Development of an Efficient Protocol to Obtain Transgenic Coffee, Coffea arabica L., Expressing the Cry10Aa Toxin of Bacillus thuringiensis
Abstract
1. Introduction
2. Results
2.1. Cry10aa Codon Optimization for Coffee C. arabica Genetic Transformation
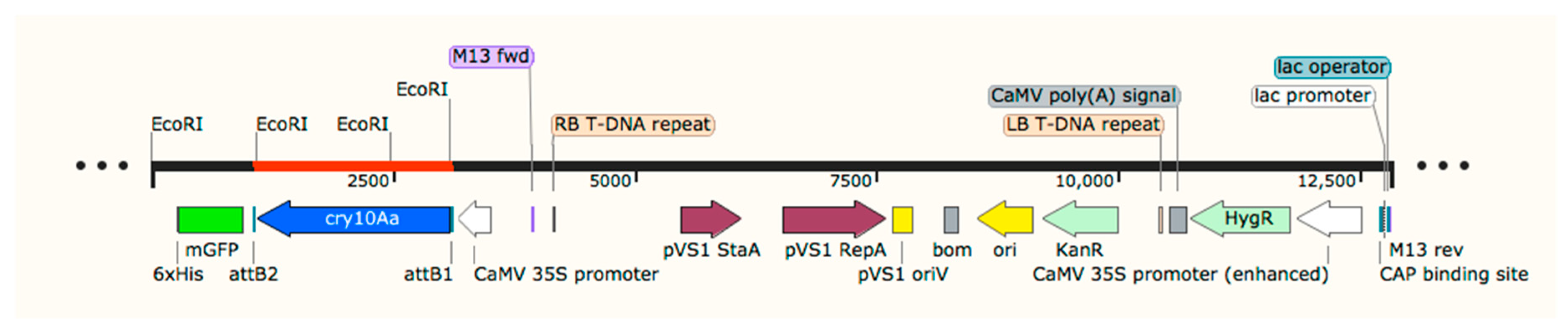
2.2. Generation of Plasmid pMDC85/Cry10Aa
2.3. Induction of Somatic Embryogenesis
2.4. Somatic Embryo Maturation
2.5. Selection of Transgenic Clones
2.6. Regeneration of Transgenic Plants
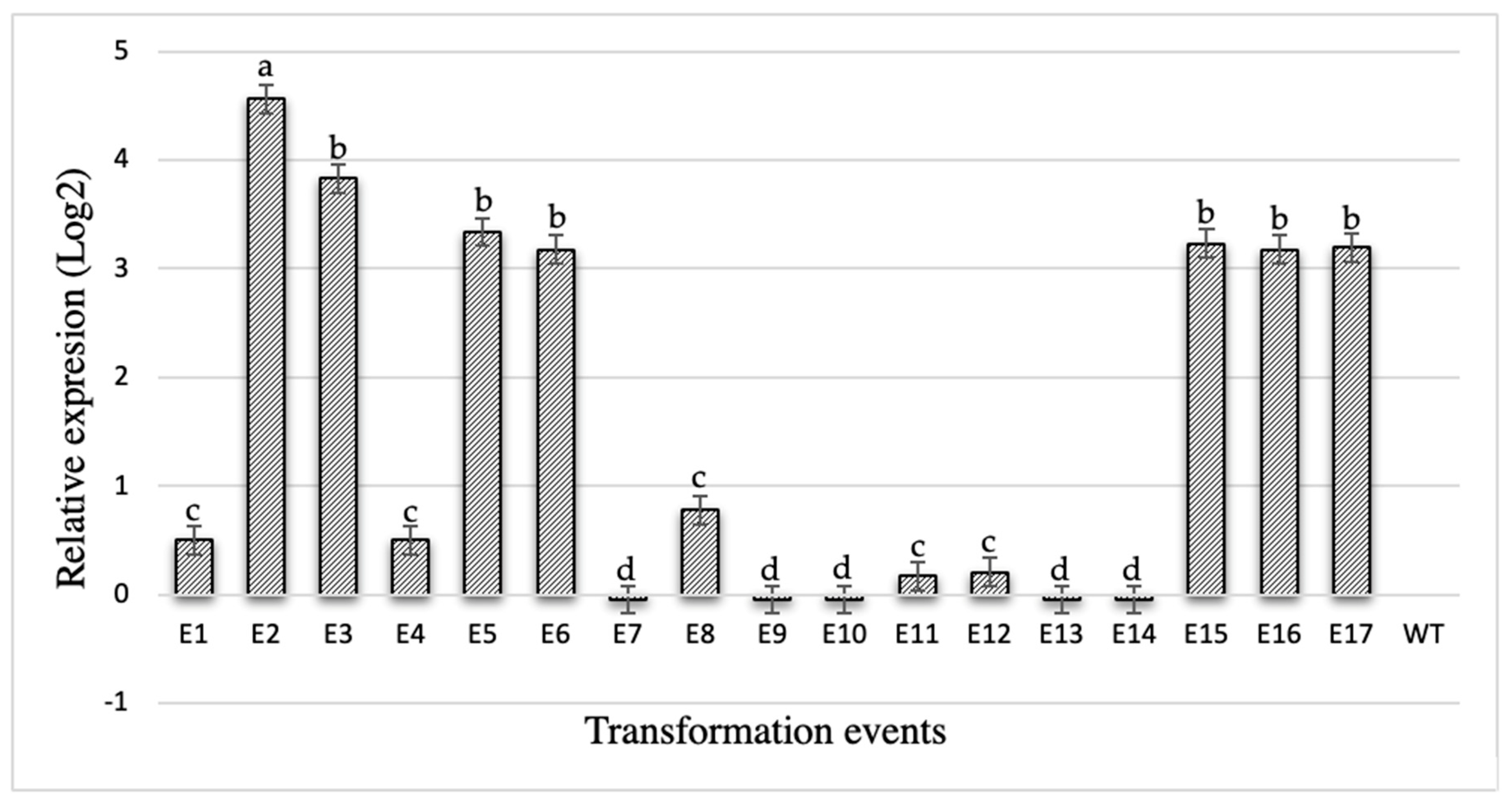
2.7. PCR and qPCR Analysis of Cry10Aa
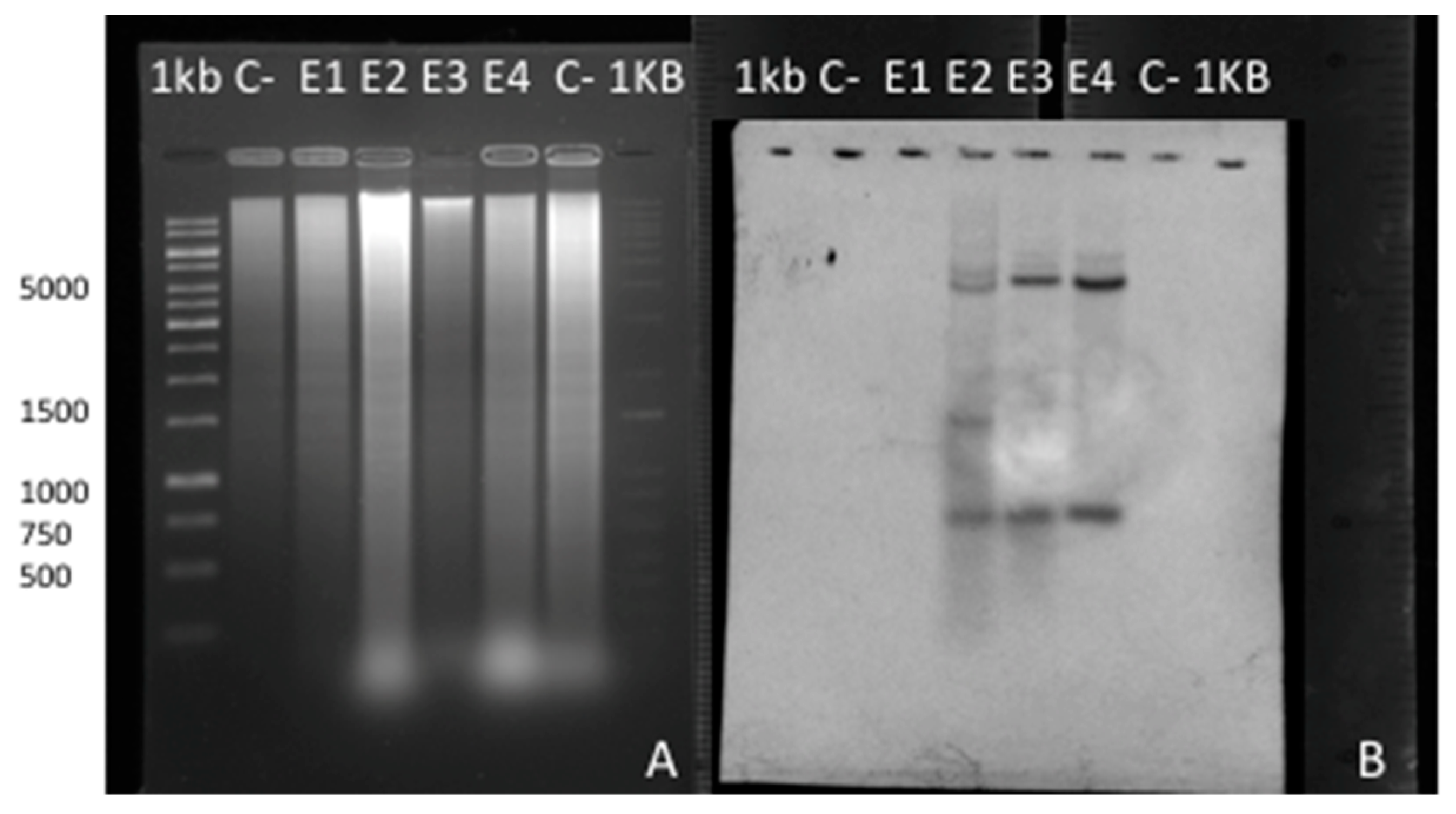
2.8. Southern Blot and Hybridization Analysis
2.9. Immunodetection of the Cry10Aa Protein
3. Discussion
4. Materials and Methods
4.1. Cry10aa Gene Codon Optimization for C. arabica
4.2. Cry10Aa Expression Vector
4.3. Induction of Somatic Embryogenesis
4.4. Somatic Embryo Maturation of C. arabica
4.5. Isolation of RNA and qPCR Analysis
4.6. Preparation of the DNA Plasmid Construct (pMDC85/Cry10Aa)
4.7. Osmotic Treatment
4.8. Particle Bombardment
4.9. Selection of Transgenic Embryogenic Lines
4.10. Transgenic Somatic Embryo Maturation
4.11. Regeneration of Transgenic Plants
4.12. Analysis of GFP Expression
4.13. Southern Blot Analysis
4.14. Immunodetection of the Cry10Aa Protein
4.15. Mass-Spectrometry Analysis of the Cry10Aa Protein
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| CBB | Coffee Berry Borer |
| SE | Somatic Embryos |
| SEG | Somatic Embryogenesis |
| ABA | Abscisic acid |
| CBW | Cotton Boll Weevil |
References
- International Coffee Organization (ICO, 2018). Available online: http://www.coffeeclubnetwork.com/redes/form/post?pub_id=2533 (accessed on 26 March 2018).
- Vega, F.E.; Rosenquist, E.; Collins, W. Global project needed to tackle coffee crisis. Nature 2003, 425, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Damon, A. A review of the biology and control of the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae). Bull. Entomol. Res. 2000, 90, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Baker, P.S.; Jackson, J.A.F.; Murphy, S.T. Natural Enemies, Natural Allies; Project completion report of the integrated management of coffee berry borer project, CFC/ICO/02 (1998–2002); CABI Commodities Press: Egham, UK, 2002. [Google Scholar]
