Matrix Metalloproteinase-3 is Key Effector of TNF-α-Induced Collagen Degradation in Skin
Abstract
1. Introduction
2. Results
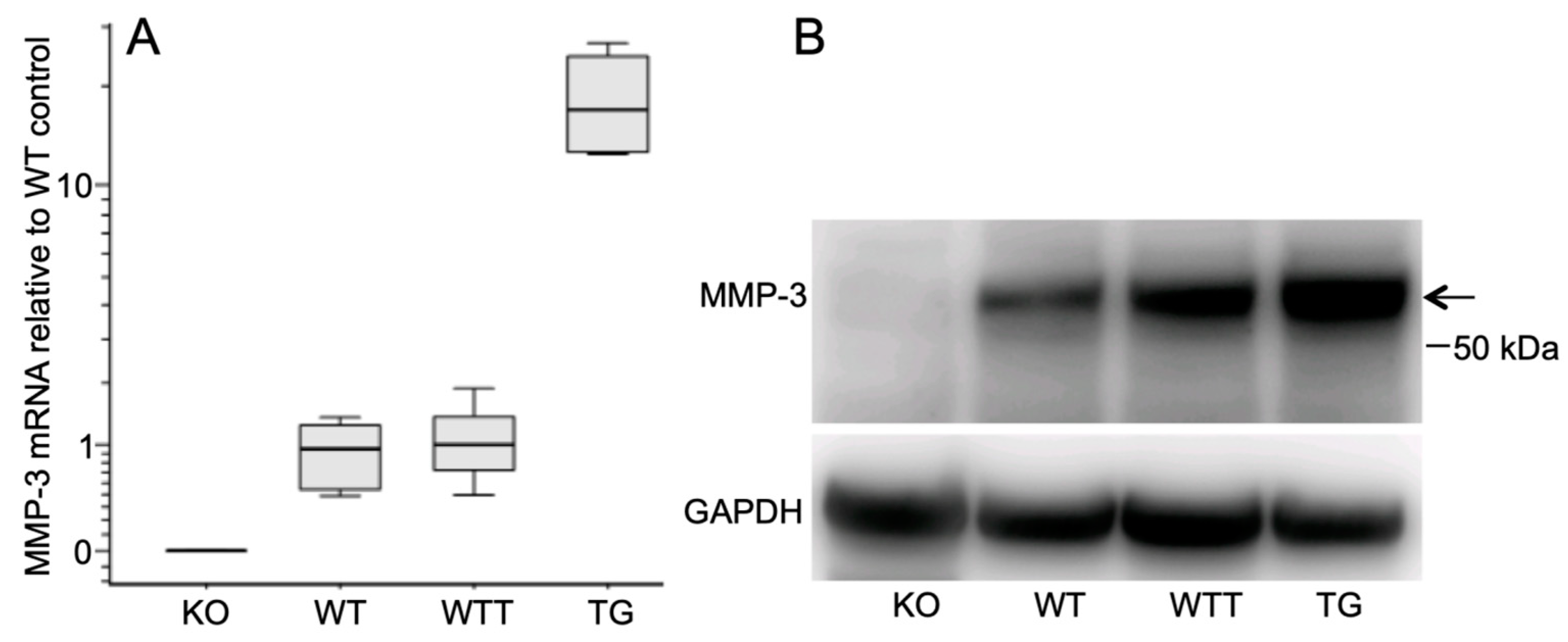
2.1. MMP-3 Expression in the Skin of the Four Murine Genotypes
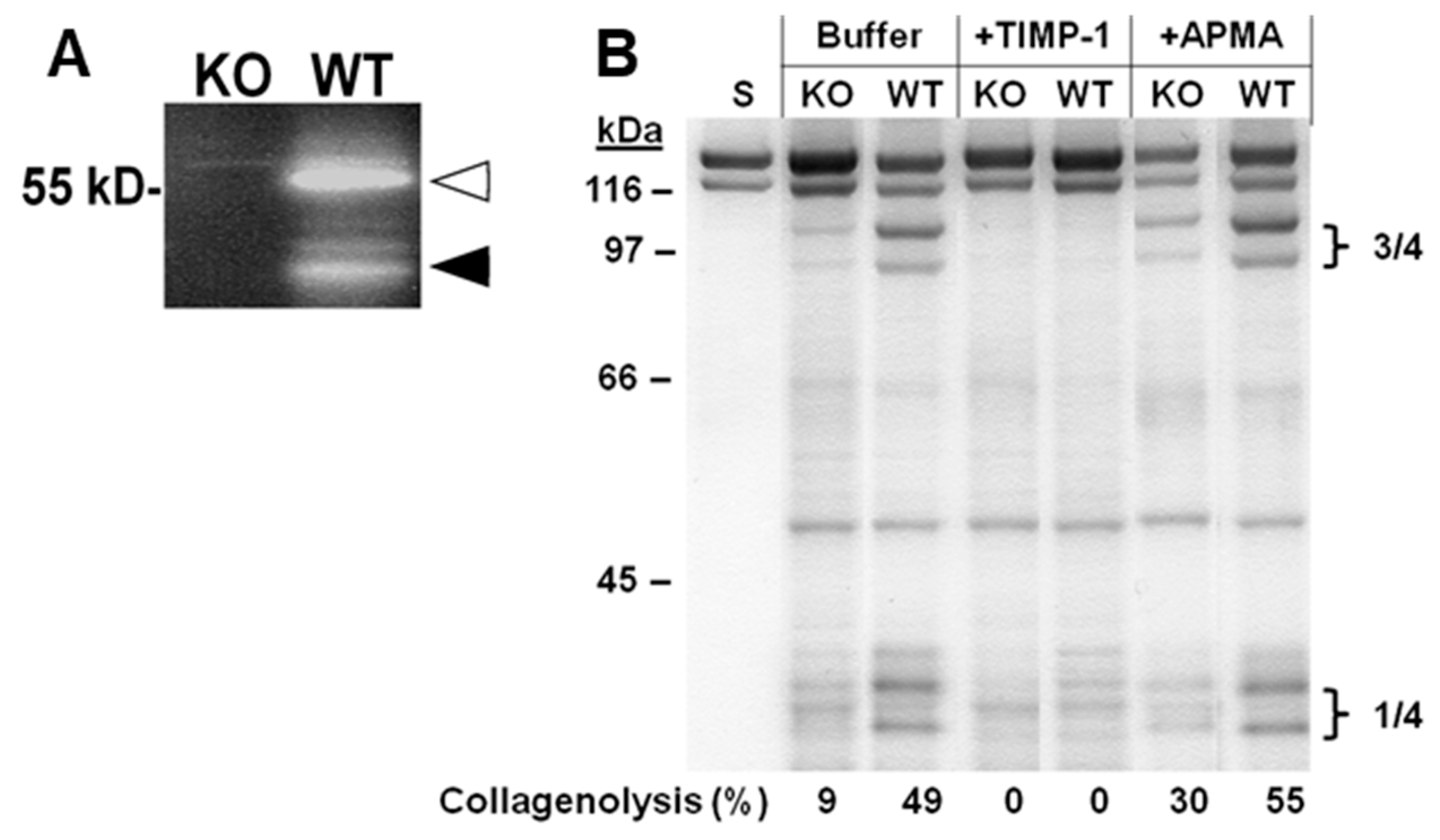
2.2. Collagen Degradation in Incubated Skin Explants of the Four Murine Genotypes
2.3. MMP-13 in MMP-3-Deficient and MMP-3-Overexpressing Conditions
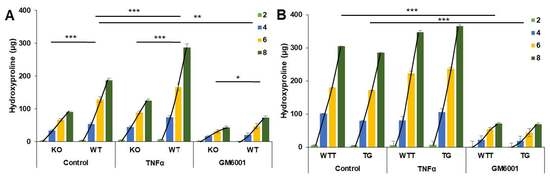
2.4. Effect of Pro-Inflammatory TNF-α on MMP-3 Tissue Levels, MMP-13 Secretion and Collagen Degradation
2.5. Effect of Pro-Inflammatory TNF-α on MMP-2 Secretion in MMP-3-Deficient and -Overexpressing Conditions
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Model and Treatment Groups
4.3. Analyses
4.3.1. Hydroxyproline Assay
4.3.2. MMP-3 and MMP-13 mRNA Determined by qPCR
4.3.3. Casein and Gelatin Zymography
4.3.4. Collagenase Activity
4.3.5. Western Blot Analyses
4.4. Statistics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| APMA | Aminophenylmercuric acetate |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| IL-1β | Interleukin-1β |
| LRP | Lipoprotein receptor-related protein |
| MMP | Matrix metalloproteinase |
| PVDF | Polyvinylidene fluoride |
| RAP | Receptor-associated protein |
| TIMP | Tissue inhibitor of metalloproteinases |
| TNF-α | Tumor necrosis factor-α |
References
- Gross, J.; Lapiere, C.M. Collagenolytic activity in amphibian tissues: A tissue culture assay. Proc. Natl. Acad Sci. USA 1962, 48, 1014–1022. [Google Scholar] [CrossRef] [PubMed]
- Rohani, M.G.; Parks, W.C. Matrix remodeling by MMPs during wound repair. Matrix Biol. 2015, 44–46, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Piperi, C.; Papavassiliou, A.G. Molecular mechanisms regulating matrix metalloproteinases. Curr. Top. Med. Chem. 2012, 12, 1095–1112. [Google Scholar] [CrossRef] [PubMed]
