Abstract
Hematopoietic stem cell transplantation (HSCT) remains the only curative treatment for several malignant and non-malignant diseases at the cost of serious treatment-related toxicities (TRTs). Recent research on extending the benefits of HSCT to more patients and indications has focused on limiting TRTs and improving immunological effects following proper mobilization and engraftment. Increasing numbers of studies report associations between HSCT outcomes and the expression or the manipulation of G protein-coupled receptors (GPCRs). This large family of cell surface receptors is involved in various human diseases. With ever-better knowledge of their crystal structures and signaling dynamics, GPCRs are already the targets for one third of the current therapeutic arsenal. The present paper assesses the current status of animal and human research on GPCRs in the context of selected HSCT outcomes via a systematized survey and analysis of the literature.
1. Introduction
1.1. Hematopoietic Stem Cell Transplantation (HSCT)
The field of hematopoietic stem cell transplantation (HSCT) has witnessed t remendous progress since its origins in the 1950s [1]#. The number of HSCTs has exploded, along with its range of indications, candidates, and donor sources [2,3]#. HSCT remains indispensable for treating several malignant and non-malignant disorders. The use of peripheral blood stem cells (PBSCs) is well established in autologous transplantation [4]#, and they have become the preferred source of allogeneic hematopoietic stem cells (HSCs), at least in adults [5,6]#. In both scenarios, the number of circulating HSCs mobilized from the bone marrow is closely associated with the engraftment outcome [7]#. Before the graft infusion, most HSCT protocols require a preparation phase, which aims to kill malignant cells to make room for the newly infused HSCs to engraft or to induce immunosuppression. The latter is important to avoid graft rejection and graft-versus-host disease (GvHD) in allogeneic settings. This so-called conditioning regimen comprises high doses of chemotherapeutic drugs and/or radiotherapy that cause a cytotoxic burst of tumor and/or normal cells. This results in a pro-inflammatory status [8,9]#, which is desired when treating malignant conditions with allogeneic HSCT, as it promotes a graft-versus-leukemia (GvL) effect. On the other hand, uncontrolled inflammation results in serious treatment-related toxicities (TRTs), such as sinusoidal obstruction syndrome (SOS), lung toxicity, or GvHD, the negative counterpart of GvL. Recent research efforts have focused on limiting transplantation- or treatment-related mortality (TRM) and TRTs while improving the beneficial immunological effects after adequate mobilization and engraftment.
1.2. G Protein-Coupled Receptors (GPCRs)
Human cells express some 400 non-olfactory G protein-coupled receptors (GPCRs) [10]# that respond to a large variety of ligands and thereby affect key cellular functions such as survival or proliferation [11]#. GPCRs, also known as seven-transmembrane spanning receptors (7TMRs), are classified into five subfamilies based on sequence homology: glutamate, rhodopsin, adhesion, frizzled/taste2, and secretin [12]#. Mechanistically, a ligand binding to its cognate GPCR induces the dissociation and the activation of α and βγ subunits in the associated G protein. This signaling ceases upon GPCR phosphorylation by a GPCR kinase (GRK), which causes the binding of a β-arrestin and GPCR endocytosis [13]#. Regulation occurs at multiple levels, whereas GRKs and β-arrestins can also generate G protein-independent GPCR signaling [14,15,16,17]#. GPCRs or their ligands are targeted by a third of all approved drugs, and many more are in development [18]#. Their ubiquity and location at the cell surface make them attractive targets. In addition, many GPCR-ligand crystal structures are already available, and steady progress in the fields of crystallization and molecular modeling has facilitated GPCR drug development [19,20]#. For instance, the discovery of “biased” GPCR agonists, which can differentially activate G protein-dependent and G protein-independent signaling, holds the promise of fine-tuning the pharmacological modulation of GPCRs [21,22]#. Importantly, GPCRs have been linked to multiple human diseases [23,24,25,26]#, and their roles in regulating inflammation are increasingly recognized. Lipid mediators produced by innate immune cells such as the eicosanoids, which include prostaglandins, thromboxanes, and leukotrienes, signal via GPCRs to initiate acute inflammation [27]#. Other related classes of GPCR lipid ligands, such as endocannabinoids [28]#, sphingolipids [29]#, or even the so-called specialized pro-resolving mediators (SPMs) [30]#, also participate in the regulation of inflammation. In turn, chemokines can activate, via GPCRs, both innate and adaptive immune cells and regulate their traffic between lymphoid organs and inflammatory sites [31]#. Finally, although the Beta-2 adrenergic receptor (B2AR) was the first GPCR ever cloned [32]#, the role of adrenergic receptors in modulating immunity and inflammation has only recently been brought to light [33,34,35]#. Discussing time and tissue-specific expression patterns of each GPCR is beyond the scope of this review, but this type of information can be found in part in the references above or in public databases such as the Human Protein Atlas (HPA) or the Genotype-Tissue Expression (GTEx) Portal.
1.3. HSCT and GPCR: Plerixafor and Beyond
C-X-C receptor 4 (CXCR4) is a noticeable example of the convergence between GPCRs and HSCT. C-X-C ligand 12 (CXCL12) is a chemokine produced by the stromal cells populating the hematopoietic niche in the bone marrow (BM) [36]#. CXCL12 exerts its function by binding CXCR4, a GPCR present on the surface of hematopoietic cells. That interaction is essential for the homing and the maintenance of HSCs in the BM [37]#. In 2008, the Food and Drug Administration (FDA) approved the use of the first-in-class CXCR4-inhibitor, plerixafor (AMD3100; Mozobil®), in association with granulocyte colony-stimulating factor (G-CSF) for the mobilization and the collection of PBSC in patients with non-Hodgkin’s lymphoma (NHL) and multiple myeloma (MM) [38,39,40]#. In this regard, the present paper assesses the new developments concerning plerixafor, which have occurred since the last systematic review on the topic [41]#. Importantly, this paper also surveys the available evidence linking other GPCRs to HSCT outcomes, for which there was not any existing comprehensive review (see the Appendix A1). With this in mind, we prepared a systematized search of the medical literature.
2. Results and Discussion
2.1. Mobilization
Mobilization of HSC from the BM into peripheral blood (PB) is usually measured by the number of circulating CD34+ and/or nucleated blood cells harvested using leukapheresis [42]#. The standard mobilization agent is recombinant granulocyte colony-stimulating factor (G-CSF; filgrastim or lenograstim), an endogenous growth factor responsible for inducing granulocyte expansion and maturation in times of infection or stress [43]#.
Within the group of chemokines (Table 1), the CXCL12 3’UTR A allele (rs1801157; g.44372809G>A) has shown positive correlation with mobilization in both healthy donors and patients undergoing autologous transplantation [44,45,46]. The functional consequence of this CXCL12 (SDF-1) polymorphism is still unclear, but it may lead to lower protein levels [47]#. This would concur with abounding evidence on CXCR4, the CXCL12 receptor, whose blockade promotes mobilization when using plerixafor.

Table 1.
Mobilization in human (H) studies. Mobilization is measured by the number of circulating CD34+ (HSC) and/or nucleated blood cells harvested using leukapheresis. See the Methods section regarding the reporting of results (Section 3.2).
As expected, several publications on plerixafor (47) were relevant, with most assessing mobilization for autologous HSCT in MM and lymphoma patients. Although an improvement may be achieved by increasing the dose [48]#, plerixafor has been demonstrated to be less efficient as a monotherapy than in combination with G-CSF [49]. Interestingly, in patients responding poorly to G-CSF (< 20 × 106/L CD34+ cells in PB), pre-emptive plerixafor treatment led to a final yield equivalent to a rescue strategy administered to patients with insufficient leukapheresis [50]. Several additional studies have endorsed the use of plerixafor in autologous transplantation for diabetic patients [51] and pediatric patients [52,53,54], whereas other articles have supported its use in elderly patients and those with renal insufficiency [55,56]#.
Two early studies also showed plerixafor to be efficient in mobilizing healthy allogeneic donors with a reasonable safety profile [57,58], and this was later reported by a phase I/II trial [59]#. Examining these varied studies, an extension of plerixafor indications is to be expected in the coming years, as are new pharmacological alternatives. Indeed, new compounds targeting CXCR4 are in development: small molecules (TG-0054 [60,61,62]) such as plerixafor, but also peptides (BL-8040 [63], (BK)T140 [64], POL6326 [65], LY2510924 [66]), or oligonucleotides (NOX-A12 [67]). All have already been tested in humans as part of phase I or early phase II clinical trials.
Finally, although the CD34+ count in PB remains the most used predictor for guiding cost-efficient mobilization regimens [68]#, new biomarkers are being eagerly sought to improve individualized prescriptions. Nonetheless, the expression of CXCR4 in CD34+ HSC in correlation with mobilization has thus far shown discordant findings [69,70,71], and additional studies are needed.
2.2. Engraftment
Engraftment in humans is assessed in PB and defined by the stable recovery of blood cell counts after myeloablative conditioning and graft infusion: platelets > 50 × 109/L in the absence of transfusion (platelet engraftment); or neutrophils > 500 × 106/L (neutrophil engraftment) [105]#. In allogeneic HSCT, additional genetic testing for chimerism is performed to confirm the donor origin of the hematopoietic recovery [106,107]#. The absence of engraftment or the loss of donor cells after initial engraftment constitute primary and secondary graft failure (GF), respectively [108]#. In animal studies, mostly on mice, competitive repopulation assays allow for a much larger toolkit of measurements of HSC engraftment capacity [109]#.
The use of anti-CXCR4 compounds for mobilization in the donor did not preclude engraftment in humans [61,85,94,95,110,111] or mice [112], with some studies reporting even better engraftment in mice [113,114] (Table 2). Targeting CXCR4 could also improve engraftment by vacating the hematopoietic niches in the recipient before HSCT, either via chimeric antigen receptor (CAR) T cells co-expressing CXCR4 and C-kit or via plerixafor [115,116,117]. Despite discordant results in mice [118], plerixafor administration post-HSCT in human recipients improved engraftment in one phase I/II clinical trial [119]. In this study, “mobilizing” doses of plerixafor were started from day 2 post-HSCT and continued until day 21 or neutrophil engraftment.

Table 2.
Engraftment in animal (A) or human (H) studies. In humans, engraftment is measured by either the time to platelet/neutrophil recovery, chimerism, or the absence of graft failure. In animals, genetic manipulation allows for various measures of engraftment. See the Methods section regarding the reporting of results (Section 3.2).
Conversely, CXCR4 expression in both mice and human cells correlated positively with autologous and xeno-engraftment [120,121]. In humans, following G-CSF mobilization, CXCR4 expression showed a positive correlation with engraftment [122,123,124]. Surprisingly, here, the CXCL12 3’UTR A polymorphism whose occurrence had been associated with increased mobilization (see the Mobilization subsection) was associated with faster hematopoietic recovery in autologous transplant patients [125]. Indeed, if it really decreased protein expression, one would expect reduced homing of the graft CXCR4+ HSC by CXCL12-expressing stromal cells. However, more research seems warranted to define the timing of CXCR4 requirements both before and during the course of engraftment.
CXCL12-CXCR4 may also act indirectly. Prostaglandin E2 (PGE2) ex vivo treatment of murine HSC improved their BM homing and engraftment through increased expression of CXCR4 [126,127,128,129]. Similarly, inhibition of Bone Morphogenetic Protein (BMP) signaling in recipients increased CXCL12 levels and engraftment [130]. In a zebrafish model, CXCL8/CXCR1 expression by endothelial cells in the hematopoietic niche helped HSC engraftment, partly via CXCL12 upregulation [131].
Concerning other chemokines, high levels of interferon gamma-dependent CXCL9 [132,133] have been associated with GF in humans. In mice, knocking out (CXCR2) delayed hematopoietic recovery [134]. On the other hand, CCR1 expression marked human HSC as responsible for high levels of xeno-engraftment in mice [135]. These are some examples of the contribution of chemokines to hematopoietic-niche integrity.
There is less evidence available for other classes of GPCR. For instance, the engraftment of cells mobilized by cannabinoid receptor 2 (CB2) agonism [136] in animals or Beta-3 adrenergic receptor (B3AR) agonism [102] in humans was equivalent to those mobilized by G-CSF. Frizzled-6 (Fzd-6), a class F GPCR for Wnt protein ligands [137]#, is another potential contributor, as it was shown to be necessary for BM reconstitution beyond the homing phase [138]. A potentially clinically relevant finding is the presence of auto-antibodies activating Angiotensin 1 receptor (AT1R) in human allogeneic HSCT recipients, described in auto-immune settings [139]# and solid organ allo-rejection [140]#, and their association with decreased engraftment[141].
2.3. Sinusoidal Obstruction Syndrome (SOS)
Some early HSCT complications such as thrombotic microangiopathy and SOS are initiated by endothelial cell damage [156,157]#. SOS, formerly called veno-occlusive disease of the liver (VOD), occurs in 5–60% of HSCT patients, depending on prophylaxis and risk factors [158,159,160]# such as the underlying disease, the use of alkylating agents for conditioning, patient age, or liver disease. Sinusoidal endothelial cell damage is the key step in the pathophysiology of SOS, leading to the activation of the coagulation cascade, centrilobular thrombosis and consequent post-sinusoidal hepatic hypertension and, potentially, multiple-organ failure [157]#. Clinically, SOS is characterized by jaundice, fluid retention, painful hepatomegaly, and often thrombocytopenia refractory to transfusion [160,161]#.
Our review strategy identified no direct associations between any GPCRs and SOS occurrence or severity, yet some additional reports caught our attention. For example, recombinant thrombomodulin (rTM) is approved in Japan to treat disseminated intravascular coagulation (DIC) and has been shown to reduce SOS and the occurrence of thrombotic microangiopathy in HSCT patients [162,163]#. In two murine SOS models, one using monocrotaline (MCT) and the other using busulfan/cyclophosphamide conditioning followed by HSCT, rTM’s cytoprotective effect was demonstrated to depend on its fifth epidermal growth factor-like region (TME5) [164,165]#. A murine model of tacrolimus-induced vascular injury showed that the pro-angiogenic functions of TME5 depended on its binding to G protein-coupled receptor (GPR) 15 [165,166]#. rTM was able to mitigate aGvHD in mice in a GPR15-dependent manner [167]#. However, this GPR15 dependency has yet to be demonstrated directly for SOS in vivo. Interestingly, the oligonucleotide—defibrotide—the only FDA/European Medicines Agency (EMA)-approved drug for the treatment of SOS [168]#, was shown to increase thrombomodulin expression in humans [169]#.
A traditional Japanese medicine called Dai-kenchu-to (DKT) was able to attenuate liver damage but not prevent the development of SOS induced by MCT [170]#. As a potential mechanism, MCT-induced CXCL1 (or CINC1) upregulation was suppressed in the DKT-treatment group, which could be a potential mechanism for explaining the associated reduction of neutrophil accumulation in the liver.
2.4. Graft-Versus-Host Disease (GvHD)
2.4.1. Acute GvHD
Acute GvHD (aGvHD) occurs when naïve T cells from an allogeneic donor are activated by recipient or donor antigen-presenting cells to attack recipient cells [171]#. This process is triggered by the inflammatory setting of HSCT. Once activated within lymph nodes, the alloreactive effector T cells migrate to the skin, the gastrointestinal (GI) tract, or the liver, causing further inflammation and damage [172]#. Some of the main determinants of aGvHD risk are the sources of HSCs themselves, donor–recipient HLA mismatches, the intensity of the conditioning regimen, and the absence of any GvHD prophylaxis [173]#. Immunosuppression is systematically used to prevent and treat aGvHD [174]#. Like other immune cells, T cell trafficking is regulated by myriad chemo-attractants, including chemokines. A study of the expression kinetics of a panel of chemokines and receptors in GvHD-target organs following allo-HSCT compared that expression to the histopathological changes occurring in the same organs [175]#. Characterization of the individual contributions of each chemokine/receptor would be needed to make further conclusions, but it highlights that aGvHD is a dynamic process with a complex spatiotemporal network of chemo-attractants at play.
A number of chemokines or their receptors are associated with the development of aGvHD (Table 3). For instance, higher CCL8 levels correlated with more severe murine aGvHD [176], and CCR2 expression on CD8+ effector T cells was necessary for their migration to the murine gut and the liver and for the generation of aGvHD [177]. In contrast, broad inhibition of CCL2, CCL3, and CCL5 reduced murine liver aGvHD [178]. Also in mice, anti-CD3 treatment during preconditioning reduced aGvHD by limiting both CCR7+ dendritic cells homing to lymph nodes and CCR9+ effector T cells homing to aGvHD target organs without reducing GvL [179]. In humans, both a CCL5 (RANTES; Regulated on Activation, Normal T Cell Expressed and Secreted) haplotype of three polymorphisms [180] and the expression of the CX3CL1/CX3CR1 pair [181] positively correlated with the occurrence of aGvHD. Depending on the cells bearing GPCRs, other chemokine receptors can prevent aGvHD. The presence of CCR8 on regulatory T cells (Tregs) is crucial to their anti-GvHD action in mice [182], whereas Chem23R, another chemo-attractant receptor [183], prevents intestinal aGvHD in mice [183]. The CXCL12 3’UTR A allele previously discussed for mobilization and engraftment was here associated with reduced risk and severity of aGvHD [184], highlighting the favorable prognosis carried by this allele. The anti-CCR4 antibody, mogamulizumab, is currently approved for human use before HSCT to treat certain adult T-cell leukemias. This might accelerate subsequent aGvHD because it not only targets CCR4+ tumor cells but also CCR4+ Tregs [185,186]. Higher CCR5 and CCR9 levels were detected on children’s memory effector T cells before they developed GI aGvHD [187].

Table 3.
Acute GvHD occurrence/severity in animal (A) or human (H) studies. See the Methods section regarding the reporting of results (Section 3.2).
