Treadmill Running in Established Phase Arthritis Inhibits Joint Destruction in Rat Rheumatoid Arthritis Models
Abstract
1. Introduction
2. Results
2.1. Kinetic Change in Body Weight and Paw Volume
2.2. Effect of Treadmill Running on Articular Cartilage
2.3. Influence of Treadmill Running on the Production of TNF-α and Cx43 in the Synovium
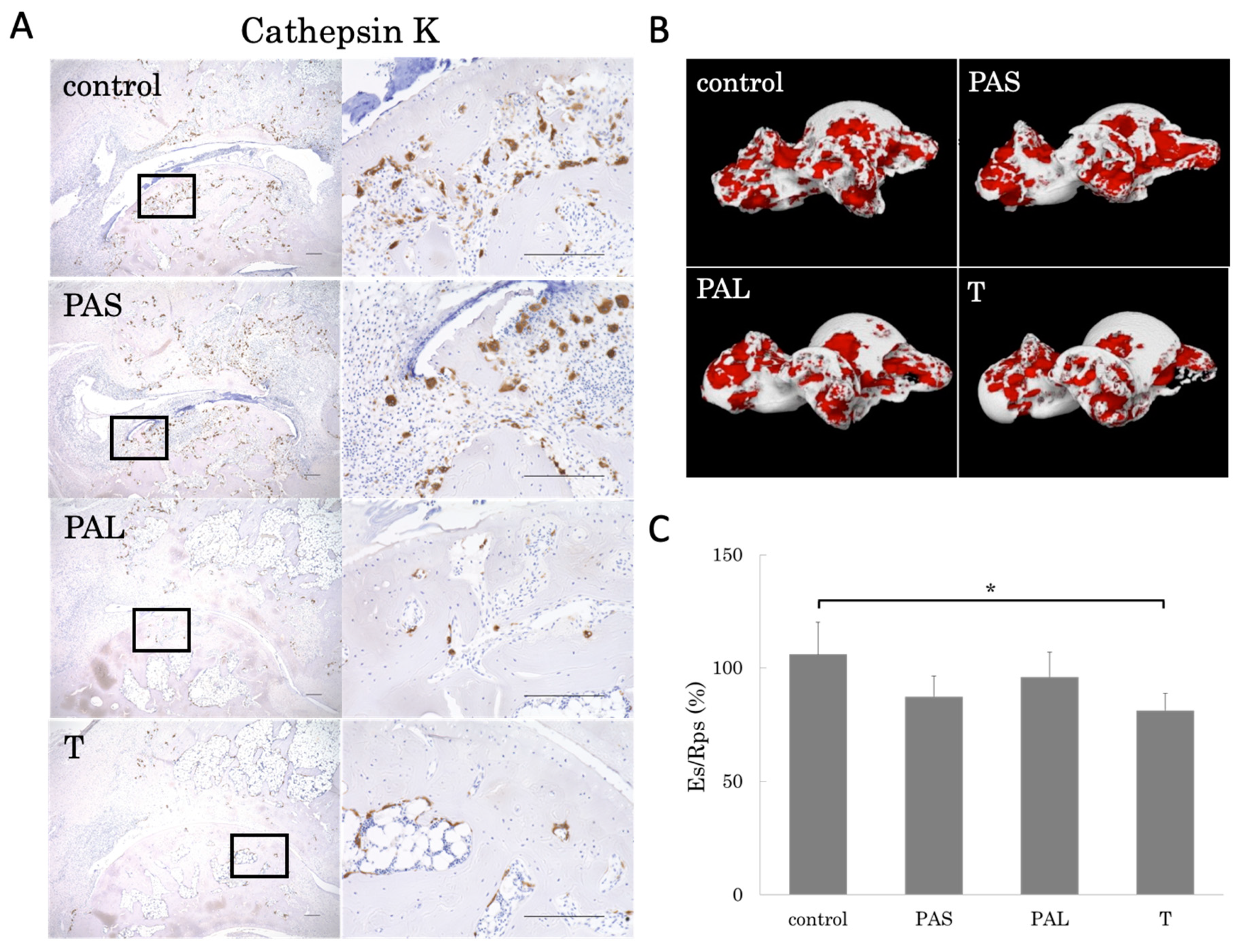
2.4. Prevention of Bone Loss by Treadmill Running
2.5. Effects of Treadmill Running on Bone Erosion
3. Discussion
4. Materials and Methods
4.1. CIA Model
4.2. Treadmill Running Protocol
4.3. Body Weight, Paw Volume, and Clinical Score
4.4. Histochemical Analyses and Semi-Quantitative Analyses
4.5. Immunohistochemical Analyses
4.6. Micro-Computed Tomography Analysis
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| µ-CT | micro-computed tomography |
| ADL | activities of daily living |
| bDMARDs | biological disease-modifying anti-rheumatic drugs |
| BMC/TV | bone mineral content per tissue volume |
| BV/TV | bone volume fraction |
| CIA | collagen-induced arthritis |
| Cx43 | connexin 43 |
| Es/Rps | eroded bone surface per repaired bone surface |
| H&E | hematoxylin and eosin |
| IL | interleukin |
| MSV | marrow star volume |
| OA | osteoarthritis |
| PAL | pre-arthritis intervention long |
| PAS | pre-arthritis intervention short |
| RA | rheumatoid arthritis |
| SPSS | Statistical Package for Social Sciences |
| T | therapeutic intervention |
| Tb.Th | trabecular thickness |
| TNF | tumor necrosis factor |
References
- Nanki, T.; Hayashida, K.; El-Gabalawy, H.S.; Suson, S.; Shi, K.; Girschick, H.J.; Yavuz, S.; Lipsky, P.E. Stromal cell-derived factor-1-CXC chemokine receptor 4 interactions play a central role in CD4+ T cell accumulation in rheumatoid arthritis synovium. J. Immunol. 2000, 165, 6590–6598. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Mcknnes, I.B.; Schett, G. The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 2011, 365, 2205–2219. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Jantan, I.; Bukhari, S.N.A. Rheumatoid arthritis: Recent advances on its etiology, role of cytokines and pharmacotherapy. Biomed. Pharm. 2017, 92, 615–633. [Google Scholar] [CrossRef]
- Maini, R.; St Clair, E.W.; Breedveld, F.; Furst, D.; Kalden, J.; Weisman, M.; Smolen, J.; Emery, P.; Harriman, G.; Feldmann, M.; et al. Infliximab (chimeric anti-tumour necrosis factor α monoclonal antibody) versus placebo in rheumatoid arthritis patients receiving concomitant methotrexate: A randomised phase III trial. Lancet 1999, 354, 1932–1939. [Google Scholar] [CrossRef]