- Parks, W.C.; Wilson, C.L.; Lopez-Boado, Y.S. Matrix metalloproteinases as modulators of inflammation and innate immunity. Nat. Rev. Immunol. 2004, 4, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Witty, J.P.; Wright, J.H.; Matrisian, L.M. Matrix metalloproteinases are expressed during ductal and alveolar mammary morphogenesis, and misregulation of stromelysin-1 in transgenic mice induces unscheduled alveolar development. Mol. Biol. Cell 1995, 6, 1287–1303. [Google Scholar] [CrossRef] [PubMed]
- Saarialho-Kere, U.K.; Pentland, A.P.; Birkedal-Hansen, H.; Parks, W.C.; Welgus, H.G. Distinct populations of basal keratinocytes express stromelysin-1 and stromelysin-2 in chronic wounds. J. Clin. Invest. 1994, 94, 79–88. [Google Scholar] [CrossRef]
- Haro, H.; Crawford, H.C.; Fingleton, B.; MacDougall, J.R.; Shinomiya, K.; Spengler, D.M.; Matrisian, L.M. Matrix metalloproteinase-3-dependent generation of a macrophage chemoattractant in a model of herniated disc resorption. J. Clin. Invest. 2000, 105, 133–141. [Google Scholar] [CrossRef]
- Nerusu, K.C.; Warner, R.L.; Bhagavathula, N.; McClintock, S.D.; Johnson, K.J.; Varani, J. Matrix metalloproteinase-3 (stromelysin-1) in acute inflammatory tissue injury. Exp. Mol. Pathol. 2007, 83, 169–176. [Google Scholar] [CrossRef]
- Lochter, A.; Galosy, S.; Muschler, J.; Freedman, N.; Werb, Z.; Bissell, M.J. Matrix metalloproteinase stromelysin-1 triggers a cascade of molecular alterations that leads to stable epithelial-to-mesenchymal conversion and a premalignant phenotype in mammary epithelial cells. J. Cell Biol. 1997, 139, 1861–1872. [Google Scholar] [CrossRef]
- Brenneisen, P.; Sies, H.; Scharffetter-Kochanek, K. Ultraviolet-B irradiation and matrix metalloproteinases: From induction via signaling to initial events. Ann. N Y Acad Sci. 2002, 973, 31–43. [Google Scholar] [CrossRef]
- Kurz, B.; Lemke, A.K.; Fay, J.; Pufe, T.; Grodzinsky, A.J.; Schünke, M. Pathomechanisms of cartilage destruction by mechanical injury. Ann. Anat. 2005, 187, 473–485. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D.; Khalil, R.A. Matrix metalloproteinases and their inhibitors in vascular remodeling and vascular disease. Biochem. Pharmacol. 2008, 75, 346–359. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Rodriguez Perez, C.E.; Nie, W.; Sinnett-Smith, J.; Rozengurt, E. Protein kinase D1 mediates synergistic MMP-3 expression induced by TNF-α and bradykinin in human colonic myofibroblasts. Biochem. Biophys Res. Commun. 