CCR5 is particularly interesting and is used by human immunodeficiency virus (HIV) as a co-receptor for entry into CD4+ T cells, thus partly explaining the genetic susceptibility to HIV infection [188]#. Maraviroc, a CCR5-antagonist, was approved in 2016 for the treatment of HIV. In the context of HSCT, the CCR5 ∆32 mutation was first associated with lower aGvHD [189,190]. Several related studies subsequently showed different subgroups of CCR5+/CD4+ T cells could be associated with intestinal aGvHD [191,192,193]. Similarly, dendritic cells expressing CCR5 could be associated with aGvHD [194,195,196], showing that CCR5 could be a chemo-attractant for several causative immune cell types. Two phase I/II trials have now tested the safety and the efficacy of CCR5 blockade using maraviroc for the prevention of aGvHD. The first trial, conducted in adults [197], proved successful and led to follow-up studies by the same group of researchers [198,199,200,201,202] as well as an ongoing phase II study (NCT01785810). Another trial [203], published in 2019, included adults and children but had inconclusive findings due to unrelated toxicities. According to its authors, CCR5 blockade could prevent lymphocyte homing but not their activation, highlighting the temporal complexity of immune activation. In mice, three studies have shown the absence of CCR5 to accelerate aGvHD [204,205,206]. Nevertheless, more recent studies demonstrated that another anti-CCR5 antibody, (PRO-140) [207], or maraviroc combined with either cyclosporine A [208,209] or CXCR3 blockade [210], could indeed prevent aGvHD in mice. CXCR3 also demonstrates a compelling case. One study showed that CXCR3-expressing Tregs could mitigate aGvHD [211], and more recent studies indicated a positive correlation between CXCR3 expression and aGvHD in mice [210,212,213,214,215,216,217] and humans [200,218]. One of these proposed CXCR3-signaling as a resistance mechanism to CCR5 blockade [200]. As for CCR6 and 7 [179,194,195,219,220,221,222,223,224] or CXCR2 and 4 [225,226,227,228,229], the evidence has been too heterogeneous and conflicting to draw any conclusions. Using combined blockade at several steps in the immune activation underlying aGvHD could create a synergy to reduce its severity. However, additional studies are needed, especially to assess the potential of abrogating the GvL effect with such an approach.
Among adrenergic receptors, alpha-2 adrenergic receptor (A2AR) agonism [230,231] or beta-adrenergic receptor (BAR) activation under stressful conditions [232,233] was associated with lower aGvHD in mice, and so was P2Y2 knock-out [234]. The previously mentioned AT1R auto-antibodies were also revealed to be associated with increased aGvHD in humans [141]. Some interesting candidates have also emerged from the class of lipid mediators. The role played by the endocannabinoid system (ECS) in inflammation is now established [28]#, and the ECS was previously implicated in solid organ rejection [235,236]#. In mice, CB1/2 activation with tetrahydrocannabinol (THC) was able to mitigate aGvHD [237], whereas transplants where CB2 was knocked-down induced higher aGvHD [238]. In a human phase II trial, cannabidiol was also able to prevent aGvHD [239]#. The broad S1P1 agonist, fingolimod, is approved for the treatment of multiple sclerosis and works by sequestering lymphocytes in secondary lymphoid organs [240]#. A more specific agonist (CYM-5442) was shown to reduce the severity of murine aGvHD by inhibiting macrophage recruitment via a reduction of CCL2 and CCL7 expression on endothelial cells [241].
Among the other GPCR classes, complement 3/5 activator fragments receptors (C3aR/C5aR) [242] or platelet-activating factor receptor (PAFR) [243] in mice, as well as a microsatellite in human EGF, Latrophilin, and Seven Transmembrane Domain-Containing Protein 1 (ELTD1) [244], have all shown positive correlation with aGvHD. A frizzled agonist was able to rescue LGr5+ gastric stem cells from murine aGvHD [245], underlining the importance of each target organ’s microenvironment. Activated protein C (aPC) signaling using protease-activated receptor 2/3 (PAR 2 and 3) expanded Tregs and mitigated aGvHD in mice [246]. rTM depends on GPR15 to mitigate murine aGvHD [167], whereas human patients receiving rTM were shown to have lower CCL5 levels and aGvHD [247]. The case of GPR43 merits further discussion; it is a sensor of gut microbiota-derived metabolites, such as short-chain fatty acids (SCFAs). These metabolites limit a number of inflammatory processes via action on endothelial cells [248]# by modulating neutrophil recruitment [249]# or the CD8+ T cell’s effector function [250]#. In the intestine, GPR43 contributes to epithelial integrity, and GPR43 knock-out in mice was associated with the increased severity of aGvHD [251].
2.4.2. Chronic GvHD
Chronic cGvHD (cGvHD) historically develops from 100 days after allogeneic HSCT, but it can nevertheless overlap with aGvHD, as it shares some initiating events, although it has different pathophysiology and clinical manifestations [257]#. Although it has not been completely elucidated, cGvHD pathogenesis involves chronic inflammation, aberrant tissue repair, and fibrosis, while the underlying immune dysregulation affects multiple cell types [258]#. The therapeutic arsenal against cGvHD is limited [259,260]#, making cGvHD the main contributor to TRM in long-term HSCT survivors [261]#. Due to its timescale, cGvHD overlaps with other chronic and/or age-related conditions, such as metabolic syndrome, chronic infections, or second primary cancers [262]#. cGvHD can affect virtually any organ but strikes the following systems in particular: skin and its appendages, mucosae, muscles and joints, and lungs.
High levels of several CCL and CXCL chemokines [263,264,265,266,267] have shown positive correlation with cGvHD, although most of the evidence originated from a single animal study [263] (Table 4). The expression of CXCL9 [267,268,269,270,271], CXCL10 [265,266,269,270,272,273], and CXCL11 [270], as well as their common receptor, CXCR3 [269,270,271,272,273,274], correlated positively with cGvHD in humans. Some chemokine receptors (CCR1, 3, 6, 7, 9) [263,275,276,277] showed positive correlation, whereas the correlation with some others (CXCR5, CX3CR1) [278,279] was negative. The available evidence on CCR4 and CCR5 is conflicting. High CCR4/5 levels in the buccal mucosa and salivary glands have been associated with higher T cell infiltrates and cGvHD [265]. However, in another study, CCR4+ CD4+ T cells were associated with lower cGvHD [280], although the authors did not specify the subset of CD4+ T cells in question. Similarly, lower CCR5 on monocytes could be associated with cGvHD in joints [279]. Considering the large variety of immune cells involved in cGvHD, chemokines are naturally expected to play different roles during its course. It is still interesting to note that, as for aGvHD, CB2 knock-out is associated with more severe cGvHD. To date, evidence for the other GPCRs [Prostaglandin D2 receptor (PGD2R), AT1R, smoothened (SMO), CB2] is either conflicting or based on single studies.

Table 4.
Chronic GvHD occurrence/severity in animal (A) or human (H)studies. See the Methods section regarding the reporting of results (Section 3.2).
2.5. Lung Toxicity
Pulmonary complications following HSCT are a cause of morbidity and mortality. They arise from infections, iatrogenic fluid overload, idiopathic pneumonia syndrome (IPS), and as a consequence of renal or cardiac failure or cGvHD [285]#. IPS is an early complication of allogeneic HSCT that encompasses a spectrum of clinical presentations arising from acute, widespread alveolar injury [286]#. The type and the intensity of conditioning medication, especially cyclophosphamide, and the activation and the migration of donor T cells are important contributors to that injury [287,288]#. Various cellular and soluble inflammatory mediators are thought to play a role in the development of IPS [286,289]#. Our systematized search of the literature found several animal studies reporting associations between chemokines and/or receptors and the occurrence of IPS (Table 5). CXCL 9 and 10, and their receptor, CXCR3 [290], in addition to CCL5 (RANTES) [178,291], showed positive correlation with IPS. For CCL2 [178,292,293] and CCL3 [178,294], the evidence was more conflicting. As with GvHD, specific chemokines probably correlate with distinct immune cell functions during the course of IPS [286]#. No reports of associations were found for any other functional classes of GPCR. It is interesting to note that no new studies on this have been published in the last ten years.

Table 5.
Lung toxicity occurrence/severity in animals (A) or humans (H). See the Methods section regarding the reporting of results (Section 3.2).
2.6. Treatment-Related Mortality (TRM)
TRM comprises deaths not due to the underlying disease. In cases of malignant diagnoses, this means death not due to a relapse of the disease, also sometimes called non-relapse mortality (NRM) [295]#. GvHD, VOD, lung toxicity, and infections due to HSCT-related immunosuppression are important causes of TRM [285]#. Mortality rates are tightly linked to responses to the initial treatments for each one of those complications; refractory aGvHD, for instance, is fatal in up to 80% of cases [285]#. Animal transplantation models do not usually recapitulate the underlying diseases for which human HSCTs are indicated, thus defining TRM seems futile in animal studies. In contrast, in human studies, two human polymorphisms in CCL2 (rs1024610, NG_012123.1:g.2936T>A) [296] and CXCL10 (rs3921, NM_001565.3:c.*140G>C) [297] have been associated with increased and decreased TRM, respectively (Table 6). The study on CCL2 found no significant associations between the variant and aGvHD, suggesting that another TRT could be the cause of the observed TRM. In contrast, in the study on the CXCL10 variant, lower TRM was associated with lower organ failure. CXCL9 was part of a four-biomarker panel associated with TRM [268]. High CCR5 expression in recipient T cells increased TRM [198,254], whereas a CD4+ CCR5+ cell population was associated with higher TRM [191]. In another study, CCR7+ CD4+ T cells were associated with death from cGvHD [298]. No reports of associations were found for any other functional class of GPCR.

Table 6.
Treatment-related mortality (TRM) in humans (H). See the Methods section regarding the reporting of results (Section 3.2).
3. Methods
3.1. Systematized Search
We used MEDLINE (www.ncbi.nlm.nih.gov/pubmed/) and EMBASE (www.embase.com/) databases to carry out a systematized review [299]# of articles published in English up to 4 March 2019 (see Appendix A4). The search extended to in vivo models and human interventional and non-interventional studies (see Appendix A3). The following HSCT outcomes were selected: mobilization, engraftment, SOS, acute GvHD, chronic GvHD, lung toxicity, and TRM. The rationale for this selection and the measurement methods are explained in the Appendix A8. Due to the advances in research on plerixafor and the existence of another recent systematic report reviewing its use for its approved indications [41]#, we restricted our search to human studies where mobilization was the measured outcome. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA; www.prisma-statement.org/) guidelines were followed to ensure a systematized search, although some of the requirements were not applicable due to the quite inclusive selection criteria used [300]#, as explained in the Appendix A6, Appendix A7, Appendix A8, Appendix A9, Appendix A10 and Appendix A11. The selection and data collection processes are described in the Appendix A6 and Appendix A7 as well. The search workflow and its output are reported in Figure 1 below, whereas Figure 2 details the number of published articles per year. In the Results and Discussion section, we report on and discuss the results of the search.
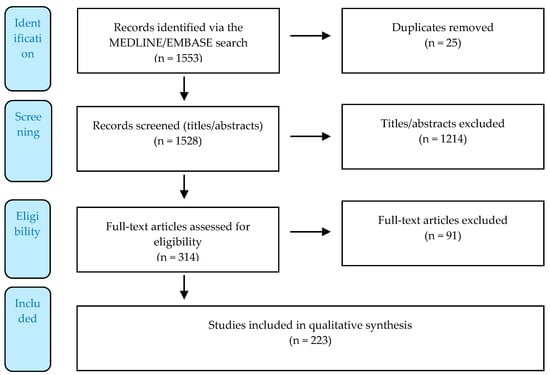
Figure 1.
Flow diagram displaying the number of records identified, included, and excluded via a systematized literature review following Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [300]#.
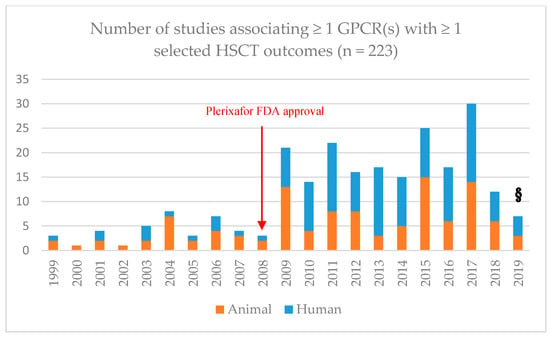
Figure 2.
Number of animal (orange) or human (blue) studies per year reporting any association between at least one G protein-coupled receptor (GPCR) and at least one of the selected hematopoietic stem cell transplantation (HSCT) outcomes: mobilization, engraftment, treatment-related toxicities (TRTs) [veno-occlusive disease (VOD), acute graft-versus-host disease (aGvHD), chronic graft-versus-host disease (cGvHD), lung toxicity], and transplantation- or treatment-related mortality (TRM). N = 223. These records were selected via two rounds of systematized screening for eligibility/exclusion criteria (see the Appendix A6). §: up to 4 March 2019.
3.2. Reporting of the Results
In the Results and Discussion section, each table lists the GPCRs, GPCR ligands, or related proteins whose expression/activity was reported to correlate with the HSCT outcome under consideration, as well as the corresponding reference(s) from the systematized search. Whenever an increase in gene/protein expression or activity of a GPCR, a GPCR ligand, or a related protein was associated with an increase in the incidence/level/severity of the outcome under consideration, the correlation is described as positive (+). The same applies whenever a decrease in a GPCR expression/activity was associated with a decrease in the outcome. Conversely, whenever an increase or a decrease in the GPCR expression/activity was associated with a decrease or, respectively, an increase in the outcome, the correlation is negative (−). Whenever there was no association between a GPCR expression/activity and the outcome, the correlation is null (0). As for polymorphisms (identified as “haplotype”, “microsatellite”, or by the variant number), their presence can correlate either positively (+) or negatively (−) with the outcome, yet their effect on protein level/function is not necessarily known. GPCRs or their ligands are grouped according to functional classes: chemokines [C-C ligand/receptor (CCL/R), C-X-C ligand/receptor (CXCL/R), C-X3-C ligand/receptor (CX3CL/R) blue], adrenergic receptors (orange), lipid mediators/receptors (green), and “others” (gray). To introduce topics or to enrich the discussion, we considered additional studies, which were not selected by the research query and/or criteria, as well as reviews. These references, along with those cited in the introduction, are specifically identified (#).
4. Conclusions and Perspectives
This systematized review reports on a significant number of GPCRs showing consistent associations with mobilization and engraftment and for which research has moved on to more advanced stages. Although there is some evidence that GPCRs play a role in SOS, GvHD, lung toxicity, and TRM in HSCT settings, there is a flagrant paucity of clinical associations. For several target GPCRs, the evidence is lacking or conflicting. In contrast, chemokines and their receptors make promising potential targets/biomarkers, as there are numerous potential candidates in various settings. Despite the difficulties in isolating the contributions of individual GPCRs, research has made significant progress for several of them. Targeting CXCR4 for mobilization has proven its utility, with the marketing authorization of plerixafor coming in 2008. Further work is needed to extend plerixafor’s indications, and the new anti-CXCR compounds in development could offer interesting pharmacological alternatives. The timing of CXCR4′s role during engraftment remains unclear, but CXCR4 blocking during mobilization does not seem to prevent engraftment, and CXCR4 could be manipulated so that it vacates the recipient niche or stimulates engraftment. No direct link between a GPCR and SOS has been consistently demonstrated in vivo. As for aGvHD, CCR5 blockade, such as with the anti-HIV drug, maraviroc, is on track to become a therapeutic option for its prophylaxis. Combined or alternated blockade using CXCR3 and CCR5 might bring further benefits. Activating cannabinoid receptors could be another prospect. GPR43 also merits further investigation as the importance of the gut’s microbiota in inflammatory processes is increasingly recognized.
It seems that research on GPCRs in the context of cGvHD is less advanced than in that of aGVHD. The current state of knowledge involves multiple chemokines but is either based on single studies or reports with conflicting findings. Studies on lung toxicity and IPS were scarce, and no relevant contribution to this field has been made in the last ten years. For both cGvHD and IPS, a better understanding of the molecular pathogenesis will probably be required before any useful biomarkers are revealed. As for TRM, it is often multifactorial and may thus prove more challenging to associate death with a single biomarker than individual or even combined toxicities. The absence of an assumed common toxicity-related pathway may explain the paucity of studies revealed by our literature search strategy.
The methodology used in the present paper strived to follow the PRISMA protocols, which, due to the nature of the search performed, could not be followed strictly. However, given the broad range of GPCRs, using the PRISMA methodology helped the authors to guide their search, resulting in a systematized review [299]. Because our search included both pre-clinical and clinical studies, quality, precision, and developmental stage of the evidence was inevitably heterogeneous and could not be reported or summarized using quantitative measures. Despite our best efforts to cover all GPCRs, certain reports that we judged significant enough to mention were missed by the search strategy. Nevertheless, the structure of this article allowed the authors to include such papers in the Results and Discussion section in order to properly cover the subject. Also, time and human resources limitations did not allow for quality or bias assessment by multiple unbiased reviewers, as should be expected from a completely systematic review. Regardless of the limitations to our systematized approach, it did allow for comprehensive scope and was meant to inform scientists and clinicians of the latest developments in a field that is (re-)gaining momentum.
Author Contributions
M.A. is the guarantor and the corresponding author. T.N., S.J.M., and H.G. developed the inclusion/exclusion criteria, the list of outcomes and their measurement, outlined the search strategy, and drafted the piloting form for data collection. H.G. developed and carried out the search strategy, deduplicated and screened the results, collected the data, prepared the summary table, and drafted the manuscript. M.A., S.J.M., V.M., and T.N. supervised the process and provided regular feedback. All the authors revised the manuscript critically. M.A. had final responsibility for the decision to submit for publication. All the authors read and approved the final manuscript.
Funding
H.G., S.J.M., V.M., and T.N.’s work is financed by the CANSEARCH foundation, based in Geneva, Switzerland. M.A. is employed by the Department of Women-Children-Teenagers, at Geneva University Hospitals (HUG). For part of this work, H.G. was also supported by a MIMOSA scholarship from the University of Geneva’s Department of Pediatrics, Gynecology, and Obstetrics.
Acknowledgments
Mafalda Burri, a librarian at the University of Geneva Medical Center (CMU), was consulted for feedback on our methodology. Darren Hart professionally edited the manuscript for the English language.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| aGvHD | acute graft-versus-host disease |
| cGvHD | chronic graft-versus-host disease |
| aPC | activated protein C |
| AT1R | angiotensin 1 receptor |
| AR | adrenergic receptor |
| BM | bone marrow |
| BMP | bone morphogenetic protein |
| CAR | chimeric antigen receptor |
| CB | cannabinoid receptor |
| DIC | disseminated intravascular coagulation |
| DKT | Dai-kenchu-to |
| ECS | endocannabinoid system |
| G-CSF | granulocyte colony-stimulating factor |
| GTEx | Genotype-Tissue expression |
| GF | graft failure |
| GPCR | G protein-coupled receptor |
| GRK | GPCR-related kinases |
| GvL | graft-versus-leukemia |
| HPA | Human Protein Atlas |
| HSC | hematopoietic stem cell |
| HSCT | hematopoietic stem cell transplantation |
| IPS | idiopathic pneumonia syndrome |
| MCT | Monocrotaline |
| MM | multiple myeloma |
| NHL | non-Hodgkin lymphoma |
| NRM | non-relapse mortality |
| P2Y2 | P2Y purinoreceptor 2 |
| PAF | platelet-activating factor |
| PAR | protease-activated receptor |
| PB | peripheral blood |
| PBSC | peripheral blood stem cell |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| RANTES | Regulated on Activation, Normal T Cell Expressed and Secreted (CCL5) |
| 7TMR | seven trans-membrane spanning receptor |
| SCFA | short-chain fatty acid |
| SMO | Smoothened |
| SOS | sinusoidal obstruction syndrome |
| SPM | specialized pro-resolving mediators |
| THC | tetrahydrocannabinol |
| TM(E5) | thrombomodulin (fifth epidermal growth factor-like region) |
| Tregs | regulatory T cells |
| TRM | transplantation- or treatment-related mortality |
| TRTs | treatment-related toxicities |
| VOD | veno-occlusive disease (the term formerly used for SOS) |
Appendix A. Methodology
Appendix A1. Administrative Information
This manuscript is a review using some of the systematic elements recommended in the latest PRISMA guidelines [300]#. Its protocol was not registered prior to its completion. Cochrane, Prospero, and Epistemonikos databases were searched (see Appendix A4) to verify that this manuscript was not repeating any existing review.
