Radio-Enhancing Properties of Bimetallic Au:Pt Nanoparticles: Experimental and Theoretical Evidence
Abstract
1. Introduction
2. Results
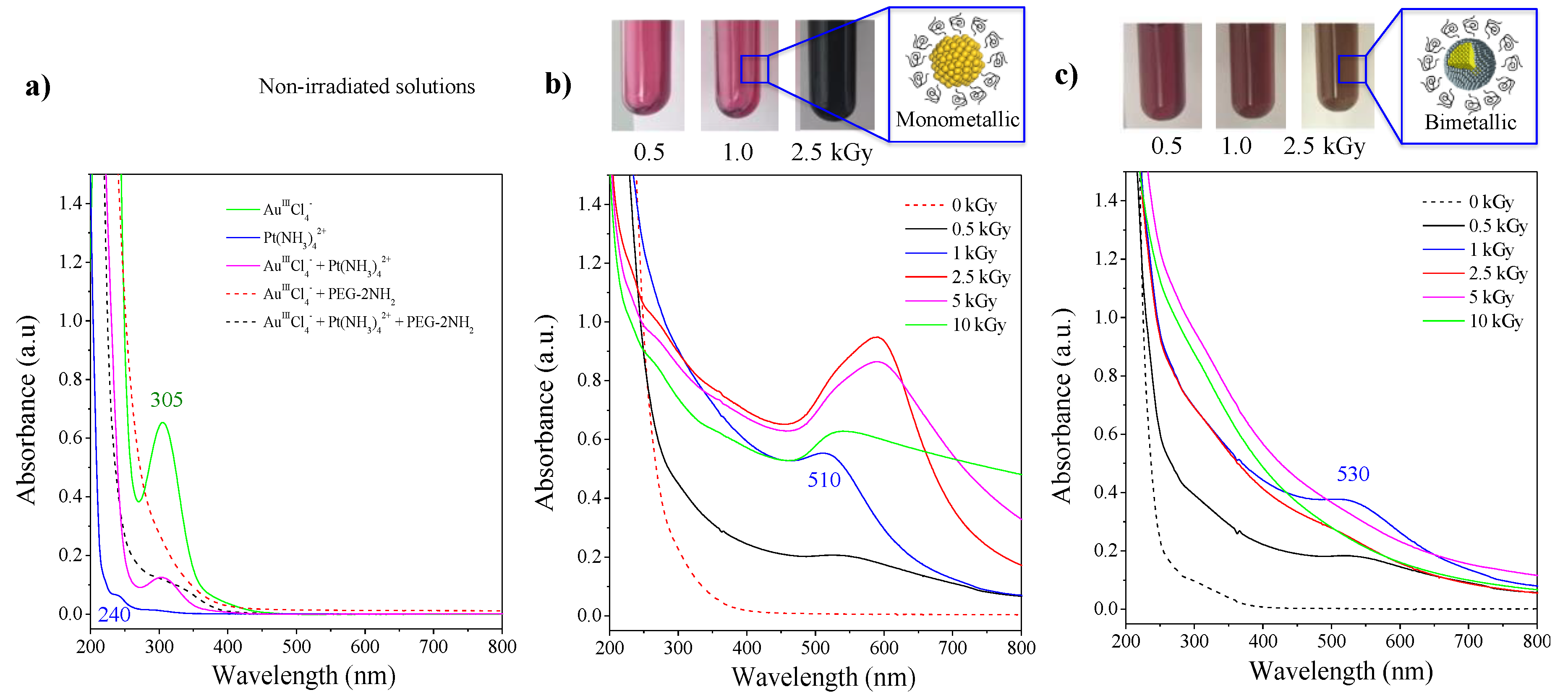
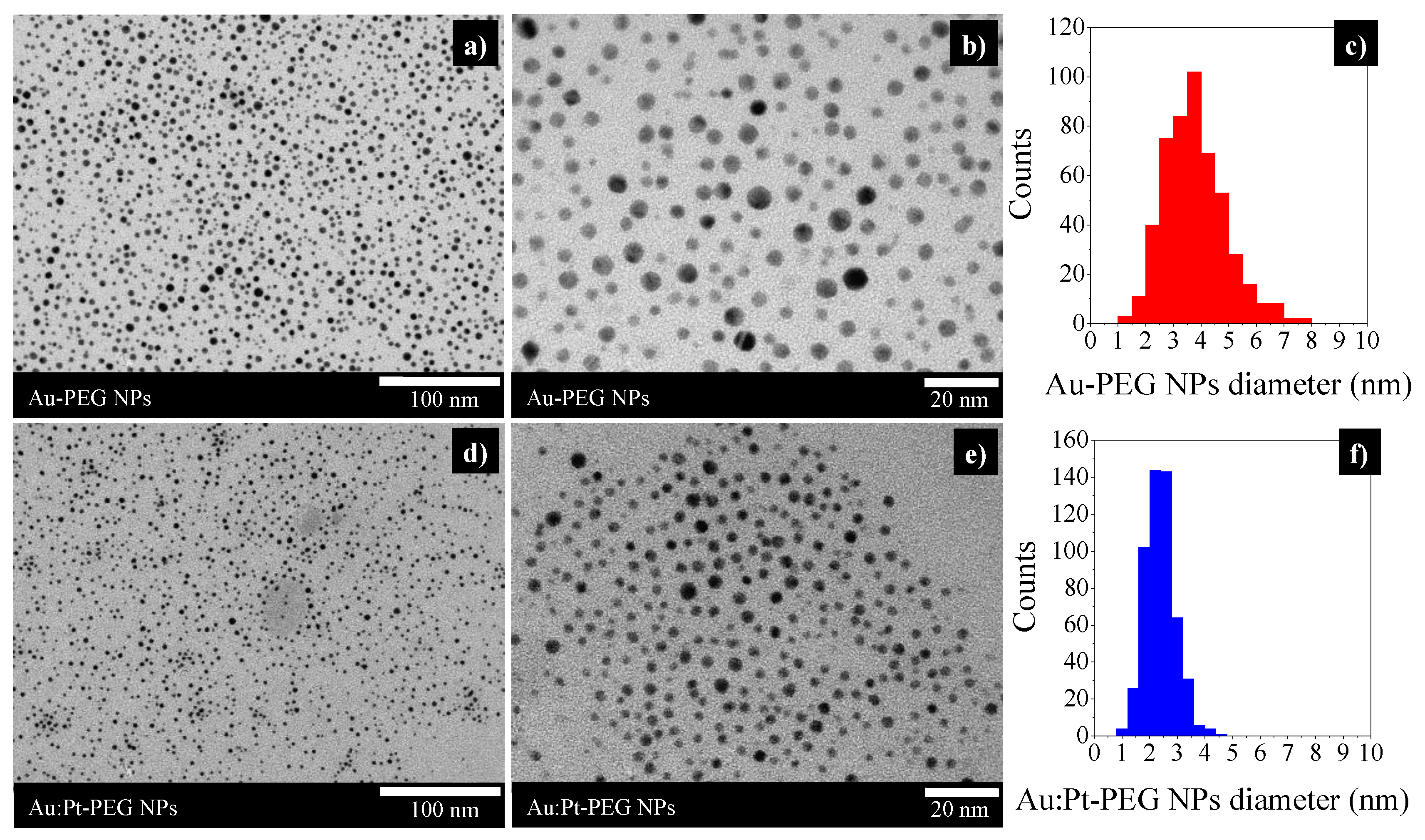
2.1. Characterization of Nanoparticles
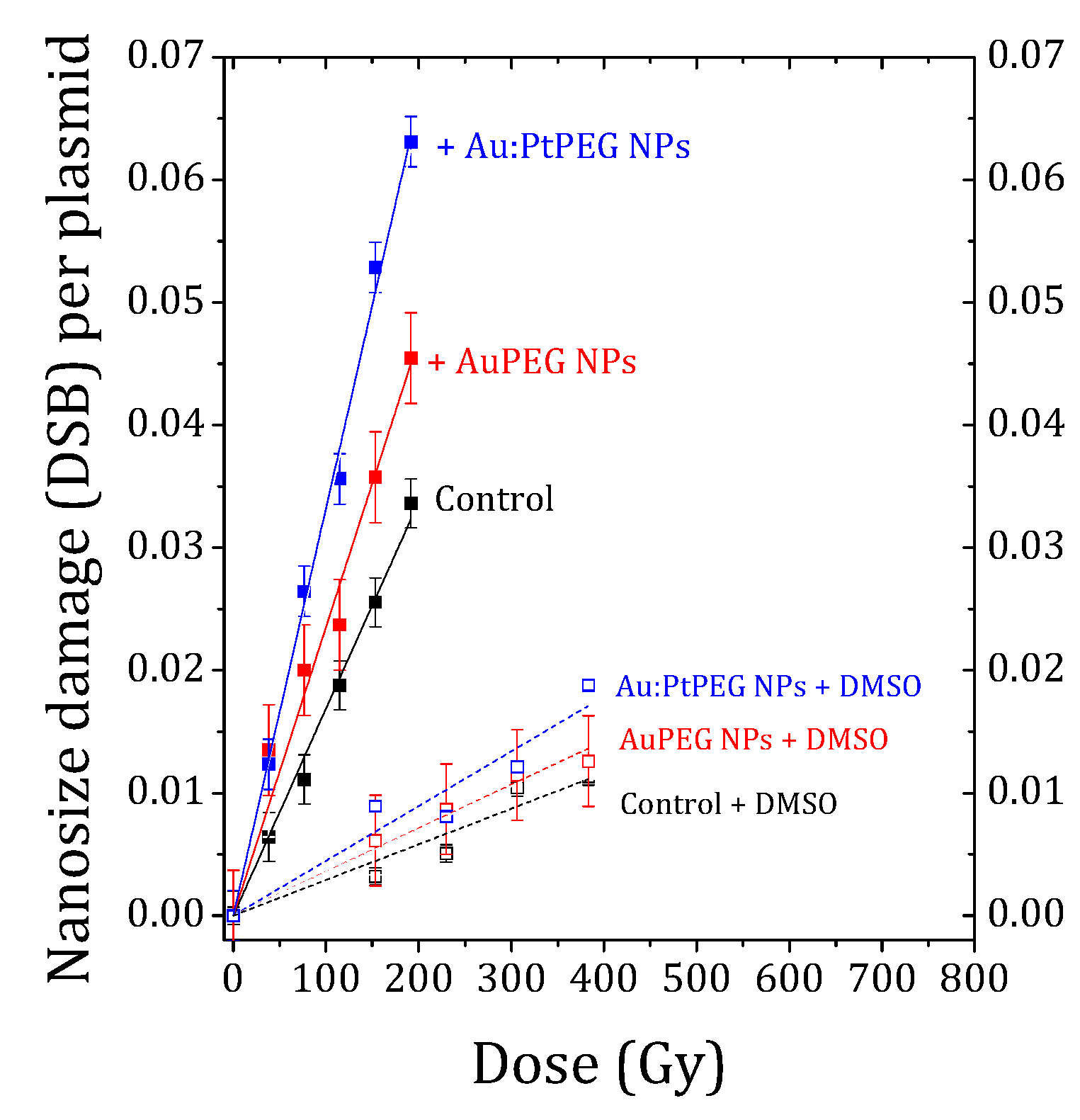
2.2. Effect of Nanoparticles on Complex Biodamage Induced by Radiation
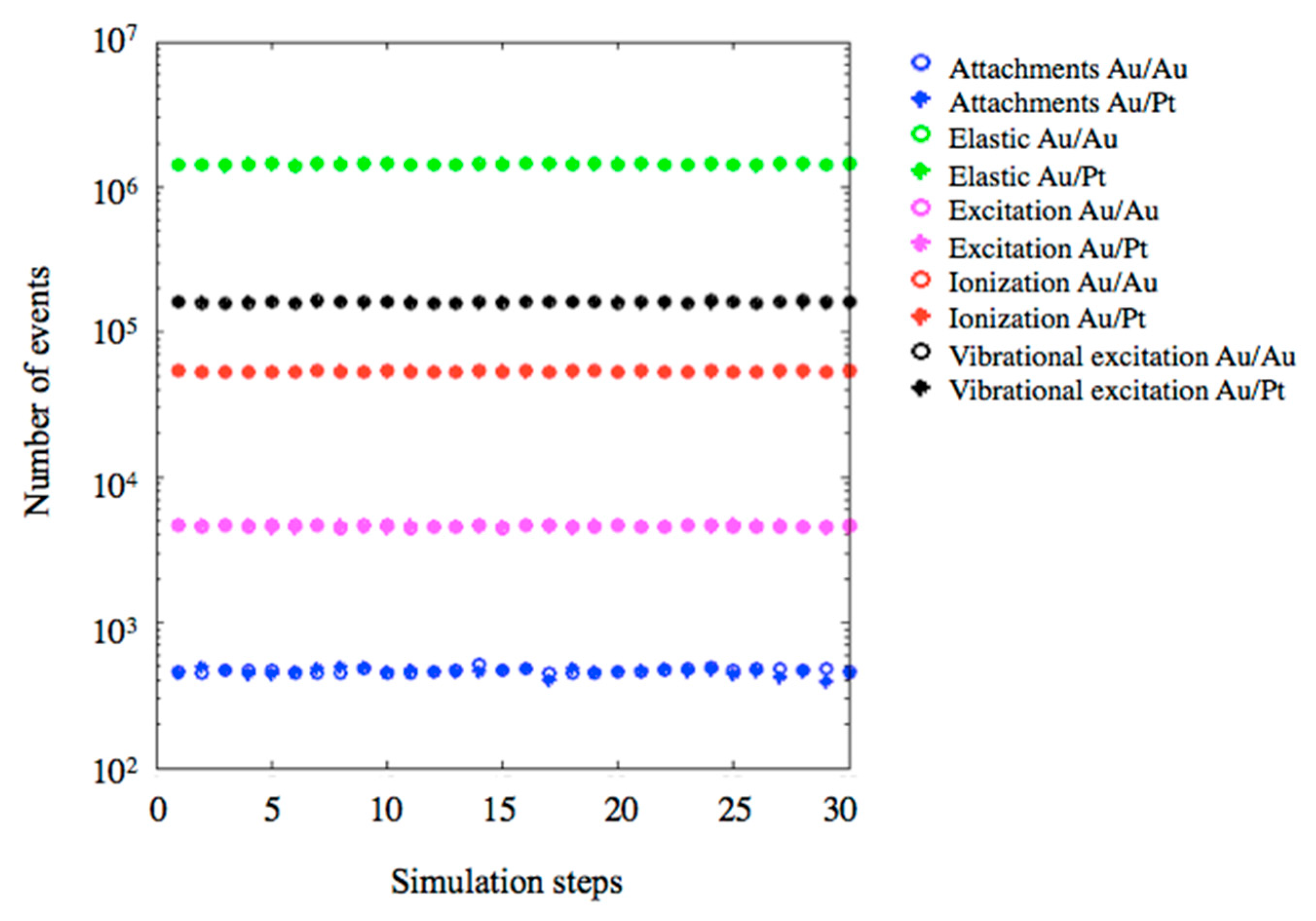
2.3. Monte Carlo Simulations
3. Discussion
4. Materials and Methods
4.1. Experimental Section
4.1.1. Materials
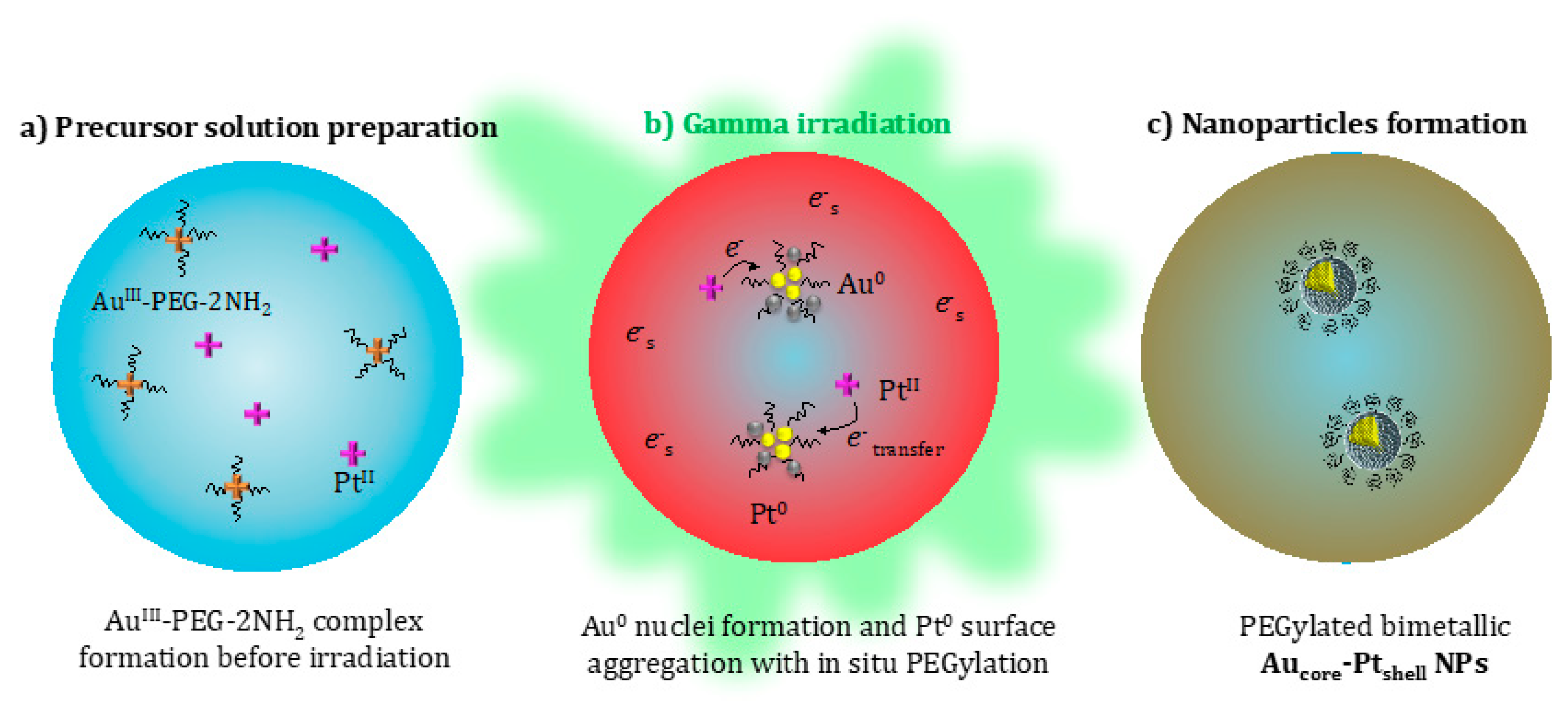
4.1.2. Radiolytic Synthesis
4.1.3. Physico-Chemical Characterization
4.1.4. Preparation and Analysis of the Biomolecular Samples
4.2. Theoretical Section
4.2.1. Calculation of the Platinum Shell Thickness
4.2.2. Monte Carlo Simulations
4.2.3. Geant4-Penelope
4.2.4. Geant4-DNA
5. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AFDSB | Amplification factor of radio-induced double-strand breaks (nanosize biodamage) |
| Au NPs | Gold nanoparticles |
| Au-PEG NPs | PEGylated gold nanoparticles |
| Au:Pt-PEG NPs | PEGylated bimetallic Aucore/Ptshell nanoparticles |
| BE | Binding energy |
| dH | Hydrodynamic diameter |
| DMSO | Dimethylsulfoxide |
| DSBs | Double-strand breaks |
| EDTA | Ethylenediaminetetraacetic acid |
| EPR | Enhanced permeability and retention effect |
| FWHM | Full width at half maximum |
| GAMOS | Geant4-based architecture for medicine oriented simulations tool |
| LEPTS | Low energy particle track structure code |
| LMCT | Ligand-to-metal charge transfer |
| mDSB | yields of double-strand breaks (nanosize biodamage) |
| PdI | Polidispersity index |
| PEG | Poly (ethylene glycol) |
| PEG-2NH2 | Homobifunctional poly (ethylene glycol) diamine |
| PENELOPE | Penetration and energy loss of positrons and electrons |
| SPR | Surface plasmon resonance |
| SSB | Single-strand breaks |
| TPS | Treatment planning system |
| XPS | X-ray photoelectron spectroscopy |
References
- Kuncic, Z.; Lacombe, S. Nanoparticle radio-enhancement: Principles, progress and application to cancer treatment. Phys. Med. Biol. 2018, 63, 02TR01. [Google Scholar] [CrossRef] [PubMed]
- Chou, L.Y.T.; Zagorovsky, K.; Chan, W.C.W. DNA assembly of nanoparticle superstructures for controlled biological delivery and elimination. Nat. Nanotechnol. 2014, 9, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Antosh, M.P.; Wijesinghe, D.D.; Shrestha, S.; Lanou, R.; Huang, Y.H.; Hasselbacher, T.; Fox, D.; Neretti, N.; Sun, S.; Katenka, N.; et al. Enhancement of radiation effect on cancer cells by gold-pHLIP. Proc. Natl. Acad. Sci. USA 2015, 112, 5372–5376. [Google Scholar] [CrossRef] [PubMed]
