Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties
Abstract
1. Introduction
2. Natural Polysaccharides and Polysaccharide Nanomaterials
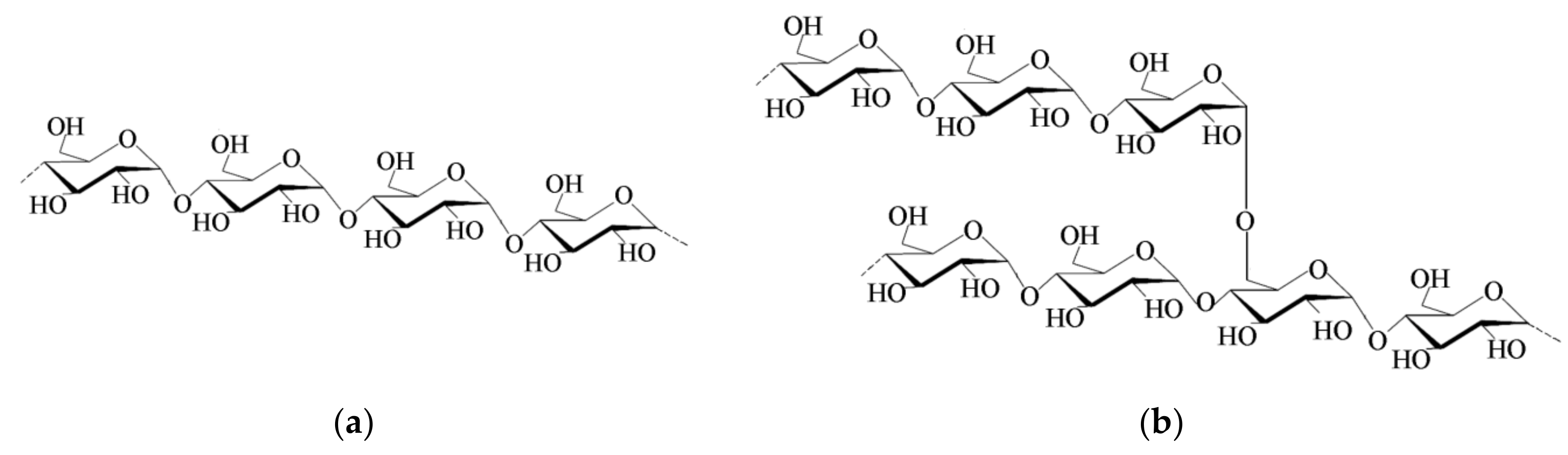
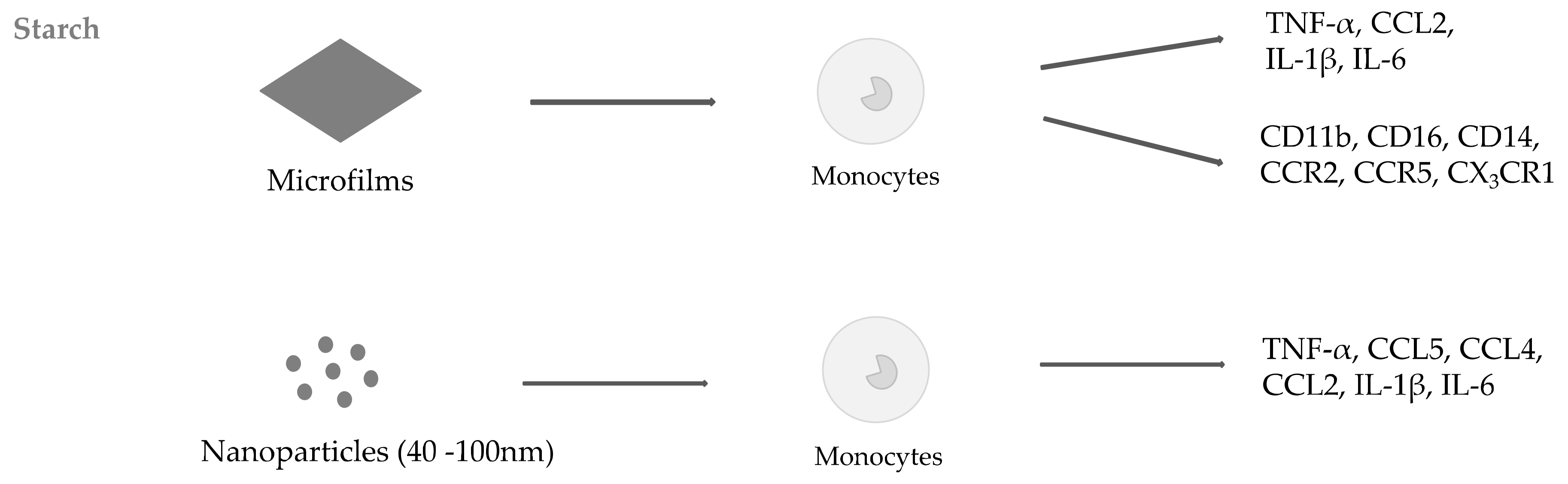
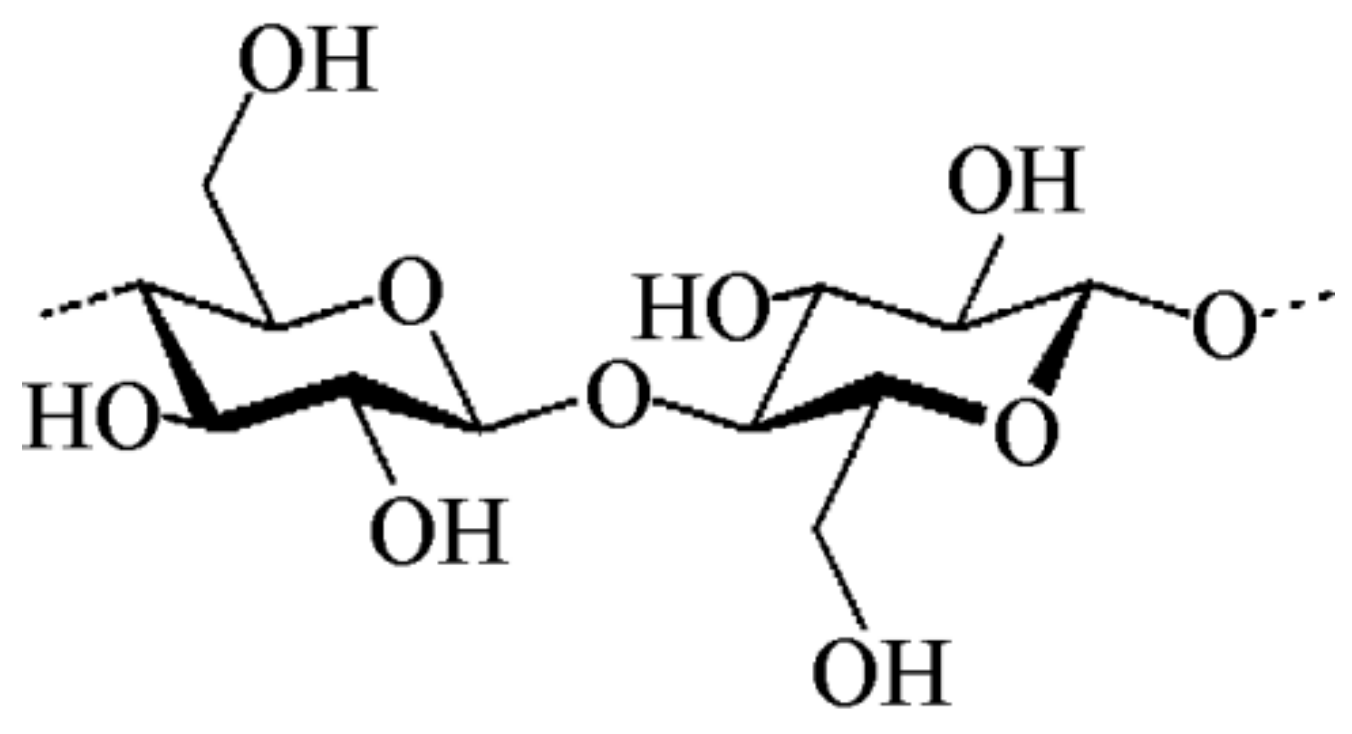
2.1. Starch
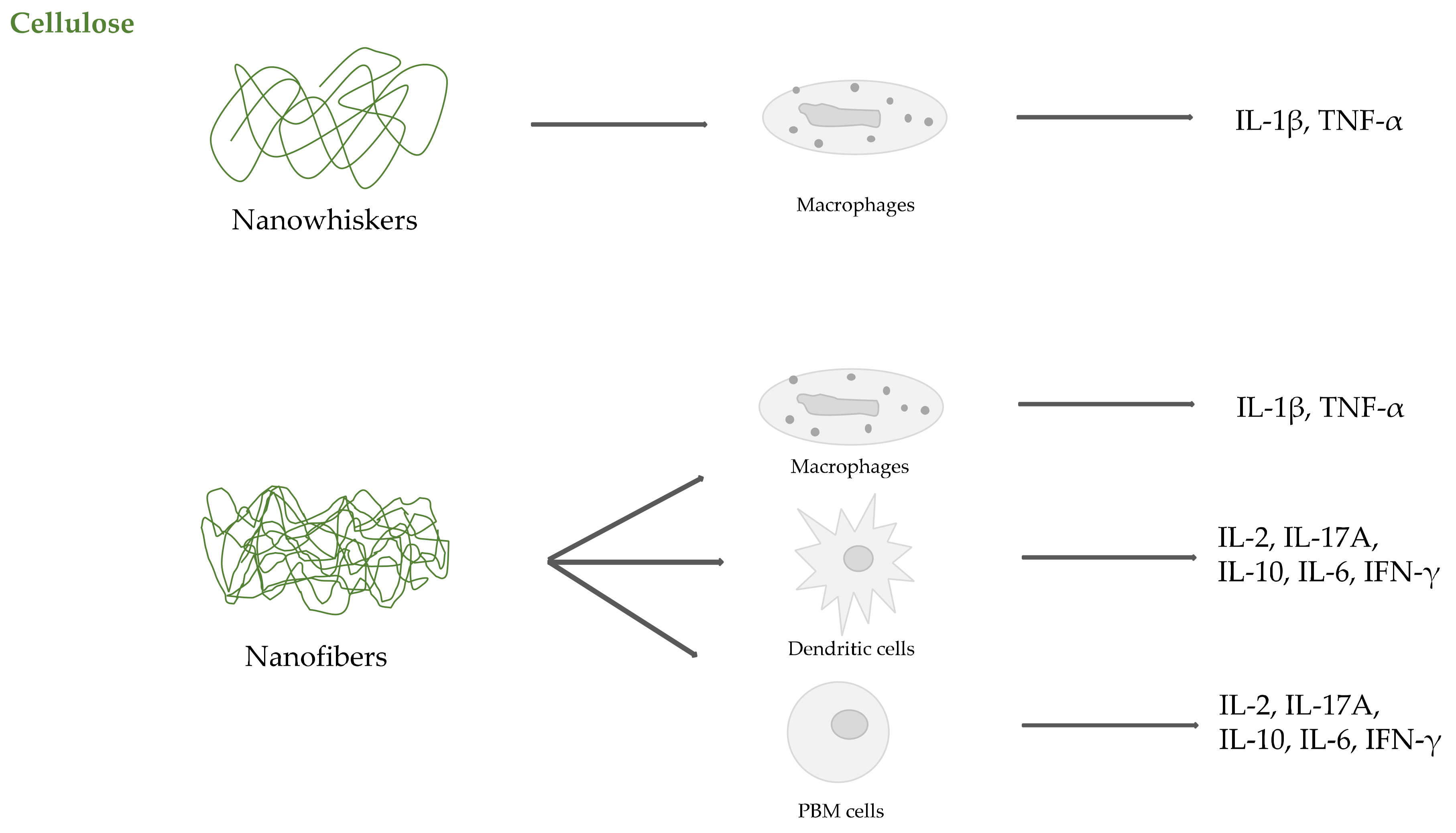
2.2. Cellulose
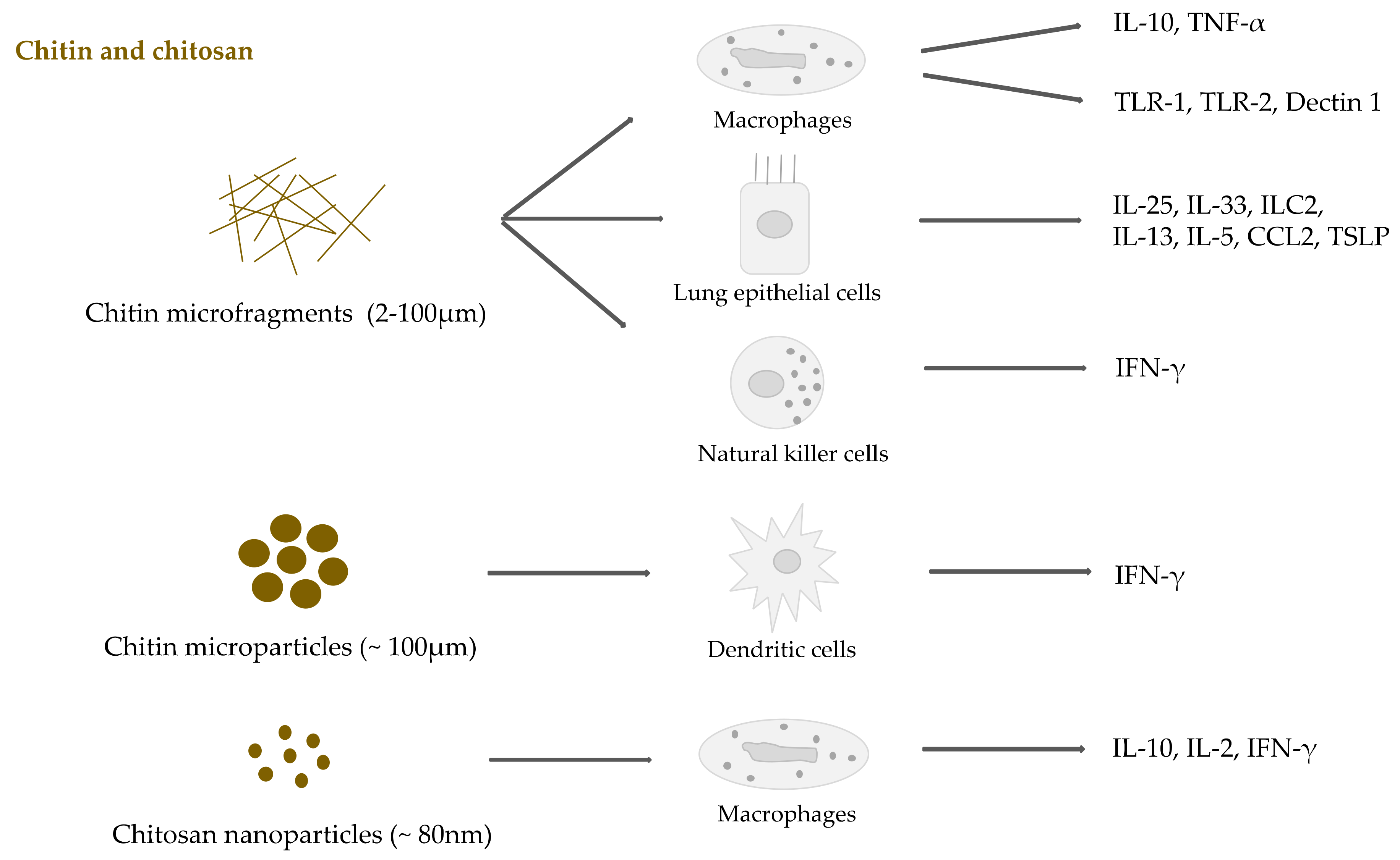
2.3. Chitin and Chitosan
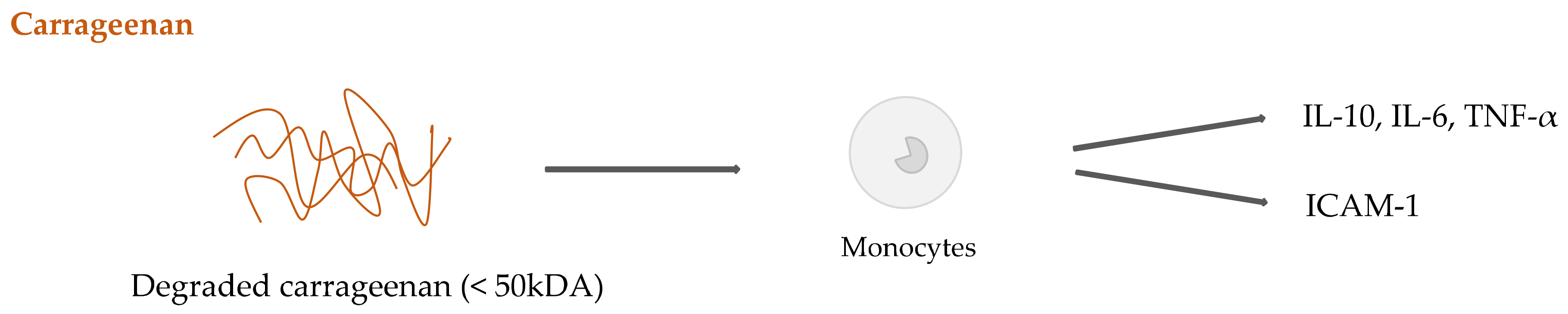
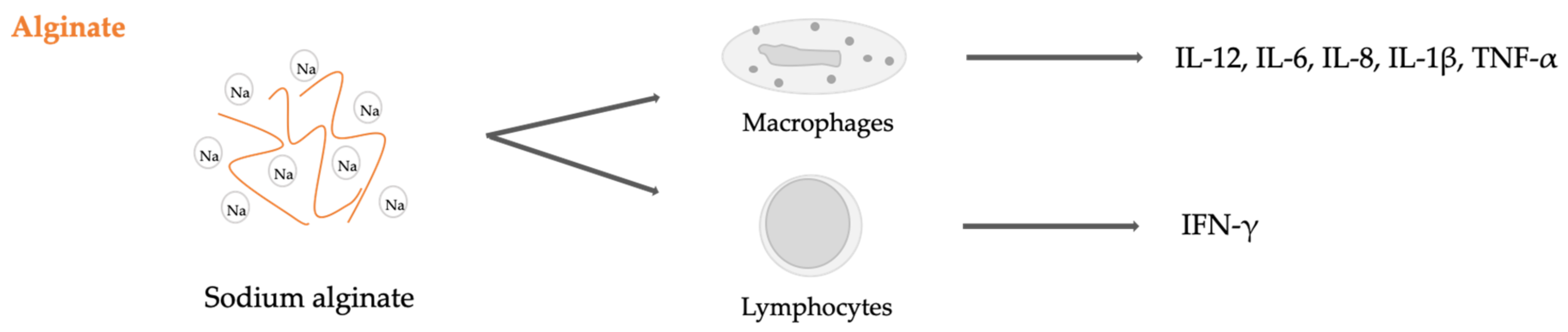
2.4. Seaweed Derived Carrageenan and Alginate