Appendix A2. Rationale and Objectives
The rationale was explained in the main text. The general objective was to genuinely report on the state of knowledge regarding the identification and the targeting of GPCRs in the management of hematopoietic stem cell transplantation (HSCT) outcomes. Hence, we used an inclusive approach in selecting the types of studies under consideration.
Appendix A3. Eligibility/Exclusion Criteria
Appendix A3.1. Study Designs
Clinical studies, both observational and interventional, prospective randomized clinical trials, and retrospective cohort or case-control studies were included in the search. Both adult and pediatric studies were considered. Preclinical studies were included provided they used animal models (mice, rats, primates, zebrafish) that reproduced conditioning and hematopoietic stem cell transplantation comparable to humans. We thus excluded animal studies that only investigated the mobilization stage of HSCT. Conference abstracts were included, whereas reviews, editorials, and case reports were excluded. Only English language literature was included. As there was no existing comprehensive review of the field to start from (see Appendix A1), we did not set any anterior limit on the time of publication. We ran the search for the last time on 4 March 2019.
Appendix A3.2. Interventions/Observations
Studies were considered for this review if they reported:
- any association between the expression of a GPCR, a GPCR ligand, or a related protein (e.g. GPCR kinase, beta-arrestins) and one of the selected outcomes (see Appendix A8) of autologous and/or allogeneic HSCT;
- any intervention on a GPCR, a GPCR ligand, or a related protein to change one of the selected outcomes of autologous/allogeneic HSCT.
The list of GPCRs provided by Uniprot (Available online: www.uniprot.org/docs/7tmrlist.txt) was used as a reference and was sometimes cross-checked with other public databases.
Appendix A4. Information Sources and Search Strategy
- Cochrane (www.cochranelibrary.com/), Prospero (www.crd.york.ac.uk/prospero/) and Epistomonikos (www.epistemonikos.org/): queried using Medical Subject Headings (MeSH®) descriptors (explode all trees) or free text terms, to probe for already existing systematic reviews.
- MEDLINE (Pubmed interface, 1966 onwards; www.ncbi.nlm.nih.gov/pubmed/) database: queried using either MeSH® or free text terms.
- EMBASE (Elsevier interface, 1947 onwards; www.embase.com/) database: queried using either Embase Subject Headings (Emtree ®) or free text terms.
With the help of SJM and TN, HG created a search equation stepwise for each database, as shown in Table A1 for MEDLINE (Pubmed interface), and all searches were run for the last time on 4 March 2019.

Table A1.
Search equation built for MEDLINE (Pubmed interface).
Table A1.
Search equation built for MEDLINE (Pubmed interface).
| Key Concepts | G Protein-Coupled Receptor | Hematopoietic Stem Cell Transplantation |
|---|---|---|
| Free text terms | “G protein-coupled receptor”[Title/Abstract] OR “GPCR” [Title/Abstract] OR “G protein coupled receptor” [Title/Abstract] OR “chemokine” [Text Word] | “Hematopoietic stem cell transplantation” [Title/Abstract] OR “HSCT” [Title/Abstract] OR “haematopoietic stem cell therapy” [Title/Abstract] OR “haematopoietic stem cell transplantation” [Title/Abstract] OR “hematopoietic stem cell (hsc) transplantation” [Title/Abstract] OR “hematopoietic stem cell therapy” [Title/Abstract] OR “hsc therapy” [Title/Abstract] OR “hsc transplantation” [Title/Abstract] OR “Hematopoietic cell transplantation” [Title/Abstract] |
| MeSH terms | “Receptors, G-protein-coupled”[MeSH Terms] OR “beta-Arrestins”[Mesh Terms] OR “G-Protein-Coupled Receptor Kinases”[Mesh Terms] OR “Receptors, Thyrotropin”[Mesh Terms] OR “Receptors, Thyrotropin-Releasing Hormone”[Mesh Terms] | (“Hematopoietic Stem Cell Transplantation”[Mesh Terms] OR “Bone Marrow Transplantation”[Mesh Terms] OR “Hematopoietic Stem Cell Mobilization”[Mesh Terms] OR “Transplantation Conditioning”[Mesh Terms] OR “Cord Blood Stem Cell Transplantation”[Mesh Terms] |
| Others | “MSH receptor” [Supplementary Concept]NOT “editorial”[Publication Type] NOT “review”[Publication Type] AND “english”[Language] |
Appendix A5. Data Management
The references were assembled and screened using EndNote X9.1.1 Desktop software for MacOS. The extracted data were stored in an Excel form.
Appendix A6. Selection Process
Appendix A6.1. De-Duplications
HG used EndNote’s automatic duplication search function and also conducted a manual curation of the assembled articles to remove obvious duplicates before screening. As this review was not completely systematic for the reasons previously explained, we decided against running a thorough de-duplication algorithm [301]#.
Appendix A6.2. Screening
HG screened the title and the abstract of each article found using the search strategy described above for whether they fulfilled the eligibility/exclusion criteria. A rating system was used to discuss the least obvious exclusions with TN and SJM. Records for which important information was missing, typically the abstract, but for which titles indicated a likely match to our topic were further screened, along with all the included records. HG performed this second screening step based on full-text articles, at the time as he collected the data.
Appendix A7. Data Collection Process
HG extracted the data from the full-text records using an Excel piloting form drafted with TN and SJM. The investigators undertook no data verification. No systematic publication quality assessment was conducted. The item variables sought were as follows:
- General information: Article ID (1st author’s name, 2nd author’s name if ambiguity), year of publication, PDF retrievability;
- Study types (if applicable): animal, human, observational, interventional, prospective/retrospective, gene manipulation;
- Intervention (if applicable): drug used, drug type, mode of action, polymorphism;
- Outcome, effect of GPCR, and direction of the effect: mobilization, engraftment, VOD, acute GvHD, chronic GvHD, lung toxicity, treatment-related mortality.
Appendix A8. Outcomes and Measurement
The search and the ensuing data collection considered the following outcomes, as they were identified as being the most common or the most important issues in HSCT in the latest European Society for Blood and Marrow Transplantation (EBMT) Handbook [285]. The measurement method is indicated in brackets, when appropriate.
- -
- Stem cell mobilization in donors (allogeneic) or hosts (autologous), as measured using circulating CD34+ (HSC) and/or nucleated blood cells harvested through leukapheresis.
- -
- Engraftment (neutrophil and/or platelet recovery, lab diagnosis). Better engraftment was measured by shorter recovery times or lower rates of graft failure.
- -
- Hepatic sinusoidal obstruction syndrome (SOS), formerly known as hepatic veno-occlusive disease (VOD) or liver inflammation (clinical diagnosis).
- -
- Acute graft-versus-host disease (aGvHD) (clinical diagnosis).
- -
- Chronic graft-versus-host disease (cGvHD) (clinical diagnosis).
- -
- Treatment-related mortality.
Appendix A9. Risk of Bias
Due to the heterogeneity of the studies found and the fact that the screening was executed by one reviewer only, the risk of bias in individual studies was not properly assessed. It is obviously an important shortcoming of this review.
Appendix A10. Data Synthesis
Appendix A11. Meta-Biases and Cumulative Evidence
Due to the heterogeneity of the studies reviewed, no meta-bias analysis was undertaken, and this is a shortcoming of this review. No systematic approach was undertaken to assess the quality of individual studies due to the constraints and heterogeneity previously underlined.
References
- Thomas, E.D.; Lochte, H.L., Jr.; Lu, W.C.; Ferrebee, J.W. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N. Engl. J. Med. 1957, 257, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Gratwohl, A. Hematopoietic Stem Cell TransplantationA Global Perspective. JAMA 2010, 303, 1617–1624. [Google Scholar] [CrossRef] [PubMed]
- Gratwohl, A.; Pasquini, M.C.; Aljurf, M.; Atsuta, Y.; Baldomero, H.; Foeken, L.; Gratwohl, M.; Bouzas, L.F.; Confer, D.; Frauendorfer, K.; et al. One million haemopoietic stem-cell transplants: A retrospective observational study. Lancet Haematol. 2015, 2, e91–e100. [Google Scholar] [CrossRef]
- Korbling, M.; Freireich, E.J. Twenty-five years of peripheral blood stem cell transplantation. Blood 2011, 117, 6411–6416. [Google Scholar] [CrossRef] [PubMed]
- Niederwieser, D.; Baldomero, H.; Szer, J.; Gratwohl, M.; Aljurf, M.; Atsuta, Y.; Bouzas, L.F.; Confer, D.; Greinix, H.; Horowitz, M.; et al. Hematopoietic stem cell transplantation activity worldwide in 2012 and a SWOT analysis of the Worldwide Network for Blood and Marrow Transplantation Group including the global survey. Bone Marrow Transplant. 2016, 51, 778–785. [Google Scholar] [CrossRef] [PubMed]
- Cottler-Fox, M.H. Stem Cell Mobilization. Hematology (Amsterdam Netherlands) 2003, 2003, 419–437. [Google Scholar] [CrossRef]
- Bender, J.G.; To, L.B.; Williams, S.; Schwartzberg, L.S. Defining a therapeutic dose of peripheral blood stem cells. J. Hematother. 1992, 1, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Blazar, B.R.; Murphy, W.J.; Abedi, M. Advances in graft-versus-host disease biology and therapy. Nat. Rev. Immunol. 2012, 12, 443–458. [Google Scholar] [CrossRef] [PubMed]
- Apostolova, P.; Zeiser, R. The role of danger signals and ectonucleotidases in acute graft-versus-host disease. Hum. Immunol. 2016, 77, 1037–1047. [Google Scholar] [CrossRef]
- Pándy-Szekeres, G.; Munk, C.; Tsonkov, T.M.; Mordalski, S.; Harpsøe, K.; Hauser, A.S.; Bojarski, A.J.; Gloriam, D.E. GPCRdb in 2018: Adding GPCR structure models and ligands. Nucleic Acids Res. 2018, 46, D440–D446. [Google Scholar] [CrossRef]
- Gurevich, V.; Gurevich, E. Molecular Mechanisms of GPCR Signaling: A Structural Perspective. Int. J. Mol. Sci. 2017, 18, 2519. [Google Scholar] [CrossRef] [PubMed]
- Fredriksson, R. The G-Protein-Coupled Receptors in the Human Genome Form Five Main Families. Phylogenetic Analysis, Paralogon Groups, and Fingerprints. Mol. Pharmacol. 2003, 63, 1256–1272. [Google Scholar] [CrossRef] [PubMed]
- Weis, W.I.; Kobilka, B.K. The Molecular Basis of G Protein–Coupled Receptor Activation. Annu. Rev. Biochem. 2018, 87, 897–919. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Rajagopal, S. The β-Arrestins: Multifunctional Regulators of G Protein-coupled Receptors. J. Biol. Chem. 2016, 291, 8969–8977. [Google Scholar] [CrossRef] [PubMed]
- Okamoto, Y.; Shikano, S. Differential phosphorylation signals control endocytosis of GPR15. Mol. Biol. Cell 2017, 28, 2267–2281. [Google Scholar] [CrossRef]
- Nogués, L.; Reglero, C.; Rivas, V.; Neves, M.; Penela, P.; Mayor, F. G-Protein–Coupled Receptor Kinase 2 as a Potential Modulator of the Hallmarks of Cancer. Mol. Pharmacol. 2017, 91, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Hanyaloglu, A.C. Advances in Membrane Trafficking and Endosomal Signaling of G Protein-Coupled Receptors. In International Review of Cell and Molecular Biology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 339, pp. 93–131. [Google Scholar]
- Hauser, A.S.; Attwood, M.M.; Rask-Andersen, M.; Schiöth, H.B.; Gloriam, D.E. Trends in GPCR drug discovery: New agents, targets and indications. Nat. Rev. Drug Discov. 2017, 16, 829–842. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, Y. Recent Trends and Applications of Molecular Modeling in GPCR–Ligand Recognition and Structure-Based Drug Design. Int. J. Mol. Sci. 2018, 19, 2105. [Google Scholar] [CrossRef]
- Shimada, I.; Ueda, T.; Kofuku, Y.; Eddy, M.T.; Wüthrich, K. GPCR drug discovery: Integrating solution NMR data with crystal and cryo-EM structures. Nat. Rev. Drug Discov. 2018, 18, 59–82. [Google Scholar] [CrossRef]
- Smith, J.S.; Lefkowitz, R.J.; Rajagopal, S. Biased signalling: From simple switches to allosteric microprocessors. Nat. Rev. Drug Discov. 2018, 17, 243–260. [Google Scholar] [CrossRef]
- Wisler, J.W.; Rockman, H.A.; Lefkowitz, R.J. Biased G Protein–Coupled Receptor Signaling: Changing the Paradigm of Drug Discovery. Circulation 2018, 137, 2315–2317. [Google Scholar] [CrossRef] [PubMed]
- Bar-Shavit, R.; Maoz, M.; Kancharla, A.; Nag, J.K.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G Protein-Coupled Receptors in Cancer. Int. J. Mol. Sci. 2016, 17, 1320. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Gareri, C.; Rockman, H.A. G-Protein-Coupled Receptors in Heart Disease. Circ. Res. 2018, 123, 716–735. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Todd, N.; Thathiah, A. The role of GPCRs in neurodegenerative diseases: Avenues for therapeutic intervention. Curr. Opin. Pharmacol. 2017, 32, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.D.; Hendy, G.N.; Percy, M.E.; Bichet, D.G.; Cole, D.E. G protein-coupled receptor mutations and human genetic disease. Methods Mol. Biol. (Clifton N.J.) 2014, 1175, 153–187. [Google Scholar] [CrossRef]
- Umamaheswaran, S.; Dasari, S.K.; Yang, P.; Lutgendorf, S.K.; Sood, A.K. Stress, inflammation, and eicosanoids: An emerging perspective. Cancer Metastasis Rev. 2018, 37, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Witkamp, R.; Meijerink, J. The endocannabinoid system: An emerging key player in inflammation. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 130–138. [Google Scholar] [CrossRef]
- Albi, E.; Alessenko, A.; Grösch, S. Sphingolipids in Inflammation. Mediat. Inflamm. 2018, 2018, 7464702. [Google Scholar] [CrossRef]
- Chiurchiù, V.; Leuti, A.; Maccarrone, M. Bioactive Lipids and Chronic Inflammation: Managing the Fire Within. Front. Immunol. 2018, 9, 38. [Google Scholar] [CrossRef]
- Charo, I.F.; Ransohoff, R.M. The Many Roles of Chemokines and Chemokine Receptors in Inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Dixon, R.A.F.; Kobilka, B.K.; Strader, D.J.; Benovic, J.L.; Dohlman, H.G.; Frielle, T.; Bolanowski, M.A.; Bennett, C.D.; Rands, E.; Diehl, R.E.; et al. Cloning of the gene and cDNA for mammalian β-adrenergic receptor and homology with rhodopsin. Nature 1986, 321, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Marino, F.; Cosentino, M. Adrenergic modulation of immune cells: An update. Amino Acids 2013, 45, 55–71. [Google Scholar] [CrossRef] [PubMed]
- Kolmus, K.; Tavernier, J.; Gerlo, S. β2-Adrenergic receptors in immunity and inflammation: Stressing NF-κB. Brain Behav. Immun. 2015, 45, 297–310. [Google Scholar] [CrossRef] [PubMed]
- Scanzano, A.; Cosentino, M. Adrenergic regulation of innate immunity: A review. Front. Pharmacol. 2015, 6, 171. [Google Scholar] [CrossRef] [PubMed]
- Bendall, L. Extracellular molecules in hematopoietic stem cell mobilisation. Int. J. Hematol. 2017, 105, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, T. CXCL12/SDF-1 and CXCR4. Front. Immunol. 2015, 6, 301. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, J.F.; Micallef, I.N.; Stiff, P.J.; Bolwell, B.J.; Maziarz, R.T.; Jacobsen, E.; Nademanee, A.; McCarty, J.; Bridger, G.; Calandra, G.; et al. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2009, 27, 4767–4773. [Google Scholar] [CrossRef]
- Brave, M.; Farrell, A.; Ching Lin, S.; Ocheltree, T.; Pope Miksinski, S.; Lee, S.L.; Saber, H.; Fourie, J.; Tornoe, C.; Booth, B.; et al. FDA review summary: Mozobil in combination with granulocyte colony-stimulating factor to mobilize hematopoietic stem cells to the peripheral blood for collection and subsequent autologous transplantation. Oncology 2010, 78, 282–288. [Google Scholar] [CrossRef] [PubMed]