- Jaramillo, J.; Borgemeister, C.; Baker, P. Coffee berry borer Hypothenemus hampei (Coleoptera: Curculionidae): Searching for sustainable control strategies. Bull. Entomol. Res. 2006, 96, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Brun, L.O.; Marcillaud, C.; Gaudichon, V.; Suckling, D.M. Endosulfan resistance in Hypothenemus hampei (Coleoptera: Scolytidae) in New Caledonia. J. Econ. Entomol. 1989, 82, 1311–1316. [Google Scholar] [CrossRef]
- Murphy, S.T.; Moore, D. Biological control of the coffee berry borer, Hypothenemus hampei (Ferrari) (Coleoptera, Scolytidae): Previous programmed and possibilities for the future. Biocontrol News Inf. 1990, 11, 107–117. [Google Scholar]
- Brun, L.O.; Stuart, J.; Gaudichon, V.; Aronstein, K.; French-Constant, R.H. Functional haplodiploidy: A mechanism for the spread of insecticide resistance in an important international insect pest. Proc. Natl. Acad. Sci. USA 1995, 92, 9861–9865. [Google Scholar] [CrossRef]
- Gingerich, D.P.; Borsa, P.; Suckling, D.M.; Brun, L.O. Inbreeding in the coffee berry borer, Hypothenemus hampei (Coleoptera: Scolytidae) estimated from endosulfan resistance phenotype frequencies. Bull. Entomol. Res. 1996, 186, 667–674. [Google Scholar] [CrossRef]
- Bustillo, A.E.; Cárdenas, R.; Villalba, D.A.; Benavides, P.; Orozco, J.; Posada, F.J. Manejo integrado de la broca del café: Hypothenemus hampei Ferrari en Colombia; Centro Nacional de Investigaciones de Café: Caldas, Colombia, 1998. [Google Scholar]
- Heimpel, A.M.; Angus, T.A. Bacterial insecticides. Bacteriol. Rev. 1960, 24, 266–288. [Google Scholar]
- Crickmore, N.; Zeigler, D.R.; Feitelson, J.; Schnepf, E.; Van Rie, J.; Lereclus, D.; Baum, J.; Dean, D.H. Revision of the nomenclature for the Bacillus thuringiensis pesticidal crystal proteins. Microbiol. Mol. Biol. Rev. 1998, 62, 807–813. [Google Scholar]
- Fischhoff, D.A.; Bowdish, K.S.; Perlak, F.J.; Marrone, P.G.; McCormick, S.M.; Niedermeyer, J.G.; Dean, D.A.; Kusano-Kretzmer, K.; Mayer, E.J.; Rochester, D.E.; et al. Insect tolerant transgenic tomato plants. Biotechnology 1987, 5, 807–813. [Google Scholar] [CrossRef]
- Vaeck, M.; Reynaerts, A.; Höfte, H.; Jansens, S.; De Beuckeleer, M.; Dean, C.; Zabeau, M.; Van Montagu, M.; Leemans, J. Transgenic plants protected from insect attack. Nature 1987, 328, 33–37. [Google Scholar] [CrossRef]
- Perlak, F.J.; Deaton, R.W.; Armstrong, T.A.; Fuchs, R.L.; Sims, S.R.; Greenplate, J.T.; Fischhoff, D.A. Insect resistant cotton plants. Biotechnology 1990, 8, 939–943. [Google Scholar] [CrossRef] [PubMed]
