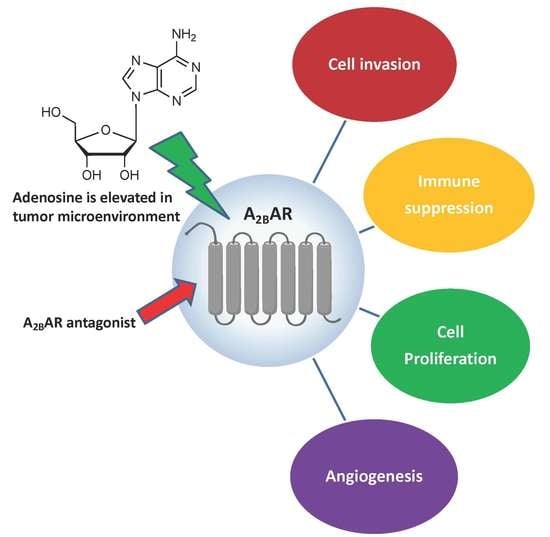
A2B Adenosine Receptor and Cancer
Abstract
:1. Introduction
2. A2BAR Distribution and Expression
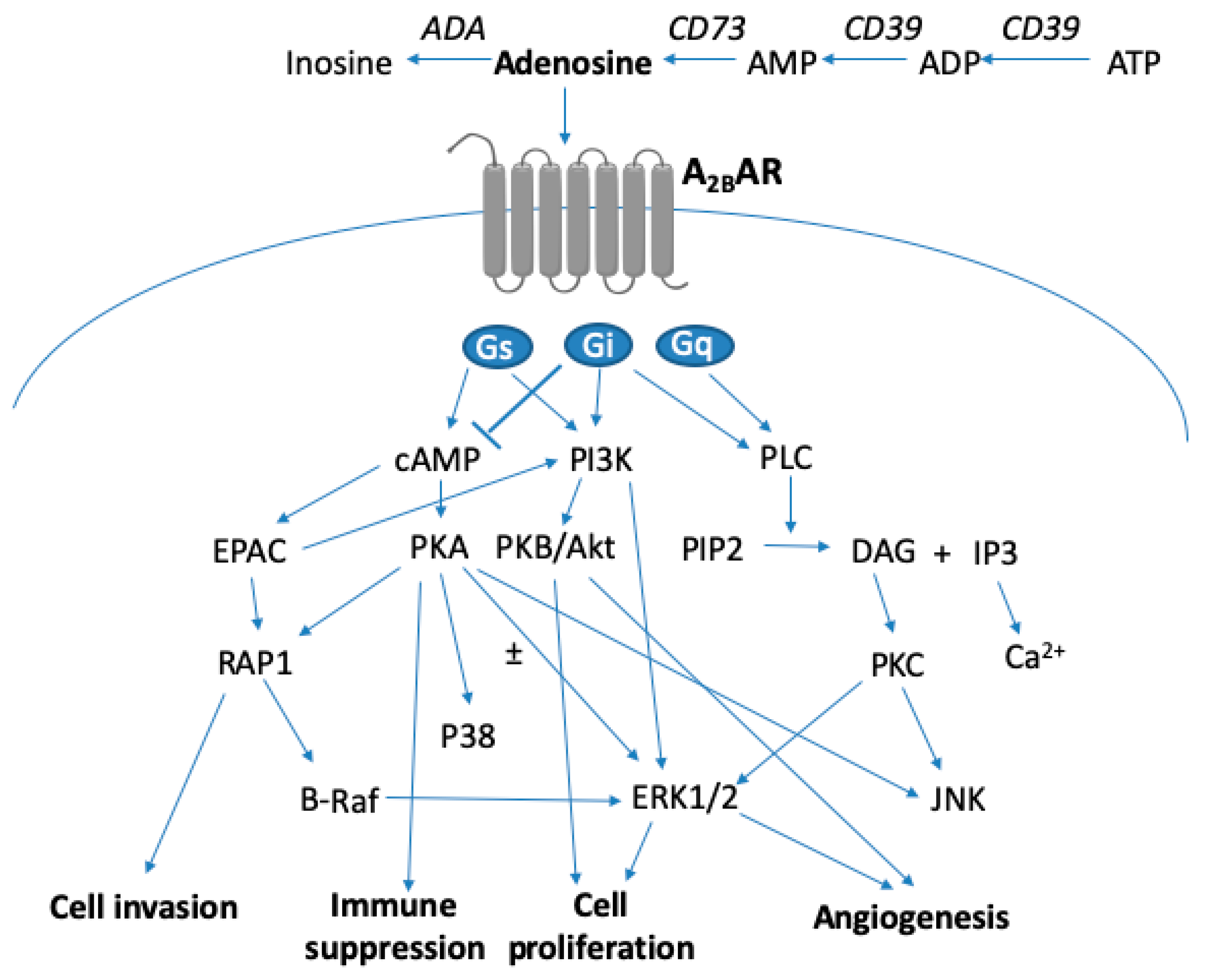
3. A2BAR Signaling
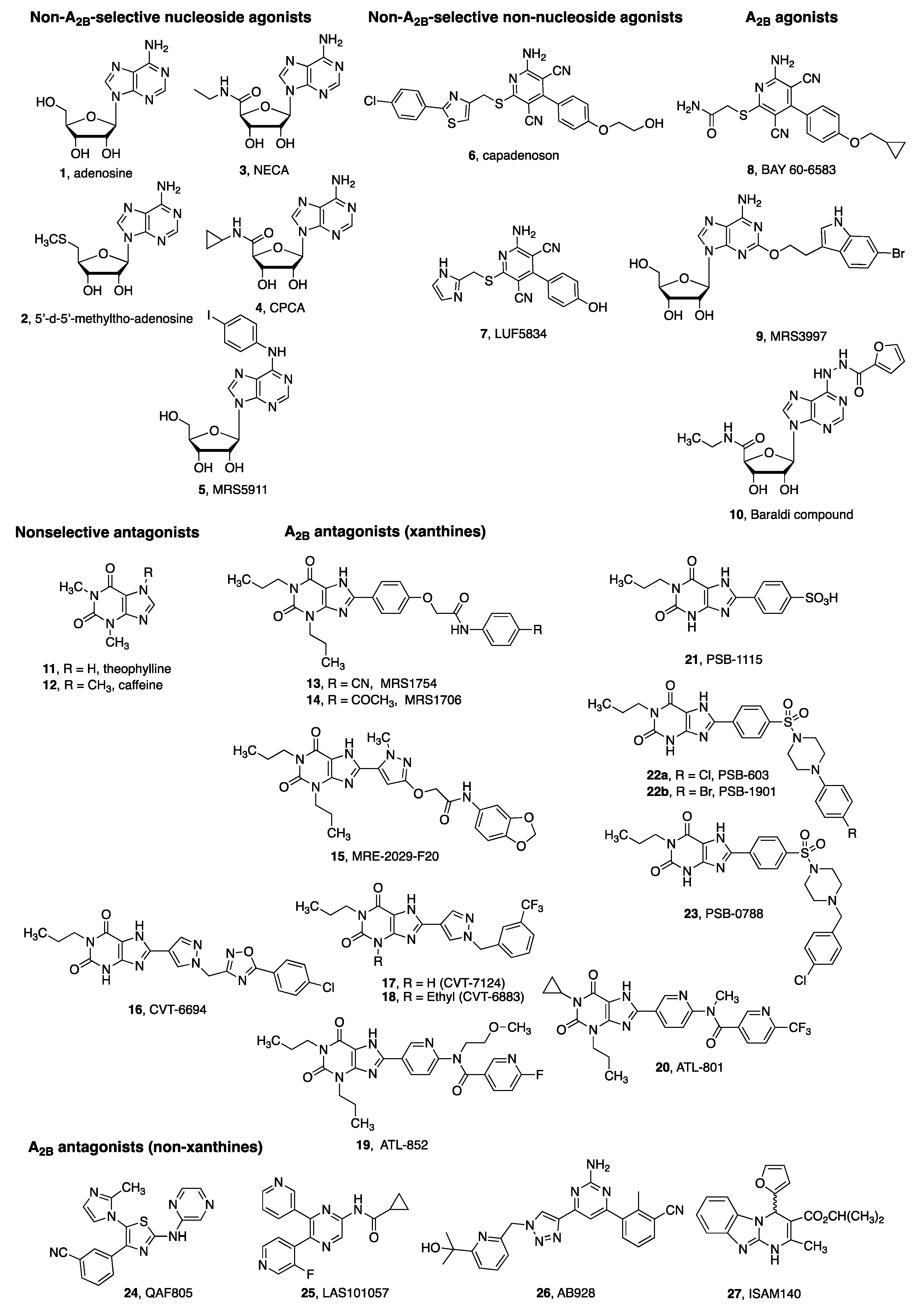
4. A2BAR Agonists and Antagonists as Pharmacological Tools
5. A2BAR in Cell Proliferation and Tumor Growth
6. A2BAR and Tumor Metastasis
7. A2BAR and Angiogenesis
8. A2BAR and Immunity
9. A2BAR Antagonists as Novel Anticancer Agents
10. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, Q.; Costanzi, S.; Liu, Q.Z.; Gao, Z.G.; Jacobson, K.A. Activation of the P2Y1 receptor induces apoptosis and inhibits proliferation of prostate cancer cells. Biochem. Pharmacol. 2011, 82, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Burnstock, G.; Di Virgilio, F. Purinergic signalling and cancer. Purinergic Signal. 2013, 9, 491–540. [Google Scholar] [CrossRef] [PubMed]
- Seitz, L.; Jin, L.; Leleti, M.; Ashok, D.; Jeffrey, J.; Rieger, A.; Tiessen, R.G.; Arold, G.; Tan, J.B.L.; Powers, J.P.; et al. Safety, tolerability, and pharmacology of AB928, a novel dual adenosine receptor antagonist, in a randomized, phase 1 study in healthy volunteers. Invest. New Drugs 2019, 37, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Costanzi, S.; Balasubramanian, R.; Gao, Z.G.; Jacobson, K.A. A2B adenosine receptor blockade inhibits growth of prostate cancer cells. Purinergic Signal. 2013, 9, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Cekic, C.; Sag, D.; Li, Y.; Theodorescu, D.; Strieter, R.M.; Linden, J. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J. Immunol. 2012, 188, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kasama, H.; Sakamoto, Y.; Kasamatsu, A.; Okamoto, A.; Koyama, T.; Minakawa, Y.; Ogawara, K.; Yokoe, H.; Shiiba, M.; Tanzawa, H.; et al. Adenosine A2b receptor promotes progression of human oral cancer. Clin. Cancer Res. 2016, 22, 158–166. [Google Scholar] [CrossRef]
- Mittal, D.; Sinha, D.; Barkauskas, D.; Young, A.; Kalimutho, M.; Stannard, K.; Caramia, F.; Haibe-Kains, B.; Stagg, J.; Khanna, K.K.; et al. Adenosine 2B Receptor Expression on Cancer Cells Promotes Metastasis. Cancer Res. 2016, 76, 4372–4382. [Google Scholar] [CrossRef]
- Sepúlveda, C.; Palomo, I.; Fuentes, E. Role of adenosine A2b receptor overexpression in tumor progression. Life Sci. 2016, 166, 92–99. [Google Scholar] [CrossRef]
- Ryzhov, S.; Novitskiy, S.V.; Zaynagetdinov, R.; Goldstein, A.E.; Carbone, D.P.; Biaggioni, I.; Dikov, M.M.; Feoktistov, I. Host A2B adenosine receptors promote carcinoma growth. Neoplasia 2008, 10, 987–995. [Google Scholar] [CrossRef]
- Iannone, R.; Miele, L.; Maiolino, P.; Pinto, A.; Morello, S. Blockade of A2b adenosine receptor reduces tumor growth and immune suppression mediated by myeloid-derived suppressor cells in a mouse model of melanoma. Neoplasia 2013, 15, 1400–1409. [Google Scholar] [CrossRef]
- Fredholm, B.B.; IJzerman, A.P.; Jacobson, K.A.; Linden, J.; Müller, C.E. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors—An update. Pharmacol. Rev. 2011, 63, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drury, A.N.; Szent-Györgyi, A. The physiological activity of adenine compounds with especial reference to their action upon the mammalian heart. J. Physiol. 1929, 68, 213–237. [Google Scholar] [CrossRef] [PubMed]
- Daly, J.W.; Butts-Lamb, P.; Padgett, W. Subclasses of adenosine receptors in the central nervous system: Interaction with caffeine and related methylxanthines. Cell Mol. Neurobiol. 1983, 3, 69–80. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Varani, K. Adenosine as a Multi-Signalling Guardian Angel in Human Diseases: When, Where and How does it Exert its Protective Effects? Trends Pharmacol. Sci. 2016, 37, 419–434. [Google Scholar] [CrossRef]
- Borea, P.A.; Gessi, S.; Merighi, S.; Vincenzi, F.; Varani, K. Pharmacology of Adenosine Receptors: The State of the Art. Physiol. Rev. 2018, 98, 1591–1625. [Google Scholar] [CrossRef]
- Cekic, C.; Linden, J. Purinergic regulation of the immune system. Nat. Rev. Immunol. 2016, 16, 177–192. [Google Scholar] [CrossRef]
- Ramakers, B.P.; Riksen, N.P.; van der Hoeven, J.G.; Smits, P.; Pickkers, P. Modulation of innate immunity by adenosine receptor stimulation. Shock 2011, 36, 208–215. [Google Scholar] [CrossRef]