- DiPersio, J.F.; Stadtmauer, E.A.; Nademanee, A.; Micallef, I.N.; Stiff, P.J.; Kaufman, J.L.; Maziarz, R.T.; Hosing, C.; Fruehauf, S.; Horwitz, M.; et al. Plerixafor and G-CSF versus placebo and G-CSF to mobilize hematopoietic stem cells for autologous stem cell transplantation in patients with multiple myeloma. Blood 2009, 113, 5720–5726. [Google Scholar] [CrossRef]
- Hartmann, T.; Hübel, K.; Monsef, I.; Engert, A.; Skoetz, N. Additional plerixafor to granulocyte colony-stimulating factors for haematopoietic stem cell mobilisation for autologous transplantation in people with malignant lymphoma or multiple myeloma. Cochrane Database Syst. Rev. 2015, CD010615. [Google Scholar] [CrossRef]
- Hopman, R.K.; DiPersio, J.F. Advances in stem cell mobilization. Blood Rev. 2014, 28, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Bendall, L.J.; Bradstock, K.F. G-CSF: From granulopoietic stimulant to bone marrow stem cell mobilizing agent. Cytokine Growth Factor Rev. 2014, 25, 355–367. [Google Scholar] [CrossRef] [PubMed]
- Ben Nasr, M.; Reguaya, Z.; Berraies, L.; Maamar, M.; Ladeb, S.; Ben Othmen, T.; Mellouli, F.; Bejaoui, M.; Domenech, J.; Jenhani, F. Association of stromal cell-derived factor-1-3′A polymorphism to higher mobilization of hematopoietic stem cells CD34+ in Tunisian population. Transplant. Proc. 2011, 43, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Benboubker, L.; Watier, H.; Carion, A.; Georget, M.T.; Desbois, I.; Colombat, P.; Bardos, P.; Binet, C.; Domenech, J. Association between the SDF1-3′A allele and high levels of CD34(+) progenitor cells mobilized into peripheral blood in humans. Br. J. Haematol. 2001, 113, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Bogunia-Kubik, K.; Gieryng, A.; Dlubek, D.; Lange, A. The CXCL12-3′A allele is associated with a higher mobilization yield of CD34 progenitors to the peripheral blood of healthy donors for allogeneic transplantation. Bone Marrow Transplant. 2009, 44, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Soriano, A.; Martinez, C.; Garcia, F.; Plana, M.; Palou, E.; Lejeune, M.; Arostegui, J.I.; De Lazzari, E.; Rodriguez, C.; Barrasa, A.; et al. Plasma stromal cell-derived factor (SDF)-1 levels, SDF1-3′A genotype, and expression of CXCR4 on T lymphocytes: Their impact on resistance to human immunodeficiency virus type 1 infection and its progression. J. Infect. Dis. 2002, 186, 922–931. [Google Scholar] [CrossRef] [PubMed]
- Pantin, J.; Purev, E.; Tian, X.; Cook, L.; Donohue-Jerussi, T.; Cho, E.; Reger, R.; Hsieh, M.; Khuu, H.; Calandra, G.; et al. Effect of high-dose plerixafor on CD34(+) cell mobilization in healthy stem cell donors: Results of a randomized crossover trial. Haematologica 2017, 102, 600–609. [Google Scholar] [CrossRef]
- Flomenberg, N.; Comenzo, R.L.; Badel, K.; Calandra, G. Plerixafor (Mozobil) alone to mobilize hematopoietic stem cells from multiple myeloma patients for autologous transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 695–700. [Google Scholar] [CrossRef][Green Version]
- Cheng, J.; Schmitt, M.; Wuchter, P.; Buss, E.C.; Witzens-Harig, M.; Neben, K.; Hundemer, M.; Hillengass, J.; Alexi, R.; Goldschmidt, H.; et al. Plerixafor is effective given either preemptively or as a rescue strategy in poor stem cell mobilizing patients with multiple myeloma. Transfusion 2015, 55, 275–283. [Google Scholar] [CrossRef]
- Fadini, G.P.; Fiala, M.; Cappellari, R.; Danna, M.; Park, S.; Poncina, N.; Menegazzo, L.; Albiero, M.; DiPersio, J.; Stockerl-Goldstein, K.; et al. Diabetes Limits Stem Cell Mobilization Following G-CSF but Not Plerixafor. Diabetes 2015, 64, 2969–2977. [Google Scholar] [CrossRef]
- Patel, B.; Pearson, H.; Zacharoulis, S. Mobilisation of haematopoietic stem cells in paediatric patients, prior to autologous transplantation following administration of plerixafor and G-CSF. Pediatr. Blood Cancer 2015, 62, 1477–1480. [Google Scholar] [CrossRef] [PubMed]
- Teusink, A.; Pinkard, S.; Davies, S.; Mueller, M.; Jodele, S. Plerixafor is safe and efficacious for mobilization of peripheral blood stem cells in pediatric patients. Transfusion 2016, 56, 1402–1405. [Google Scholar] [CrossRef]
- Bitan, M.; Eshel, R.; Sadot, E.; Friedman, S.; Pinhasov, A.; Levin, D.; Dvir, R.; Manisterski, M.; Berger-Achituv, S.; Rosenfeld-Keidar, H.; et al. Combined plerixafor and granulocyte colony-stimulating factor for harvesting high-dose hematopoietic stem cells: Possible niche for plerixafor use in pediatric patients. Pediatr. Transplant. 2016, 20, 565–571. [Google Scholar] [CrossRef] [PubMed]
- Douglas, K.W.; Parker, A.N.; Hayden, P.J.; Rahemtulla, A.; D’Addio, A.; Lemoli, R.M.; Rao, K.; Maris, M.; Pagliuca, A.; Uberti, J.; et al. Plerixafor for PBSC mobilisation in myeloma patients with advanced renal failure: Safety and efficacy data in a series of 21 patients from Europe and the USA. Bone Marrow Transplant. 2012, 47, 18–23. [Google Scholar] [CrossRef] [PubMed][Green Version]
- MacFarland, R.; Hard, M.L.; Scarborough, R.; Badel, K.; Calandra, G. A pharmacokinetic study of plerixafor in subjects with varying degrees of renal impairment. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 95–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liles, W.C.; Broxmeyer, H.E.; Rodger, E.; Wood, B.; Hubel, K.; Cooper, S.; Hangoc, G.; Bridger, G.J.; Henson, G.W.; Calandra, G.; et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003, 102, 2728–2730. [Google Scholar] [CrossRef] [PubMed]
- Devine, S.M.; Vij, R.; Rettig, M.; Todt, L.; McGlauchlen, K.; Fisher, N.; Devine, H.; Link, D.C.; Calandra, G.; Bridger, G.; et al. Rapid mobilization of functional donor hematopoietic cells without G-CSF using AMD3100, an antagonist of the CXCR4/SDF-1 interaction. Blood 2008, 112, 990–998. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.A.; Rettig, M.P.; Lopez, S.; Christ, S.; Fiala, M.; Eades, W.; Mir, F.A.; Shao, J.; McFarland, K.; Trinkaus, K.; et al. Mobilization of allogeneic peripheral blood stem cell donors with intravenous plerixafor mobilizes a unique graft. Blood 2017, 129, 2680–2692. [Google Scholar] [CrossRef]
- Chung, D.T.; Chang, L.W.; Huang, Y.H.; Tsai, C.Y.; Hsu, C.H.; King, C.H.R.; Yuan, J.H.; Yen, C.F.; Chen, Y.M.; Lu, Y.C.; et al. TG-0054, a novel and potent stem cell mobilizer, displays excellent PK/PD and safety profile in phase I trial. Blood 2009, 114, 866. [Google Scholar]
- Schuster, M.W.; Hagog, N.; Jalilizeinali, B.; Funkhauser, S.; Yohannan, M.S.; Sadler, J.; Wood, S.; Carey, S.; Kelleher, K.; Tsai, C.E.; et al. Rapid mobilization of CD34+ progenitor cells with TG0054-03, a novel CXC chemokine receptor 4 (CXCR4) antagonoist. Blood 2013, 122, 905. [Google Scholar]
- Setia, G.; Hagog, N.; Jalilizeinali, B.; Funkhouser, S.; Pierzchanowski, L.; Lan, F.; Gabig, T.G.; Kiner-Strachan, B.; Kelleher, K.; Hsu, M.C.; et al. A phase ii, open-label pilot study to evaluate the hematopoietic stem cell mobilization of TG-0054 combined with G-CSF in 12 patients with multiple myeloma, non-hodgkin lymphoma or hodgkin lymphoma—An interim analysis. Blood 2015, 126, 515. [Google Scholar]
- Abraham, M.; Pereg, Y.; Bulvik, B.; Klein, S.; Mishalian, I.; Wald, H.; Eizenberg, O.; Beider, K.; Nagler, A.; Golan, R.; et al. Single Dose of the CXCR4 Antagonist BL-8040 Induces Rapid Mobilization for the Collection of Human CD34(+) Cells in Healthy Volunteers. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2017, 23, 6790–6801. [Google Scholar] [CrossRef] [PubMed]
- Peled, A.; Abraham, M.; Avivi, I.; Rowe, J.M.; Beider, K.; Wald, H.; Tiomkin, L.; Ribakovsky, L.; Riback, Y.; Ramati, Y.; et al. The high-affinity CXCR4 antagonist BKT140 is safe and induces a robust mobilization of human CD34+ cells in patients with multiple myeloma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2014, 20, 469–479. [Google Scholar] [CrossRef] [PubMed]
- Karpova, D.; Brauninger, S.; Wiercinska, E.; Kramer, A.; Stock, B.; Graff, J.; Martin, H.; Wach, A.; Escot, C.; Douglas, G.; et al. Mobilization of hematopoietic stem cells with the novel CXCR4 antagonist POL6326 (balixafortide) in healthy volunteers-results of a dose escalation trial. J. Transl. Med. 2017, 15, 2. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.B.; Van Horn, R.D.; Yin, T.; Brown, R.M.; Roell, W.C.; Obungu, V.H.; Ruegg, C.; Wroblewski, V.J.; Raddad, E.; Stille, J.R. Distinct mobilization of leukocytes and hematopoietic stem cells by CXCR4 peptide antagonist LY2510924 and monoclonal antibody LY2624587. Oncotarget 2017, 8, 94619–94634. [Google Scholar] [CrossRef] [PubMed]
- Vater, A.; Sahlmann, J.; Kroger, N.; Zollner, S.; Lioznov, M.; Maasch, C.; Buchner, K.; Vossmeyer, D.; Schwoebel, F.; Purschke, W.G.; et al. Hematopoietic stem and progenitor cell mobilization in mice and humans by a first-in-class mirror-image oligonucleotide inhibitor of CXCL12. Clin. Pharmacol. Ther. 2013, 94, 150–157. [Google Scholar] [CrossRef]
- Costa, L.J.; Alexander, E.T.; Hogan, K.R.; Schaub, C.; Fouts, T.V.; Stuart, R.K. Development and validation of a decision-making algorithm to guide the use of plerixafor for autologous hematopoietic stem cell mobilization. Bone Marrow Transplant. 2011, 46, 64–69. [Google Scholar] [CrossRef]
- Dabusti, M.; Lanza, F.; Campioni, D.; Castagnari, B.; Tieghi, A.; Moretti, S.; Punturieri, M.; De Angeli, C.; Spanedda, R.; Ferrazzi, E.; et al. CXCR-4 expression on bone marrow CD34+ cells prior to mobilization can predict mobilization adequacy in patients with hematologic malignancies. J. Hematother. Stem Cell Res. 2003, 12, 425–434. [Google Scholar] [CrossRef]
- Cecyn, K.Z.; Schimieguel, D.M.; Kimura, E.Y.; Yamamoto, M.; Oliveira, J.S. Plasma levels of FL and SDF-1 and expression of FLT-3 and CXCR4 on CD34+ cells assessed pre and post hematopoietic stem cell mobilization in patients with hematologic malignancies and in healthy donors. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapher. 2009, 40, 159–167. [Google Scholar] [CrossRef]
- Dlubek, D.; Drabczak-Skrzypek, D.; Lange, A. Low CXCR4 membrane expression on CD34(+) cells characterizes cells mobilized to blood. Bone Marrow Transplant. 2006, 37, 19–23. [Google Scholar] [CrossRef][Green Version]
- Watanabe, T.; Kawano, Y.; Kanamaru, S.; Onishi, T.; Kaneko, S.; Wakata, Y.; Nakagawa, R.; Makimoto, A.; Kuroda, Y.; Takaue, Y.; et al. Endogenous interleukin-8 (IL-8) surge in granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Blood 1999, 93, 1157–1163. [Google Scholar] [PubMed]
- Kozuka, T.; Ishimaru, F.; Fujii, K.; Masuda, K.; Kaneda, K.; Imai, T.; Fujii, N.; Ishikura, H.; Hongo, S.; Watanabe, T.; et al. Plasma stromal cell-derived factor-1 during granulocyte colony-stimulating factor-induced peripheral blood stem cell mobilization. Bone Marrow Transplant. 2003, 31, 651–654. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Devine, S.M.; Flomenberg, N.; Vesole, D.H.; Liesveld, J.; Weisdorf, D.; Badel, K.; Calandra, G.; DiPersio, J.F. Rapid mobilization of CD34+ cells following administration of the CXCR4 antagonist AMD3100 to patients with multiple myeloma and non-Hodgkin’s lymphoma. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2004, 22, 1095–1102. [Google Scholar] [CrossRef] [PubMed]
- Gazitt, Y.; Freytes, C.O.; Akay, C.; Badel, K.; Calandra, G. Improved mobilization of peripheral blood CD34+ cells and dendritic cells by AMD3100 plus granulocyte-colony-stimulating factor in non-Hodgkin’s lymphoma patients. Stem Cells Dev. 2007, 16, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Stiff, P.; Micallef, I.; McCarthy, P.; Magalhaes-Silverman, M.; Weisdorf, D.; Territo, M.; Badel, K.; Calandra, G. Treatment with plerixafor in non-Hodgkin’s lymphoma and multiple myeloma patients to increase the number of peripheral blood stem cells when given a mobilizing regimen of G-CSF: Implications for the heavily pretreated patient. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2009, 15, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Modak, S.; Cheung, I.Y.; Kushner, B.H.; Kramer, K.; Reich, L.; Cheung, N.K. Plerixafor plus granulocyte-colony stimulating factor for autologous hematopoietic stem cell mobilization in patients with metastatic neuroblastoma. Pediatr. Blood Cancer 2012, 58, 469–471. [Google Scholar] [CrossRef] [PubMed]
- Teusink, A.; Pinkard, S.L.; Davies, S.M.; Mueller, M.; Jodele, S. Safety and efficacy of plerixafor for mobilization of peripheral blood stem cells in pediatric patients. Biol. Blood Marrow Transplant. 2014, 20, S294–S295. [Google Scholar] [CrossRef][Green Version]
- Bekadja, M.A.; Bouhass, R.; Osmani, S.; Brahimi, M.; Talhi, S.; Yafour, N.; Arabi, A. Plerixafor in the treatment of progenitor cell mobilization failure: First experience in Algeria. Hematol. Oncol. Stem Cell Ther. 2015, 8, 93–94. [Google Scholar] [CrossRef]
- Danylesko, I.; Sareli, R.; Varda-Bloom, N.; Yerushalmi, R.; Shem-Tov, N.; Shimoni, A.; Nagler, A. Plerixafor (Mozobil): A Stem Cell-Mobilizing Agent for Transplantation in Lymphoma Patients Predicted to Be Poor Mobilizers—A Pilot Study. Acta Haematol. 2016, 135, 29–36. [Google Scholar] [CrossRef]
- López-Parra, M.; López Villar, O.; Bastida Bermejo, J.M.; López-Godino, O.; Cabrero, M.; Ramos Sevillano, M.I.; Oreja Martín, B.; López Cadenas, F.; Dávila Vals, J.; Nieto, M.J.; et al. Preemptive use of plerixafor on day 5 of G-CSF treatment. Experience of University Hospital of Salamanca. Bone Marrow Transplant. 2016, 51, S334–S335. [Google Scholar] [CrossRef][Green Version]
- Andritsos, L.A.; Huang, Y.; Fan, T.; Huff, K.; Drea, E.; McBride, A. A retrospective evaluation of the impact of pre-emptive plerixafor administration on collection efficiency in patients with myeloma undergoing stem cell mobilization. Blood 2016, 128, 5740. [Google Scholar]
- Basak, G.W.; Knopinska-Posluszny, W.; Matuszak, M.; Kisiel, E.; Hawrylecka, D.; Szmigielska-Kaplon, A.; Urbaniak-Kujda, D.; Dybko, J.; Zielinska, P.; Dabrowska-Iwanicka, A.; et al. Hematopoietic stem cell mobilization with the reversible CXCR4 receptor inhibitor plerixafor (AMD3100)-Polish compassionate use experience. Ann. Hematol. 2011, 90, 557–568. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Boulad, F.; Shore, T.B.; Van Besien, K.; Guarneri, D.; Greenberg, J.; Minniti, C.; Fedus, S.W.; Perna, F.; Wang, X.; Riviere, I.; et al. Safety and efficacy trial of escalation of plerixafor for mobilization of CD34+ hematopoietic progenitor cells (HPCS) for globin gene transfer in patients with sickle cell disease. Blood 2017, 130, 3531. [Google Scholar]
- Haen, S.; Schober-Melms, I.; Schumm, M.; Henes, J.; Möhle, R.; Bethge, W.; Kanz, L.; Vogel, W. Addition of plerixafor overcomes poor mobilization in autologous and allogeneic stem cell grafts and leads to efficient and sustained engraftment. Blood 2017, 130, 5457. [Google Scholar]
- Ogunniyi, A.; Rodriguez, M.; Devlin, S.; Adel, N.; Landau, H.; Chung, D.J.; Lendvai, N.; Lesokhin, A.; Koehne, G.; Mailankody, S.; et al. Upfront use of plerixafor and granulocyte-colony stimulating factor (GCSF) for stem cell mobilization in patients with multiple myeloma: Efficacy and analysis of risk factors associated with poor stem cell collection efficiency. Leuk. Lymphoma 2017, 58, 1123–1129. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lanza, F.; Lemoli, R.M.; Olivieri, A.; Laszlo, D.; Martino, M.; Specchia, G.; Pavone, V.; Imola, M.; Pasini, A.; Milone, G.; et al. Factors affecting successful mobilization with plerixafor: An Italian prospective survey in 215 patients with multiple myeloma and lymphoma. Transfusion 2014, 54, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Haen, S.P.; Schober-Melms, I.; Bethge, W.A.; Möhle, R.; Schumm, M.; Kanz, L.; Vogel, W. Plerixafor significantly increases the hematopoietic stem cell yield in documented poor mobilizers in the autologous and allogeneic setting. Onkologie 2013, 36. [Google Scholar] [CrossRef]