- Retif, P.; Pinel, S.; Toussaint, M.; Frochot, C.; Chouikrat, R.; Bastogne, T.; Barberi-Heyob, M. Nanoparticles for Radiation Therapy Enhancement: The Key Parameters. Theranostics 2015, 5, 1030–1044. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Hyland, W.B.; Muir, M.F.; Coulter, J.A.; Jain, S.; Butterworth, K.T.; Schettino, G.; Dickson, G.R.; Hounsell, A.R.; O’Sullivan, J.M.; et al. Biological consequences of nanoscale energy deposition near irradiated heavy atom nanoparticles. Sci. Rep. 2011, 1, 18. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Hirst, D.G.; O’Sullivan, J.M. Gold nanoparticles as novel agents for cancer therapy. Br. J. Radiol. 2012, 85, 101–113. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Focella, T.M.; Smilowitz, H.M. Gold nanoparticles: A new X-ray contrast agent. Br. J. Radiol. 2006, 79, 248–253. [Google Scholar] [CrossRef] [PubMed]
- Butterworth, K.T.; Nicol, J.R.; Ghita, M.; Rosa, S.; Chaudhary, P.; McGarry, C.K.; McCarthy, H.O.; Jimenez-Sanchez, G.; Bazzi, R.; Roux, S.; et al. Preclinical evaluation of gold-DTDTPA nanoparticles as theranostic agents in prostate cancer radiotherapy. Nanomedicine 2016, 11, 2035–2047. [Google Scholar] [CrossRef] [PubMed]
- Mieszawska, A.J.; Mulder, W.J.M.; Fayad, Z.A.; Cormode, D.P. Multifunctional Gold Nanoparticles for Diagnosis and Therapy of Disease. Mol. Pharm. 2013, 10, 831–847. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Slatkin, D.N.; Smilowitz, H.M. The use of gold nanoparticles to enhance radiotherapy in mice. Phys. Med. Biol. 2004, 49, N309–N315. [Google Scholar] [CrossRef] [PubMed]
- Hainfeld, J.F.; Dilmanian, F.A.; Slatkin, D.N.; Smilowitz, H.M. Radiotherapy enhancement with gold nanoparticles. J. Pharm. Pharmacol. 2008, 60, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Bonvalot, S.; Le Pechoux, C.; De Baere, T.; Kantor, G.; Buy, X.; Stoeckle, E.; Terrier, P.; Sargos, P.; Coindre, J.M.; Lassau, N.; et al. First-in-Human Study Testing a New Radioenhancer Using Nanoparticles (NBTXR3) Activated by Radiation Therapy in Patients with Locally Advanced Soft Tissue Sarcomas. Clin. Cancer Res. 2017, 23, 908–917. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Ma, H.; Liu, J.; Huo, S.; Kumar, A.; Wei, T.; Zhang, X.; Jin, S.; Gan, Y.; Wang, P.C.; et al. Size-dependent localization and penetration of ultrasmall gold nanoparticles in cancer cells, multicellular spheroids, and tumors in vivo. ACS Nano 2012, 6, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- McQuaid, H.N.; Muir, M.F.; Taggart, L.E.; McMahon, S.J.; Coulter, J.A.; Hyland, W.B.; Jain, S.; Butterworth, K.T.; Schettino, G.; Prise, K.M.; et al. Imaging and radiation effects of gold nanoparticles in tumour cells. Sci. Rep. 2016, 6, 19442. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.L.; Krishnan, S.; Cho, S.H. Estimation of microscopic dose enhancement factor around gold nanoparticles by Monte Carlo calculations. Med. Phys. 2010, 37, 3809–3816. [Google Scholar] [CrossRef] [PubMed]
- Lechtman, E.; Chattopadhyay, N.; Cai, Z.; Mashouf, S.; Reilly, R.; Pignol, J.P. Implications on clinical scenario of gold nanoparticle radiosensitization in regards to photon energy, nanoparticle size, concentration and location. Phys. Med. Biol. 2011, 56, 4631–4647. [Google Scholar] [CrossRef] [PubMed]