- Philip, K.; Mills, T.W.; Davies, J.; Chen, N.Y.; Karmouty-Quintana, H.; Luo, F.; Molina, J.G.; Amione-Guerra, J.; Sinha, N.; Guha, A.; et al. HIF1A up-regulates the ADORA2B receptor on alternatively activated macrophages and contributes to pulmonary fibrosis. FASEB J. 2017, 31, 4745–4748. [Google Scholar] [CrossRef]
- Lan, J.; Lu, H.; Samanta, D.; Salman, S.; Lu, Y.; Semenza, G.L. Hypoxia-inducible factor 1-dependent expression of adenosine receptor 2B promotes breast cancer stem cell enrichment. Proc. Natl. Acad. Sci. USA 2018, 115, E9640–E9648. [Google Scholar] [CrossRef]
- Ma, D.F.; Kondo, T.; Nakazawa, T.; Niu, D.F.; Mochizuki, K.; Kawasaki, T.; Yamane, T.; Katoh, R. Hypoxia-inducible adenosine A2B receptor modulates proliferation of colon carcinoma cells. Hum. Pathol. 2010, 41, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Kong, T.; Westerman, K.A.; Faigle, M.; Eltzschig, H.K.; Colgan, S.P. HIF-dependent induction of adenosine A2B receptor in hypoxia. FASEB J. 2006, 20, 2242–2250. [Google Scholar] [CrossRef] [PubMed]
- Feoktistov, I.; Goldstein, A.E.; Ryzhov, S.; Zeng, D.; Belardinelli, L.; Voyno-Yasenetskaya, T.; Biaggioni, I. Differential expression of adenosine receptors in human endothelial cells: Role of A2B receptors in angiogenic factor regulation. Circ. Res. 2002, 90, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Chu, X.; Deng, F.; Tong, L.; Tong, G.; Yi, Y.; Liu, J.; Tang, J.; Tang, Y.; Xia, Y.; et al. The adenosine A2b receptor promotes tumor progression of bladder urothelial carcinoma by enhancing MAPK signaling pathway. Oncotarget 2017, 8, 48755–48768. [Google Scholar] [CrossRef]
- Horigome, E.; Fujieda, M.; Handa, T.; Katayama, A.; Ito, M.; Ichihara, A.; Tanaka, D.; Gombodorj, N.; Yoshiyama, S.; Yamane, A.; et al. Mutant TP53 modulates metastasis of triple negative breast cancer through adenosine A2b receptor signaling. Oncotarget 2018, 9, 34554–34566. [Google Scholar] [CrossRef]
- Li, S.; Huang, S.; Peng, S.B. Overexpression of G protein-coupled receptors in cancer cells: Involvement in tumor progression. Int. J. Oncol. 2005, 27, 1329–1339. [Google Scholar] [CrossRef]
- Xiang, H.J.; Liu, Z.C.; Wang, D.S.; Chen, Y.; Yang, Y.L.; Dou, K.F. Adenosine A2b receptor is highly expressed in human hepatocellular carcinoma. Hepatol. Res. 2006, 36, 56–60. [Google Scholar] [CrossRef]
- Cohen, S.; Fishman, P. Targeting the A3 adenosine receptor to treat cytokine release syndrome in cancer immunotherapy. Drug Des. Devel. Ther. 2019, 13, 491–497. [Google Scholar] [CrossRef]
- Koszałka, P.; Gołuńska, M.; Urban, A.; Stasiłojć, G.; Stanisławowski, M.; Majewski, M.; Składanowski, A.C.; Bigda, J. Specific Activation of A3, A2A and A1 Adenosine Receptors in CD73-Knockout Mice Affects B16F10 Melanoma Growth, Neovascularization, Angiogenesis and Macrophage Infiltration. PLoS ONE 2016, 11, e0151420. [Google Scholar] [CrossRef]
- Marwein, S.; Mishra, B.; Chandra, D.U.; Acharya, P.C. Recent progress of adenosine receptor modulators in the development of anticancer chemotherapeutic agents. Curr. Pharm. Des. 2019, 25, 2842–2858. [Google Scholar] [CrossRef]
- Gorain, B.; Choudhury, H.; Yee, G.S.; Bhattamisra, S.K. Adenosine receptors as novel targets for the treatment of various cancers. Curr. Pharm. Des. 2019. [Epub ahead of print]. [Google Scholar] [CrossRef] [PubMed]
- Allard, B.; Beavis, P.A.; Darcy, P.K.; Stagg, J. Immunosuppressive activities of adenosine in cancer. Curr. Opin. Pharmacol. 2016, 29, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Cekic, C.; Linden, J. Adenosine A2A receptors intrinsically regulate CD8+ T cells in the tumor microenvironment. Cancer Res. 2014, 74, 7239–7249. [Google Scholar] [CrossRef] [PubMed]
- Vecchio, E.A.; White, P.J.; May, L.T. The adenosine A2B G protein-coupled receptor: Recent advances and therapeutic implications. Pharmacol. Ther. 2019, 198, 20–33. [Google Scholar] [CrossRef]
- Gao, Z.G.; Inoue, A.; Jacobson, K.A. On the G protein-coupling selectivity of the native A2B adenosine receptor. Biochem. Pharmacol. 2018, 151, 201–213. [Google Scholar] [CrossRef]