- Fink, G.; Kleber, M.; Scherer, E.; Gross, B.; Müller-Schmah, C.; Duyster, J.; Wäsch, R.; Engelhardt, M. Plerixafor (AMD3100) for stem cell mobilization: Once dosing in poor mobilizers: Efficacious and economically feasible? Onkologie 2013, 36, 236–237. [Google Scholar] [CrossRef]
- Khaled, Y.; Solh, M.; Lamontagne, D.; Batista, A.; Sullivan, J.; Chan-Fong, S.; Fondaw, M.; Reddy, V. Successful stem cell mobilization and engraftment in heavily pretreated multiple myeloma patients with prior high dose melphalan and autologous stem cell transplantation. Biol. Blood Marrow Transplant. 2012, 18, S300. [Google Scholar] [CrossRef][Green Version]
- Abusin, G.A.; Abu-Arja, R.F.; Gingrich, R.D.; Silverman, M.D.; Zamba, G.K.; Schlueter, A.J. An algorithm for utilizing peripheral blood CD34 count as a predictor of the need for plerixafor in autologous stem cell mobilization--cost-effectiveness analysis. J. Clin. Apher. 2013, 28, 293–300. [Google Scholar] [CrossRef]
- Sunu, C.; Onder Savas, O.; Ozet, G.; Dagdas, S.; Ceran, F.; Falay, M.; Koyuncu, N. Stem cell mobilization with plerixafor: A single center experience. Transfus. Apher. Sci. 2012, 47, S53. [Google Scholar] [CrossRef]
- Kasparu, H.; Kolb, A.; Böhm, A.; Hauser, H.; Weltermann, A. Overcoming poor stem cell mobilization and long term recovery after autologous transplantation with plerixafor primed stem cells. Eur. Surg.-Acta Chir. Austriaca 2012, 44. [Google Scholar]
- Worel, N.; Rosskopf, K.; Neumeister, P.; Kasparu, H.; Nachbaur, D.; Russ, G.; Namberger, K.; Witt, V.; Schloegl, E.; Zojer, N.; et al. Plerixafor and granulocyte-colony-stimulating factor (G-CSF) in patients with lymphoma and multiple myeloma previously failing mobilization with G-CSF with or without chemotherapy for autologous hematopoietic stem cell mobilization: The Austrian experience on a named patient program. Transfusion 2011, 51, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Selleslag, D.; Dierickx, D.; Breems, D.A.; Huynh, P.; Van De Velde, A.; Meers, S.; Brouwer, E.; Mertens, A. Plerixafor in poor stem cell mobilizers: The Belgian Compassionate Use Program. Acta Clin. Belg. 2011, 66, 200–204. [Google Scholar] [CrossRef] [PubMed]
- Basak, G.W.; Jaksic, O.; Koristek, Z.; Mikala, G.; Basic-Kinda, S.; Mayer, J.; Masszi, T.; Giebel, S.; Labar, B.; Wiktor-Jedrzejczak, W. Haematopoietic stem cell mobilization with plerixafor and G-CSF in patients with multiple myeloma transplanted with autologous stem cells. Eur. J. Haematol. 2011, 86, 488–495. [Google Scholar] [CrossRef] [PubMed]
- Nademanee, A.P.; DiPersio, J.F.; Maziarz, R.T.; Stadtmauer, E.A.; Micallef, I.N.; Stiff, P.J.; Hsu, F.J.; Bridger, G.; Bolwell, B.J. Plerixafor plus granulocyte colony-stimulating factor versus placebo plus granulocyte colony-stimulating factor for mobilization of CD34(+) hematopoietic stem cells in patients with multiple myeloma and low peripheral blood CD34(+) cell count: Results of a subset analysis of a randomized trial. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2012, 18, 1564–1572. [Google Scholar] [CrossRef]
- Jantunen, E.; Kuittinen, T.; Mahlamaki, E.; Pyorala, M.; Mantymaa, P.; Nousiainen, T. Efficacy of pre-emptively used plerixafor in patients mobilizing poorly after chemomobilization: A single centre experience. Eur. J. Haematol. 2011, 86, 299–304. [Google Scholar] [CrossRef]
- Jing, D.; Alakel, N.; Bornhauser, M.; Ehninger, G.; Ordemann, R. SDF-1/CXCR4 blockade to mobilize hematopoietic progenitor cells from the placenta. Bone Marrow Transplant. 2010, 45, 1661–1662. [Google Scholar] [CrossRef][Green Version]
- Horwitz, M.; Khan, T.; Long, G.; Gasparetto, C.; Sullivan, K.; Chute, J.; Rizzieri, D.; Drago, S.; Chao, N. Plerixafor given “just in time” for peripheral blood stem cell mobilization of patients with suboptimal response to G-CSF. Biol. Blood Marrow Transplant. 2010, 16, S208. [Google Scholar] [CrossRef][Green Version]
- Flomenberg, N.; Devine, S.M.; Dipersio, J.F.; Liesveld, J.L.; McCarty, J.M.; Rowley, S.D.; Vesole, D.H.; Badel, K.; Calandra, G. The use of AMD3100 plus G-CSF for autologous hematopoietic progenitor cell mobilization is superior to G-CSF alone. Blood 2005, 106, 1867–1874. [Google Scholar] [CrossRef]
- Shastri, A.; Budhathoki, A.; Barta, S.K.; Kornblum, N.; Derman, O.; Battini, R.; Raghupathy, R.; Verma, A.K.; Frenette, P.S.; Braunschweig, I.; et al. Stimulation of adrenergic activity by desipramine enhances hematopoietic stem and progenitor cell mobilization along with G-CSF in multiple myeloma: A pilot study. Am. J. Hematol. 2017, 92, 1047–1051. [Google Scholar] [CrossRef] [PubMed]
- Nevo, N.; Gur-Cohen, S.; Kollet, O.; Avemaria, F.; Avci, S.; Itkin, T.; Chakrabarty, S.; Zuckerman, T.; Brenner, B.; Nadir, Y.; et al. Inverse PAR1 activity of hematopoietic stem cells and BM stromal cells mediates G-CSF-induced mobilization by regulation of nitric oxide generation. Blood 2016, 128, 3370. [Google Scholar]
- Shin, S.; Kim, J.; Kim-Wanner, S.Z.; Bonig, H.; Cho, S.R.; Kim, S.; Choi, J.R.; Lee, K.A. A novel association between relaxin receptor polymorphism and hematopoietic stem cell yield after mobilization. PLoS ONE 2017, 12, e0179986. [Google Scholar] [CrossRef] [PubMed]
- Olsson, R.; Remberger, M.; Schaffer, M.; Berggren, D.M.; Svahn, B.M.; Mattsson, J.; Ringden, O. Graft failure in the modern era of allogeneic hematopoietic SCT. Bone Marrow Transplant. 2013, 48, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Bach, C.; Steffen, M.; Roesler, W.; Winkler, J.; Mackensen, A.; Stachel, K.D.; Metzler, M.; Spriewald, B.M. Systematic comparison of donor chimerism in peripheral blood and bone marrow after hematopoietic stem cell transplantation. Blood Cancer J. 2017, 7, e566. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.I. Engraftment and Chimerism. Hematop. Stem Cell Transplant. Pediatr. Hematol./Oncol. 2017, 177–186. [Google Scholar] [CrossRef]
- Ozdemir, Z.N.; Civriz Bozdag, S. Graft failure after allogeneic hematopoietic stem cell transplantation. Transfus. Apher. Sci. Off. J. World Apher. Assoc. Off. J. Eur. Soc. Haemapher. 2018, 57, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Purton, L.E.; Scadden, D.T. Limiting factors in murine hematopoietic stem cell assays. Cell Stem Cell 2007, 1, 263–270. [Google Scholar] [CrossRef]
- Girbl, T.; Lunzer, V.; Greil, R.; Namberger, K.; Hartmann, T.N. The CXCR4 and adhesion molecule expression of CD34+ hematopoietic cells mobilized by “on-demand” addition of plerixafor to granulocyte-colony-stimulating factor. Transfusion 2014, 54, 2325–2335. [Google Scholar] [CrossRef]
- Varmavuo, V.; Rimpilainen, J.; Kuitunen, H.; Nihtinen, A.; Vasala, K.; Mikkola, M.; Kutila, A.; Lehtonen, P.; Kuittinen, T.; Mantymaa, P.; et al. Engraftment and outcome after autologous stem cell transplantation in plerixafor-mobilized non-Hodgkin’s lymphoma patients. Transfusion 2014, 54, 1243–1250. [Google Scholar] [CrossRef]
- Bonig, H.; Chudziak, D.; Priestley, G.; Papayannopoulou, T. Insights into the biology of mobilized hematopoietic stem/progenitor cells through innovative treatment schedules of the CXCR4 antagonist AMD3100. Exp. Hematol. 2009, 37, 402–415. [Google Scholar] [CrossRef] [PubMed]
- Hess, D.A.; Bonde, J.; Craft, T.P.; Wirthlin, L.; Hohm, S.; Lahey, R.; Todt, L.M.; Dipersio, J.F.; Devine, S.M.; Nolta, J.A. Human progenitor cells rapidly mobilized by AMD3100 repopulate NOD/SCID mice with increased frequency in comparison to cells from the same donor mobilized by granulocyte colony stimulating factor. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2007, 13, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Lidonnici, M.R.; Aprile, A.; Frittoli, M.; Mandelli, G.; Paleari, Y.; Spinelli, A.; Gentner, B.; Zambelli, M.; Parisi, C.; Bellio, L.; et al. Human CD34+ cells from different sources disclose a specific stemness signature. Blood 2016, 128, 4709. [Google Scholar]
- Arai, Y.; Choi, U.; Corsino, C.I.; Koontz, S.M.; Tajima, M.; Sweeney, C.L.; Black, M.A.; Feldman, S.A.; Dinauer, M.C.; Malech, H.L. Enhanced expression of CXCR4 facilitates C-Kit-targeted CAR-T cell trafficking to bone marrow and enables donor stem cell engraftment. Biol. Blood Marrow Transplant. 2018, 24, S311. [Google Scholar] [CrossRef]
- Arai, Y.; Corsino, C.I.; Koontz, S.M.; Tajima, M.; Black, M.A.; Sweeney, C.L.; Choi, U.; Feldman, S.A.; Dinauer, M.C.; Malech, H.L. Enhanced expression of CXCR4 facilitates the trafficking of anti-C-kit chimeric antigen receptor (CAR) T cells to bone marrow and achieves effective donor stem cell engraftment. Blood 2017, 130, 4446. [Google Scholar]
- Chen, J.; Larochelle, A.; Fricker, S.; Bridger, G.; Dunbar, C.E.; Abkowitz, J.L. Mobilization as a preparative regimen for hematopoietic stem cell transplantation. Blood 2006, 107, 3764–3771. [Google Scholar] [CrossRef]
- Jiang, Y.; Ulyanova, T.; Papayannopoulou, T. Is the post-transplantation treatment with AMD beneficial? Blood Cells Mol. Dis. 2012, 49, 29–31. [Google Scholar] [CrossRef][Green Version]
- Green, M.M.; Chao, N.; Chhabra, S.; Corbet, K.; Gasparetto, C.; Horwitz, A.; Li, Z.; Venkata, J.K.; Long, G.; Mims, A.; et al. Plerixafor (a CXCR4 antagonist) following myeloablative allogeneic hematopoietic stem cell transplantation enhances hematopoietic recovery. J. Hematol. Oncol. 2016, 9, 71. [Google Scholar] [CrossRef]
- Lai, C.Y.; Otsu, M.; Okabe, M.; Suzuki, S.; Yamazaki, S.; Onodera, M.; Ema, H.; Kakuta, S.; Iwakura, Y.; Nakauchi, H. Evidence for the involvement of CXCR4 signaling in in vivo self-renewal of transplanted hematopoietic stem cells. Blood 2011, 118. [Google Scholar]
- Lai, C.Y.; Suzuki, S.; Okabe, M.; Yamazaki, S.; Otsu, M.; Nakauchi, H. Role of cxcr4 signaling in hematopoietic stem cell repopulation. J. Gene Med. 2012, 14, 682–683. [Google Scholar] [CrossRef]
- Asfour, I.; Afify, H.; Elkourashy, S.; Ayoub, M.; Kamal, G.; Gamal, M.; Elgohary, G. CXCR4 (CD184) expression on stem cell harvest and CD34(+) cells post-transplant. Hematol./Oncol. Stem Cell Ther. 2017, 10, 63–69. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.; Jackson, J.; Baulch-Brown, C. Enumeration of bone marrow ‘homing’ haemopoietic stem cells from G-CSF-mobilised normal donors and influence on engraftment following allogeneic transplantation. Bone Marrow Transplant. 2001, 28, 1019–1022. [Google Scholar] [CrossRef] [PubMed]
- Hicks, C.; Isaacs, A.; Wong, R.; Chong, B.H. CXCR4 expression on transplanted peripheral blood CD34+ cells: Relationship to engraftment after autologous transplantation in a cohort of multiple myeloma patients. Ann. Hematol. 2011, 90, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Gieryng, A.; Bogunia-Kubik, K.; Lange, A. CXCL12 gene polymorphism and hematologic recovery after transplantation of peripheral blood progenitor cells. Transplant. Proc. 2010, 42, 3280–3283. [Google Scholar] [CrossRef] [PubMed]
- Hoggatt, J.; Singh, P.; Sampath, J.; Pelus, L.M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 2009, 113, 5444–5455. [Google Scholar] [CrossRef] [PubMed]
- Pelus, L.M.; Hoggatt, J.; Singh, P. Pulse exposure of haematopoietic grafts to prostaglandin E2 in vitro facilitates engraftment and recovery. Cell Prolif. 2011, 44, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Speth, J.M.; Hoggatt, J.; Pelus, L.M. Enhanced homing of hspcs after treatment with prostaglandin E2 (PGE2) may be an effect of transcriptional regulation of CXC chemokine receptor 4 (CXCR4) through hypoxia inducible factor 1α (HIF1α). Blood 2011, 118, 918. [Google Scholar]
- Speth, J.M.; Hoggatt, J.; Singh, P.; Pelus, L.M. Pharmacologic increase in HIF1alpha enhances hematopoietic stem and progenitor homing and engraftment. Blood 2014, 123, 203–207. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Melacarne, A.; Yadak, R.; Schouteden, S.; Notelaers, T.; Pistoni, M.; Maes, C.; Verfaillie, C.M. SMAD signaling regulates CXCL12 expression in the bone marrow niche, affecting homing and mobilization of hematopoietic progenitors. Stem Cells (Dayton Ohio) 2014, 32, 3012–3022. [Google Scholar] [CrossRef]
- Blaser, B.W.; Moore, J.L.; Hagedorn, E.J.; Li, B.; Riquelme, R.; Lichtig, A.; Yang, S.; Zhou, Y.; Tamplin, O.J.; Binder, V.; et al. CXCR1 remodels the vascular niche to promote hematopoietic stem and progenitor cell engraftment. J. Exp. Med. 2017, 214, 1011–1027. [Google Scholar] [CrossRef]
- Merli, P.; Caruana, I.; De Vito, R.; Del Bufalo, F.; Strocchio, L.; Weber, G.A.; Pitisci, A.; Cirillo, V.; Galaverna, F.; Algeri, M.; et al. Role of IFN-gamma in immune-mediated graft failure after allogeneic hematopoietic stem cell transplantation. HemaSphere 2018, 2, 676–677. [Google Scholar] [CrossRef]
- Merli, P.; Caruana, I.; De Vito, R.; Strocchio, L.; Weber, G.; Del Bufalo, F.; Buatois, V.; Montanari, P.; Cefalo, M.G.; Pitisci, A.; et al. Role of IFNgamma in immune-mediated graft failure occurring after allogeneic hematopoietic stem cell transplantation. Haematologica 2019. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.J.; Hale, A.B.; Zhang, Y.; Sweeney, D.; Fisher, N.; van der Garde, M.; Grabowska, R.; Pepperell, E.; Channon, K.; Martin-Rendon, E.; et al. CXCR2 modulates bone marrow vascular repair and haematopoietic recovery post-transplant. Br. J. Haematol. 2015, 169, 552–564. [Google Scholar] [CrossRef] [PubMed]
- de Wynter, E.A.; Heyworth, C.M.; Mukaida, N.; Matsushima, K.; Testa, N.G. NOD/SCID repopulating cells but not LTC-IC are enriched in human CD34+ cells expressing the CCR1 chemokine receptor. Leukemia 2001, 15, 1092–1101. [Google Scholar] [CrossRef] [PubMed]
- Jorda, M.A.; Jiang, S.; Zagozdzon, R.; Parmar, K.; Mauch, P.; Fu, Y.; Makriyannis, A.; Zvonok, A.M.; Tenen, D.G.; Avraham, S.; et al. The peripheral cannabinoid receptor regulates human and mouse hematopoiesis, bone marrow recovery, and hematopoietic stem and progenitor cell mobilization. Haematologica 2009, 94, 208. [Google Scholar]
- Schulte, G. International Union of Basic and Clinical Pharmacology. LXXX. The class Frizzled receptors. Pharmacol. Rev. 2010, 62, 632–667. [Google Scholar] [CrossRef]
- Abidin, B.M.; Owusu Kwarteng, E.; Heinonen, K.M. Frizzled-6 Regulates Hematopoietic Stem/Progenitor Cell Survival and Self-Renewal. J. Immunol. (Baltimore Md. 1950) 2015, 195, 2168–2176. [Google Scholar] [CrossRef] [PubMed]
- Zong, W.N.; Chen, X.M.; Yang, Y.Q.; Cao, J.L.; Zou, H.Y.Y.; Sun, H.W.; Hou, M.H.; Huang, H.J.; Zheng, H.J.; Qin, X.Y.; et al. Plasma auto-antibodies to angiotensin II receptors are correlated with blood pressure and inflammatory factors in hypertension patients. Eur. Heart J. Suppl. 2015, 17, B65–B70. [Google Scholar] [CrossRef][Green Version]
- Dragun, D.; Philippe, A.; Catar, R. Role of non-HLA antibodies in organ transplantation. Curr. Opin. Organ Transplant. 2012, 17, 440–445. [Google Scholar] [CrossRef]
- Taniguchi, M.; Gendzekhadze, K.; Lee, J.H.; Senitzer, D. Anti-angiotensin II type-1 receptor antibodies in failed chimerism after hematopoietic stem cell transplantation. Hum. Immunol. 2017, 78, 89. [Google Scholar] [CrossRef]