2011, 413, 30–35. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, M.; Qin, X.; Mudgett, J.S.; Ferguson, T.A.; Senior, R.M.; Welgus, H.G. Matrix metalloproteinase deficiencies affect contact hypersensitivity: Stromelysin-1 deficiency prevents the response and gelatinase B deficiency prolongs the response. Proc. Natl. Acad Sci. USA 1999, 96, 6885–6889. [Google Scholar] [CrossRef]
- Warner, R.L.; Beltran, L.; Younkin, E.M.; Lewis, C.S.; Weiss, S.J.; Varani, J.; Johnson, K.J. Role of stromelysin 1 and gelatinase B in experimental acute lung injury. Am. J. Respir Cell Mol. Biol. 2001, 24, 537–544. [Google Scholar] [CrossRef]
- Bullard, K.M.; Lund, L.; Mudgett, J.S.; Mellin, T.N.; Hunt, T.K.; Murphy, B.; Ronan, J.; Werb, Z.; Banda, M.J. Impaired wound contraction in stromelysin-1-deficient mice. Ann. Surg. 1999, 230, 260–265. [Google Scholar] [CrossRef]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef]
- Steenport, M.; Khan, K.M.; Du, B.; Barnhard, S.E.; Dannenberg, A.J.; Falcone, D.J. Matrix metalloproteinase (MMP)-1 and MMP-3 induce macrophage MMP-9: Evidence for the role of TNF-alpha and cyclooxygenase-2. J. Immunol. 2009, 183, 8119–8127. [Google Scholar] [CrossRef]
- Lee, E.J.; Moon, P.G.; Baek, M.C.; Kim, H.S. Comparison of the effects of matrix metalloproteinase inhibitors on TNF-alpha release from activated microglia and TNF-alpha converting enzyme activity. Biomol. Ther. 2014, 22, 414–419. [Google Scholar] [CrossRef]
- Gearing, A.J.; Beckett, P.; Christodoulou, M.; Churchill, M.; Clements, J.; Davidson, A.H.; Drummond, A.H.; Galloway, W.A.; Gilbert, R.; Gordon, J.L.; et al. Processing of tumour necrosis factor-alpha precursor by metalloproteinases. Nature 1994, 370, 555–557. [Google Scholar] [CrossRef]