- Müller, C.E.; Baqi, Y.; Hinz, S.; Namasivayam, V. Chapter 6, Medicinal chemistry of A2B adenosine receptors. Adenosine Recept. 2018, 34, 137–168. [Google Scholar]
- Gao, Z.G.; Balasubramanian, R.; Kiselev, E.; Wei, Q.; Jacobson, K.A. Probing biased/partial agonism at the G protein-coupled A2B adenosine receptor. Biochem. Pharmacol. 2014, 90, 297–306. [Google Scholar] [CrossRef]
- Alnouri, M.W.; Jepards, S.; Casari, A.; Schiedel, A.C.; Hinz, S.; Müller, C.E. Selectivity is species-dependent: Characterization of standard agonists and antagonists at human, rat, and mouse adenosine receptors. Purinergic Signal. 2015, 11, 389–407. [Google Scholar] [CrossRef] [Green Version]
- Dixon, A.K.; Gubitz, A.K.; Sirinathsinghji, D.J.; Richardson, P.J.; Freeman, T.C. Tissue distribution of adenosine receptor mRNAs in the rat. Br. J. Pharmacol. 1996, 118, 1461–1468. [Google Scholar] [CrossRef] [Green Version]
- Yang, D.; Zhang, Y.; Nguyen, H.G.; Koupenova, M.; Chauhan, A.K.; Makitalo, M.; Jones, M.R.; St Hilaire, C.; Seldin, D.C.; Toselli, P.; et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Invest. 2006, 116, 1913–1923. [Google Scholar] [CrossRef]
- Haskó, G.; Csóka, B.; Németh, Z.H.; Vizi, E.S.; Pacher, P. A2B adenosine receptors in immunity and inflammation. Trends Immunol. 2009, 30, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Neumann, C.A.; Levine, K.; Oesterreich, S. Targeting adenosine receptor 2B in triple negative breast cancer. J. Cancer Metastasis Treat. 2018, 4, 13. [Google Scholar] [CrossRef] [Green Version]
- Fiebich, B.L.; Biber, K.; Gyufko, K.; Berger, M.; Bauer, J.; van Calker, D. Adenosine A2b receptors mediate an increase in interleukin (IL)-6 mRNA and IL-6 protein synthesis in human astroglioma cells. J. Neurochem. 1996, 66, 1426–1431. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, H.; Ichimura, M.; Takeda, M.; Nonaka, Y.; Shimada, J.; Suzuki, F.; Yamaguchi, K.; Kase, H. KF17837 ((E)-8-(3,4-dimethoxystyryl)-1,3-dipropyl-7-methylxanthine), a potent and selective adenosine A2 receptor antagonist. Eur. J. Pharmacol. 1994, 267, 335–341. [Google Scholar] [CrossRef]
- Kalhan, A.; Gharibi, B.; Vazquez, M.; Jasani, B.; Neal, J.; Kidd, M.; Modlin, I.M.; Pfragner, R.; Rees, D.A.; Ham, J. Adenosine A2A and A2B receptor expression in neuroendocrine tumours: Potential targets for therapy. Purinergic Signal. 2012, 8, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Merighi, S.; Simioni, C.; Gessi, S.; Varani, K.; Mirandola, P.; Tabrizi, M.A.; Baraldi, P.G.; Borea, P.A. A2B and A3 adenosine receptors modulate vascular endothelial growth factor and interleukin-8 expression in human melanoma cells treated with etoposide and doxorubicin. Neoplasia 2009, 11, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Rao, X.; Deiuliis, J.; Braunstein, Z.; Narula, V.; Hazey, J.; Mikami, D.; Needleman, B.; Satoskar, A.R.; Rajagopalan, S. A potential role for dendritic cell/macrophage-expressing DPP4 in obesity-induced visceral inflammation. Diabetes 2013, 62, 149–157. [Google Scholar] [CrossRef]
- Yan, A.; Joachims, M.L.; Thompson, L.F.; Miller, A.D.; Canoll, P.D.; Bynoe, M.S. CD73 Promotes Glioblastoma Pathogenesis and Enhances Its Chemoresistance via A2B Adenosine Receptor Signaling. J. Neurosci. 2019, 39, 4387–4402. [Google Scholar] [CrossRef]
- Giacomelli, C.; Daniele, S.; Romei, C.; Tavanti, L.; Neri, T.; Piano, I.; Celi, A.; Martini, C.; Trincavelli, M.L. The A2B adenosine receptor modulates the epithelial– mesenchymal transition through the balance of cAMP/PKA and MAPK/ERK pathway activation in human epithelial lung cells. Front Pharmacol. 2018, 9, 54. [Google Scholar] [CrossRef]
- Rivkees, S.A.; Reppert, S.M. RFL9 encodes an A2b-adenosine receptor. Mol. Endocrinol. 1992, 6, 1598–1604. [Google Scholar]
- Pierce, K.D.; Furlong, T.J.; Selbie, L.A.; Shine, J. Molecular cloning and expression of an adenosine A2b receptor from human brain. Biochem. Biophys. Res. Commun. 1992, 187, 86–93. [Google Scholar] [CrossRef]
- Schulte, G.; Fredholm, B.B. The Gs-coupled adenosine A2B receptor recruits divergent pathways to regulate ERK1/2 and p38. Exp. Cell Res. 2003, 290, 168–176. [Google Scholar] [CrossRef]