- Tomita, H.; Kai, M.; Kusama, T.; Ito, A. Monte Carlo simulation of DNA strand-break induction in supercoiled plasmid pBR322 DNA from indirect effects. Radiat. Environ. Biophys. 1998, 36, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Semenenko, V.A.; Stewart, R.D. Monte Carlo Simulation of Base and Nucleotide Excision Repair of Clustered DNA Damage Sites. II. Comparisons of Model Predictions to Measured Data. Radiat. Res. 2005, 164, 194–201. [Google Scholar] [CrossRef] [PubMed]
- Aydogan, B.; Bolch, W.E.; Swarts, S.G.; Turner, J.E.; Marshall, D.T. Monte Carlo Simulations of Site-Specific Radical Attack to DNA Bases. Radiat. Res. 2008, 169, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Francis, Z.; Incerti, S.; Capra, R.; Mascialino, B.; Montarou, G.; Stepan, V.; Villagrasa, C. Molecular scale track structure simulations in liquid water using the Geant4-DNA Monte-Carlo processes. Appl. Radiat. Isot. 2011, 69, 220–226. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Paganetti, H.; McMahon, S.J.; Schuemann, J. Gold nanoparticle induced vasculature damage in radiotherapy: Comparing protons, megavoltage photons, and kilovoltage photons. Med. Phys. 2015, 42, 5890–5902. [Google Scholar] [CrossRef] [PubMed]
- Sung, W.; Ye, S.-J.; McNamara, A.L.; McMahon, S.J.; Hainfeld, J.; Shin, J.; Smilowitz, H.M.; Paganetti, H.; Schuemann, J. Dependence of gold nanoparticle radiosensitization on cell geometry. Nanoscale 2017, 9, 5843–5853. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.J.; Paganetti, H.; Prise, K.M. Optimising element choice for nanoparticle radiosensitisers. Nanoscale 2016, 8, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Cao, L.; Wang, Z. Platinum-Coated Gold Nanoporous Film Surface: Electrodeposition and Enhanced Electrocatalytic Activity for Methanol Oxidation. Langmuir 2008, 24, 5932–5936. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, L.; Mott, D.; Njoki, P.N.; Lin, Y.; He, T.; Xu, Z.; Wanjana, B.N.; Lim, I.-I.S.; Zhong, C.-J. Core/Shell Nanoparticles as Electrocatalysts for Fuel Cell Reactions. Adv. Mater. 2008, 20, 4342–4347. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; He, W.; Huang, Q.; Yang, H. Influence of the composition of core-shell Au-Pt nanoparticle electrocatalysts for the oxygen reduction reaction. J. Colloid Interface Sci. 2010, 344, 132–136. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chemie Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Belloni, J.; Mostafavi, M.; Remita, H.; Marignier, J.; Delcourt, M.-O. Radiation-induced synthesis of mono- and multi-metallic clusters and nanocolloids. New J. Chem. 1998, 22, 1239–1255. [Google Scholar] [CrossRef]
- Remita, H.; Lampre, I.; Mostafavi, M.; Balanzat, E.; Bouffard, S. Comparative study of metal clusters induced in aqueous solutions by γ-rays, electron or C6+ ion beam irradiation. Radiat. Phys. Chem. 2005, 72, 575–586. [Google Scholar] [CrossRef]
