Effects of Early Exposure of Isoflurane on Chronic Pain via the Mammalian Target of Rapamycin Signal Pathway
Abstract
:1. Introduction
2. Results
2.1. Effect of Early Isoflurane Exposure on Chronic Pain Behaviors
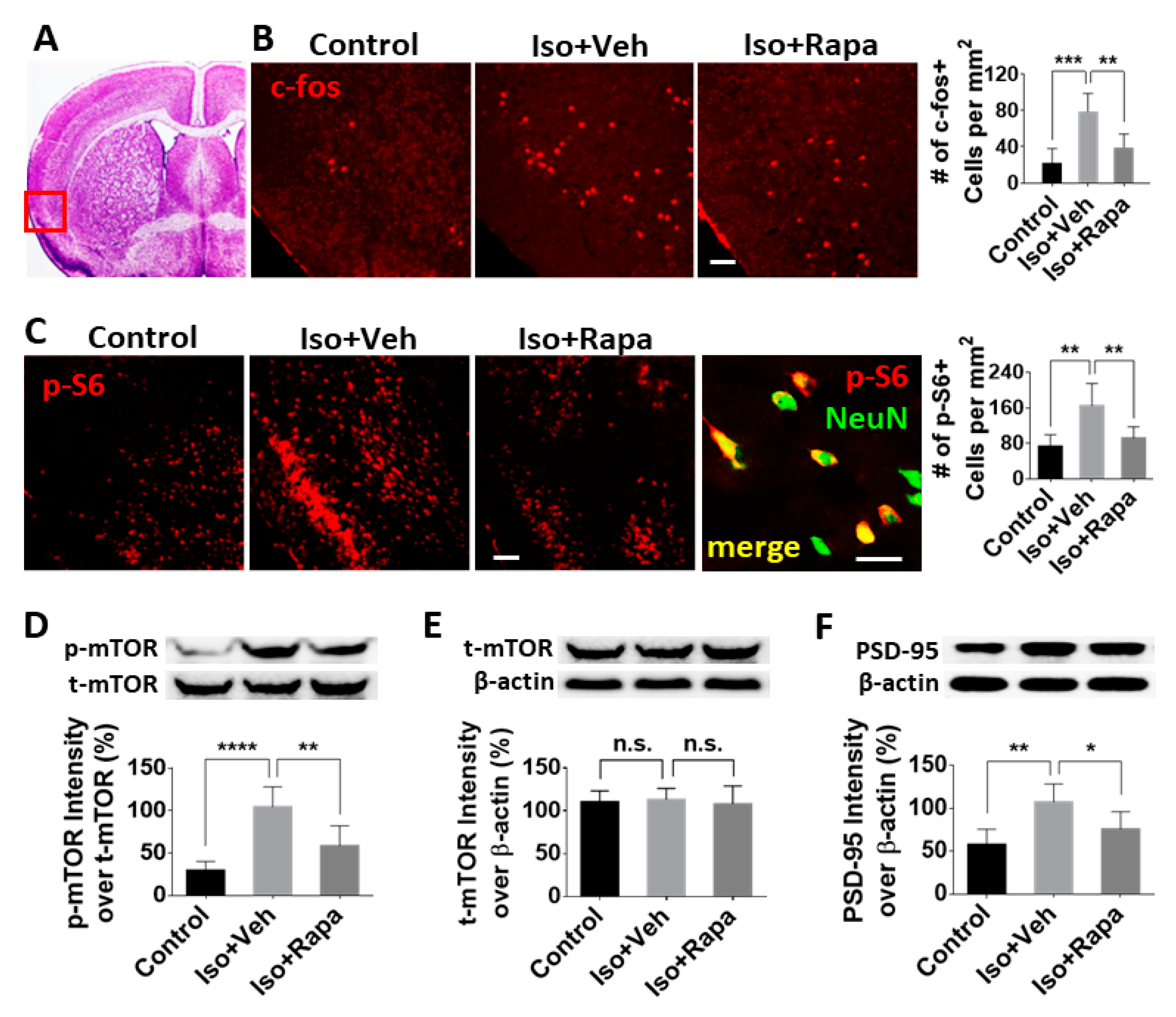
2.2. Effect of Isoflurane Exposure on Expression of mTOR Pathway and Neuronal Activity in Insular Cortex (IC)
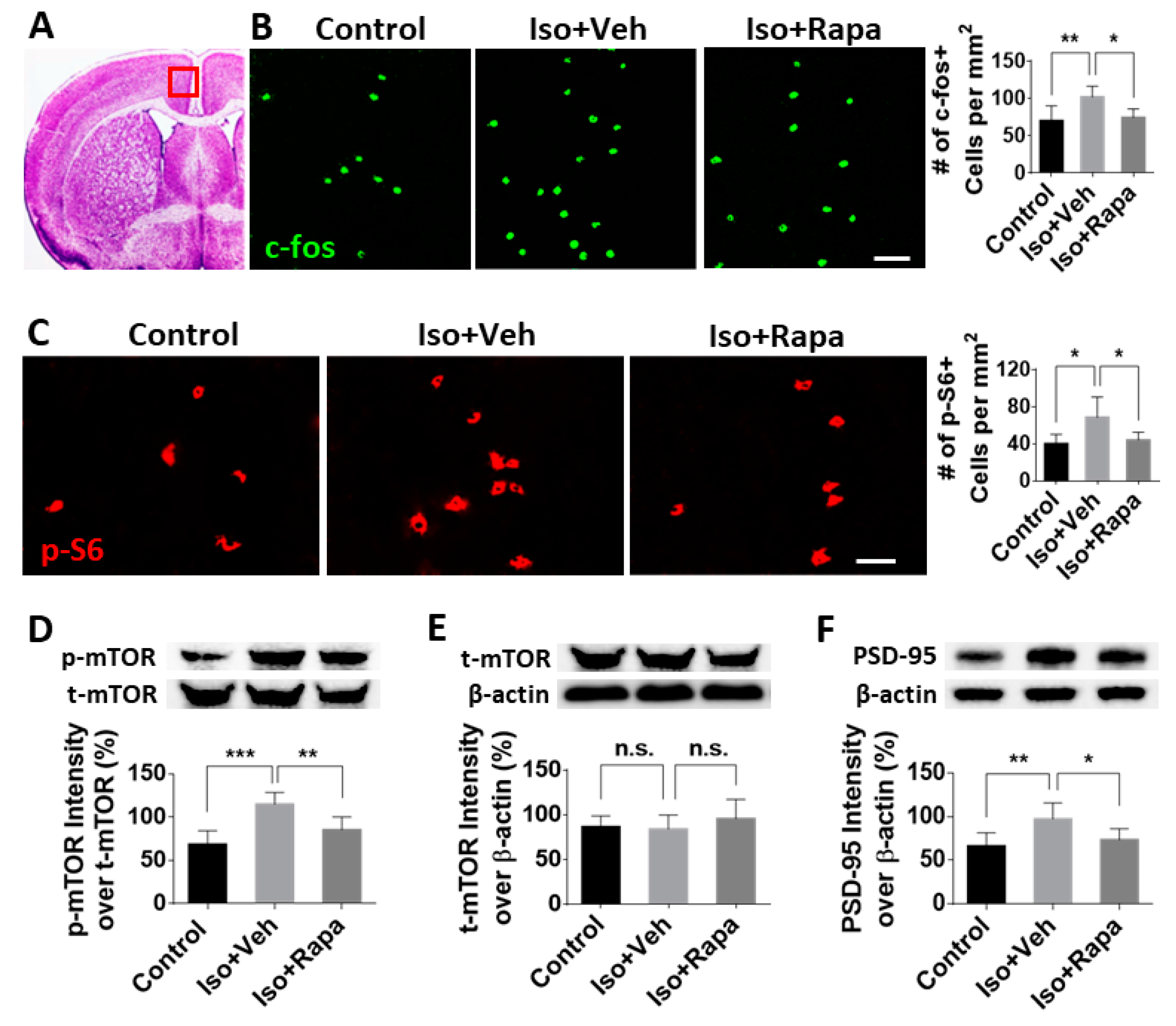
2.3. Effect of Early Isoflurane Exposure on Expression of mTOR and Neuronal Activity in Anterior Cingulate Cortex (ACC)
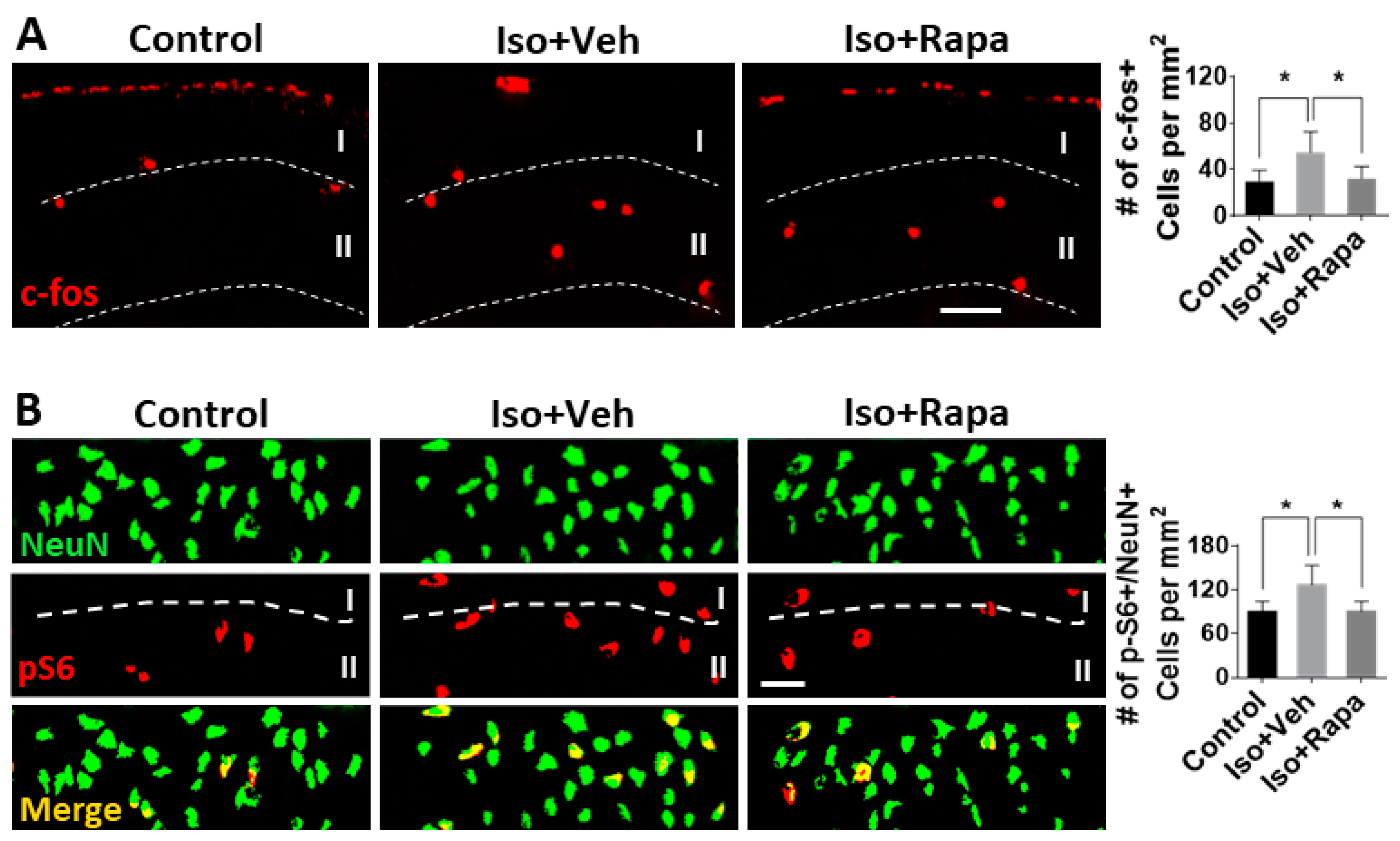
2.4. Effect of Isoflurane Exposure on Neuronal Activity and pS6 Expression in Superficial Spinal Dorsal Horn (SDH)
3. Discussion
4. Materials and Methods
4.1. Animal Paradigm and Experimental Timeline
4.2. Isoflurane Exposure
4.3. Rapamycin Injection
4.4. Behavior Tests
4.4.1. Tail Flick Test
4.4.2. The von Frey Test
4.4.3. Formalin Test
4.5. Immunohistochemistry (IHC)