- Xu, Y.; Ravid, K.; Smith, B.D. Major histocompatibility class II transactivator expression in smooth muscle cells from A2b adenosine receptor knock-out mice: Cross-talk between the adenosine and interferon-gamma signaling. J. Biol. Chem. 2008, 283, 14213–14220. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Tamplin, O.J.; Chen, M.J.; Deng, Q.; Patterson, S.; Kim, P.G.; Durand, E.M.; McNeil, A.; Green, J.M.; Matsuura, S.; et al. Adenosine signaling promotes hematopoietic stem and progenitor cell emergence. J. Exp. Med. 2015, 212, 649–663. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.; Olah, M.E. Cyclic AMP-dependent.; protein kinase A-independent activation of extracellular signal-regulated kinase 1/2 following adenosine receptor stimulation in human umbilical vein endothelial cells: Role of exchange protein activated by cAMP 1 (Epac1). J. Pharmacol. Exp. Ther. 2007, 322, 1189–1200. [Google Scholar] [CrossRef] [PubMed]
- Ntantie, E.; Gonyo, P.; Lorimer, E.L.; Hauser, A.D.; Schuld, N.; McAllister, D.; Kalyanaraman, B.; Dwinell, M.B.; Auchampach, J.A.; Williams, C.L. An adenosine-mediated signaling pathway suppresses prenylation of the GTPase Rap1B and promotes cell scattering. Sci. Signal. 2013, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Mundell, S.J.; Olah, M.E.; Panettieri, R.A.; Benovic, J.L.; Penn, R.B. Regulation of G protein-coupled receptor-adenylyl cyclase responsiveness in human airway smooth muscle by exogenous and autocrine adenosine. Am. J. Respir. Cell Mol. Biol. 2001, 24, 155–163. [Google Scholar] [CrossRef]
- Koussémou, M.; Lorenz, K.; Klotz, K.N. The A2B adenosine receptor in MDA-MB-231 breast cancer cells diminishes ERK1/2 phosphorylation by activation of MAPK-phosphatase-1. PLoS ONE 2018, 13, e0202914. [Google Scholar] [CrossRef]
- Ou, Y.; Chan, G.; Zuo, J.; Rattner, J.B.; van der Hoorn, F.A. Purinergic A2b Receptor Activation by Extracellular Cues Affects Positioning of the Centrosome and Nucleus and Causes Reduced Cell Migration. J. Biol. Chem. 2016, 291, 15388–15403. [Google Scholar] [CrossRef]
- Phosri, S.; Arieyawong, A.; Bunrukchai, K.; Parichatikanond, W.; Nishimura, A.; Nishida, M.; Mangmool, S. Stimulation of Adenosine A2B Receptor Inhibits Endothelin-1-Induced Cardiac Fibroblast Proliferation and α-Smooth Muscle Actin Synthesis Through the cAMP/Epac/PI3K/Akt-Signaling Pathway. Front Pharmacol. 2017, 8, 428. [Google Scholar] [CrossRef]
- Lim, B.; Jung, K.; Gwon, Y.; Oh, J.G.; Roh, J.I.; Hong, S.H.; Kho, C.; Park, W.J.; Lee, H.W.; Bae, J.W.; et al. Cardioprotective role of APIP in myocardial infarction through ADORA2B. Cell Death Dis. 2019, 10, 511. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Liang, D.; Tian, Y.; Kron, I.L.; French, B.A.; Yang, Z. Infarct-Sparing Effect of Adenosine A2B Receptor Agonist Is Primarily Due to Its Action on Splenic Leukocytes Via a PI3K/Akt/IL-10 Pathway. J. Surg. Res. 2018, 232, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Tang, G.; Gao, P.; Zhang, B.; Xiao, H.; Si, L.Y. Activation of adenosine A2b receptor attenuates high glucose-induced apoptosis in H9C2 cells via PI3K/Akt signaling. In Vitro Cell. Dev. Biol. Anim. 2018, 54, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Hinz, S.; Alnouri, W.M.; Pleiss, U.; Müller, C.E. Tritium-labeled agonists as tools for studying adenosine A2B receptors. Purinergic Signal. 2018, 14, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, M.; Hinz, S.; Deuther-Conrad, W.; Namasivayam, V.; Dukic-Stefanovic, S.; Teodoro, R.; Toussaint, M.; Kranz, M.; Juhl, C.; Steinbach, J.; et al. Radiosynthesis and in vivo evaluation of a fluorine-18 labeled pyrazine based radioligand for PET imaging of the adenosine A2B receptor. Bioorg. Med. Chem. 2018, 26, 4650–4663. [Google Scholar] [CrossRef] [PubMed]
- Barresi, E.; Giacomelli, C.; Daniele, S.; Tonazzini, I.; Robello, M.; Salerno, S.; Piano, I.; Cosimelli, B.; Greco, G.; Da Settimo, F.; et al. Novel fluorescent triazinobenzimidazole derivatives as probes for labelling human A1 and A2B adenosine receptor subtypes. Bioorg. Med. Chem. 2018, 26, 5885–5895. [Google Scholar] [CrossRef]
- Köse, M.; Gollos, S.; Karcz, T.; Fiene, A.; Heisig, F.; Behrenswerth, A.; Kieć-Kononowicz, K.; Namasivayam, V.; Müller, C.E. Fluorescent-Labeled Selective Adenosine A2B Receptor Antagonist Enables Competition Binding Assay by Flow Cytometry. J. Med. Chem. 2018, 61, 4301–4316. [Google Scholar] [CrossRef]