- Voigt, H.; Lemke, A.K.; Mentlein, R.; Schunke, M.; Kurz, B. Tumor necrosis factor alpha-dependent aggrecan cleavage and release of glycosaminoglycans in the meniscus is mediated by nitrous oxide-independent aggrecanase activity in vitro. Arthritis Res. Ther. 2009, 11, R141. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.J.; Rhim, E.M.; Kim, J.Y.; Kim, K.H.; Lee, H.W.; Kim, E.C.; Park, S.H. Tumor necrosis factor-alpha induces matrix metalloproteinases-3, -10, and -13 in human periodontal ligament cells. J. Periodontol. 2014, 85, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Ågren, M.S.; Schnabel, R.; Christensen, L.H.; Mirastschijski, U. Tumor necrosis factor-alpha-accelerated degradation of type I collagen in human skin is associated with elevated matrix metalloproteinase (MMP)-1 and MMP-3 ex vivo. Eur. J. Cell Biol. 2015, 94, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Walker, E.J.; Rosenberg, G.A. TIMP-3 and MMP-3 contribute to delayed inflammation and hippocampal neuronal death following global ischemia. Exp. Neurol. 2009, 216, 122–131. [Google Scholar] [CrossRef]
- McCawley, L.J.; Wright, J.; LaFleur, B.J.; Crawford, H.C.; Matrisian, L.M. Keratinocyte expression of MMP3 enhances differentiation and prevents tumor establishment. Am. J. Pathol. 2008, 173, 1528–1539. [Google Scholar] [CrossRef]
- Henriet, P.; Rousseau, G.G.; Eeckhout, Y. Cloning and sequencing of mouse collagenase cDNA. Divergence of mouse and rat collagenases from the other mammalian collagenases. FEBS letters 1992, 310, 175–178. [Google Scholar] [CrossRef]
- Balbin, M.; Fueyo, A.; Knauper, V.; Lopez, J.M.; Alvarez, J.; Sanchez, L.M.; Quesada, V.; Bordallo, J.; Murphy, G.; Lopez-Otin, C. Identification and enzymatic characterization of two diverging murine counterparts of human interstitial collagenase (MMP-1) expressed at sites of embryo implantation. J. Biol. Chem. 2001, 276, 10253–10262. [Google Scholar] [CrossRef]
- Nagase, H. Chapter 158: Matrix Metalloproteinase-3/Stromelysin-1. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: London, UK, 2013; Volume 1, pp. 763–774. [Google Scholar]