4.6. Western Blotting (WB)
4.7. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| PPSP | Persistent post-surgical pain |
| mTOR | Mammalian target of rapamycin |
| WB | Western blot |
| IC | Insular cortex |
| ACC | Anterior cingulate cortex |
| SDH | Spinal cord dorsal horn |
| CNS | Central nervous system |
| p(number) | Post-natal day |
| IHC | Immunohistochemistry |
| pS6 | Phospho-s6 |
| p-mTOR | Phosphorylated mTOR |
| t-mTOR | Total mTOR |
| PSD95 | Post synaptic density 95 |
| PFC | Pre-frontal cortex |
| 4E-BP | Eukaryotic inhibition factor 4E-binding protein |
| p70S6K | p70 ribosomal S6 protein kinase |
| DRG | Dorsal root ganglion |
| i.P. | Intraperitoneal injection |
| CG | Carrier gas |
| PBS | Phosphate-buffered saline |
References
- Macrae, W.A. Chronic post-surgical pain: 10 years on. Br. J. Anaesth. 2008, 101, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Werner, M.U.; Kongsgaard, U.E.I. Defining persistent post-surgical pain: Is an update required? Br. J. Anaesth. 2014, 113, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Williams, G.; Howard, R.F.; Liossi, C. Persistent postsurgical pain in children and young people. PAIN Rep. 2017, 2, e616. [Google Scholar] [CrossRef] [PubMed]
- Nikolajsen, L.; Brix, L.D. Chronic pain after surgery in children. Curr. Opin. Anaesthesiol. 2014, 27, 507–712. [Google Scholar] [CrossRef] [PubMed]
- Rabbitts, J.A.; Fisher, E.; Rosenbloom, B.N.; Palermo, T.M. Prevalence and Predictors of Chronic Postsurgical Pain in Children: A Systematic Review and Meta-Analysis. J. Pain 2017, 18, 605–614. [Google Scholar] [CrossRef] [PubMed]
- Richebé, P.; Capdevila, X.; Rivat, C. Persistent Postsurgical Pain. Anesthesiology 2018, 129, 590–607. [Google Scholar] [CrossRef] [PubMed]
- Batoz, H.; Semjen, F.; Bordes-Demolis, M.; Bénard, A.; Nouette-Gaulain, K. Chronic postsurgical pain in children: Prevalence and risk factors. A prospective observational study. Br. J. Anaesth. 2016, 117, 489–496. [Google Scholar] [CrossRef]
- Mossetti, V.; Boretsky, K.; Astuto, M.; Locatelli, B.G.; Zurakowski, D.; Lio, R.; Nicoletti, R.; Sonzogni, V.; Maffioletti, M.; Vicchio, N.; et al. Persistent pain following common outpatient surgeries in children: A multicenter study in Italy. Pediatr. Anesth. 2018, 28, 231–236. [Google Scholar] [CrossRef]
- Kristensen, A.D.; Ahlburg, P.; Lauridsen, M.C.; Jensen, T.S.; Nikolaisen, L. Chronic pain after inguinal hernia repair in children. Br. J. Anaesth. 2012, 109, 603–608. [Google Scholar] [CrossRef] [Green Version]
- FDA. FDA Drug Safety Communication: FDA Review Results in New Warnings about Using General Anesthetics and Sedation Drugs in Young Children and Pregnant Women; FDA: White Oak, MD, USA, 2016. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-review-results-new-warnings-about-using-general-anesthetics-and (accessed on 21 August 2019).