- Fredholm, B.B.; Irenius, E.; Kull, B.; Schulte, G. Comparison of the potency of adenosine as an agonist at human adenosine receptors expressed in Chinese hamster ovary cells. Biochem. Pharmacol. 2001, 61, 443–448. [Google Scholar] [CrossRef]
- Hinz, S.; Lacher, S.K.; Seibt, B.F.; Müller, C.E. BAY60-6583 acts as a partial agonist at adenosine A2B receptors. J. Pharmacol. Exp. Ther. 2014, 349, 427–436. [Google Scholar] [CrossRef]
- Baltos, J.A.; Vecchio, E.A.; Harris, M.A.; Qin, C.X.; Ritchie, R.H.; Christopoulos, A.; White, P.J.; May, L.T. Capadenoson, a clinically trialed partial adenosine A1 receptor agonist, can stimulate adenosine A2B receptor biased agonism. Biochem. Pharmacol. 2017, 135, 79–89. [Google Scholar] [CrossRef]
- Beukers, M.W.; Chang, L.C.; von Frijtag Drabbe Künzel, J.K.; Mulder-Krieger, T.; Spanjersberg, R.F.; Brussee, J.; IJzerman, A.P. New, non-adenosine, high-potency agonists for the human adenosine A2B receptor with an improved selectivity profile compared to the reference agonist N-ethylcarboxamidoadenosine. J. Med. Chem. 2004, 47, 3707–3709. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.C.; Ji, X.D.; Melman, N.; Linden, J.; Jacobson, K.A. Anilide derivatives of an 8-phenylxanthine carboxylic congener are highly potent and selective antagonists at human A2B adenosine receptors. J. Med. Chem. 2000, 43, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Seel, C.J.; Temirak, A.; Namasivayam, V.; Arridu, A.; Schabikowski, J.; Baqi, Y.; Hinz, S.; Hockemeyer, J.; Müller, C.E. A2B adenosine receptor antagonists with picomolar potency. J. Med. Chem. 2019, 62, 4032–4055. [Google Scholar] [CrossRef] [PubMed]
- Elzein, E.; Kalla, R.V.; Li, X.; Perry, T.; Gimbel, A.; Zeng, D.; Lustig, D.; Leung, K.; Zablocki, J. Discovery of a novel A2B adenosine receptor antagonist as a clinical candidate for chronic inflammatory airway diseases. J. Med. Chem. 2008, 51, 2267–2278. [Google Scholar] [CrossRef]
- Eastwood, P.; Esteve, C.; González, J.; Fonquerna, S.; Aiguadé, J.; Carranco, I.; Doménech, T.; Aparici, M.; Miralpeix, M.; Albertí, J.; et al. Discovery of LAS101057: A potent, selective, and orally efficacious A2B adenosine receptor antagonist. ACS Med. Chem. Lett. 2011, 2, 213–218. [Google Scholar] [CrossRef]
- Walters, M.J.; Piovesan, D.; Tan, J.B.; DiRenzo, D.; Yin, F.; Miles, D.; Leleti, M.R.; Park, T.; Soriano, F.; Sharif, E.; et al. Combining adenosine receptor inhibition with AB928 and chemotherapy results in greater immune activation and tumor control. Cancer Res. 2018, 78, 5556. [Google Scholar]
- El Maatougui, A.; Azuaje, J.; González-Gómez, M.; Miguez, G.; Crespo, A.; Carbajales, C.; Escalante, L.; García-Mera, X.; Gutiérrez-de-Terán, H.; Sotelo, E. Discovery of potent and highly selective A2B adenosine receptor antagonist chemotypes. J. Med. Chem. 2016, 59, 1967–1983. [Google Scholar] [CrossRef]
- Fernandez-Gallardo, M.; González-Ramírez, R.; Sandoval, A.; Felix, R.; Monjaraz, E. Adenosine Stimulate Proliferation and Migration in Triple Negative Breast Cancer Cells. PLoS ONE 2016, 11, e0167445. [Google Scholar] [CrossRef]
- Vecchio, E.A.; Tan, C.Y.; Gregory, K.J.; Christopoulos, A.; White, P.J.; May, L.T. Ligand-Independent Adenosine A2B receptor constitutive activity as a promoter of prostate cancer cell proliferation. J. Pharmacol. Exp. Ther. 2016, 357, 36–44. [Google Scholar] [CrossRef]
- Stagg, J.; Divisekera, U.; McLaughlin, N.; Sharkey, J.; Pommey, S.; Denoyer, D.; Dwyer, K.M.; Smyth, M.J. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl. Acad. Sci. USA 2010, 107, 1547–1552. [Google Scholar] [CrossRef] [Green Version]
- Thimm, D.; Schiedel, A.C.; Sherbiny, F.F.; Hinz, S.; Hochheiser, K.; Bertarelli, D.C.; Maass, A.; Müller, C.E. Ligand-specific binding and activation of the human adenosine A2B receptor. Biochemistry 2013, 52, 726–740. [Google Scholar] [CrossRef] [PubMed]