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bassas-Galia, M.; Follonier, S.; Pusnik, M.; Zinn, M. Natural polymers: A source of inspiration. Bioresorbable Polym. Biomed. Appl. 2017, 31–64. [Google Scholar] [CrossRef]
- Galaev, I.Y.; Mattiasson, B. “Smart” polymers and what they could do in biotechnology and medicine. Trends Biotechnol. 1999, 17, 335–340. [Google Scholar] [CrossRef]
- Lefèvre, T.; Subirade, M. Molecular structure and interaction of biopolymers as viewed by Fourier transform infrared spectroscopy: Model studies on β-lactoglobulin. Food Hydrocoll. 2001, 15, 365–376. [Google Scholar] [CrossRef]
- Diener, M.; Adamcik, J.; Sánchez-Ferrer, A.; Jaedig, F.; Schefer, L.; Mezzenga, R. Primary, Secondary, Tertiary and Quaternary Structure Levels in Linear Polysaccharides: From Random Coil, to Single Helix to Supramolecular Assembly. Biomacromolecules 2019, 20, 1731–1739. [Google Scholar] [CrossRef]
- Gupta, P.; Nayak, K.K. Characteristics of protein-based biopolymer and its application. Polym. Eng. Sci. 2015, 55, 485–498. [Google Scholar] [CrossRef]
- Kumar, P.; Sandeep, K.P.; Alavi, S.; Truong, V.D. A Review of Experimental and Modeling Techniques to Determine Properties of Biopolymer-Based Nanocomposites. J. Food Sci. 2011, 76, E2–E14. [Google Scholar] [CrossRef]