- Arce, P.; Ignacio Lagares, J.; Harkness, L.; Pérez-Astudillo, D.; Cañadas, M.; Rato, P.; de Prado, M.; Abreu, Y.; de Lorenzo, G.; Kolstein, M.; et al. Gamos: A framework to do Geant4 simulations in different physics fields with an user-friendly interface. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrom. Detect. Assoc. Equip. 2014, 735, 304–313. [Google Scholar] [CrossRef]
- Delage, E.; Pham, Q.T.; Karamitros, M.; Payno, H.; Stepan, V.; Incerti, S.; Maigne, L.; Perrot, Y. PDB4DNA: Implementation of DNA geometry from the Protein Data Bank (PDB) description for Geant4-DNA Monte-Carlo simulations. Comput. Phys. Commun. 2015, 192, 282–288. [Google Scholar] [CrossRef]
- Remita, S.; Picq, G.; Khatouri, J.; Mostafavi, M. Radiolytic formation of bilayered Ptcore/Aushell and Aucore/Ptshell clusters in aqueous solution. Radiat. Phys. Chem. 1999, 54, 463–473. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Yamahira, A.; Torigoe, K. Role of Poly(amidoamine) Dendrimers for Preparing Nanoparticles of Gold, Platinum, and Silver. Langmuir 2000, 16, 2604–2608. [Google Scholar] [CrossRef]
- Gharibshahi, E.; Saion, E. Influence of Dose on Particle Size and Optical Properties of Colloidal Platinum Nanoparticles. Int. J. Mol. Sci. 2012, 13, 14723–14741. [Google Scholar] [CrossRef] [PubMed]
- Polte, J.; Ahner, T.T.; Delissen, F.; Sokolov, S.; Emmerling, F.; Thünemann, A.F.; Kraehnert, R. Mechanism of Gold Nanoparticle Formation in the Classical Citrate Synthesis Method Derived from Coupled In Situ XANES and SAXS Evaluation. J. Am. Chem. Soc. 2010, 132, 1296–1301. [Google Scholar] [CrossRef] [PubMed]
- Kim, F.; Song, J.H.; Yang, P. Photochemical Synthesis of Gold Nanorods. J. Am. Chem. Soc. 2002, 124, 14316–14317. [Google Scholar] [CrossRef] [PubMed]
- Henglein, A.; Ershov, B.G.; Malow, M. Absorption Spectrum and Some Chemical Reactions of Colloidal Platinum in Aqueous Solution. J. Phys. Chem. 1995, 99, 14129–14136. [Google Scholar] [CrossRef]
- Abidi, W.; Remita, H. Gold based Nanoparticles Generated by Radiolytic and Photolytic Methods. Recent Patents Eng. 2010, 4, 170–188. [Google Scholar] [CrossRef]
- Mirdamadi-Esfahani, M.; Mostafavi, M.; Keita, B.; Nadjo, L.; Kooyman, P.; Remita, H. Bimetallic Au-Pt nanoparticles synthesized by radiolysis: Application in electro-catalysis. Gold Bull. 2010, 43, 49–56. [Google Scholar] [CrossRef]
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Chastain, J., Ed.; Perkin-Elmer Corporation: Waltham, MA, USA, 1992. [Google Scholar]
- Citrin, P.H.; Wertheim, G.K.; Baer, Y. Surface-atom x-ray photoemission from clean metals: Cu, Ag, and Au. Phys. Rev. B 1983, 27, 3160–3175. [Google Scholar] [CrossRef]
- Hong, R.; Fischer, N.O.; Emrick, T.; Rotello, V.M. Surface PEGylation and Ligand Exchange Chemistry of FePt Nanoparticles for Biological Applications. Chem. Mater. 2005, 17, 4617–4621. [Google Scholar] [CrossRef]