- Lipsky, P.E.; Van der Heijde, D.M.; St Clair, E.W.; Furst, D.E.; Breedveid, F.C.; Kalden, J.R.; Smolen, J.R.; Weisman, M.; Emery, P.; Feldmann, M.; et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-tumor necrosis factor trial in rheumatoid arthritis with concomitant therapy study group. N. Engl. J. Med. 2000, 343, 1594–1602. [Google Scholar] [CrossRef]
- Smolen, J.S.; Weinblatt, M.E.; Sheng, S.; Zhuang, Y.; Hsu, B. Sirukumab. A human anti-interleukin-6 monoclonal antibody: A randomised, 2-part (proof-of-concept and dose-finding), phase II study in patients with active rheumatoid arthritis despite methotrexate therapy. Ann. Rheum. Dis. 2014, 73, 1616–1625. [Google Scholar] [CrossRef]
- Genovese, M.C.; McKay, J.D.; Nasonov, E.L.; Mysler, E.F.; da Silva, N.A.; Alecock, E.; Woodworth, T.; Gomez-Reino, J.J. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arthritis with inadequate response to disease-modifying antirheumatic drugs: The tocilizumab in combination with traditional disease-modifying antirheumatic drug therapy study. Arthritis Rheum. 2008, 58, 2968–2980. [Google Scholar]
- Singh, J.A.; Wells, G.A.; Christensen, R.; Tanjong Ghogomu, E.; Maxwell, L.; Macdonald, J.K.; Filippini, G.; Skoetz, N.; Francis, D.; Lopes, L.C.; et al. Adverse effects of biologics: A network meta-analysis and Cochrane overview. Cochrane Database Syst. Rev. 2011, CD008794. [Google Scholar] [CrossRef]
- Zhang, W.; Nuki, G.; Moskowitz, R.W.; Abramson, S.; Altman, R.D.; Arden, N.K.; Bierma-Zeinstra, S.; Brandt, K.D.; Croft, P.; Doherty, M.; et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part III: Changes in evidence following systematic cumulative update of research published through January 2009. Osteoarthr. Cartil. 2010, 18, 476–499. [Google Scholar] [CrossRef]
- Iijima, H.; Aoyama, T.; Ito, A.; Yamaguchi, S.; Nagai, M.; Tajino, J.; Zhang, X.; Kuroki, H. Effects of short-term gentle treadmill walking on subchondral bone in a rat model of instability-induced osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Ingle, L. Theoretical rationale and practical recommendations for cardiopulmonary exercise testing in patients with chronic heart failure. Heart Fail. Rev. 2007, 12, 12–22. [Google Scholar] [CrossRef] [PubMed]
- Salcedo, P.A.; Lindheimer, J.B.; Klein-Adams, J.C.; Sotolongo, A.M.; Falvo, M.J. Effects of exercise training on pulmonary function in adults with chronic lung disease: A meta-analysis of randomized controlled trials. Arch. Phys. Med. Rehabil. 2018, 99, 2561–2569. [Google Scholar] [CrossRef] [PubMed]