- Jevtovic-Todorovic, V.; Hartman, R.E.; Izumi, Y.; Benshoff, N.D.; Dikranian, K.; Zorumski, C.F.; Olney, J.W.; Wozniak, D.F. Early exposure to common anesthetic agents causes widespread neurodegeneration in the developing rat brain and persistent learning deficits. J. Neurosci. 2003, 23, 876–882. [Google Scholar] [CrossRef]
- Ing, C.; Brambrink, A.M. Mayo Anesthesia Safety in Kids continued: Two new studies and a potential redirection of the field. Br. J. Anaesth. 2019, 122, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Raper, J.; Alvarado, M.C.; Murphy, K.L.; Baxter, M.G. Multiple Anesthetic Exposure in Infant Monkeys Alters Emotional Reactivity to an Acute Stressor. Anesthesiology 2015, 123, 1084–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raper, J.; De Biasio, J.C.; Murphy, K.L.; Alvarado, M.C.; Baxter, M.G. Persistent alteration in behavioural reactivity to a mild social stressor in rhesus monkeys repeatedly exposed to sevoflurane in infancy. Br. J. Anaesth. 2018, 120, 761–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, D.O.; Zaccariello, M.J.; Katusic, S.K.; Schroeder, D.R.; Hanson, A.C.; Schulte, P.J.; Buenvenida, S.L.; Gleich, S.J.; Wilder, R.T.; Sprung, J.; et al. Neuropsychological and Behavioral Outcomes after Exposure of Young Children to Procedures Requiring General Anesthesia: The Mayo Anesthesia Safety in Kids (MASK) Study. Anesthesiology 2018, 129, 89–105. [Google Scholar] [CrossRef] [PubMed]
- Coleman, K.; Robertson, N.D.; Dissen, G.A.; Nueringer, M.D.; Martin, L.D.; Cuzon Carlson, V.C.; Kroenke, C.; Fair, D.; Brambrink, A.M. Isoflurane Anesthesia Has Long-term Consequences on Motor and Behavioral Development in Infant Rhesus Macaques. Anesthesiology 2017, 126, 74–84. [Google Scholar] [CrossRef] [Green Version]
- Jackson, W.M.; Gray, C.D.B.; Jiang, D.; Schaefer, M.L.; Connor, C.; Mintz, C.D. Molecular Mechanisms of Anesthetic Neurotoxicity. J. Neurosurg. Anesthesiol. 2016, 28, 361–372. [Google Scholar] [CrossRef]
- Yu, D.; Li, L.; Yuan, W. Neonatal anesthetic neurotoxicity: Insight into the molecular mechanisms of long-term neurocognitive deficits. Biomed. Pharmacother. 2017, 87, 196–199. [Google Scholar] [CrossRef]
- Jevtovic-Todorovic, V. Anesthesia and the developing brain: Are we getting closer to understanding the truth? Curr. Opin. Anaesthesiol. 2011, 24, 395–399. [Google Scholar] [CrossRef]
- Vutskits, L.; Xie, Z. Lasting impact of general anaesthesia on the brain: Mechanisms and relevance. Nat. Rev. Neurosci. 2016, 17, 705–717. [Google Scholar] [CrossRef]
- Kuner, R.; Flor, H. Structural plasticity and reorganisation in chronic pain. Nat. Rev. Neurosci. 2016, 18, 20–30. [Google Scholar] [CrossRef]
- Kwon, M.; Han, J.; Kim, U.J.; Cha, M.; Um, S.W.; Bai, S.J.; Hong, S.-K.; Lee, B.H. Inhibition of Mammalian Target of Rapamycin (mTOR) Signaling in the Insular Cortex Alleviates Neuropathic Pain after Peripheral Nerve Injury. Front. Mol. Neurosci. 2017, 10, 79. [Google Scholar] [CrossRef] [PubMed]
- Coffeenl, U.; Ortega-Legaspil, J.M.; López-Muñozl, F.J.; Simón-Arceol, K.; Jaimesl, O.; Pellicerl, F. Insular cortex lesion diminishes neuropathic and inflammatory pain-like behaviours. Eur. J. Pain 2011, 15, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Um, S.W.; Kim, M.J.; Leem, J.W.; Bai, S.J.; Lee, B.H. Pain-Relieving Effects of mTOR Inhibitor in the Anterior Cingulate Cortex of Neuropathic Rats. Mol. Neurobiol. 2019, 56, 2482–2494. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Taniguchi, W.; Chen, Q.-Y.; Tozaki-Saitoh, H.; Song, Q.; Lio, R.-H.; Koga, K.; Matsuda, T.; Kaito-Sugimura, Y.; Wang, J.; et al. Top-down descending facilitation of spinal sensory excitatory transmission from the anterior cingulate cortex. Nat. Commun. 2018, 9, 1886. [Google Scholar] [CrossRef] [PubMed]
