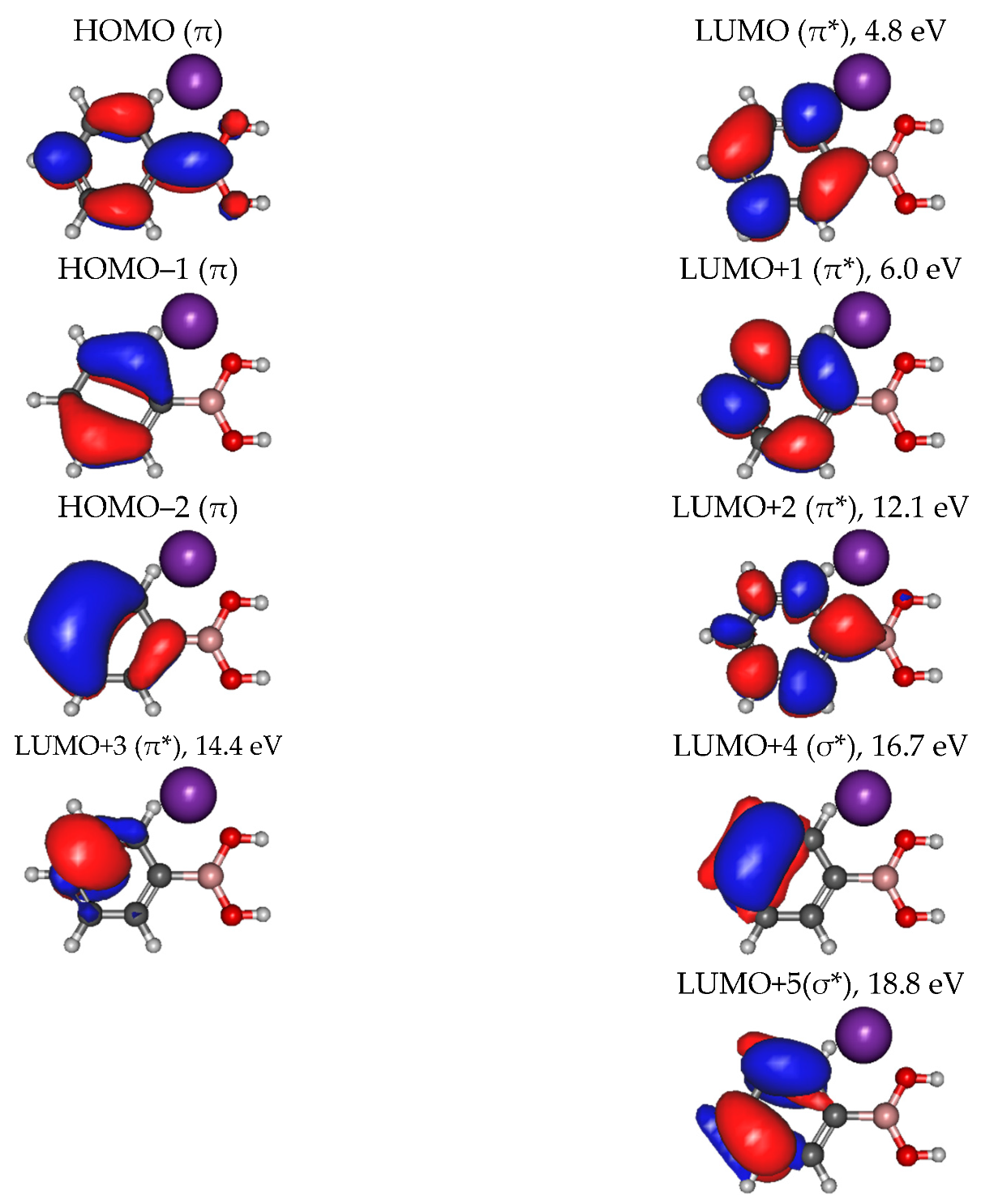
| Mass (a. m. u.) | PBA | CHBA |
|---|---|---|
| 103 | C6H4BO− | |
| 99 | C6H11O− | |
| 97 | C5H10BO−/C4H6BO2− | |
| 91 | C5H411BO− | |
| 90 | C5H410BO− | |
| 89 | C5H211BO−/C6H611B− | |
| 77 | C6H5− | |
| 75 | C6H3− | |
| 66 | C3H311BO− | |
| 65 | C5H5− | |
| 64 | C5H4−/C3H210BO−/C3H11BO−/C4H511B− | |
| 63 | C5H3−/C3H10BO−/C4H510B− | |
| 51 | C211BO−/C3H411B− | |
| 50 | C210BO−/C3H410B− | |
| 49 | C3H211B− | |
| 46 | 11BO2H3− | |
| 43 | 11BO2− | 11BO2− |
| 42 | 10BO2− | 10BO2− |
| 41 | CH2BO−/C3H5− | |
| 40 | C11BOH−/C3H4− | C11BOH−/C3H4− |
| 39 | C11BO−/C3H3− | C11BO−/C3H3− |
| 38 | C2H311B−/C3H2− | |
| 37 | C2H211B−/C3H− | |
| 32 | O2− | O2− |
| 30 | CH2O− | |
| 27 | 11BO− | 11BO− |
| 26 | 10BO−/C2H2− | 10BO− |
| 25 | C2H− | C2H− |
| 24 | CH11B−/C2− | |
| 17 | OH− | OH− |
| 16 | O− | O− |
| 14 | CH2− | CH2− |
| 13 | CH− | CH− |
| 12 | C− | C− |
| Neutral Species | Electron Affinity (eV) |
|---|---|
| BO | 2.510 ± 0.015 |
| BO2 | 4.460 ± 0.030 |
| C2H• | 2.9689 ± 0.0011 |
| C2H2 | 0.480 ± 0.010 |
| C5H5• | 1.786 ± 0.020 |
| C5H4 | 1.750 ± 0.047 |
| C6H5• | 1.0960 ± 0.0060 |
| O | 1.4610 ± 0.0010 |
| OH• | 1.829 ± 0.010 |
| O2 | 0.4480 ± 0.0060 |
| C6H11• | −0.24 ± 0.11 |
| Bond | Dissociation Energy (eV) |
|---|---|
| C–B | 4.640 ± 0.030 |
| O–B | 8.38 |
| O–H | 4.460 ± 0.003 |