- Loi, S.; Dushyanthen, S.; Beavis, P.A.; Salgado, R.; Denkert, C.; Savas, P.; Combs, S.; Rimm, D.L.; Giltnane, J.M.; Estrada, M.V.; et al. RAS/MAPK Activation Is Associated with Reduced Tumor-Infiltrating Lymphocytes in Triple-Negative Breast Cancer: Therapeutic Cooperation Between MEK and PD-1/PD-L1 Immune Checkpoint Inhibitors. Clin. Cancer Res. 2019, 25, 1437. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.B.; Davis, M.I.; Caballero, S.; Feoktistov, I.; Biaggioni, I.; Belardinelli, L. Proliferation, migration, and ERK activation in human retinal endothelial cells through A2B adenosine receptor stimulation. Invest. Ophthalmol Vis. Sci. 2001, 42, 2068–2073. [Google Scholar] [PubMed]
- Liu, T.Z.; Wang, X.; Bai, Y.F.; Liao, H.Z.; Qiu, S.C.; Yang, Y.Q.; Yan, X.H.; Chen, J.; Guo, H.B.; Zhang, S.Z. The HIF-2alpha dependent induction of PAP and adenosine synthesis regulates glioblastoma stem cell function through the A2B adenosine receptor. Int. J. Biochem. Cell Biol. 2014, 49, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, W.; Yu, X.; Liu, Z.; Tarran, R.; Ravid, K.; Huang, P. Actinin-1 binds to the C-terminus of A2B adenosine receptor (A2BAR) and enhances A2BAR cell-surface expression. Biochem. J. 2016, 473, 2179–2186. [Google Scholar] [CrossRef]
- Rodrigues, S.; De Wever, O.; Bruyneel, E.; Rooney, R.J.; Gespach, C. Opposing roles of netrin-1 and the dependence receptor DCC in cancer cell invasion, tumor growth and metastasis. Oncogene 2007, 26, 5615–5625. [Google Scholar] [CrossRef] [Green Version]
- Desmet, C.J.; Gallenne, T.; Prieur, A.; Reyal, F.; Visser, N.L.; Wittner, B.S.; Smit, M.A.; Geiger, T.R.; Laoukili, J.; Iskit, S.; et al. Identification of a pharmacologically tractable Fra-1/ADORA2B axis promoting breast cancer metastasis. Proc. Natl. Acad. Sci. USA 2013, 110, 5139–5144. [Google Scholar] [CrossRef] [Green Version]
- Wilson, J.M.; Lorimer, E.; Tyburski, M.D.; Williams, C.L. β-Adrenergic receptors suppress Rap1B prenylation and promote the metastatic phenotype in breast cancer cells. Cancer Biol. Ther. 2015, 16, 1364–1374. [Google Scholar] [CrossRef]
- Belguise, K.; Kersual, N.; Galtier, F.; Chalbos, D. FRA-1 expression level regulates proliferation and invasiveness of breast cancer cells. Oncogene 2005, 24, 1434–1444. [Google Scholar] [CrossRef]
- Adiseshaiah, P.; Lindner, D.J.; Kalvakolanu, D.V.; Reddy, S.P. FRA-1 proto-oncogene induces lung epithelial cell invasion and anchorage-independent growth in vitro, but is insufficient to promote tumor growth in vivo. Cancer Res. 2007, 67, 6204–6211. [Google Scholar] [CrossRef]
- Zhao, C.; Qiao, Y.; Jonsson, P.; Wang, J.; Xu, L.; Rouhi, P.; Sinha, I.; Cao, Y.; Williams, C.; Dahlman-Wright, K. Genome-wide profiling of AP-1-regulated transcription provides insights into the invasiveness of triple-negative breast cancer. Cancer Res. 2014, 74, 3983–3994. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.N.; Youkey, R.; Liu, X.; Jia, L.; Blatt, R.; Day, Y.J.; Sullivan, G.W.; Linden, J.; Tucker, A.L. A1 adenosine receptor activation promotes angiogenesis and release of VEGF from monocytes. Circ. Res. 2007, 101, 1130–1138. [Google Scholar] [CrossRef] [PubMed]
- Feoktistov, I.; Feoktistov, I.; Ryzhov, S.; Zhong, H.; Goldstein, A.E.; Matafonov, A.; Zeng, D.; Biaggioni, I. Hypoxia modulates adenosine receptors in human endothelial and smooth muscle cells toward an A2B angiogenic phenotype. Hypertension 2004, 44, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Adair, T.H. Growth regulation of the vascular system: An emerging role for adenosine. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R283–R296. [Google Scholar] [CrossRef]
- Novitskiy, S.V.; Ryzhov, S.; Zaynagetdinov, R.; Goldstein, A.E.; Huang, Y.; Tikhomirov, O.Y.; Blackburn, M.R.; Biaggioni, I.; Carbone, D.P.; Feoktistov, I.; et al. Adenosine receptors in regulation of dendritic cell differentiation and function. Blood 2008, 112, 1822–1831. [Google Scholar] [CrossRef]
- Acurio, J.; Troncoso, F.; Bertoglia, P.; Salomon, C.; Aguayo, C.; Sobrevia, L.; Escudero, C. Potential role of A2B adenosine receptors on proliferation/migration of fetal endothelium derived from preeclamptic pregnancies. Biomed. Res. Int. 2014, 2014, 274507. [Google Scholar]
- Gu, J.W.; Ito, B.R.; Sartin, A.; Frascogna, N.; Moore, M.; Adair, T.H. Inhibition of adenosine kinase induces expression of VEGF mRNA and protein in myocardial myoblasts. Am. J. Physiol. Heart Circ. Physiol. 2000, 279, H2116–H2123. [Google Scholar] [CrossRef]