- Barmina, O.Y.; Walling, H.W.; Fiacco, G.J.; Freije, J.M.; Lopez-Otin, C.; Jeffrey, J.J.; Partridge, N.C. Collagenase-3 binds to a specific receptor and requires the low density lipoprotein receptor-related protein for internalization. J. Biol. Chem. 1999, 274, 30087–30093. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, K.; Okano, H.; Miyagawa, W.; Visse, R.; Shitomi, Y.; Santamaria, S.; Dudhia, J.; Troeberg, L.; Strickland, D.K.; Hirohata, S.; et al. MMP-13 is constitutively produced in human chondrocytes and co-endocytosed with ADAMTS-5 and TIMP-3 by the endocytic receptor LRP1. Matrix Biol. 2016, 56, 57–73. [Google Scholar] [CrossRef]
- Yamamoto, K.; Troeberg, L.; Scilabra, S.D.; Pelosi, M.; Murphy, C.L.; Strickland, D.K.; Nagase, H. LRP-1-mediated endocytosis regulates extracellular activity of ADAMTS-5 in articular cartilage. FASEB J. 2013, 27, 511–521. [Google Scholar] [CrossRef]
- Knäuper, V.; Lopez-Otin, C.; Smith, B.; Knight, G.; Murphy, G. Biochemical characterization of human collagenase-3. J. Biol. Chem. 1996, 271, 1544–1550. [Google Scholar] [CrossRef] [PubMed]
- Knäuper, V.; Will, H.; López-Otin, C.; Smith, B.; Atkinson, S.J.; Stanton, H.; Hembry, R.M.; Murphy, G. Cellular mechanisms for human procollagenase-3 (MMP-13) activation. Evidence that MT1-MMP (MMP-14) and gelatinase A (MMP-2) are able to generate active enzyme. J. Biol. Chem. 1996, 271, 17124–17131. [Google Scholar] [CrossRef] [PubMed]
- Eeckhout, Y.; Vaes, G. Further studies on the activation of procollagenase, the latent precursor of bone collagenase. Effects of lysosomal cathepsin B, plasmin and kallikrein, and spontaneous activation. Biochem. J. 1977, 166, 21–31. [Google Scholar] [CrossRef]
- Van Meurs, J.; van Lent, P.; Stoop, R.; Holthuysen, A.; Singer, I.; Bayne, E.; Mudgett, J.; Poole, R.; Billinghurst, C.; van der Kraan, P.; et al. Cleavage of aggrecan at the Asn341-Phe342 site coincides with the initiation of collagen damage in murine antigen-induced arthritis: A pivotal role for stromelysin 1 in matrix metalloproteinase activity. Arthritis Rheum 1999, 42, 2074–2084. [Google Scholar] [CrossRef]
- Henriet, P.; Eeckhout, Y. Chapter 154: Matrix Metallopeptidase-13/Collagenase-3. In Handbook of Proteolytic Enzymes, 3rd ed.; Rawlings, N.D., Salvesen, G., Eds.; Academic Press: London, UK, 2013; Volume 1, pp. 734–744. [Google Scholar]