- Crispin, X.; Lazzaroni, R.; Crispin, A.; Geskin, V.; Brédas, J.; Salaneck, W. Understanding the initial stages of polymer grafting on metals: A photoelectron spectroscopy study of acrylonitrile adsorption on transition metal surfaces. J. Electron Spectros. Relat. Phenomena 2001, 121, 57–74. [Google Scholar] [CrossRef]
- Kumar, R.; Ghosh, A.; Patra, C.R.; Mukherjee, P.; Sastry, M. Gold nanoparticles formed within ordered mesoporous silica and on amorphous silica. In Nanotechnology in Catalysis; Zhou, B., Hermans, S., Somorjai, G.A., Eds.; Kluwer Academic/Plenum Publishers: New York, NY, USA, 2004; pp. 111–136. [Google Scholar]
- Li, S.; Dong, Y.; Bi, X.; Tang, M. Synthesis of Au @ Pt Core-Shell Nanoparticles with High Electro-catalytic Activity of Methanol Oxidation by Photochemical Seeding Growth. Int. J. Electrochem. Sci. 2013, 8, 8662–8668. [Google Scholar]
- Ilayaraja, N.; Prabu, N.; Lakshminarasimhan, N.; Murugan, P.; Jeyakumar, D. Au–Pt graded nano-alloy formation and its manifestation in small organics oxidation reaction. J. Mater. Chem. A 2013, 1, 4048–4056. [Google Scholar] [CrossRef]
- Tan, C.; Sun, Y.; Zheng, J.; Wang, D.; Li, Z.; Zeng, H.; Guo, J.; Jing, L.; Jiang, L. A self-supporting bimetallic Au@Pt core-shell nanoparticle electrocatalyst for the synergistic enhancement of methanol oxidation. Sci. Rep. 2017, 7, 6347. [Google Scholar] [CrossRef] [PubMed]
- Acharya, S.; Sahoo, S.K. PLGA nanoparticles containing various anticancer agents and tumour delivery by EPR effect. Adv. Drug Deliv. Rev. 2011, 63, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Klochkov, S.G.; Neganova, M.E.; Nikolenko, V.N.; Chen, K.; Somasundaram, S.G.; Kirkland, C.E.; Aliev, G. Implications of nanotechnology for the treatment of cancer: Recent advances. Semin. Cancer Biol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Porcel, E.; Liehn, S.; Remita, H.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Le Sech, C.; Lacombe, S. Platinum nanoparticles: A promising material for future cancer therapy? Nanotechnology 2010, 21, 85103. [Google Scholar] [CrossRef] [PubMed]
- Porcel, E.; Tillement, O.; Lux, F.; Mowat, P.; Usami, N.; Kobayashi, K.; Furusawa, Y.; Le Sech, C.; Li, S.; Lacombe, S. Gadolinium-based nanoparticles to improve the hadrontherapy performances. Nanomedicine 2014, 10, 1601–1608. [Google Scholar] [CrossRef] [PubMed]
- Schlatholter, T.; Eustache, P.; Porcel, E.; Salado, D.; Stefancikova, L.; Tillement, O.; Lux, F.; Mowat, P.; van Goethem, M.-J.; Remita, H.; et al. Improving proton therapy by metal-containing nanoparticles: Nanoscale insights. Int. J. Nanomed. 2016, 11, 1549. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.A.; Bordage, M.C.; Brown, J.M.C.; Davídková, M.; Delage, E.; El Bitar, Z.; Enger, S.A.; Francis, Z.; Guatelli, S.; Ivanchenko, V.N.; et al. Track structure modeling in liquid water: A review of the Geant4-DNA very low energy extension of the Geant4 Monte Carlo simulation toolkit. Phys. Medica 2015, 31, 861–874. [Google Scholar] [CrossRef] [PubMed]