- Leibovich, S.J.; Chen, J.F.; Pinhal-Enfield, G.; Belem, P.C.; Elson, G.; Rosania, A.; Ramanathan, M.; Montesinos, C.; Jacobson, M.; Schwarzschild, M.A.; et al. Synergistic up-regulation of vascular endothelial growth factor expression in murine macrophages by adenosine A2A receptor agonists and endotoxin. Am. J. Pathol. 2002, 160, 2231–2244. [Google Scholar] [CrossRef]
- Ryzhov, S.; Biktasova, A.; Goldstein, A.E.; Zhang, Q.; Biaggioni, I.; Dikov, M.M.; Feoktistov, I. Role of JunB in adenosine A2B receptor-mediated vascular endothelial growth factor production. Mol. Pharmacol. 2014, 85, 62–73. [Google Scholar] [CrossRef]
- Sorrentino, C.; Morello, S. Role of adenosine in tumor progression: Focus on A2B receptor as potential therapeutic target. J. Cancer Metastasis Treat. 2017, 3, 127–138. [Google Scholar] [CrossRef]
- Du, X.; Ou, X.; Song, T.; Zhang, W.; Cong, F.; Zhang, S.; Xiong, Y. Adenosine A2B receptor stimulates angiogenesis by inducing VEGF and eNOS in human microvascular endothelial cells. Exp. Biol. Med. (Maywood) 2015, 240, 1472–1479. [Google Scholar] [CrossRef] [PubMed]
- Xaus, J.; Mirabet, M.; Lloberas, J.; Soler, C.; Lluis, C.; Franco, R.; Celada, A. IFN-gamma up-regulates the A2B adenosine receptor expression in macrophages: A mechanism of macrophage deactivation. J. Immunol. 1999, 162, 3607–3614. [Google Scholar] [PubMed]
- Herrera, C.; Casadó, V.; Ciruela, F.; Schofield, P.; Mallol, J.; Lluis, C.; Franco, R. Adenosine A2B receptors behave as an alternative anchoring protein for cell surface adenosine deaminase in lymphocytes and cultured cells. Mol. Pharmacol. 2001, 59, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.P.; Kameoka, J.; Hegen, M.; Tanaka, T.; Xu, Y.; Schlossman, S.F.; Morimoto, C. Characterization of adenosine deaminase binding to human CD26 on T cells and its biologic role in immune response. J. Immunol. 1996, 156, 1349–1355. [Google Scholar] [PubMed]
- Morimoto, C.; Schlossman, S.F. The structure and function of CD26 in the T-cell immune response. Immunol. Rev. 1998, 161, 55–70. [Google Scholar] [CrossRef]
- Hoskin, D.W.; Mader, J.S.; Furlong, S.J.; Conrad, D.M.; Blay, J. Inhibition of T cell and natural killer cell function by adenosine and its contribution to immune evasion by tumor cells (Review). Int. J. Oncol. 2008, 32, 527–535. [Google Scholar] [CrossRef]
- Kjaergaard, J.; Hatfield, S.; Jones, G.; Ohta, A.; Sitkovsky, M. A2A Adenosine Receptor Gene Deletion or Synthetic A2A Antagonist Liberate Tumor-Reactive CD8+ T Cells from Tumor-Induced Immunosuppression. J. Immunol. 2018, 201, 782–791. [Google Scholar] [CrossRef]
- Erdmann, A.A.; Gao, Z.G.; Jung, U.; Foley, J.; Borenstein, T.; Jacobson, K.A.; Fowler, D.H. Activation of Th1 and Tc1 cell adenosine A2A receptors directly inhibits IL-2 secretion in vitro and IL-2-driven expansion in vivo. Blood 2005, 105, 4707–4714. [Google Scholar] [CrossRef]
- Sun, X.; Liu, X.; Xia, M.; Shao, Y.; Zhang, X.D. Multicellular gene network analysis identifies a macrophage-related gene signature predictive of therapeutic response and prognosis of gliomas. J. Transl. Med. 2019, 17, 159. [Google Scholar] [CrossRef]
- Jang, J.H.; Janker, F.; De Meester, I.; Arni, S.; Borgeaud, N.; Yamada, Y.; Gil Bazo, I.; Weder, W.; Jungraithmayr, W. The CD26/DPP4-inhibitor vildagliptin suppresses lung cancer growth via macrophage-mediated NK cell activity. Carcinogenesis 2019, 40, 324–334. [Google Scholar] [CrossRef]
- Cohen, H.B.; Ward, A.; Hamidzadeh, K.; Ravid, K.; Mosser, D.M. IFN-γ Prevents Adenosine Receptor (A2bR) Upregulation to Sustain the Macrophage Activation Response. J. Immunol. 2015, 195, 3828–3837. [Google Scholar] [CrossRef] [PubMed]