- Richter, R.; Rüster, B.; Bistrian, R.; Forssmann, W.G.; Seifried, E.; Henschler, R. Beta-chemokine CCL15 affects the adhesion and migration of hematopoietic progenitor cells. Transfus. Med. Hemother. 2015, 42, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Hoggatt, J.; Singh, P.; Tate, T.A.; Chou, B.K.; Datari, S.R.; Fukuda, S.; Liu, L.; Kharchenko, P.V.; Schajnovitz, A.; Baryawno, N.; et al. Rapid Mobilization Reveals a Highly Engraftable Hematopoietic Stem Cell. Cell 2018, 172, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Foguenne, J.; Di Stefano, I.; Giet, O.; Beguin, Y.; Gothot, A. Ex vivo expansion of hematopoietic progenitor cells is associated with downregulation of alpha4 integrin-and CXCR4-mediated engraftment in NOD/SCID beta2-microglobulin-null mice. Haematologica 2009, 94, 185–194. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Peled, A.; Petit, I.; Kollet, O.; Magid, M.; Ponomaryov, T.; Byk, T.; Nagler, A.; Ben-Hur, H.; Many, A.; Shultz, L.; et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science (New York N.Y.) 1999, 283, 845–848. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Huang, X.; Cooper, S.; Broxmeyer, H.E. Glucocorticoid hormone-induced chromatin remodeling enhances human hematopoietic stem cell homing and engraftment. Nat. Med. 2017, 23, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Brenner, S.; Whiting-Theobald, N.; Kawai, T.; Linton, G.F.; Rudikoff, A.G.; Choi, U.; Ryser, M.F.; Murphy, P.M.; Sechler, J.M.; Malech, H.L. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells (Dayton Ohio) 2004, 22, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Lozano, M.L.; Niño, E.A.; Cerezo, J.J.; Castilla-Llorente, C.; Anton, A.I.; Padilla, J.; Heras, I.; Garcia, V. CXCR4 allelic variations influence hematological recovery following autologous stem cell transplantation. Blood 2011, 118, 4098. [Google Scholar]
- Abraham, M.; Biyder, K.; Begin, M.; Wald, H.; Weiss, I.D.; Galun, E.; Nagler, A.; Peled, A. Enhanced unique pattern of hematopoietic cell mobilization induced by the CXCR4 antagonist 4F-benzoyl-TN14003. Stem Cells (Dayton Ohio) 2007, 25, 2158–2166. [Google Scholar] [CrossRef]
- Wiesmann, A.; Spangrude, G.J. Marrow engraftment of hematopoietic stem and progenitor cells is independent of Galphai-coupled chemokine receptors. Exp. Hematol. 1999, 27, 946–955. [Google Scholar] [CrossRef]
- Kazemi, Z.; Bergmayr, C.; Prchal-Murphy, M.; Javaheri, T.; Themanns, M.; Pham, H.T.; Strohmaier, W.; Sexl, V.; Freissmuth, M.; Zebedin-Brandl, E. Repurposing Treprostinil for Enhancing Hematopoietic Progenitor Cell Transplantation. Mol. Pharmacol. 2016, 89, 630–644. [Google Scholar] [CrossRef]
- Bergmayr, C.; Balasz, C.; Kazemi, Z.; Hussain, F.; Bauer, T.; Strobl, H.; Selzer, P.; Strohmaier, W.; Freissmuth, M.; Zebedin-Brandl, E. Pharmacological stimulation of murine and human hematopoetic stem cells. BMC Pharmacol. Toxicol. 2012, 13. [Google Scholar] [CrossRef]
- Ogle, M.E.; Olingy, C.E.; Awojoodu, A.O.; Das, A.; Ortiz, R.A.; Cheung, H.Y.; Botchwey, E.A. Sphingosine-1-Phosphate Receptor-3 Supports Hematopoietic Stem and Progenitor Cell Residence Within the Bone Marrow Niche. Stem Cells (Dayton Ohio) 2017, 35, 1040–1052. [Google Scholar] [CrossRef] [PubMed]
- Lam, B.S.; Cunningham, C.; Adams, G.B. Pharmacologic modulation of the calcium-sensing receptor enhances hematopoietic stem cell lodgment in the adult bone marrow. Blood 2011, 117, 1167–1175. [Google Scholar] [CrossRef] [PubMed]
- Ferdous, M.; Ganuza, M.; Holmfeldt, P.; Hall, T.; Walker, M.; McKinney-Freeman, S. The G protein-coupled receptor associated sorting proteins, Gprasp2 and Armcx1 are putative negative regulators of HSC engraftment and repopulation. Blood 2015, 126, 2386. [Google Scholar]
- Cutler, C.; Kim, H.T.; Ayanian, S.; Bradwin, G.; Revta, C.; Aldridge, J.; Ho, V.; Alyea, E.; Koreth, J.; Armand, P.; et al. Prediction of veno-occlusive disease using biomarkers of endothelial injury. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 1180–1185. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.; Diaz-Ricart, M. The role of the endothelium in the short-term complications of hematopoietic SCT. Bone Marrow Transplant. 2011, 46, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Corbacioglu, S.; Cesaro, S.; Faraci, M.; Valteau-Couanet, D.; Gruhn, B.; Rovelli, A.; Boelens, J.J.; Hewitt, A.; Schrum, J.; Schulz, A.S.; et al. Defibrotide for prophylaxis of hepatic veno-occlusive disease in paediatric haemopoietic stem-cell transplantation: An open-label, phase 3, randomised controlled trial. Lancet 2012, 379, 1301–1309. [Google Scholar] [CrossRef]
- Carreras, E.; Diaz-Beya, M.; Rosinol, L.; Martinez, C.; Fernandez-Aviles, F.; Rovira, M. The incidence of veno-occlusive disease following allogeneic hematopoietic stem cell transplantation has diminished and the outcome improved over the last decade. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 1713–1720. [Google Scholar] [CrossRef]
- Corbacioglu, S.; Carreras, E.; Ansari, M.; Balduzzi, A.; Cesaro, S.; Dalle, J.H.; Dignan, F.; Gibson, B.; Guengoer, T.; Gruhn, B.; et al. Diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in pediatric patients: A new classification from the European society for blood and marrow transplantation. Bone Marrow Transplant. 2018, 53, 138–145. [Google Scholar] [CrossRef]
- Mohty, M.; Malard, F.; Abecassis, M.; Aerts, E.; Alaskar, A.S.; Aljurf, M.; Arat, M.; Bader, P.; Baron, F.; Bazarbachi, A.; et al. Revised diagnosis and severity criteria for sinusoidal obstruction syndrome/veno-occlusive disease in adult patients: A new classification from the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant. 2016, 51, 906–912. [Google Scholar] [CrossRef]
- Ikezoe, T.; Togitani, K.; Komatsu, N.; Isaka, M.; Yokoyama, A. Successful treatment of sinusoidal obstructive syndrome after hematopoietic stem cell transplantation with recombinant human soluble thrombomodulin. Bone Marrow Transplant. 2010, 45, 783–785. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Nomura, S. The Preventative Effects of Recombinant Thrombomodulin on Transplantation-Associated Coagulopathy after Allogeneic Hematopoietic Stem Cell Transplantation. J. Stem Cell Res. Ther. 2014, 04. [Google Scholar] [CrossRef]
- Ikezoe, T.; Yang, J.; Nishioka, C.; Pan, B.; Xu, K.; Furihata, M.; Nakamura, K.; Yurimoto, H.; Sakai, Y.; Honda, G.; et al. The fifth epidermal growth factor-like region of thrombomodulin exerts cytoprotective function and prevents SOS in a murine model. Bone Marrow Transplant. 2017, 52, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pan, B.; Honda, G.; Wang, X.; Hashimoto, Y.; Ohkawara, H.; Xu, K.; Zeng, L.; Ikezoe, T. Cytoprotective and pro-angiogenic functions of thrombomodulin are preserved in the C loop of the fifth epidermal growth factor-like domain. Haematologica 2018, 103, 1730–1740. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Wang, X.; Nishioka, C.; Honda, G.; Yokoyama, A.; Zeng, L.; Xu, K.; Ikezoe, T. G-protein coupled receptor 15 mediates angiogenesis and cytoprotective function of thrombomodulin. Sci. Rep. 2017, 7, 692. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Wang, X.; Kojima, S.; Nishioka, C.; Yokoyama, A.; Honda, G.; Xu, K.; Ikezoe, T. The Fifth Epidermal Growth Factor-like Region of Thrombomodulin Alleviates Murine Graft-versus-Host Disease in a G-Protein Coupled Receptor 15 Dependent Manner. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 746–756. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.G.; Carreras, E.; Iacobelli, M.; Nejadnik, B. The use of defibrotide in blood and marrow transplantation. Blood Adv. 2018, 2, 1495–1509. [Google Scholar] [CrossRef]
- Zhou, Q.; Chu, X.; Ruan, C. Defibrotide Stimulates Expression of Thrombomodulin in Human Endothelial Cells. Thromb. Haemost. 1994, 72, 507–510. [Google Scholar] [CrossRef]
- Narita, M.; Hatano, E.; Tamaki, N.; Yamanaka, K.; Yanagida, A.; Nagata, H.; Asechi, H.; Takada, Y.; Ikai, I.; Uemoto, S. Dai-kenchu-to attenuates rat sinusoidal obstruction syndrome by inhibiting the accumulation of neutrophils in the liver. J. Gastroenterol. Hepatol. 2009, 24, 1051–1057. [Google Scholar] [CrossRef]
- Markey, K.A.; MacDonald, K.P.A.; Hill, G.R. The biology of graft-versus-host disease: Experimental systems instructing clinical practice. Blood 2014, 124, 354–362. [Google Scholar] [CrossRef]
- Ferrara, J.L.M.; Levine, J.E.; Reddy, P.; Holler, E. Graft-versus-host disease. Lancet 2009, 373, 1550–1561. [Google Scholar] [CrossRef]
- Jagasia, M.; Arora, M.; Flowers, M.E.; Chao, N.J.; McCarthy, P.L.; Cutler, C.S.; Urbano-Ispizua, A.; Pavletic, S.Z.; Haagenson, M.D.; Zhang, M.J.; et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood 2012, 119, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Zeiser, R.; Blazar, B.R. Acute Graft-versus-Host Disease—Biologic Process, Prevention, and Therapy. N. Engl. J. Med. 2017, 377, 2167–2179. [Google Scholar] [CrossRef] [PubMed]
- Bouazzaoui, A.; Spacenko, E.; Mueller, G.; Miklos, S.; Huber, E.; Holler, E.; Andreesen, R.; Hildebrandt, G.C. Chemokine and chemokine receptor expression analysis in target organs of acute graft-versus-host disease. Genes Immun. 2009, 10, 687–701. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, M.; Ota, A.; Hori, T.; Imai, S.; Sohma, H.; Suzuki, N.; Hatakeyama, N.; Inazawa, N.; Ito, Y.M.; Kimura, H.; et al. Early expression of plasma CCL8 closely correlates with survival rate of acute graft-vs.-host disease in mice. Exp. Hematol. 2011, 39, 1101–1112. [Google Scholar] [CrossRef]
- Terwey, T.H.; Kim, T.D.; Kochman, A.A.; Hubbard, V.M.; Lu, S.; Zakrzewski, J.L.; Ramirez-Montagut, T.; Eng, J.M.; Muriglan, S.J.; Heller, G.; et al. CCR2 is required for CD8-induced graft-versus-host disease. Blood 2005, 106, 3322–3330. [Google Scholar] [CrossRef]
- Miklos, S.; Mueller, G.; Chang, Y.; Bouazzaoui, A.; Spacenko, E.; Schubert, T.E.O.; Grainger, D.J.; Holler, E.; Andreesen, R.; Hildebrandt, G.C. Preventive usage of broad spectrum chemokine inhibitor NR58-3.14.3 reduces the severity of pulmonary and hepatic graft-versus-host disease. Int. J. Hematol. 2009, 89, 383–397. [Google Scholar] [CrossRef]
- Li, N.; Chen, Y.; He, W.; Yi, T.; Zhao, D.; Zhang, C.; Lin, C.L.; Todorov, I.; Kandeel, F.; Forman, S.; et al. Anti-CD3 preconditioning separates GVL from GVHD via modulating host dendritic cell and donor T-cell migration in recipients conditioned with TBI. Blood 2009, 113, 953–962. [Google Scholar] [CrossRef]
- Shin, D.Y.; Kim, I.; Kim, J.H.; Lee, Y.G.; Kang, E.J.; Cho, H.J.; Lee, K.H.; Kim, H.J.; Park, E.H.; Lee, J.E.; et al. RANTES polymorphisms and the risk of graft-versus-host disease in human leukocyte antigen-matched sibling allogeneic hematopoietic stem cell transplantation. Acta Haematol. 2013, 129, 137–145. [Google Scholar] [CrossRef]
- Brissot, E.; Bossard, C.; Malard, F.; Braudeau, C.; Chevallier, P.; Guillaume, T.; Delaunay, J.; Josien, R.; Gregoire, M.; Gaugler, B.; et al. Involvement of the CX3CL1 (fractalkine)/CX3CR1 pathway in the pathogenesis of acute graft-versus-host disease. J. Leukoc. Biol. 2015, 97, 227–235. [Google Scholar] [CrossRef]
- Coghill, J.M.; West, M.L.; Cook, D.N.; Serody, J.S. The absence of cc-chemokine receptor 8 on donor regulatory t cells impairs their ability to prevent lethal acute GVHD. Blood 2010, 116. [Google Scholar]
- Vinci, P.; Recordati, C.; Bardelli, D.; Cappuzzello, C.; Fumagalli, V.; Dander, E.; Del Prete, A.; Sozzani, S.; Biondi, A.; D’Amico, G. The chemerin/ChemR23 axis plays a pivotal role in the pathogenesis of intestinal damage in a murine model of graft versus host disease. Blood 2015, 126, 3077. [Google Scholar]
- Bogunia-Kubik, K.; Mizia, S.; Polak, M.; Gronkowska, A.; Nowak, J.; Kyrcz-Krzemien, S.; Markiewicz, M.; Dzierzak-Mietla, M.; Koclega, A.; Sedzimirska, M.; et al. Beneficial effect of the CXCL12-3′A variant for patients undergoing hematopoietic stem cell transplantation from unrelated donors. Cytokine 2015, 76, 182–186. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, H.; Maruyama, K.; Yamauchi, N.; Kobayashi, R.; Nagano, S.; Ishikawa, T.; Yoshii, Y.; Uoshima, N.; Hosoi, H. Effects of humanized anti-CC chemokine receptor 4 monoclonal antibody on regulatory T cells and GVHD around hematopoietic stem cell transplantation for adult T-cell leukemia lymphoma. Haematologica 2013, 98, 377. [Google Scholar]
- Sugio, T.; Kato, K.; Aoki, T.; Ota, T.; Saito, N.; Yoshida, S.; Kawano, I.; Henzan, H.; Takase, K.; Muta, T.; et al. Prior use of mogamulizumab to allogenic hematopoietic stem cell transplantation induces severe acute graft-versus-host disease. Blood 2015, 126, 1940. [Google Scholar]
- Khandelwal, P.; Davies, S.M.; Jordan, M.B.; Lake, K.E.; Litts, B.; Owsley, E.; Marsh, R.A. α4β7 Integrin Is Upregulated on CD8+ Effector Memory T-Cells in Children with Gut Gvhd Prior to Clinical Symptoms and Represents a Therapeutic Target in Pediatric Allogeneic HSCT Patients. Biol. Blood Marrow Transplant. 2019, 25, S265–S266. [Google Scholar] [CrossRef]
- Julg, B.; Goebel, F.D. Susceptibility to HIV/AIDS: An individual characteristic we can measure? Infection 2005, 33, 160–162. [Google Scholar] [CrossRef]
- Bogunia-Kubik, K.; Duda, D.; Suchnicki, K.; Lange, A. CCR5 deletion mutation and its association with the risk of developing acute graft-versus-host disease after allogeneic hematopoietic stem cell transplantation. Haematologica 2006, 91, 1628–1634. [Google Scholar] [PubMed]
- Ma, Q.; Gooley, T.A.; Storb, R.F. CCR5 expression on cells from HLA-matched unrelated marrow donors and graft-versus-host disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2010, 16, 132–133. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gomez, A.; Hammer, S.G.; Braun, T.; Hanash, S.; Ferrara, J.L.M.; Greenson, J.K.; Wang, H.; Zhang, Q.; Vander Lugt, M.; Pongtornpipat, P.; et al. A novel CD4+CD146+CCR5+T-cell population is a biomarker of intestinal graft-versus-host disease. Bone Marrow Transplant. 2013, 48, S66. [Google Scholar] [CrossRef][Green Version]
- Li, W.; Liu, L.; Gomez, A.; Zhang, J.; Ramadan, A.; Zhang, Q.; Choi, S.W.; Zhang, P.; Greenson, J.K.; Liu, C.; et al. Proteomics analysis reveals a Th17-prone cell population in presymptomatic graft-versus-host disease. JCI Insight 2016, 1, 86660. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Liu, L.; Gomez, A.; Zhang, Q.; Zhang, J.; Ramadan, A.; Mumaw, C.L.; Greenson, J.K.; Hammer, S.; Lin, J.; et al. A novel Th17-Prone CD146+CCR5+ T-Cell population as an early marker of intestinal graft-versus-host disease. Blood 2014, 124, 3. [Google Scholar]
- Sartor, M.; Lau, J.; Gottlieb, D.; Bradstock, K. CCR5 expression on circulating blood DC post-allogeneic haematopoietic cell transplant is highly predictive for the development of clinically significant acute graft-versushost disease. Bone Marrow Transplant. 2010, 45, S125–S126. [Google Scholar] [CrossRef]
- Sartor, M.M.; Lau, J.; Gottlieb, D.J.; Bradstock, K.F. CCR5 expression on circulating blood dc post-allogeneic hemopoietic cell transplant is highly predictive for the development of clinically significant acute graft versus host disease. Blood 2009, 114. [Google Scholar] [CrossRef]
- Shahin, K.; Sartor, M.; Hart, D.N.; Bradstock, K.F. Alterations in chemokine receptor CCR5 expression on blood dendritic cells correlate with acute graft-versus-host disease. Transplantation 2013, 96, 753–762. [Google Scholar] [CrossRef] [PubMed]
- Reshef, R.; Luger, S.M.; Hexner, E.O.; Loren, A.W.; Frey, N.V.; Nasta, S.D.; Goldstein, S.C.; Stadtmauer, E.A.; Smith, J.; Bailey, S.; et al. Blockade of lymphocyte chemotaxis in visceral graft-versus-host disease. N. Engl. J. Med. 2012, 367, 135–145. [Google Scholar] [CrossRef]