- Coggeshall, R.E. Fos, nociception and the dorsal horn. Prog. Neurobiol. 2005, 77, 299–352. [Google Scholar] [CrossRef]
- Brown, A.; Walters, F.; Jachuck, S.J.; Van Zwanenberg, T.D. The future of general practice in Newcastle upon Tyne. Lancet (Lond. Engl.) 1986, 1, 370–371. [Google Scholar] [CrossRef]
- Xu, J.; Mathena, R.P.; Xu, M.; Wang, Y.; Chang, C.; Fang, Y.; Zhang, P.; Mintz, C.D. Early Developmental Exposure to General Anesthetic Agents in Primary Neuron Culture Disrupts Synapse Formation via Actions on the mTOR Pathway. Int. J. Mol. Sci. 2018, 19, 2183. [Google Scholar] [CrossRef]
- Xu, J.; Kang, E.; Mintz, C.D. Anesthetics disrupt brain development via actions on the mTOR pathway. Commun. Integr. Biol. 2018, 11, 1–4. [Google Scholar] [CrossRef]
- Sabatini, D.M. Twenty-five years of mTOR: Uncovering the link from nutrients to growth. Proc. Natl. Acad. Sci. USA 2017, 114, 11818–11825. [Google Scholar] [CrossRef] [Green Version]
- Laplante, M.; Sabatini, D.M. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell. Sci. 2013, 126, 1713–1719. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.Y. Roles of mTOR Signaling in Brain Development. Exp. Neurobiol. 2015, 24, 177–185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, B.M.; Nia, S.; Xiong, M.; Tao, Y.-X.; Bekker, A. mTOR, a new potential target for chronic pain and opioid-induced tolerance and hyperalgesia. Mol. Pain 2015, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Lisi, L.; Aceto, P.; Navarra, P.; Dello Russo, C. mTOR kinase: A possible pharmacological target in the management of chronic pain. Biomed. Res. Int. 2015, 2015, 394257. [Google Scholar] [CrossRef]
- Xu, Q.; Fitzsimmons, B.; Steinauer, J.; O’Neill, A.; Newton, A.C.; Hua, X.-Y.; Yaksh, T.L. Spinal phosphinositide 3-kinase-Akt-mammalian target of rapamycin signaling cascades in inflammation-induced hyperalgesia. J. Neurosci. 2011, 31, 2113–2124. [Google Scholar] [CrossRef] [PubMed]
- Obara, I.; Tochiki, K.K.; Géranton, S.M.; Carr, F.B.; Lumb, B.M.; Liu, Q.; Hunt, S.P. Systemic inhibition of the mammalian target of rapamycin (mTOR) pathway reduces neuropathic pain in mice. Pain 2011, 152, 2582–2595. [Google Scholar] [CrossRef] [PubMed]
- Craig, A.D.B. Significance of the insula for the evolution of human awareness of feelings from the body. Ann. N. Y. Acad. Sci. 2011, 1225, 72–82. [Google Scholar] [CrossRef]
- Berret, E.; Kintscher, M.; Palchaudhuri, S.; Tang, W.; Osypenko, D.; Kochubey, O.; Schneggenburger, R. Insular cortex processes aversive somatosensory information and is crucial for threat learning. Science 2019, 364, eaaw0474. [Google Scholar] [CrossRef]
- Descalzi, G.; Li, X.-Y.; Chen, T.; Mercaldo, V.; Koga, K.; Zhuo, M. Rapid synaptic potentiation within the anterior cingulate cortex mediates trace fear learning. Mol. Brain 2012, 5, 6. [Google Scholar] [CrossRef]
- Blom, S.M.; Pfister, J.-P.; Santello, M.; Senn, W.; Nevian, T. Nerve injury-induced neuropathic pain causes disinhibition of the anterior cingulate cortex. J. Neurosci. 2014, 34, 5754–5764. [Google Scholar] [CrossRef]