- Bernal, M.A.; deAlmeida, C.E.; Sampaio, C.; Incerti, S.; Champion, C.; Nieminen, P. The invariance of the total direct DNA strand break yield. Med. Phys. 2011, 38, 4147–4153. [Google Scholar] [CrossRef] [PubMed]
- Klimczak, U.; Ludwig, D.C.; Mark, F.; Rettberg, P.; Schulte-Frohlinde, D. Irradiation of Plasmid and Phage DNA in Water—alcohol Mixtures: Strand Breaks and Lethal Damage as a Function of Scavenger Concentration. Int. J. Radiat. Biol. 1993, 64, 497–510. [Google Scholar] [CrossRef] [PubMed]
- Remita, H.; Remita, S. Metal Clusters and Nanomaterials: Contribution of Radiation Chemistry. In Recent Trends in Radiation Chemistry; Wishart, J.F., Madhava Rao, B.S., Eds.; World Scientific: Singapore, 2010; pp. 347–383. ISBN 978-981-4467-05-6. [Google Scholar]
- Spotheim-Maurizot, M.; Charlier, M.; Sabattier, R. DNA Radiolysis by Fast Neutrons. Int. J. Radiat. Biol. 1990, 57, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Royce, D.G.; Fadley, C.S.; Wagner, L.F.; Grunthaner, F.J. Properties of oxidized silicon as determined by angular-dependent X-ray photoelectron spectroscopy. Chem. Phys. Lett. 1976, 44, 225–231. [Google Scholar] [CrossRef]
- Powell, C.J.; Jablonski, A. Progress in quantitative surface analysis by X-ray photoelectron spectroscopy: Current status and perspectives. J. Electron Spectros. Relat. Phenomena 2010, 178–179, 331–346. [Google Scholar] [CrossRef]
- Shard, A.G. A Straightforward Method For Interpreting XPS Data From Core–Shell Nanoparticles. J. Phys. Chem. C 2012, 116, 16806–16813. [Google Scholar] [CrossRef]
- Muñoz, A.; Fuss, M.C.; Cortés-Giraldo, M.A.; Incerti, S.; Ivanchenko, V.; Ivanchenko, A.; Quesada, J.M.; Salvat, F.; Champion, C.; Gómez-Tejedor, G.G. Monte Carlo Methods to Model Radiation Interactions and Induced Damage. In Radiation Damage in Biomolecular Systems; García Gómez-Tejedor, G., Fuss, M., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 203–225. ISBN 978-94-007-2564-5. [Google Scholar]
- Lin, Y.; McMahon, S.J.; Scarpelli, M.; Paganetti, H.; Schuemann, J. Comparing gold nano-particle enhanced radiotherapy with protons, megavoltage photons and kilovoltage photons: A Monte Carlo simulation. Phys. Med. Biol. 2014, 59, 7675–7689. [Google Scholar] [CrossRef] [PubMed]
- Sanche, L. Nanoscopic aspects of radiobiological damage: Fragmentation induced by secondary low-energy electrons. Mass Spectrom. Rev. 2002, 21, 349–369. [Google Scholar] [CrossRef] [PubMed]
- Sanche, L. Low energy electron-driven damage in biomolecules. Eur. Phys. J. D 2005, 35, 367–390. [Google Scholar] [CrossRef]
- Bernal, M.A.; Liendo, J.A. An investigation on the capabilities of the PENELOPE MC code in nanodosimetry. Med. Phys. 2009, 36, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Pandola, L. Penelope Low-Energy Electromagnetic Models. Available online: https://twiki.cern.ch/twiki/bin/genpdf/Geant4/LowePenelope (accessed on 17 July 2019).