- Gosset, M.; Pigenet, A.; Salvat, C.; Berenbaum, F.; Jacques, C. Inhibition of matrix metalloproteinase-3 and -13 synthesis induced by IL-1beta in chondrocytes from mice lacking microsomal prostaglandin E synthase-1. J. Immunol. 2010, 185, 6244–6252. [Google Scholar] [CrossRef] [PubMed]
- Kusano, K.; Miyaura, C.; Inada, M.; Tamura, T.; Ito, A.; Nagase, H.; Kamoi, K.; Suda, T. Regulation of matrix metalloproteinases (MMP-2, -3, -9, and -13) by interleukin-1 and interleukin-6 in mouse calvaria: Association of MMP induction with bone resorption. Endocrinology 1998, 139, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Arnott, C.H.; Scott, K.A.; Moore, R.J.; Hewer, A.; Phillips, D.H.; Parker, P.; Balkwill, F.R.; Owens, D.M. Tumour necrosis factor-alpha mediates tumour promotion via a PKC alpha- and AP-1-dependent pathway. Oncogene 2002, 21, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.P.; Tuan, T.L.; Hughes, M.; Wu, H.; Garner, W.L. Transforming growth factor-beta - and tumor necrosis factor-alpha -mediated induction and proteolytic activation of MMP-9 in human skin. J. Biol. Chem. 2001, 276, 22341–22350. [Google Scholar] [CrossRef] [PubMed]
- Borden, P.; Solymar, D.; Sucharczuk, A.; Lindman, B.; Cannon, P.; Heller, R.A. Cytokine control of interstitial collagenase and collagenase-3 gene expression in human chondrocytes. J. Biol. Chem. 1996, 271, 23577–23581. [Google Scholar] [CrossRef]
- Lauridsen, H.M.; Pellowe, A.S.; Ramanathan, A.; Liu, R.; Miller-Jensen, K.; McNiff, J.M.; Pober, J.S.; Gonzalez, A.L. Tumor necrosis factor-alpha and IL-17A activation induces pericyte-mediated basement membrane remodeling in human neutrophilic dermatoses. Am. J. Pathol. 2017, 187, 1893–1906. [Google Scholar] [CrossRef]
- Lees, M.; Taylor, D.J.; Woolley, D.E. Mast cell proteinases activate precursor forms of collagenase and stromelysin, but not of gelatinases A and B. Eur. J. Biochem. 1994, 223, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.D.; Carter, K.J.; Jean-Philippe, S.R.; Chang, M.; Mobashery, S.; Thiolloy, S.; Lynch, C.C.; Matrisian, L.M.; Fingleton, B. Effect of ablation or inhibition of stromal matrix metalloproteinase-9 on lung metastasis in a breast cancer model is dependent on genetic background. Cancer Res. 2008, 68, 6251–6259. [Google Scholar] [CrossRef] [PubMed]