- Huffman, A.P.; Richman, L.P.; Crisalli, L.; Ganetsky, A.; Porter, D.L.; Vonderheide, R.H.; Reshef, R. Pharmacodynamic Monitoring Predicts Outcomes of CCR5 Blockade as Graft-versus-Host Disease Prophylaxis. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 24, 594–599. [Google Scholar] [CrossRef]
- Moy, R.H.; Huffman, A.P.; Richman, L.P.; Crisalli, L.; Hoxie, J.A.; Vonderheide, R.H.; Porter, D.L.; Reshef, R. Immunologic effects of CCR5 blockade in graft-versus-host disease prophylaxis. Blood 2015, 126, 920. [Google Scholar]
- Moy, R.H.; Huffman, A.P.; Richman, L.P.; Crisalli, L.; Wang, X.K.; Hoxie, J.A.; Mick, R.; Emerson, S.G.; Zhang, Y.; Vonderheide, R.H.; et al. Clinical and immunologic impact of CCR5 blockade in graft-versus-host disease prophylaxis. Blood 2017, 129, 906–916. [Google Scholar] [CrossRef]
- Reshef, R.; Ganetsky, A.; Acosta, E.P.; Blauser, R.; Crisalli, L.; McGraw, J.; Frey, N.V.; Hexner, E.O.; Hoxie, J.A.; Loren, A.W.; et al. Extended CCR5 Blockade for Graft-versus-Host Disease Prophylaxis Improves Outcomes of Reduced-Intensity Unrelated Donor Hematopoietic Cell Transplantation: A Phase II Clinical Trial. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2018, 25, 515–521. [Google Scholar] [CrossRef]
- Reshef, R.; Luger, S.M.; Loren, A.W.; Frey, N.V.; Goldstein, S.C.; Hexner, E.O.; Stadtmauer, E.A.; Smith, J.; Mick, R.; Heitjan, D.F.; et al. Prevention of graft-versus-host disease by inhibition of lymphocyte trafficking using a CCR5 antagonist. Blood 2010, 116. [Google Scholar]
- Khandelwal, P.; Fukuda, T.; Teusink-Cross, A.; Kashuba, A.D.; Marsh, R.A.; Mehta, P.A.; Vinks, A.; Nelson, A.S.; Myers, K.C.; Krupski, M.C.; et al. Pediatric Phase II Study of Maraviroc for Acute Graft Versus Host Disease Prophylaxis. Biol. Blood Marrow Transplant. 2019, 25, S251–S252. [Google Scholar] [CrossRef]
- Wang, Z.; Murphy, W.J. Relationship between CCR5 and acute graft-versus-host disease in murine bone marrow transplantation. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2006, 14, 934–940. [Google Scholar] [PubMed]
- Welniak, L.A.; Wang, Z.; Sun, K.; Kuziel, W.; Anver, M.R.; Blazar, B.R.; Murphy, W.J. An absence of CCR5 on donor cells results in acceleration of acute graft-vs-host disease. Exp. Hematol. 2004, 32, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, C.A.; Burkett, S.B.; Panoskaltsis-Mortari, A.; Kirby, S.L.; Luster, A.D.; McKinnon, K.; Blazar, B.R.; Serody, J.S. Differential roles for CCR5 expression on donor T cells during graft-versus-host disease based on pretransplant conditioning. J. Immunol. (Baltimore Md. 1950) 2004, 173, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Burger, D.R.; Parker, Y.; Guinta, K.; Lindner, D. PRO 140 Monoclonal Antibody to CCR5 Prevents Acute Xenogeneic Graft-versus-Host Disease in NOD-scid IL-2Rynull Mice. Biol. Blood Marrow Transplant. 2018, 24, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Ren, H.Y.; Shi, Y.J.; Liu, W. Prophylaxis of acute graft-versus-host disease by CCR5 blockade combined with cyclosporine A in a murine model. Inflamm. Res. 2015, 64, 137–144. [Google Scholar] [CrossRef]
- Tang, B.; Ren, H.; Liu, H.; Shi, Y.; Liu, W.; Dong, Y.; Yin, Y.; Miao, S. CCR5 blockade combined with cyclosporine A attenuates liver GVHD by impairing T cells function. Inflamm. Res. 2016, 65, 917–924. [Google Scholar] [CrossRef]
- Tang, B.; Liu, H.; Miao, S.; Dong, Y.; Liu, W.; Shi, Y.; Qin, C.; Wang, Z.; Zhao, M.; Ren, H. Combined CCR5 and CXCR3 blockade attenuates murine agvhd through alternating donor-derived t cell distribution and function. Blood 2017, 130, 5449. [Google Scholar]
- Hasegawa, H.; Inoue, A.; Kohno, M.; Lei, J.; Miyazaki, T.; Yoshie, O.; Nose, M.; Yasukawa, M. Therapeutic effect of CXCR3-expressing regulatory T cells on liver, lung and intestinal damages in a murine acute GVHD model. Gene Ther. 2008, 15, 171–182. [Google Scholar] [CrossRef]
- Duffner, U.; Lu, B.; Hildebrandt, G.C.; Teshima, T.; Williams, D.L.; Reddy, P.; Ordemann, R.; Clouthier, S.G.; Lowler, K.; Liu, C.; et al. Role of CXCR3-induced donor T-cell migration in acute GVHD. Exp. Hematol. 2003, 31, 897–902. [Google Scholar] [CrossRef]
- He, S.; Cao, Q.; Qiu, Y.; Mi, J.; Zhang, J.Z.; Jin, M.; Ge, H.; Emerson, S.G.; Zhang, Y.; Zhang, Y. A new approach to the blocking of alloreactive T cell-mediated graft-versus-host disease by in vivo administration of anti-CXCR3 neutralizing antibody. J. Immunol. (Baltimore Md. 1950) 2008, 181, 7581–7592. [Google Scholar] [CrossRef] [PubMed]
- Satake, A.; Inoue, T.; Kubo, S.; Taniguchi, Y.; Imado, T.; Fujioka, T.; Horiuchi, M.; Xu, Y.; Ikegame, K.; Yoshihara, S.; et al. Separation of antileukemic effects from graft-versus-host disease in MHC-haploidentical murine bone marrow transplantation: Participation of host immune cells. Int. J. Hematol. 2010, 91, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ziga, E.D.; Ritchey, J.; Collins, L.; Prior, J.L.; Cooper, M.L.; Piwnica-Worms, D.; DiPersio, J.F. IFNgammaR signaling mediates alloreactive T-cell trafficking and GVHD. Blood 2012, 120, 4093–4103. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Cooper, M.L.; Alahmari, B.; Ritchey, J.; Collins, L.; Holt, M.; DiPersio, J.F. Pharmacologic blockade of JAK1/JAK2 reduces GvHD and preserves the graft-versus-leukemia effect. PLoS ONE 2014, 9, e109799. [Google Scholar] [CrossRef]
- Miao, S.; Tang, B.; Liu, H.; Wang, Z.; Shi, Y.; Dong, Y.; Liu, W.; Qin, C.; Ren, H. CXCR3 blockade combined with cyclosporine A alleviates acute graft-versus-host disease by inhibiting alloreactive donor T cell responses in a murine model. Mol. Immunol. 2018, 94, 82–90. [Google Scholar] [CrossRef]
- O’Boyle, G.; Barker, C.; Mavin, E.; Wang, X.; Fox, C.; Walden, H.; Willet, J.; Hine, D.; Palmer, J.; Lamb, C.; et al. A small molecule agonist of the chemokine receptor CXCR3 prevents experimental graft-versus-host disease. Immunology 2013, 140, 149. [Google Scholar] [CrossRef]
- Broen, K.; van der Waart, A.B.; Greupink-Draaisma, A.; Metzig, J.; Feuth, T.; Schaap, N.P.; Blijlevens, N.M.; van der Velden, W.J.; Dolstra, H. Polymorphisms in CCR6 are associated with chronic graft-versus-host disease and invasive fungal disease in matched-related hematopoietic stem cell transplantation. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 1443–1449. [Google Scholar] [CrossRef][Green Version]
- de Jager, S.C.; Cante-Barrett, K.; Bot, I.; Husberg, C.; van Puijvelde, G.H.; van Santbrink, P.J.; Yndestad, A.; van den Oever, J.M.; Kuiper, J.; van Berkel, T.J.; et al. Impaired effector memory T-cell regulation facilitates graft versus host disease in CCR7-deficient bone marrow transplant chimeras. Transplantation 2009, 88, 631–639. [Google Scholar] [CrossRef]
- Xu, Y.J.; Chen, W.R.; Li, D.P.; Song, L.X.; Wu, J.Q.; Zhang, P.; Li, Z.Y.; Huang, Y.H. Suppression of lentivirus-mediated transgenic dendritic cells in graft-versus-host disease after allogeneic bone marrow transplantation in mice. Genet. Mol. Res. GMR 2015, 14, 11444–11455. [Google Scholar] [CrossRef]
- Wang, M.; Hu, J.; Qiu, Z.X.; Liu, W.; Wang, M.J.; Li, Y.; Sun, Y.H.; Zhu, S.N.; Ren, H.Y.; Dong, Y.J. Alterations of CCR5 and CCR7 expression on donor peripheral blood T cell subsets after mobilization with rhG-CSF correlate with acute graft-versus-host disease. Clin. Immunol. 2018, 191, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ren, H.Y.; Dong, Y.J.; Liang, Z.Y.; Qiu, Z.X.; Liu, W.; Wang, L.H. Association of chemokine receptor CCR5, CCR6 and CCR7 expressions on T lymphocyte subsets in recipients after Allo-HSCT with acute GvHD. Blood 2013, 122, 4596. [Google Scholar]
- Ren, H.Y.; Wang, M.; Ma, X.J.; Dong, Y.J.; Qiu, Z.X.; Liu, W. Differential regulation of chemokine receptor expressions on T lymphocyte subsets in healthy donors after mobilization with RHG-CSF and its correlation with acute GvHD. Blood 2013, 122. [Google Scholar]
- Goncalves, K.A.; Falahee, P.C.; Hyzy, S.L.; Li, S.; Boitano, A.E.; Morrow, D.M.; Cooke, M.P. Co-Administration of Mgta-145 and Plerixafor Rapidly Mobilizes High Numbers of Hematopoietic Stem Cells and Graft-Versus-Host Disease Inhibiting Monocytic Cells in Non-Human Primates. Biol. Blood Marrow Transplant. 2019, 25, S291–S292. [Google Scholar] [CrossRef]
- Cho, K.A.; Woo, S.Y.; Park, Y.S.; Park, M.H.; Ryu, K.H. Macrophage inflammatory protein-2 (MIP-2)/CXCR2 blockade attenuates acute graft-versus-host disease while preserving graft-versus-leukemia activity. Biochem. Biophys. Res. Commun. 2012, 426, 558–564. [Google Scholar] [CrossRef]
- Lazarus, H.M.; Sommers, S.R.; Arfons, L.M.; Fu, P.; Ataergin, S.A.; Kaye, N.M.; Liu, F.; Kindwall-Keller, T.L.; Cooper, B.W.; Laughlin, M.J.; et al. Spontaneous autologous graft-versus-host disease in plasma cell myeloma autograft recipients: Flow cytometric analysis of hematopoietic progenitor cell grafts. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2011, 17, 970–978. [Google Scholar] [CrossRef]
- Arbez, J.; Saas, P.; Lamarthee, B.; Malard, F.; Couturier, M.; Mohty, M.; Gaugler, B. Impact of donor hematopoietic cells mobilized with G-CSF and plerixafor on murine acute graft-versus-host-disease. Cytotherapy 2015, 17, 948–955. [Google Scholar] [CrossRef]
- Lundqvist, A.; Smith, A.L.; Takahashi, Y.; Wong, S.; Bahceci, E.; Cook, L.; Ramos, C.; Tawab, A.; McCoy, J.P., Jr.; Read, E.J.; et al. Differences in the phenotype, cytokine gene expression profiles, and in vivo alloreactivity of T cells mobilized with plerixafor compared with G-CSF. J. Immunol. (Baltimore Md. 1950) 2013, 191, 6241–6249. [Google Scholar] [CrossRef]
- Han, K.L.; Changpriroa, C.M.; Malech, H.L.; Kang, E.M. Activation of the adenosine A2A receptor promotes the development of donor-derived T regulatory cells in a graft-versus host disease mouse model. Blood 2009, 114. [Google Scholar]
- Lappas, C.M.; Liu, P.C.; Linden, J.; Kang, E.M.; Malech, H.L. Adenosine A2A receptor activation limits graft-versus-host disease after allogenic hematopoietic stem cell transplantation. J. Leukoc. Biol. 2010, 87, 345–354. [Google Scholar] [CrossRef][Green Version]
- Leigh, N.D.; Kokolus, K.M.; Eng, J.W.L.; Qiu, J.; Chen, G.L.; McCarthy, P.L.; Cao, X.; Repasky, E.A. The degree of adrenergic stress signaling regulates the severity of graft versus host disease following allogeneic hematopoietic cell transplantation. Cancer Immunol. Res. 2015, 3, B43. [Google Scholar] [CrossRef]
- Leigh, N.D.; Kokolus, K.M.; O’Neill, R.E.; Du, W.; Eng, J.W.; Qiu, J.; Chen, G.L.; McCarthy, P.L.; Farrar, J.D.; Cao, X.; et al. Housing Temperature-Induced Stress Is Suppressing Murine Graft-versus-Host Disease through beta2-Adrenergic Receptor Signaling. J. Immunol. (Baltimore Md. 1950) 2015, 195, 5045–5054. [Google Scholar] [CrossRef] [PubMed]
- Klambt, V.; Wohlfeil, S.A.; Schwab, L.; Hulsdunker, J.; Ayata, K.; Apostolova, P.; Schmitt-Graeff, A.; Dierbach, H.; Prinz, G.; Follo, M.; et al. A Novel Function for P2Y2 in Myeloid Recipient-Derived Cells during Graft-versus-Host Disease. J. Immunol. (Baltimore Md. 1950) 2015, 195, 5795–5804. [Google Scholar] [CrossRef] [PubMed]
- Sido, J.M.; Nagarkatti, P.S.; Nagarkatti, M. Delta(9)-Tetrahydrocannabinol attenuates allogeneic host-versus-graft response and delays skin graft rejection through activation of cannabinoid receptor 1 and induction of myeloid-derived suppressor cells. J. Leukoc. Biol. 2015, 98, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Kemter, A.M.; Scheu, S.; Huser, N.; Ruland, C.; Schumak, B.; Findeiss, M.; Cheng, Z.; Assfalg, V.; Arolt, V.; Zimmer, A.; et al. The cannabinoid receptor 2 is involved in acute rejection of cardiac allografts. Life Sci. 2015, 138, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Hegde, V.L.; Nagarkatti, M.; Nagarkatti, P.S. Targeting cannabinoid receptors as a novel approach in the treatment of graft-versus-host disease: Evidence from an experimental murine model. J. Pharmacol. Exp. Ther. 2011, 338, 819–828. [Google Scholar] [CrossRef] [PubMed]
- Yuan, V.; Belle, L.; Komorowski, R.; Hillard, C.; Drobyski, W.R. The type 2 cannabinoid receptor regulates the severity of acute and chronic graft versus host disease in mice. Biol. Blood Marrow Transplant. 2018, 24, S175. [Google Scholar] [CrossRef]
- Yeshurun, M.; Shpilberg, O.; Herscovici, C.; Shargian, L.; Dreyer, J.; Peck, A.; Israeli, M.; Levy-Assaraf, M.; Gruenewald, T.; Mechoulam, R.; et al. Cannabidiol for the Prevention of Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation: Results of a Phase II Study. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 1770–1775. [Google Scholar] [CrossRef]
- Smith, P.; O’Sullivan, C.; Gergely, P. Sphingosine 1-Phosphate Signaling and Its Pharmacological Modulation in Allogeneic Hematopoietic Stem Cell Transplantation. Int. J. Mol. Sci. 2017, 18, E2027. [Google Scholar] [CrossRef]
- Cheng, Q.; Ma, S.; Lin, D.; Mei, Y.; Gong, H.; Lei, L.; Chen, Y.; Zhao, Y.; Hu, B.; Wu, Y.; et al. The S1P1 receptor-selective agonist CYM-5442 reduces the severity of acute GVHD by inhibiting macrophage recruitment. Cell. Mol. Immunol. 2015, 12, 681–691. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Bastian, D.; Kuril, S.; Dany, M.; Fairlie, D.; Atkinson, C.; Ogretmen, B.; Tomlinson, S.; Yu, X.Z. Complement promotes GVHD through suppressing autophagy in recipient dendritic cells after allogeneic hematopoietic cell transplantation in mice. Blood 2017, 130, 1904. [Google Scholar]
- Castor, M.G.; Rezende, B.M.; Resende, C.B.; Bernardes, P.T.; Cisalpino, D.; Vieira, A.T.; Souza, D.G.; Silva, T.A.; Teixeira, M.M.; Pinho, V. Platelet-activating factor receptor plays a role in the pathogenesis of graft-versus-host disease by regulating leukocyte recruitment, tissue injury, and lethality. J. Leukoc. Biol. 2012, 91, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Harkensee, C.; Oka, A.; Onizuka, M.; Middleton, P.G.; Inoko, H.; Nakaoka, H.; Gennery, A.R.; Ando, K.; Morishima, Y. Microsatellite scanning of the immunogenome associates MAPK14 and ELTD1 with graft-versus-host disease in hematopoietic stem cell transplantation. Immunogenetics 2013, 65, 417–427. [Google Scholar] [CrossRef] [PubMed]
- Hayase, E.; Hashimoto, D.; Takahashi, S.; Ohigashi, H.; Tateno, T.; Ara, T.; Yokoyama, E.; Teshima, T. Gastric stem cells are targeted in upper gastrointestinal GVHD. Blood 2017, 130, 3173. [Google Scholar]
- Ranjan, S.; Goihl, A.; Kohli, S.; Gadi, I.; Pierau, M.; Shahzad, K.; Gupta, D.; Bock, F.; Wang, H.; Shaikh, H.; et al. Activated protein C protects from GvHD via PAR2/PAR3 signalling in regulatory T-cells. Nat. Commun. 2017, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Nomura, S.; Ishii, K.; Fujita, S.; Nakaya, A.; Satake, A.; Ito, T. Associations between acute GVHD-related biomarkers and endothelial cell activation after allogeneic hematopoietic stem cell transplantation. Transpl. Immunol. 2017, 43–44, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; van Esch, B.; Henricks, P.A.J.; Folkerts, G.; Garssen, J. The Anti-inflammatory Effects of Short Chain Fatty Acids on Lipopolysaccharide-or Tumor Necrosis Factor alpha-Stimulated Endothelial Cells via Activation of GPR41/43 and Inhibition of HDACs. Front. Pharmacol. 2018, 9, 533. [Google Scholar] [CrossRef] [PubMed]
- Kamp, M.E.; Shim, R.; Nicholls, A.J.; Oliveira, A.C.; Mason, L.J.; Binge, L.; Mackay, C.R.; Wong, C.H. G Protein-Coupled Receptor 43 Modulates Neutrophil Recruitment during Acute Inflammation. PLoS ONE 2016, 11, e0163750. [Google Scholar] [CrossRef] [PubMed]
- Luu, M.; Weigand, K.; Wedi, F.; Breidenbend, C.; Leister, H.; Pautz, S.; Adhikary, T.; Visekruna, A. Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci. Rep. 2018, 8, 14430. [Google Scholar] [CrossRef] [PubMed]