- Jelsch, C.; Mourey, L.; Masson, J.-M.; Samama, J.-P. Crystal structure of Escherichia coli TEM1 β-lactamase at 1.8 Å resolution. Proteins Struct. Funct. Genet. 1993, 16, 364–383. [Google Scholar] [CrossRef] [PubMed]
- Henson, R.; Cetto, L. The MATLAB bioinformatics toolbox. In Encyclopedia of Genetics, Genomics, Proteomics and Bioinformatics; John Wiley & Sons, Ltd.: Chichester, UK, 2005. [Google Scholar]
- Incerti, S.; Douglass, M.; Penfold, S.; Guatelli, S.; Bezak, E. Review of Geant4-DNA applications for micro and nanoscale simulations. Phys. Medica 2016, 32, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Melton, C.E. Cross Sections and Interpretation of Dissociative Attachment Reactions Producing OH−, O− and H− in H2O. J. Chem. Phys. 1972, 57, 4218–4225. [Google Scholar] [CrossRef]
- Michaud, M.; Sanche, L. Absolute vibrational excitation cross sections for slow-electron (1–18 eV) scattering in solid H2O. Phys. Rev. A 1987, 36, 4684–4699. [Google Scholar] [CrossRef] [PubMed]
- Emfietzoglou, D.; Kyriakou, I.; Garcia-Molina, R.; Abril, I.; Nikjoo, H. Inelastic Cross Sections for Low-Energy Electrons in Liquid Water: Exchange and Correlation Effects. Radiat. Res. 2013, 180, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Kyriakou, I.; Incerti, S.; Francis, Z. Technical Note: Improvements in geant 4 energy-loss model and the effect on low-energy electron transport in liquid water. Med. Phys. 2015, 42, 3870–3876. [Google Scholar] [CrossRef] [PubMed]
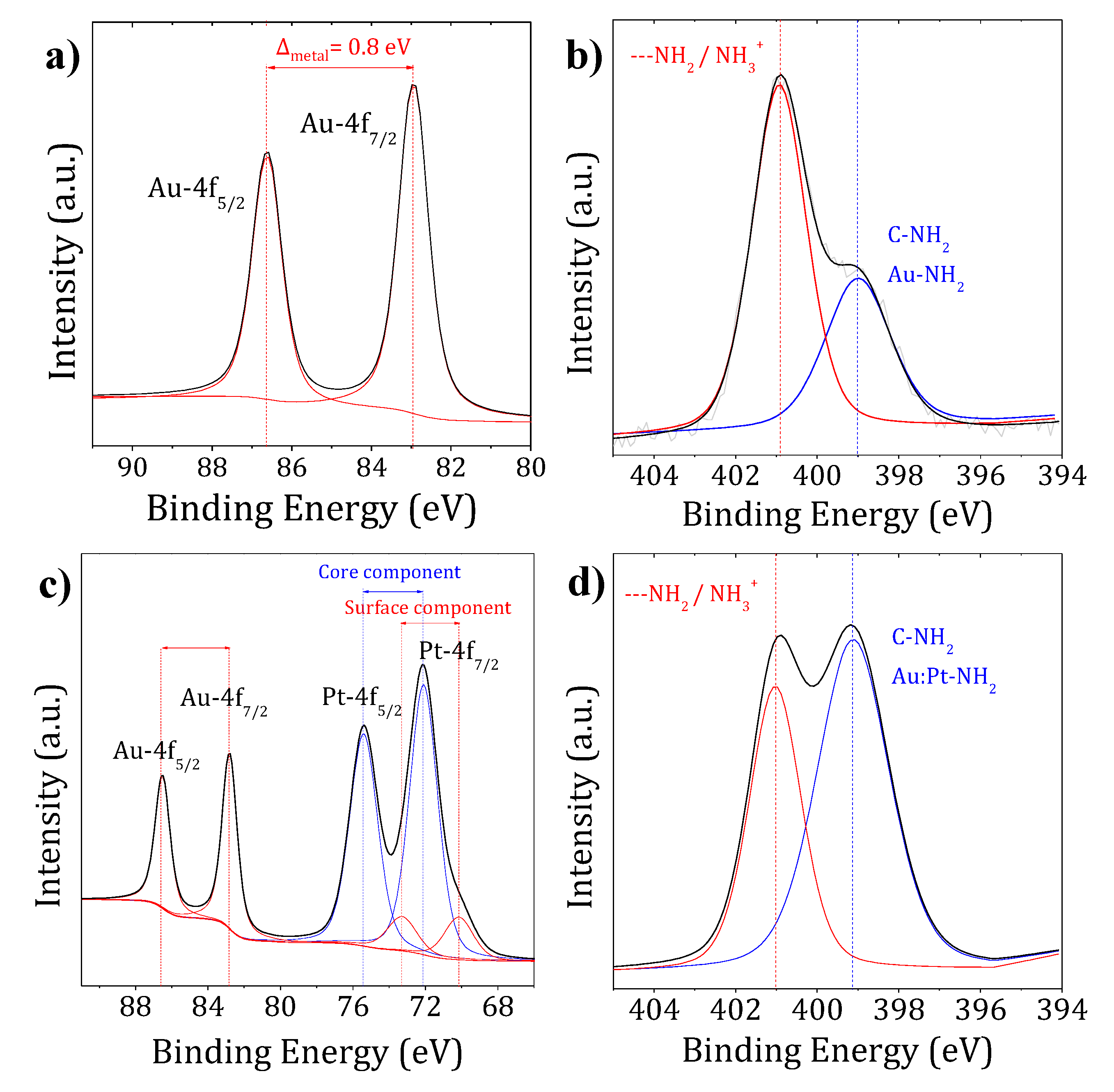

| Compound | Atomic Core Level | BE (eV) | FWHM (eV) | Assignment |
|---|---|---|---|---|
| * | Au-4f7/2 | 87.8 | 1.3 | AuIII |
| Au-4f5/2 | 91.5 | 1.3 | AuIII | |
| Pt-4f7/2 | 73.0 | 1.7 | PtII | |
| Pt-4f5/2 | 76.3 | 1.7 | PtII | |
| N-1s | 400.2 | 1.6 | Pt-NH3+ | |
| Au0 solid | Au-4f7/2 | 84.1 | 0.9 | Au0 |
| Au-4f5/2 | 87.8 | 0.9 | Au0 | |
| Pt0 solid | Pt-4f7/2 | 71.1 | 1.2 | Pt0 |
| Pt-4f5/2 | 74.5 | 1.2 | Pt0 | |
| PEG-2NH2 | C-1s | 285.9 | 1.3 | C–O and C–N |
| O-1s | 532.4 | 1.4 | C–O–H | |
| C–O–C | ||||
| N-1s | 398.8 | 1.4 | C–N | |
| N-1s | 399.8 | 1.4 | C-NH2 | |
| Au-PEG NPs | Au-4f7/2 | 82.9 | 0.8 | Au0 |
| Au-4f5/2 | 86.6 | 0.8 | Au0 | |
| C-1s | 285.9 | 1.2 | C–O and C–N | |
| C-1s | 284.4 | 1.0 | C–C | |
| O-1s | 532.2 | 1.3 | C–O–H | |
| C–O–C | ||||
| N-1s | 399.0 | 1.9 | C-NH2 | |
| Au-NH2 | ||||
| N-1s | 400.9 | 1.6 | ---NH2 | |
| Au:Pt-PEG NPs | Au-4f7/2 | 82.8 | 1.0 | Au0 |
| Au-4f5/2 | 86.5 | 1.0 | Au0 | |
| Pt-4f7/2 surf. | 70.2 | 1.9 | Pt0 | |
| Pt-4f5/2 surf. | 73.5 | 1.9 | Pt0 | |
| Pt-4f7/2 core | 72.1 | 1.8 | Pt–N | |
| Pt-4f5/2 core | 75.4 | 1.8 | Pt–N | |