- Mähler, M.; Berard, M.; Feinstein, R.; Gallagher, A.; Illgen-Wilcke, B.; Pritchett-Corning, K.; Raspa, M. FELASA recommendations for the health monitoring of mouse, rat, hamster, guinea pig and rabbit colonies in breeding and experimental units. FELASA working group on revision of guidelines for health monitoring of rodents and rabbits. Lab. Anim. 2014, 48, 178–192. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, N.A.; Sørensen, L.T.; Jorgensen, L.N.; Ågren, M.S. Circulating levels of matrix metalloproteinases and tissue inhibitor of metalloproteinases in patients with incisional hernia. Wound Repair Regen 2013, 21, 661–666. [Google Scholar] [CrossRef]
- Hinke, S.A.; Navedo, M.F.; Ulman, A.; Whiting, J.L.; Nygren, P.J.; Tian, G.; Jimenez-Caliani, A.J.; Langeberg, L.K.; Cirulli, V.; Tengholm, A.; et al. Anchored phosphatases modulate glucose homeostasis. EMBO J. 2012, 31, 3991–4004. [Google Scholar] [CrossRef]
- Mirastschijski, U.; Dinesh, N.; Baskaran, S.; Wedekind, D.; Gavrilovic, J.; Murray, M.Y.; Bevan, D.; Kelm, S. Novel specific human and mouse stromelysin-1 (MMP-3) and stromelysin-2 (MMP-10) antibodies for biochemical and immunohistochemical analyses. Wound Repair Regen 2019, 27, 309–323. [Google Scholar] [CrossRef]
- Ardestani, A.; Paroni, F.; Azizi, Z.; Kaur, S.; Khobragade, V.; Yuan, T.; Frogne, T.; Tao, W.; Oberholzer, J.; Pattou, F.; et al. MST1 is a key regulator of beta cell apoptosis and dysfunction in diabetes. Nat. Med. 2014, 20, 385–397. [Google Scholar] [CrossRef]
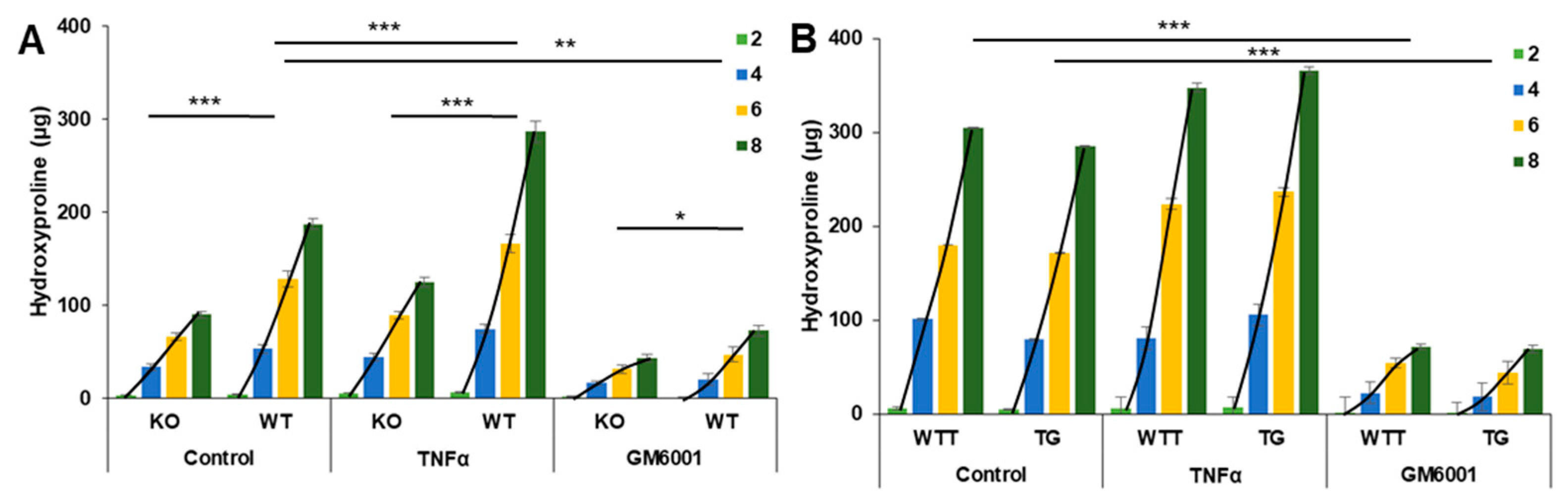
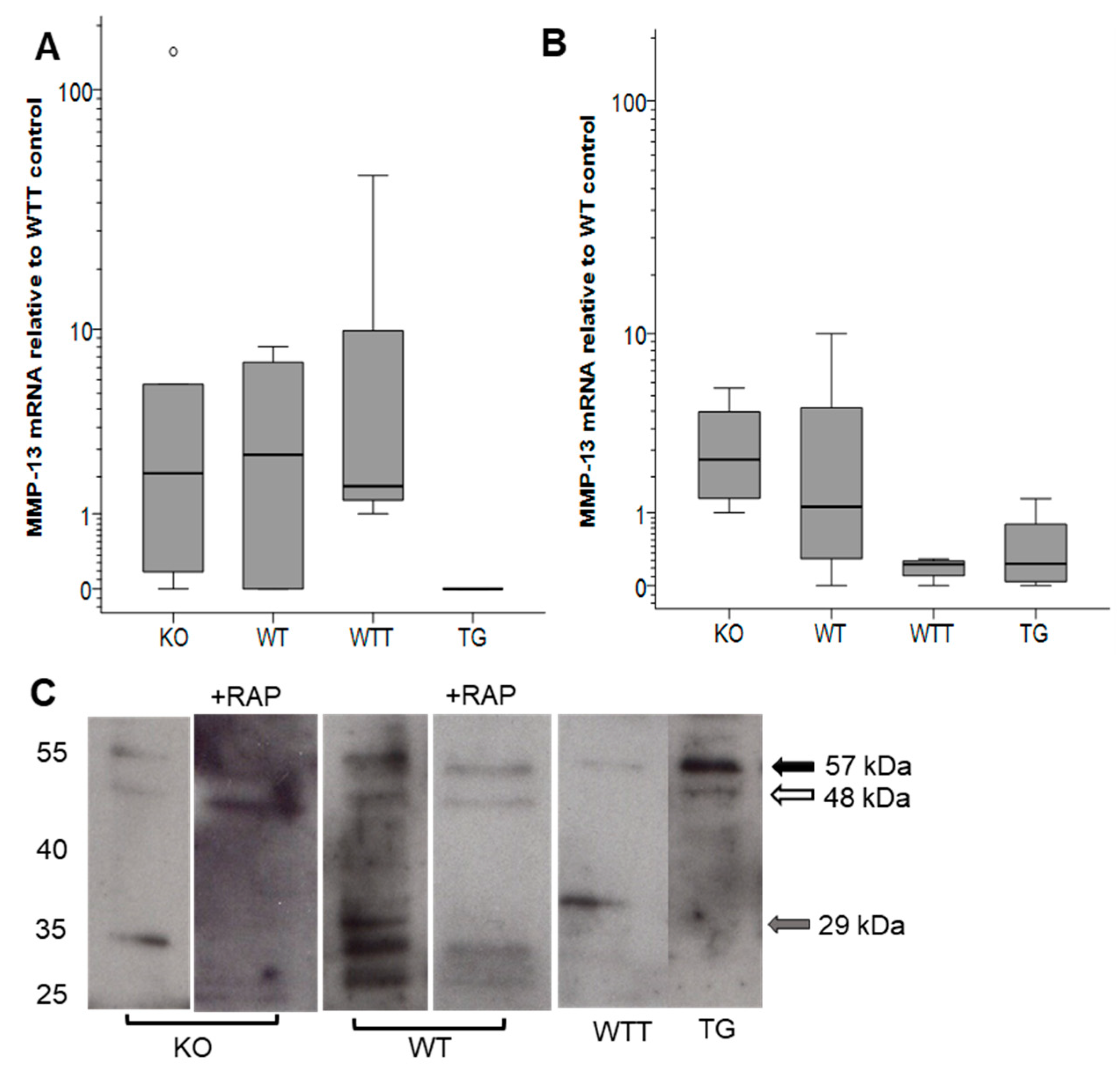
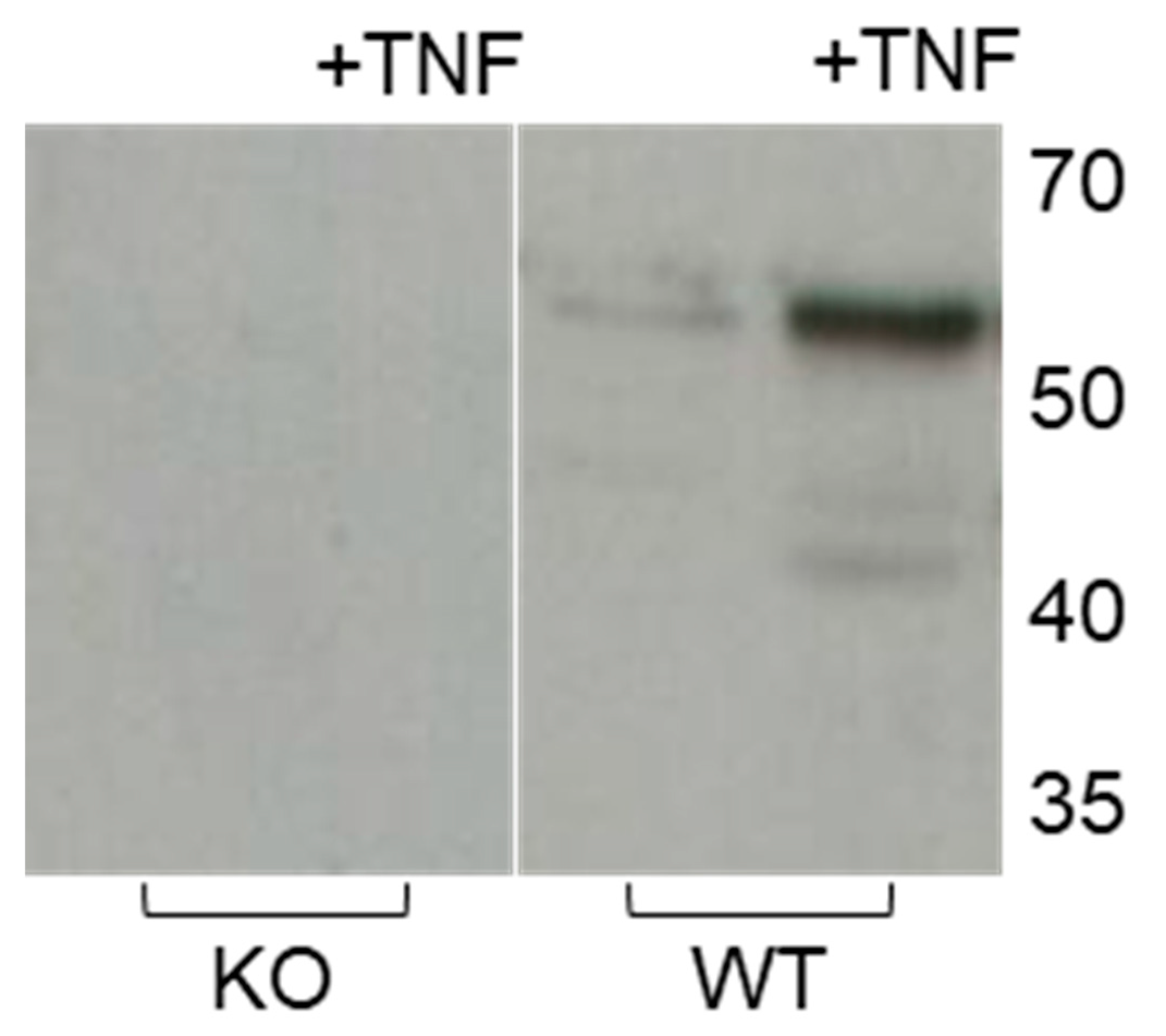
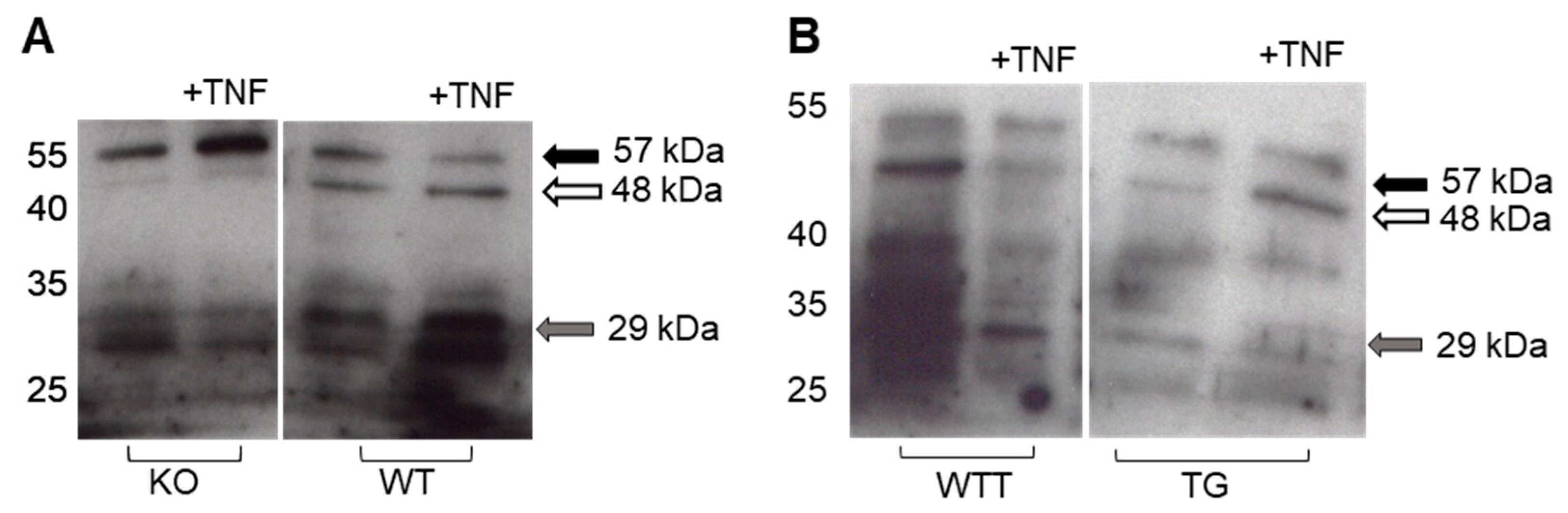
| Day | KO (n = 10) | WT (n = 10) | WTT (n = 9) | TG (n = 10) |
|---|---|---|---|---|
| 0 | 0 | 0 | 0 | 0 |
| 2 | 3.9 ± 0.2 | 4.3 ± 0.5 | 6.8 ± 1.7 | 5.0 ± 1.0 |
| 4 | 29.9 ± 3.5 | 49.3 ± 4.7 | 94.3 ± 12.0 | 74.4 ± 10.5 |
| 6 | 32.4 ± 3.9 | 74.9 ± 8.4 | 79.0 ± 5.7 | 92.4 ± 11.2 |
| 8 | 24.1 ± 3.6 | 59.0 ± 5.5 | 124.4 ± 16.7 | 113.2 ± 11.8 |