- Hurkmans, E.; van der Giesen, F.J.; Vliet Vlieland, T.P.; Schoones, J.; Van den Ende, E.C. Dynamic exercise programs (aerobic capacity and/or muscle strength training) in patients with rheumatoid arthritis. Vet. Res. Commun. 2009. [Google Scholar] [CrossRef] [PubMed]
- Van den Ende, C.H.; Hazes, J.M.; le Cessie, S.; Mulder, W.J.; Belfor, D.G.; Breedveld, F.C.; Dijkmans, B.A. Comparison of high and low intensity training in well controlled rheumatoid arthritis. Results of a randomised clinical trial. Ann. Rheum. Dis. 1996, 55, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Baillet, A.; Vaillant, M.; Guinot, M.; Juvin, R.; Gaudin, P. Efficacy of resistance exercises in rheumatoid arthritis: Meta-analysis of randomized controlled trials. Rheumatology. 2012, 51, 519–527. [Google Scholar] [CrossRef]
- Shimomura, S.; Inoue, H.; Arai, Y.; Nakagawa, S.; Fujii, Y.; Kishida, T.; Ichimaru, S.; Tsuchida, S.; Shirai, T.; Ikoma, K.; et al. Treadmill running ameliorates destruction of articular cartilage and subchondral bone, not only synovitis, in a rheumatoid arthritis rat model. Int. J. Mol. Sci. 2018, 19, 1653. [Google Scholar] [CrossRef]
- Batsalova, T.; Lindh, I.; Backlund, J.; Dzhambazov, B.; Holmdahl, R. Comparative analysis of collagen type II-specific immune responses during development of collagen-induced arthritis in two B10 mouse strains. Arthritis Res. Ther. 2012, 14, R237. [Google Scholar] [CrossRef]
- Dekkers, J.S.; Schoones, J.W.; Huizinga, T.W.; Toes, R.E.; van der Helm-van Mil, A.H. Possibilities for preventive treatment in rheumatoid arthritis? Lessons from experimental animal models of arthritis: A systematic literature review and meta-analysis. Ann. Rheum. Dis. 2017, 76, 458–467. [Google Scholar] [CrossRef]
- Bokarewa, M.; Tarkowski, A. Local infusion of infliximab for the treatment of acute joint inflammation. Ann. Rheum. Dis. 2003, 62, 783–784. [Google Scholar] [CrossRef]
- Drake, M.T.; Clarke, B.L.; Ourler, M.J.; Khosla, S. Cathepsin K inhibitors for osteoporosis: Biology, potential clinical utility, and lessons learned. Endcr. Rev. 2017, 38, 325–350. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.R.; Peters, C.; Saftig, P.; Bromme, D. Cathepsin K activity-dependent regulation of osteoclast actin ring formation and bone resorption. J. Biol. Chem. 2009, 284, 2584–2592. [Google Scholar] [CrossRef] [PubMed]