- Leroy, T.; Henry, A.M.; Royer, M.; Altosaar, I.; Frutos, R.; Duris, D.; Philippe, R. Genetically modified coffee plants expressing the Bacillus thuringiensis cry1Ac gene for resistance to leaf miner. Plant Cell Rep. 2000, 19, 382–385. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.P.; Arraes, F.B.M.; Lourenço, I.T.; Silva, M.S.; Lisei de Sá, M.E.; Lucena, W.A.; Alves Ferreira, M. Transgenic cotton expressing Cry10Aa toxin confers high resistance to the cotton boll weevil. Plant Biotechnol. J. 2007, 15, 997–1009. [Google Scholar] [CrossRef]
- Cabrera-Ponce, J.L.; Valencia-Lozano, E.; Trejo-Saavedra, D.L. Chapter 3—Genetic Modifications of Corn. In Corn, 3rd ed.; Serna-Saldivar, S.O., Ed.; AACC International Press: Oxford, UK, 2019; pp. 43–85. [Google Scholar]
- Van Rie, J.; Jansens, S. Midgut Transgenic Crops Expressing Bacillus thuringiensis Cry Proteins. Mod. Crop. Protect. Comp. 2019, 3, 1103–1136. [Google Scholar]
- Klotz, C.A.; Jans, S.; Fernandez-Cornejo, J.; McBride, W.D. Farm-level production effects related to the adoption of genetically modified cotton for pest management. AgBioForum 1999, 2, 73–84. [Google Scholar]
- Betz, F.S.; Hammond, B.G.; Fuchs, R.L. Safety and advantages of Bacillus thuringiensis-protected plants to control insect pests. Regul. Toxicol. Pharm. 2002, 32, 156–173. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Sutton, B.C.; Polonenko, D.R.; Cyr, D.R.; Stodola, T.F.U.S. Maturation of Somatic Embryos. Patent No. 6,200,809, 13 March 2001. [Google Scholar]
- Su, Y.H.; Liu, Y.B.; Bai, B.; Zhang, X.S. Establishment of embryonic shoot-root axis is involved in auxin and cytokinin response during Arabidopsis somatic embryogenesis. Front. Plant. Sci. 2015, 5, 792–801. [Google Scholar] [CrossRef]
- Wang, L.; Chong, K. The essential role of cytokinin signaling in root apical meristem formation during somatic embryogenesis. Front. Plant Sci. 2016, 6, 1196–1204. [Google Scholar] [CrossRef]
- Perthuis, B.; Vassal, J.M.; Fenouillet, C.; Leroy, T. Cry1Ac insecticidal protein levels in genetically modified Coffea canephora Pierre coffee plants were negatively correlated with the growth speed in a field experiment. Euphytica 2015, 202, 373–383. [Google Scholar] [CrossRef]
- James, C. Global Status of Commercialized Biotech/GM Crops: 2009. ISAAA. Brief 2009, 41, 304. [Google Scholar]
- Maagd, R.A. Bacillus thuringiensis-based products for insect pest control. In Principles of Plant-Microbe Interactions; Springer: Cham, The Netherlands, 2005; pp. 185–192. [Google Scholar]