- Nakatsukasa, H.; Tsukimoto, M.; Harada, H.; Kojima, S. Adenosine A2B receptor antagonist suppresses differentiation to regulatory T cells without suppressing activation of T cells. Biochem. Biophys. Res. Commun. 2011, 409, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Xia, J.; Wu, X.; Kong, H.; Wang, H.; Xie, W.; Xu, Y. Adenosine signaling inhibits CIITA-mediated MHC class II transactivation in lung fibroblast cells. Eur. J. Immunol. 2013, 43, 2162–2173. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Fang, M.; Wu, X.; Yang, Y.; Yu, L.; Xu, H.; Kong, H.; Tan, Q.; Wang, H.; Xie, W.; et al. A2b adenosine signaling represses CIITA transcription via an epigenetic mechanism in vascular smooth muscle cells. Biochim. Biophys. Acta. 2015, 1849, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Warabi, M.; Kitagawa, M.; Hirokawa, K. Loss of MHC class II expression is associated with a decrease of tumor-infiltrating T cells and an increase of metastatic potential of colorectal cancer: Immunohistological and histopathological analyses as compared with normal colonic mucosa and adenomas. Pathol. Res. Pract. 2000, 196, 807–815. [Google Scholar] [CrossRef]
- Shi, B.; Vinyals, A.; Alia, P.; Broceño, C.; Chen, F.; Adrover, M.; Gelpi, C.; Price, J.E.; Fabra, A. Differential expression of MHC class II molecules in highly metastatic breast cancer cells is mediated by the regulation of the CIITA transcription Implication of CIITA in tumor and metastasis development. Int. J. Biochem. Cell Biol. 2006, 38, 544–562. [Google Scholar] [CrossRef]
- Figueiredo, A.B.; Souza-Testasicca, M.C.; Mineo, T.W.P.; Afonso, L.C.C. Leishmania amazonensis-Induced cAMP Triggered by Adenosine A2B Receptor Is Important to Inhibit Dendritic Cell Activation and Evade Immune Response in Infected Mice. Front Immunol. 2017, 8, 849. [Google Scholar] [CrossRef]
- Sciaraffia, E.; Riccomi, A.; Lindstedt, R.; Gesa, V.; Cirelli, E.; Patrizio, M.; De Magistris, M.T.; Vendetti, S. Human monocytes respond to extracellular cAMP through A2A and A2B adenosine receptors. J. Leukoc. Biol. 2014, 96, 113–122. [Google Scholar] [CrossRef]
- Hatfield, S.M.; Sitkovsky, M. A2A adenosine receptor antagonists to weaken the hypoxia-HIF-1α driven immunosuppression and improve immunotherapies of cancer. Curr. Opin. Pharmacol. 2016, 29, 90–96. [Google Scholar] [CrossRef]
- Wiernik, P.H.; Paietta, E.; Goloubeva, O.; Lee, S.J.; Makower, D.; Bennett, J.M.; Wade, J.L.; Ghosh, C.; Kaminer, L.S.; Pizzolo, J.; et al. Eastern Cooperative Oncology Group. Phase II study of theophylline in chronic lymphocytic leukemia: A study of the Eastern Cooperative Oncology Group (E4998). Leukemia 2004, 18, 1605–1610. [Google Scholar] [CrossRef]
| Compound | A1 | A2A | A2B | A3 | Reference |
|---|---|---|---|---|---|
| Agonists | |||||
| 1, Adenosine a | 310 | 700 | 24,000 | 290 | [68] |
| 4620 c (97%) | [37] | ||||
| 3, NECA b | 14 | 20 | 1900 | 25 | [38] |
| 3, NECA a | 12 | 60 | 104 (100%) | 11 | [71] |
| 4, CPCA | 1.9b | 50b | 267 c (102%) | 108 b | [37] |
| 6, BAY68-4986 a | 0.66 | 1400 | 1.1 (93%) | 2400 | [70] |
| (Capadenoson) | 522 c,d (95%) | ||||
| 7, LUF5834 | 2.6 b | 28 b | 12 a (74%) | 538 b | [71] |
| 8, BAY60-6583 b | 390 | >10,000 | 110 | 220 | [38] |
| 242 c (73%) | [37] | ||||
| 6.1 e (102%) | [37] | ||||
| Antagonists | |||||
| 11, Theophylline b | 6200 | 1710 | 7850 | 22,300 | |
| 12, Caffeine b | 44,900 | 9560 | 33,800 | 13,300 | [38] |
| 13, MRS1754 b | 403 | 503 | 1.7 | 570 | [38] |
| 14, MRS1706 b | 157 | 112 | 1.4 | 230 | [38] |
| 18, GS6201 b (CVT-6833) | 1940 | 3280 | 22 | 1070 | [74] |
| 21, PSB-1115 b | >10,000 | 3790 | 53.4 | >10,000 | [38] |
| 22a, PSB-603 b | >10,000 | >10,000 | 0.55 | >1000 | [38] |
| 22b, PSB-1901 b | >1000 | >1000 | 0.060 | >1000 | [73] |
| 23, PSB-0788 b | 2240 | 333 | 0.39 | >1000 | [38] |
| 27, LAS101057 b | >10,000 | 2500 | 24 | >10,000 | [75] |
| 26, AB928 b | 64 | 1.5 | 2.0 | 489 | [76] |
| 27, ISAM140b | >10,000 | >10,000 | 0.55 | >1000 | [77] |
| PBF-1129 | nd | nd | nd | nd |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.-G.; Jacobson, K.A. A2B Adenosine Receptor and Cancer. Int. J. Mol. Sci. 2019, 20, 5139. https://doi.org/10.3390/ijms20205139
Gao Z-G, Jacobson KA. A2B Adenosine Receptor and Cancer. International Journal of Molecular Sciences. 2019; 20(20):5139. https://doi.org/10.3390/ijms20205139
Chicago/Turabian StyleGao, Zhan-Guo, and Kenneth A. Jacobson. 2019. "A2B Adenosine Receptor and Cancer" International Journal of Molecular Sciences 20, no. 20: 5139. https://doi.org/10.3390/ijms20205139
APA StyleGao, Z.-G., & Jacobson, K. A. (2019). A2B Adenosine Receptor and Cancer. International Journal of Molecular Sciences, 20(20), 5139. https://doi.org/10.3390/ijms20205139