| C-1s | 285.9 | 1.2 | C–H and C–N | |
| C-1s | 284.3 | 0.9 | C–C | |
| O-1s | 532.2 | 1.3 | C–O–C | |
| N-1s | 399.1 | 2.1 | C-NH2 | |
| Au:Pt-NH2 | ||||
| N-1s | 401.0 | 1.5 | ---NH2/NH3+ |
| Sample | mDSB (×10−5) (Breaks Per Plasmid Per Gy) | AFDSB (%) | OH Effect (%) |
|---|---|---|---|
| Control | 17.47 ± 0.51 | - | - |
| Au-PEG NPs | 23.49±0.94 | 34 ± 2 | - |
| Au:Pt-PEG NPs | 33.18 ± 0.54 | 90 ± 2 | - |
| Control + DMSO | 2.91 ±0.22 | - | 83 ± 1 |
| Au-PEG NPs + DMSO | 3.56 ± 0.13 | 22 ± 4 | 85 ± 1 |
| Au:Pt-PEGNPs + DMSO | 4.14 ± 0.36 | 42 ± 2 | 86 ± 1 |
| Section | Dose (Gy) | |
|---|---|---|
| Monometallic Aucore/Aushell | Bimetallic Aucore/Ptshell | |
| Water sphere | 47.8 | 47.9 |
| Shell | 143.2 | 143.9 |
| Core | 375.8 | 383.4 |
| Physical Process | Monometallic Aucore/Aushell | Bimetallic Aucore/Ptshell |
|---|---|---|
| Electronic ionization from electrons | 191 | 204 |
| Direct electron impact ionization from electrons | 50 | 59 |
| Multi Coulomb scattering from electrons | 4,874,768 | 5,008,513 |
| Compton scattering from photons | 35 | 36 |
| Photoelectric effect from photons | 9 | 7 |
| Total | 4,875,053 | 5,008,819 |
| System | Energy Deposition (MeV) | DSBs (Gy−1.Gbp−1) | AF (%) |
|---|---|---|---|
| Beta lactamase | 1.075 | 9.32 ± 0.05 | - |
| +Au NPs | 0.913 | 13.10 ± 0.03 | 40 ± 1 |
| +Au:Pt NPs | 0.916 | 13.53 ± 0.02 | 45 ± 1 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salado-Leza, D.; Traore, A.; Porcel, E.; Dragoe, D.; Muñoz, A.; Remita, H.; García, G.; Lacombe, S. Radio-Enhancing Properties of Bimetallic Au:Pt Nanoparticles: Experimental and Theoretical Evidence. Int. J. Mol. Sci. 2019, 20, 5648. https://doi.org/10.3390/ijms20225648
Salado-Leza D, Traore A, Porcel E, Dragoe D, Muñoz A, Remita H, García G, Lacombe S. Radio-Enhancing Properties of Bimetallic Au:Pt Nanoparticles: Experimental and Theoretical Evidence. International Journal of Molecular Sciences. 2019; 20(22):5648. https://doi.org/10.3390/ijms20225648
Chicago/Turabian StyleSalado-Leza, Daniela, Ali Traore, Erika Porcel, Diana Dragoe, Antonio Muñoz, Hynd Remita, Gustavo García, and Sandrine Lacombe. 2019. "Radio-Enhancing Properties of Bimetallic Au:Pt Nanoparticles: Experimental and Theoretical Evidence" International Journal of Molecular Sciences 20, no. 22: 5648. https://doi.org/10.3390/ijms20225648
APA StyleSalado-Leza, D., Traore, A., Porcel, E., Dragoe, D., Muñoz, A., Remita, H., García, G., & Lacombe, S. (2019). Radio-Enhancing Properties of Bimetallic Au:Pt Nanoparticles: Experimental and Theoretical Evidence. International Journal of Molecular Sciences, 20(22), 5648. https://doi.org/10.3390/ijms20225648