- Shah, J.V.; Shailesh, B.L.; Rakeshkumar, Y.; Sanjay, S.I. Activity of two indigenous Bacillus thuringiensis isolates on lepidopteran insect pest Amsacta albistriga (Arctiidae). Int. J. Appl. Res. 2016, 2, 20–23. [Google Scholar]
- Méndez-López, I.; Basurto-Rios, R.; Ibarra, J.E. Bacillus thuringiensis serovar israelensis is highly toxic to the coffee berry borer, Hypothenemus hampei Ferr. (Coleoptera: Scolytidae). FEMS Microbiol. Lett. 2003, 226, 73–77. [Google Scholar] [CrossRef]
- Agüiar, R.W.S.; Martins, E.S.; Ribeiro, B.M.; Monnerat, R.G. Cry10Aa protein is highly toxic to Anthonomus grandis Boheman (Coleoptera: Curculionidae), an important insect pest in Brazilian cotton crop fields. Bti. Res. 2012, 3, 20–28. [Google Scholar]
- Van Boxtel, J.; Berthouly, M. High frequency somatic embryogenesis from coffee leaves. Plant Cell Tiss. Org. Cult. 1996, 44, 7–17. [Google Scholar] [CrossRef]
- Etienne-Barry, D.; Bertrand, B.; Schlönvoigt, A.; Etienne, H. The morphological variability within a population of coffee somatic embryos produced in a bioreactor affects the regeneration and the development of plants in the nursery. Plant Cell Tiss. Org. Cult. 2002, 68, 153–162. [Google Scholar] [CrossRef]
- Albuquerque, E.V.S.; Cunha, W.G.; Barbosa, A.E.A.D.; Costa, P.M.; Teixeira, J.B.; Vianna, G.R.; Cabral, G.B.; Fernandez, D.; Grossi-de-Sa, M.F. Transgenic coffee fruits from Coffea arabica genetically modified by bombardment. In Vitro Cell Dev. Biol. Plant 2009, 45, 532–539. [Google Scholar] [CrossRef]
- Breitler, J.C.; Déchamp, E.; Campa, C.; Rodrigues, L.A.Z.; Guyot, R.; Marraccini, P.; Etienne, H. CRISPR/Cas9-mediated efficient targeted mutagenesis has the potential to accelerate the domestication of Coffea canephora. Plant Cell Tiss. Organ. Cult. 2018, 134, 383–394. [Google Scholar] [CrossRef]
- Hatanaka, T.; Arakawa, O.; Yasuda, T.; Uchida, N.; Yamaguchi, T. Effect of plant growth regulators on somatic embryogenesis in leaf cultures of Coffea canephora. Plant Cell Rep. 1991, 10, 179–182. [Google Scholar] [CrossRef]
- Berthouly, M.; Michaux-Ferrière, N. High frequency somatic embryogenesis in Coffea canephora. Plant Cell Tiss. Organ. Cult. 1996, 44, 169–176. [Google Scholar] [CrossRef]