- Miao, H.-H.; Li, X.-H.; Chen, Q.-Y.; Zhuo, M. Calcium-stimulated adenylyl cyclase subtype 1 is required for presynaptic long-term potentiation in the insular cortex of adult mice. Mol. Pain 2019, 15, 1744806919842961. [Google Scholar] [CrossRef]
- Zhuo, M. Cortical LTP: A Synaptic Model for Chronic Pain. In Advances in Experimental Medicine and Biology; Springer: New York, NY, USA, 2018; pp. 147–155. [Google Scholar] [CrossRef]
- Gao, Y.-J.; Ji, R.-R. c-Fos and pERK, which is a better marker for neuronal activation and central sensitization after noxious stimulation and tissue injury? Open Pain J. 2009, 2, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.-T.; Zhao, X.; Yaster, M.; Tao, Y.-X. Expression and distribution of mTOR, p70S6K, 4E-BP1, and their phosphorylated counterparts in rat dorsal root ganglion and spinal cord dorsal horn. Brain Res. 2010, 1336, 46–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, L.; Tao, B.; Fan, L.; Yaster, M.; Zhang, Y.; Tao, Y.X. mTOR and its downstream pathway are activated in the dorsal root ganglion and spinal cord after peripheral inflammation, but not after nerve injury. Brain Res. 2013, 1513, 17–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, S.; Frias, M.A.; Chatterjee, A.; Yellen, P.; Foster, D.A. The enigma of rapamycin dosage. Mol. Cancer Ther. 2016, 15, 347–353. [Google Scholar] [CrossRef]
- Tao, F.; Tao, Y.-X.; Mao, P.; Johns, R.A. Role of postsynaptic density protein-95 in the maintenance of peripheral nerve injury-induced neuropathic pain in rats. Neuroscience 2003, 117, 731–739. [Google Scholar] [CrossRef]
- Tao, F.; Tao, Y.X.; Gonzalez, J.A.; Fang, M.; Mao, P.; Johns, R.A. Knockdown of PSD-95/SAP90 delays the development of neuropathic pain in rats. Neuroreport 2001, 12, 3251–3255. [Google Scholar] [CrossRef]
- Yang, P.-C.; Yang, C.-H.; Huang, C.-C.; Hsu, K.S. Phosphatidylinositol 3-kinase activation is required for stress protocol-induced modification of hippocampal synaptic plasticity. J. Biol. Chem. 2008, 283, 2631–2643. [Google Scholar] [CrossRef]
- Kang, E.; Jiang, D.; Ryu, Y.K.; Lim, S.; Kwak, M.; Gray, C.D.; Xu, M.; Choi, J.H.; Junn, S.; Kim, J.; et al. Early postnatal exposure to isoflurane causes cognitive deficits and disrupts development of newborn hippocampal neurons via activation of the mTOR pathway. PLoS Biol. 2017, 15, e2001246. [Google Scholar] [CrossRef]
- Li, Q.; Mathena, R.P.; Xu, J.; Nicole, E.O.; Wen, J.; Mintz, C.D. Early Postnatal Exposure to Isoflurane Disrupts Oligodendrocyte Development and Myelin Formation in the Mouse Hippocampus. Anesthesiology 2019. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B. Paxinos and Franklin’s the Mouse Brain in Stereotaxic Coordinates, 4th ed.; Elsevier: Waltham, MA, USA, 2013. [Google Scholar]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q.; Mathena, R.P.; Eregha, O.N.; Mintz, C.D. Effects of Early Exposure of Isoflurane on Chronic Pain via the Mammalian Target of Rapamycin Signal Pathway. Int. J. Mol. Sci. 2019, 20, 5102. https://doi.org/10.3390/ijms20205102
Li Q, Mathena RP, Eregha ON, Mintz CD. Effects of Early Exposure of Isoflurane on Chronic Pain via the Mammalian Target of Rapamycin Signal Pathway. International Journal of Molecular Sciences. 2019; 20(20):5102. https://doi.org/10.3390/ijms20205102
Chicago/Turabian StyleLi, Qun, Reilley Paige Mathena, O’Rukevwe Nicole Eregha, and C. David Mintz. 2019. "Effects of Early Exposure of Isoflurane on Chronic Pain via the Mammalian Target of Rapamycin Signal Pathway" International Journal of Molecular Sciences 20, no. 20: 5102. https://doi.org/10.3390/ijms20205102