| C–C | 6.408 ± 0.160 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano, A.I.; Pamplona, B.; Kilich, T.; Łabuda, M.; Mendes, M.; Pereira-da-Silva, J.; García, G.; Gois, P.M.P.; Ferreira da Silva, F.; Limão-Vieira, P. The Role of Electron Transfer in the Fragmentation of Phenyl and Cyclohexyl Boronic Acids. Int. J. Mol. Sci. 2019, 20, 5578. https://doi.org/10.3390/ijms20225578
Lozano AI, Pamplona B, Kilich T, Łabuda M, Mendes M, Pereira-da-Silva J, García G, Gois PMP, Ferreira da Silva F, Limão-Vieira P. The Role of Electron Transfer in the Fragmentation of Phenyl and Cyclohexyl Boronic Acids. International Journal of Molecular Sciences. 2019; 20(22):5578. https://doi.org/10.3390/ijms20225578
Chicago/Turabian StyleLozano, Ana Isabel, Beatriz Pamplona, Tymon Kilich, Marta Łabuda, Mónica Mendes, João Pereira-da-Silva, Gustavo García, Pedro M. P. Gois, Filipe Ferreira da Silva, and Paulo Limão-Vieira. 2019. "The Role of Electron Transfer in the Fragmentation of Phenyl and Cyclohexyl Boronic Acids" International Journal of Molecular Sciences 20, no. 22: 5578. https://doi.org/10.3390/ijms20225578
APA StyleLozano, A. I., Pamplona, B., Kilich, T., Łabuda, M., Mendes, M., Pereira-da-Silva, J., García, G., Gois, P. M. P., Ferreira da Silva, F., & Limão-Vieira, P. (2019). The Role of Electron Transfer in the Fragmentation of Phenyl and Cyclohexyl Boronic Acids. International Journal of Molecular Sciences, 20(22), 5578. https://doi.org/10.3390/ijms20225578