- Etienne-Barry, D.; Bertrand, B.; Vasquez, N.; Etienne, H. Direct sowing of Coffea arabica somatic embryos mass-produced in a bioreactor and regeneration of plants. Plant Cell Rep. 1999, 19, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Etienne-Barry, D.; Bertrand, B.; Vasquez, N.; Etienne, H. Comparison of Somatic Embryogenesis‚ derived Coffee (Coffea arabica L.) Plantlets Regenerated in vitro or ex vitro: Morphological, Mineral and Water Characteristics. Ann. Bot. 2002, 90, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Berthouly, M.; Etienne, H. Temporary immersion system: A new concept for use liquid medium in mass propagation. In Liquid Culture Systems for In Vitro Plant Propagation; Hvoslef-Eide, A.K., Preil, W., Eds.; Springer: Dordrecht, The Netherlands, 2005. [Google Scholar]
- Linossier, L.; Veisseire, P.; Cailloux, F.; Coudret, A. Effects of abscisic acid and high concentrations of PEG on Hevea brasiliensis somatic embryos development. Plant Sci. 1997, 124, 183–191. [Google Scholar] [CrossRef]
- Svobodová, H.; Albrechtová, J.; Kumstýřová, L.; Lipavská, H.; Vágner, M.; Vondráková, Z. Somatic embryogenesis in Norway spruce: Anatomical study of embryo development and influence of polyethylene glycol on maturation process. Plant Physiol. Biochem. 1999, 37, 209–221. [Google Scholar] [CrossRef]
- Walker, D.R.; Parrot, W.A. Effect of polyethylene glycol and sugar alcohols on soybean somatic embryo germination and conversion. Plant Cell Tiss. Org. Cult. 2001, 64, 55–62. [Google Scholar] [CrossRef]
- Troch, V.; Werbrouck, S.; Geelen, D.; Van Labeke, M.C. Optimization of horse chestnut (Aesculus hippocastanum L.) somatic embryo conversion. Plant Cell Tiss. Org. Cult. 2009, 98, 115–123. [Google Scholar] [CrossRef]
- Mishra, M.; Shukla, N.; Chandra, R. Role of polyethylene glycol in maturation and germination of transformed somatic embryos of papaya (Carica papaya L.). Acta horticul. 2010, 851, 227–230. [Google Scholar] [CrossRef]
- Heringer, A.S.; Vale, E.M.; Barroso, T.; Santa-Catarina, C.; Silveira, V. Polyethylene glycol effects on somatic embryogenesis of papaya hybrid UENF/CALIMAN 01 seeds. Theor. Exp. Plant Physiol. 2013, 25, 116–124. [Google Scholar] [CrossRef]
- De Moura Vale, E.; Heringer, A.S.; Barroso, T.; da Silva Ferreira, A.T.; da Costa, M.N.; Perales, J.E.A.; Santa-Catarina, C.; Silveira, V. Comparative proteomic analysis of somatic embryo maturation in Carica papaya L. Proteome Sci. 2014, 12, 37–42. [Google Scholar] [CrossRef]
- Attree, S.M.; Pomeroy, M.K.; Fowke, L.C. Development of white spruce (Picea glauca (Moench.) Voss) somatic embryos during culture with abscisic acid and osmoticum, and their tolerance to drying and frozen storage. J. Exp. Bot. 1995, 46, 433–439. [Google Scholar]