- Tsuchida, S.; Arai, Y.; Kishida, T.; Takahashi, K.A.; Honjo, K.; Terauchi, R.; Inoue, H.; Oda, R.; Mazda, O.; Kubo, T. Silencing the expression of connexin 43 decreases inflammation and joint destruction in experimental arthritis. J. Orthop. Res. 2013, 31, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Matsuki, T.; Arai, Y.; Tsuchida, S.; Terauchi, R.; Oda, R.; Fujiwara, H.; Mazda, O.; Kubo, T. Expression of connexin 43 in synovial tissue of patients with rheumatoid arthritis. Arch. Rheumatol. 2016, 31, 55–63. [Google Scholar] [CrossRef]
- Manuel, A.R.; Sirisha, B.; Rekha, K.; Paul, D.L.; Jean, X.J. Mitogen-activated protein kinase (MAPK) activated by prostaglandin E2 phosphorylates connexin 43 and closes osteocytic hemichannels in response to continuous flow shear stress. J. Biol. Chem. 2015, 47, 28321–28328. [Google Scholar]
- Bivi, N.; Pacheco-Costa, R.; Brun, L.R.; Murphy, T.R.; Farlow, N.R.; Robling, A.G.; Bellido, T.; Plotkin, L.I. Absence of Cx43 selectively from osteocytes enhances responsiveness to mechanical force in mice. J. Orthop. Res. 2013, 31, 1075–1081. [Google Scholar] [CrossRef]
- Yokota, K.; Sato, K.; Miyazaki, T.; Kitaura, H.; Kayama, H.; Miyoshi, F.; Araki, Y.; Akiyama, Y.; Takeda, K.; Mimura, T. Combination of tumor necrosis factor and interleukin-6 induces mouse osteoclast-like cells with bone resorption activity both in vitro and in vivo. Arthritis Rheum. 2014, 66, 121–129. [Google Scholar] [CrossRef]
- Suzuki, N.; Yoshimura, Y.; Deyama, Y.; Suzuki, K.; Kitagawa, Y. Mechanical stress directly suppresses osteoclast differentiation in RAW264.7 cells. Int. J. Mol. Med. 2008, 21, 291–296. [Google Scholar] [CrossRef]
- Sato, K.; Suematsu, A.; Okamoto, K.; Yamaguchi, A.; Morishita, Y.; Kadono, Y.; Tanaka, S.; Kodama, T.; Akira, S.; Iwakura, Y.; et al. Th17 functions as an osteoclastogenic helper T cell subset that links T cell activation and bone destruction. J. Exp. Med. 2006, 203, 2673–2682. [Google Scholar] [CrossRef]
- Bakharevski, O.; Stein-Oakley, A.N.; Thomson, N.M.; Ryan, P.F. Collagen induced arthritis in rats. Contrasting effect of subcutaneous versus intradermal inoculation of type II collagen. J. Rheumatol. 1998, 25, 1945–1952. [Google Scholar]
- Nam, J.; Perera, P.; Liu, J.; Wu, L.C.; Rath, B.; Butterfield, T.A.; Agarwal, S. Transcriptome-wide gene regulation by gentle treadmill walking during the progression of monoiodoacetate-induced arthritis. Arthritis Rheum. 2011, 63, 1613–1625. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, N.; Li, X.; Kang, M.; Guo, M.; Song, L. Application of GC/MS-based metabonomic profiling in studying the therapeutic effects of Aconitum carmichaeli with Ampelopsis japonica extract on collagen-induced arthritis in rats. Molecules 2019, 24, 1934. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, A.; Halpern, M.; Zahalka, M.A.; Quintana, F.; Traub, L.; Moroz, C. Placental immunomodulator ferritin, a novel immunoregulator, suppresses experimental arthritis. Arthritis Rheum. 2003, 48, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Mane, D.R.; Kale, A.K.; Belaldavar, C. Validation of immunoexpression of tenascin-C in oral precancerous and cancerous tissues using ImageJ analysis with novel immunohistochemistry profiler plugin: An immunohistochemical quantitative analysis. J. Oral Maxillofac. Pathol. 2017, 21, 211–217. [Google Scholar] [CrossRef]
- Munemoto, M.; Kido, A.; Sakamoto, Y.; Inoue, K.; Yokoi, K.; Shinohara, Y.; Tanaka, Y. Analysis of trabecular bone microstructure in osteoporotic femoral heads in human patients: In vivo study using multidetector row computed tomography. BMC Musculoskelet Disord. 2016, 17, 13. [Google Scholar] [CrossRef]
- Vesterby, A.; Gundersen, H.J.; Melsen, F. Star volume of marrow space and trabeculae of the first lumber vertebra: Sampling efficiency and biological variation. Bone 1989, 10, 7–13. [Google Scholar] [CrossRef]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, Y.; Inoue, H.; Arai, Y.; Shimomura, S.; Nakagawa, S.; Kishida, T.; Tsuchida, S.; Kamada, Y.; Kaihara, K.; Shirai, T.; et al. Treadmill Running in Established Phase Arthritis Inhibits Joint Destruction in Rat Rheumatoid Arthritis Models. Int. J. Mol. Sci. 2019, 20, 5100. https://doi.org/10.3390/ijms20205100
Fujii Y, Inoue H, Arai Y, Shimomura S, Nakagawa S, Kishida T, Tsuchida S, Kamada Y, Kaihara K, Shirai T, et al. Treadmill Running in Established Phase Arthritis Inhibits Joint Destruction in Rat Rheumatoid Arthritis Models. International Journal of Molecular Sciences. 2019; 20(20):5100. https://doi.org/10.3390/ijms20205100
Chicago/Turabian StyleFujii, Yuta, Hiroaki Inoue, Yuji Arai, Seiji Shimomura, Shuji Nakagawa, Tsunao Kishida, Shinji Tsuchida, Yoichiro Kamada, Kenta Kaihara, Toshiharu Shirai, and et al. 2019. "Treadmill Running in Established Phase Arthritis Inhibits Joint Destruction in Rat Rheumatoid Arthritis Models" International Journal of Molecular Sciences 20, no. 20: 5100. https://doi.org/10.3390/ijms20205100
APA StyleFujii, Y., Inoue, H., Arai, Y., Shimomura, S., Nakagawa, S., Kishida, T., Tsuchida, S., Kamada, Y., Kaihara, K., Shirai, T., Terauchi, R., Toyama, S., Ikoma, K., Mazda, O., & Mikami, Y. (2019). Treadmill Running in Established Phase Arthritis Inhibits Joint Destruction in Rat Rheumatoid Arthritis Models. International Journal of Molecular Sciences, 20(20), 5100. https://doi.org/10.3390/ijms20205100