- Fujiwara, H.; Docampo, M.D.; Riwes, M.; Peltier, D.; Toubai, T.; Henig, I.; Wu, S.J.; Kim, S.; Taylor, A.; Brabbs, S.; et al. Microbial metabolite sensor GPR43 controls severity of experimental GVHD. Nat. Commun. 2018, 9, 3674. [Google Scholar] [CrossRef] [PubMed]
- Lamarthee, B.; Malard, F.; Gamonet, C.; Bossard, C.; Couturier, M.; Renauld, J.C.; Mohty, M.; Saas, P.; Gaugler, B. Donor interleukin-22 and host type I interferon signaling pathway participate in intestinal graft-versus-host disease via STAT1 activation and CXCL10. Mucosal Immunol. 2016, 9, 309–321. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Qin, X.; Wang, N.; Jiang, E.; Han, M.; Ma, Y.; Liang, B.; Yang, K.; Yu, K.; Ye, H. B Lymphocyte Chemoattractant (CXCL13) Is an Indicator of Acute Gastrointestinal GVHD in Murine Model. Inflammation 2017, 40, 1678–1687. [Google Scholar] [CrossRef] [PubMed]
- McDermott, D.H.; Conway, S.E.; Wang, T.; Ricklefs, S.M.; Agovi, M.A.; Porcella, S.F.; Tran, H.T.; Milford, E.; Spellman, S.; Abdi, R. Donor and recipient chemokine receptor CCR5 genotype is associated with survival after bone marrow transplantation. Blood 2010, 115, 2311–2318. [Google Scholar] [CrossRef] [PubMed]
- Mapara, M.Y.; Leng, C.; Kim, Y.M.; Bronson, R.; Lokshin, A.; Luster, A.; Sykes, M. Expression of chemokines in GVHD target organs is influenced by conditioning and genetic factors and amplified by GVHR. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2006, 12, 623–634. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Liu, T.; Gu, L.; Huang, C.; Zhu, H.; Meng, W.; Xi, Y.; Li, S.; Liu, Y. Immunomodulatory effects of mesenchymal stem cells involved in favoring type 2 T cell subsets. Transpl. Immunol. 2009, 22, 55–61. [Google Scholar] [CrossRef]
- Lee, S.J. Classification systems for chronic graft-versus-host disease. Blood 2017, 129, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Cooke, K.R.; Luznik, L.; Sarantopoulos, S.; Hakim, F.T.; Jagasia, M.; Fowler, D.H.; van den Brink, M.R.M.; Hansen, J.A.; Parkman, R.; Miklos, D.B.; et al. The Biology of Chronic Graft-versus-Host Disease: A Task Force Report from the National Institutes of Health Consensus Development Project on Criteria for Clinical Trials in Chronic Graft-versus-Host Disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2017, 23, 211–234. [Google Scholar] [CrossRef]
- Kroger, N.; Solano, C.; Wolschke, C.; Bandini, G.; Patriarca, F.; Pini, M.; Nagler, A.; Selleri, C.; Risitano, A.; Messina, G.; et al. Antilymphocyte Globulin for Prevention of Chronic Graft-versus-Host Disease. N. Engl. J. Med. 2016, 374, 43–53. [Google Scholar] [CrossRef]
- Kanakry, C.G.; Tsai, H.L.; Bolanos-Meade, J.; Smith, B.D.; Gojo, I.; Kanakry, J.A.; Kasamon, Y.L.; Gladstone, D.E.; Matsui, W.; Borrello, I.; et al. Single-agent GVHD prophylaxis with posttransplantation cyclophosphamide after myeloablative, HLA-matched BMT for AML, ALL, and MDS. Blood 2014, 124, 3817–3827. [Google Scholar] [CrossRef]
- Arai, S.; Arora, M.; Wang, T.; Spellman, S.R.; He, W.; Couriel, D.R.; Urbano-Ispizua, A.; Cutler, C.S.; Bacigalupo, A.A.; Battiwalla, M.; et al. Increasing incidence of chronic graft-versus-host disease in allogeneic transplantation: A report from the Center for International Blood and Marrow Transplant Research. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2015, 21, 266–274. [Google Scholar] [CrossRef]
- Baker, K.S.; Ness, K.K.; Steinberger, J.; Carter, A.; Francisco, L.; Burns, L.J.; Sklar, C.; Forman, S.; Weisdorf, D.; Gurney, J.G.; et al. Diabetes, hypertension, and cardiovascular events in survivors of hematopoietic cell transplantation: A report from the bone marrow transplantation survivor study. Blood 2007, 109, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Sugerman, P.B.; Faber, S.B.; Willis, L.M.; Petrovic, A.; Murphy, G.F.; Pappo, J.; Silberstein, D.; van den Brink, M.R. Kinetics of gene expression in murine cutaneous graft-versus-host disease. Am. J. Pathol. 2004, 164, 2189–2202. [Google Scholar] [CrossRef]
- Martinu, T.; Kinnier, C.V.; Sun, J.; Kelly, F.L.; Nelson, M.E.; Garantziotis, S.; Foster, W.M.; Palmer, S.M. Allogeneic splenocyte transfer and lipopolysaccharide inhalations induce differential T cell expansion and lung injury: A novel model of pulmonary graft-versus-host disease. PLoS ONE 2014, 9, e97951. [Google Scholar] [CrossRef] [PubMed]
- Hayashida, J.N.; Nakamura, S.; Toyoshima, T.; Moriyama, M.; Sasaki, M.; Kawamura, E.; Ohyama, Y.; Kumamaru, W.; Shirasuna, K. Possible involvement of cytokines, chemokines and chemokine receptors in the initiation and progression of chronic GVHD. Bone Marrow Transplant. 2013, 48, 115–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Piccari, S.; Chirumbolo, G.; Nicolini, B.; Ulbar, F.; Tolomelli, G.; Bandini, G.; Bonifazi, F.; Stanzani, M.; Lemoli, R.M.; Baccarani, M.; et al. Increased serum concentration of the inflammatory chemokines IL-8, MIP1-alpha and IP-10 at 3 months after allogeneic hsct correlate with the development of chronic GVHD. Haematologica 2011, 96, 154–155. [Google Scholar]
- Abu Zaid, M.; Wu, J.; Wu, C.; Logan, B.R.; Yu, J.; Cutler, C.; Antin, J.H.; Paczesny, S.; Choi, S.W. Plasma biomarkers of risk for death in a multicenter phase 3 trial with uniform transplant characteristics post-allogeneic HCT. Blood 2017, 129, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Storer, B.E.; Kushekhar, K.; Abu Zaid, M.; Zhang, Q.; Gafken, P.R.; Ogata, Y.; Martin, P.J.; Flowers, M.E.; Hansen, J.A.; et al. Biomarker Panel for Chronic Graft-Versus-Host Disease. Biol. Blood Marrow Transplant. J. Am. Soc. Blood Marrow Transplant. 2016, 34, 2583–2590. [Google Scholar] [CrossRef]
- Westekemper, H.; Meller, S.; Citak, S.; Schulte, C.; Steuhl, K.P.; Homey, B.; Meller, D. Differential chemokine expression in chronic GVHD of the conjunctiva. Bone Marrow Transplant. 2010, 45, 1340–1346. [Google Scholar] [CrossRef]
- Croudace, J.E.; Inman, C.F.; Abbotts, B.E.; Nagra, S.; Nunnick, J.; Mahendra, P.; Craddock, C.; Malladi, R.; Moss, P.A. Chemokine-mediated tissue recruitment of CXCR3+ CD4+ T cells plays a major role in the pathogenesis of chronic GVHD. Blood 2012, 120, 4246–4255. [Google Scholar] [CrossRef][Green Version]
- Imanguli, M.M.; Swaim, W.D.; League, S.C.; Gress, R.E.; Pavletic, S.Z.; Hakim, F.T. Increased T-bet+ cytotoxic effectors and type I interferon-mediated processes in chronic graft-versus-host disease of the oral mucosa. Blood 2009, 113, 3620–3630. [Google Scholar] [CrossRef]
- Croudace, J.; Malladi, R.; Nagra, S.; Craddock, C.; Moss, P. CXCL10 contributes to the pathogenesis of chronic skin GVHD by recruiting CXCR3+ T cells. Haematologica 2011, 96, 190–191. [Google Scholar]
- Croudace, J.; Inman, C.F.; Abbotts, B.; Nagra, S.; Malladi, R.; Craddock, C.; Moss, P. CD4+ T cells, recruited via CXCL10 and CXCR3 interactions mediate chronic skin GVHD and distinguish acute from chronic disease post allogeneic stem cell transplantation. Immunology 2011, 135, 122. [Google Scholar] [CrossRef][Green Version]
- Imanguli, M.M.; Cowen, E.W.; Rose, J.; Dhamala, S.; Swaim, W.; Lafond, S.; Yagi, B.; Gress, R.E.; Pavletic, S.Z.; Hakim, F.T. Comparative analysis of FoxP3+ regulatory T cells in the target tissues and blood in chronic graft versus host disease. Leukemia 2014, 28, 2016–2027. [Google Scholar] [CrossRef]
- Klimczak, A.; Lach, A.; Jaskula, E.; Lange, A. Epithelial expression of CCR6 chemokine receptor and the presence of IL-17 producing cells is associated with skin pathology in chronic GvHD. Bone Marrow Transplant. 2011, 46, S102. [Google Scholar] [CrossRef]
- Cromvik, J.; Johnsson, M.; Vaht, K.; Johansson, J.E.; Wenneras, C. Eosinophils in the blood of hematopoietic stem cell transplanted patients are activated and have different molecular marker profiles in acute and chronic graft-versus-host disease. Immun. Inflamm. Dis. 2014, 2, 99–113. [Google Scholar] [CrossRef]
- Inamoto, Y.; Murata, M.; Katsumi, A.; Kuwatsuka, Y.; Tsujimura, A.; Ishikawa, Y.; Sugimoto, K.; Onizuka, M.; Terakura, S.; Nishida, T.; et al. Donor single nucleotide polymorphism in the CCR9 gene affects the incidence of skin GVHD. Bone Marrow Transplant. 2010, 45, 363–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- McDonald-Hyman, C.; Flynn, R.; Panoskaltsis-Mortari, A.; Peterson, N.; MacDonald, K.P.; Hill, G.R.; Luznik, L.; Serody, J.S.; Murphy, W.J.; Maillard, I.; et al. Therapeutic regulatory T-cell adoptive transfer ameliorates established murine chronic GVHD in a CXCR5-dependent manner. Blood 2016, 128, 1013–1017. [Google Scholar] [CrossRef] [PubMed]
- Konuma, T.; Kohara, C.; Watanabe, E.; Mizukami, M.; Nagai, E.; Oiwa-Monna, M.; Tanoue, S.; Isobe, M.; Jimbo, K.; Kato, S.; et al. Circulating monocyte subsets in human chronic graft-versus-host disease. Bone Marrow Transplant. 2018, 53, 1532–1540. [Google Scholar] [CrossRef] [PubMed]
- Vasu, S.; Jaglowski, S.M.; Geyer, S.; Bingman, A.; Elder, P.; Yu, J.; Andritsos, L.A.; Blum, W.; Klisovic, R.B.; Penza, S.; et al. Differential distribution of activated innate and adaptive immune subsets in G-CSF mobilized hematopoietic stem cell allografts may influence incidence of acute (AGVHD) and chronic graft-versus-host disease (CGVHD). Blood 2012, 120, 4192. [Google Scholar]
- Martinu, T.; Gowdy, K.M.; Nugent, J.L.; Sun, J.; Kinnier, C.V.; Nelson, M.E.; Lyes, M.A.; Kelly, F.L.; Foster, W.M.; Gunn, M.D.; et al. Role of C-C motif ligand 2 and C-C motif receptor 2 in murine pulmonary graft-versus-host disease after lipopolysaccharide inhalations. Am. J. Respir. Cell Mol. Biol. 2014, 51, 810–821. [Google Scholar] [CrossRef]
- Kalra, A.; Dharmani-Khan, P.; Patel, S.; Faridi, R.M.; Lewis, V.; Daly, A.; Mansoor, A.; Shabani-Rad, M.T.; Storek, J.; Khan, F.M. Differential Expression of Immunity Related Genes Can Predict Moderate-Severe Chronic GvHD Early after Myeloablative Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2019, 25, S237. [Google Scholar] [CrossRef]
- Chiron, A.; Bouaziz, J.D.; Carmagnat, M.; Peffault de Latour, R.; Lafaurie-Bergeron, A.; Robin, M.; Xhaard, A.; Toubert, A.; Charron, D.; Guigue, N.; et al. Anti-Angiotensin type 1 receptor antibodies in chronic graft-versus-host disease. Transplantation 2014, 98, 470–474. [Google Scholar] [CrossRef] [PubMed]
- Zerr, P.; Palumbo-Zerr, K.; Distler, A.; Tomcik, M.; Vollath, S.; Munoz, L.E.; Beyer, C.; Dees, C.; Egberts, F.; Tinazzi, I.; et al. Inhibition of hedgehog signaling for the treatment of murine sclerodermatous chronic graft-versus-host disease. Blood 2012, 120, 2909–2917. [Google Scholar] [CrossRef] [PubMed]
- Carreras, E.; Dufour, C.; Mohty, M.; Kröger, N. The EBMT Handbook; Springer Nature Switzerland: Cham, Switzerland, 2019. [Google Scholar]
- Panoskaltsis-Mortari, A.; Griese, M.; Madtes, D.K.; Belperio, J.A.; Haddad, I.Y.; Folz, R.J.; Cooke, K.R.; American Thoracic Society Committee on Idiopathic Pneumonia, S. An Official American Thoracic Society research statement: Noninfectious lung injury after hematopoietic stem cell transplantation: Idiopathic pneumonia syndrome. Am. J. Respir. Crit. Care Med. 2011, 183, 1262–1279. [Google Scholar] [CrossRef] [PubMed]
- Panoskaltsis-Mortari, A.; Taylor, P.A.; Yaeger, T.M.; Wangensteen, O.D.; Bitterman, P.B.; Ingbar, D.H.; Vallera, D.A.; Blazar, B.R. The critical early proinflammatory events associated with idiopathic pneumonia syndrome in irradiated murine allogeneic recipients are due to donor T cell infusion and potentiated by cyclophosphamide. J. Clin. Investig. 1997, 100, 1015–1027. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Hackman, R.C.; Guthrie, K.A.; Sandmaier, B.M.; Boeckh, M.; Maris, M.B.; Maloney, D.G.; Deeg, H.J.; Martin, P.J.; Storb, R.F.; et al. Risks and outcomes of idiopathic pneumonia syndrome after nonmyeloablative and conventional conditioning regimens for allogeneic hematopoietic stem cell transplantation. Blood 2003, 102, 2777–2785. [Google Scholar] [CrossRef] [PubMed]
- Yanik, G.; Kitko, C. Management of noninfectious lung injury following hematopoietic cell transplantation. Curr. Opin. Oncol. 2013, 25, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.C.; Corrion, L.A.; Olkiewicz, K.M.; Lu, B.; Lowler, K.; Duffner, U.A.; Moore, B.B.; Kuziel, W.A.; Liu, C.; Cooke, K.R. Blockade of CXCR3 receptor:ligand interactions reduces leukocyte recruitment to the lung and the severity of experimental idiopathic pneumonia syndrome. J. Immunol. (Baltimore Md. 1950) 2004, 173, 2050–2059. [Google Scholar] [CrossRef]
- Hildebrandt, G.C.; Olkiewicz, K.M.; Choi, S.; Corrion, L.A.; Clouthier, S.G.; Liu, C.; Serody, J.S.; Cooke, K.R. Donor T-cell production of RANTES significantly contributes to the development of idiopathic pneumonia syndrome after allogeneic stem cell transplantation. Blood 2005, 105, 2249–2257. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, G.C.; Duffner, U.A.; Olkiewicz, K.M.; Corrion, L.A.; Willmarth, N.E.; Williams, D.L.; Clouthier, S.G.; Hogaboam, C.M.; Reddy, P.R.; Moore, B.B.; et al. A critical role for CCR2/MCP-1 interactions in the development of idiopathic pneumonia syndrome after allogeneic bone marrow transplantation. Blood 2004, 103, 2417–2426. [Google Scholar] [CrossRef] [PubMed]
- Panoskaltsis-Mortari, A.; Hermanson, J.R.; Taras, E.; Wangensteen, O.D.; Charo, I.F.; Rollins, B.J.; Blazar, B.R. Post-BMT lung injury occurs independently of the expression of CCL2 or its receptor, CCR2, on host cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 286, L284–L292. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panoskaltsis-Mortari, A.; Hermanson, J.R.; Taras, E.; Wangensteen, O.D.; Serody, J.S.; Blazar, B.R. Acceleration of idiopathic pneumonia syndrome (IPS) in the absence of donor MIP-1 alpha (CCL3) after allogeneic BMT in mice. Blood 2003, 101, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.; Pole, J.D.; Gibson, P.; Lee, M.; Hesser, T.; Chi, S.N.; Dvorak, C.C.; Fisher, B.; Hasle, H.; Kanerva, J.; et al. Classification of treatment-related mortality in children with cancer: A systematic assessment. Lancet Oncol. 2015, 16, e604–e610. [Google Scholar] [CrossRef]
- Ambruzova, Z.; Mrazek, F.; Raida, L.; Jindra, P.; Vidan-Jeras, B.; Faber, E.; Pretnar, J.; Indrak, K.; Petrek, M. Association of IL6 and CCL2 gene polymorphisms with the outcome of allogeneic haematopoietic stem cell transplantation. Bone Marrow Transplant. 2009, 44, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Nakata, K.; Takami, A.; Espinoza, J.L.; Matsuo, K.; Morishima, Y.; Onizuka, M.; Fukuda, T.; Kodera, Y.; Akiyama, H.; Miyamura, K.; et al. The recipient CXCL10 + 1642C>G variation predicts survival outcomes after HLA fully matched unrelated bone marrow transplantation. Clin. Immunol. (Orlando F.L.) 2013, 146, 104–111. [Google Scholar] [CrossRef] [PubMed]
- De Soria, V.G.G.; Portero, I.; Fernandez Arandojo, C.; Muñoz, C. GVHD-related mortality is associated with high levels of CCR7+ CD4 T lymphocytes in the graft. Bone Marrow Transplant. 2016, 51, S387. [Google Scholar] [CrossRef][Green Version]
- Grant, M.J.; Booth, A. A typology of reviews: An analysis of 14 review types and associated methodologies. Health Inf. Libr. J. 2009, 26, 91–108. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: Elaboration and explanation. BMJ 2015, 350, g7647. [Google Scholar] [CrossRef]
- Bramer, W.M.; Giustini, D.; de Jonge, G.B.; Holland, L.; Bekhuis, T. De-duplication of database search results for systematic reviews in EndNote. J. Med. Libr. Assoc. 2016, 104, 240–243. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).