- Lelu, M.A.; Label, P. Changes in the levels of abscisic acid and its glucose ester conjugate during maturation of hybrid larch (Larix x leptoeuropaea) somatic embryos, in relation to germination and plantlet recovery. Physiol. Plant 1994, 92, 53–60. [Google Scholar] [CrossRef]
- Lelu, M.A.; Klimaszewska, K.; Pflaum, G.; Bastien, C. Effect of maturation duration on desiccation tolerance in hybrid larch (Larix x leptoeuropaea Dengler) somatic embryos. In Vitro Cell Dev. Biol. Plant 1995, 31, 15–20. [Google Scholar] [CrossRef]
- Vahdati, K.; Bayat, S.; Ebrahimzadeh, H.; Jariteh, M.; Mirmasoumi, M. Effect of exogenous ABA on somatic embryo maturation and germination in Persian walnut (Juglans regia L.). Plant Cell Tissue Organ. Cult. 2008, 93, 163–171. [Google Scholar] [CrossRef]
- Klimaszewska, K.; Trontin, J.F.; Becwar, M.R.; Devillard, C.; Park, Y.S.; Lelu-Walter, M.A. Recent progress in somatic embryogenesis of four Pinus spp. Tree Sci. Biotech. 2007, 1, 11–25. [Google Scholar]
- Perán-Quesada, R.; Sánchez-Romero, C.; Barceló-Muñoz, A.; Pliego-Alfaro, F. Factors affecting maturation of avocado somatic embryos. Sci. Hortic. 2004, 102, 61–73. [Google Scholar] [CrossRef]
- Sánchez-Romero, C.; Perán-Quesada, R.; Márquez-Martín, B.; Barceló-Muñoz, A.; Pliego-Alfaro, F. In vitro rescue of immature avocado (Persea americana Mill.) embryos. Sci. Hort. 2007, 111, 365–370. [Google Scholar] [CrossRef]
- Sánchez-Romero, C.; Márquez-Marín, B.; Pliego-Alfaro, F. Somatic and Zygotic Embryogenesis in Avocado. In Somatic Embryogenesis. Plant Cell Monographs; Mujib, A., Šamaj, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 271–284. [Google Scholar]
- Teyssier, C.; Grondin, C.; Bonhomme, L.; Lomenech, A.M.; Vallance, M.; Morabito, D.; Label, P.; Lelu-Walter, A.M. Increased gelling agent concentration promotes somatic embryo maturation in hybrid larch (Larix x eurolepsis): A 2-DE proteomic analysis. Physiol. Plant 2011, 141, 156–165. [Google Scholar] [CrossRef]
- Ribas, A.F.; Cenci, A.; Combes, M.C.; Etienne, H.; Lashermes, P. Organization and molecular evolution of a disease-resistance gene cluster in coffee trees. BMC 2011, 12, 240–252. [Google Scholar] [CrossRef]
- Monnerat, R.G.; Batista, A.C.; de Medeiros, P.T.; Martins, E.S.; Melatti, V.M.; Praça, L.B.; Dumas, V.F.; Morinaga, C.; Demo, C.; Menezes, A.C.; et al. Screening of Brazilian Bacillus thuringiensis isolates active against Spodoptera frugiperda, Plutella xylostella and Anticarsia gemmatalis. Biol. Control. 2007, 41, 291–295. [Google Scholar] [CrossRef]