| Accumulated | 90.3 | 187.5 | 304.5 | 285.0 |
| Regression analysis | ||||
| Slope | 3.16 | 9.49 | 16.9 | 17.1 |
| p | <0.001 | 0.47 | ||
| Group | Control | TNF-α 1 | GM6001 2 |
|---|---|---|---|
| KO | n = 10 | n = 10 | n = 5 |
| WT | n = 10 | n = 10 | n = 5 |
| WTT | n = 9 | n = 9 | n = 4 |
| TG | n = 10 | n = 10 | n = 5 |
| Group | Control | TNF-α 1 | GM6001 2 | |||
|---|---|---|---|---|---|---|
| 0 | +RAP 3 | 0 | +RAP 3 | 0 | +RAP 3 | |
| KO | n = 4 | n = 4 | n = 4 | n = 4 | n = 4 | n = 4 |
| WT | n = 4 | n = 4 | n = 4 | n = 4 | n = 4 | n = 4 |
| WTT | n = 4 | n = 4 | n = 4 | n = 4 | n = 4 | n = 4 |
| TG | n = 4 | n = 4 | n = 4 | n = 4 | n = 4 | n = 4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mirastschijski, U.; Lupše, B.; Maedler, K.; Sarma, B.; Radtke, A.; Belge, G.; Dorsch, M.; Wedekind, D.; McCawley, L.J.; Boehm, G.; et al. Matrix Metalloproteinase-3 is Key Effector of TNF-α-Induced Collagen Degradation in Skin. Int. J. Mol. Sci. 2019, 20, 5234. https://doi.org/10.3390/ijms20205234
Mirastschijski U, Lupše B, Maedler K, Sarma B, Radtke A, Belge G, Dorsch M, Wedekind D, McCawley LJ, Boehm G, et al. Matrix Metalloproteinase-3 is Key Effector of TNF-α-Induced Collagen Degradation in Skin. International Journal of Molecular Sciences. 2019; 20(20):5234. https://doi.org/10.3390/ijms20205234
Chicago/Turabian StyleMirastschijski, Ursula, Blaž Lupše, Kathrin Maedler, Bhavishya Sarma, Arlo Radtke, Gazanfer Belge, Martina Dorsch, Dirk Wedekind, Lisa J. McCawley, Gabriele Boehm, and et al. 2019. "Matrix Metalloproteinase-3 is Key Effector of TNF-α-Induced Collagen Degradation in Skin" International Journal of Molecular Sciences 20, no. 20: 5234. https://doi.org/10.3390/ijms20205234
APA StyleMirastschijski, U., Lupše, B., Maedler, K., Sarma, B., Radtke, A., Belge, G., Dorsch, M., Wedekind, D., McCawley, L. J., Boehm, G., Zier, U., Yamamoto, K., Kelm, S., & Ågren, M. S. (2019). Matrix Metalloproteinase-3 is Key Effector of TNF-α-Induced Collagen Degradation in Skin. International Journal of Molecular Sciences, 20(20), 5234. https://doi.org/10.3390/ijms20205234