- Ma, H.; Zhao, M.; Wang, H.; Wang, Z.; Wang, Q.; Dong, H. Comparative incidence of cotton spider mites on transgenic Bt versus conventional cotton in relation to contents of secondary metabolites. Arthropod Plant Inte. 2014, 8, 1–7. [Google Scholar] [CrossRef]
- Olsen, K.M.; Daly, J.C.; Holt, H.E.; Finnegan, E.J. Season-long variation in expression of Cry1Ac gene and efficacy of Bacillus thuringiensis toxin in transgenic cotton against Helicoverpa armigera (Lepidoptera: Noctuidae). J. Econ. Entomol. 2005, 98, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Huang, H.; Yang, Z.; Chen, T.; Liu, L.; Li, X.; Chen, H.; Lin, Y. Development of insect-resistant transgenic rice with Cry1C* free endosperm. Pest. Manag. Sci. 2009, 65, 1015–1020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC 2008, 9, 40. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Ponce, J.L.; López, L.; León-Ramírez, C.G.; Jofre-Garfias, A.E.; Verver-y-Vargas, A. Stress induced acquisition of somatic embryogenesis in common bean Phaseolus vulgaris L. Protoplasma 2014, 252, 559–570. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Bustin, S.A.; Beaulieu, J.F.; Huggett, J.; Jaggi, R.; Kibenge, F.S.; Olsvik, P.A.; Penning, L.C.; Toegel, S. MIQE precis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time. PCR Exp. 2010, 11, 74–82. [Google Scholar] [CrossRef]
- Birnboim, H.C.; Doly, J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucl. Acids. Res. 1979, 7, l5l3–l523. [Google Scholar]
- Lee-Stadelmann, O.Y.; Stadelmann, E.J. Plasmolysis and deplasmolysis. In Method Enzymol; Academic Press: New York, NY, USA, 1989; pp. 225–246. [Google Scholar]
- Vain, P.; McMullen, M.D.; Finer, J.J. Osmotic treatment enhances particle bombardment-mediated transient and stable transformation of maize. Plant Cell. Rep. 1993, 12, 84–88. [Google Scholar] [CrossRef]
- Tomes, D.T.; Ross, M.C.; Songstad, D.D. Direct DNA transfer into intact Plant Cells Via Microprojectile Bombardment. In Plant Cell Tissue Organ Cult; Springer: Berlin/Heidelberg, Germany, 1995; pp. 197–213. [Google Scholar]
- Cabrera-Ponce, J.L.; Lopez, L.; Assad-Garcia, N.; Medina-Arevalo, C.; Bailey, A.M.; Herrera-Estrella, L. An efficient particle bombardment system for the genetic transformation of asparagus (Asparagus officianalis L.). Plant Cell Rep. 1997, 16, 255–260. [Google Scholar]
- Sanford, J.C.; Devit, M.J.; Russell, J.A.; Smith, F.D.; Harpending, P.R.; Roy, M.K.; Johnston, S.A. An improved, helium-driven biolistic device. Technique 1991, 3, 3–16. [Google Scholar]
- Chumpookam, J.; Lin, H.L.; Shiesh, C.C. Effect of smoke-water derived from burnt dry rice straw (Oryza sativa) on seed germination and growth of papaya seedling (Carica papaya) Cultivar “Tainung No. 2”. Hort. Sci. 2002, 47, 741–744. [Google Scholar] [CrossRef]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning: A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 1989. [Google Scholar]
- Aebersold, R.; Mann, M. Mass spectrometry-based proteomics. Nature 2003, 422, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Pedrioli, P.G. Trans-proteomic pipeline: A pipeline for proteomic analysis. Meth. Mol. Biol. 2010, 604, 213–238. [Google Scholar]

| Treatment | Bombarded Plates | Transgenic Embryogenic Lines | Transformation Efficiency (%) | Transformed Events/Plate | Plants Grown (%) |
|---|---|---|---|---|---|
| CP2 (control) | 50 | 85 | 8.5 | 1.7 | 98 |
| Sucrose 6% | 50 | 256 | 25.6 | 5.12 | 100 |
| Sucrose 12% | 50 | 133 | 13.3 | 2.56 | 87 |
| Mannitol 0.15 M + Sorbitol 0.15 M | 50 | 197 | 19.7 | 3.94 | 95 |
| Mannitol 0.3 M + Sorbitol 0.3 M | 50 | 164 | 16.4 | 3.28 | 80 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valencia-Lozano, E.; Cabrera-Ponce, J.L.; Gómez-Lim, M.A.; Ibarra, J.E. Development of an Efficient Protocol to Obtain Transgenic Coffee, Coffea arabica L., Expressing the Cry10Aa Toxin of Bacillus thuringiensis. Int. J. Mol. Sci. 2019, 20, 5334. https://doi.org/10.3390/ijms20215334
Valencia-Lozano E, Cabrera-Ponce JL, Gómez-Lim MA, Ibarra JE. Development of an Efficient Protocol to Obtain Transgenic Coffee, Coffea arabica L., Expressing the Cry10Aa Toxin of Bacillus thuringiensis. International Journal of Molecular Sciences. 2019; 20(21):5334. https://doi.org/10.3390/ijms20215334
Chicago/Turabian StyleValencia-Lozano, Eliana, José L. Cabrera-Ponce, Miguel A. Gómez-Lim, and Jorge E. Ibarra. 2019. "Development of an Efficient Protocol to Obtain Transgenic Coffee, Coffea arabica L., Expressing the Cry10Aa Toxin of Bacillus thuringiensis" International Journal of Molecular Sciences 20, no. 21: 5334. https://doi.org/10.3390/ijms20215334
APA StyleValencia-Lozano, E., Cabrera-Ponce, J. L., Gómez-Lim, M. A., & Ibarra, J. E. (2019). Development of an Efficient Protocol to Obtain Transgenic Coffee, Coffea arabica L., Expressing the Cry10Aa Toxin of Bacillus thuringiensis. International Journal of Molecular Sciences, 20(21), 5334. https://doi.org/10.3390/ijms20215334