- Cheng, S.; Xie, S.-J.; Carrillo, J.-M.Y.; Carroll, B.; Martin, H.; Cao, P.-F.; Dadmun, M.D.; Sumpter, B.G.; Novikov, V.N.; Schweizer, K.S.; et al. Big Effect of Small Nanoparticles: A Shift in Paradigm for Polymer Nanocomposites. ACS Nano 2017, 11, 752–759. [Google Scholar] [CrossRef]
- Balazs, A.C.; Emrick, T.; Russell, T.P. Nanoparticle polymer composites: Where two small worlds meet. Science 2006, 314, 1107–1110. [Google Scholar] [CrossRef]
- Jancar, J.; Douglas, J.F.; Starr, F.W.; Kumar, S.K.; Cassagnau, P.; Lesser, A.J.; Sternstein, S.S.; Buehler, M.J. Current issues in research on structure–property relationships in polymer nanocomposites. Polymer (Guildf). 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Kulkarni, A.A.; Rao, P.S. Synthesis of polymeric nanomaterials for biomedical applications. Nanomater. Tissue Eng. 2013, 27–63. [Google Scholar] [CrossRef]
- Chaplin, D.D. Overview of the immune response. J. Allergy Clin. Immunol. 2010, 125, S3–S23. [Google Scholar] [CrossRef] [PubMed]
- Nesargikar, P.N.; Spiller, B.; Chavez, R. The complement system: History, pathways, cascade and inhibitors. Eur. J. Microbiol. Immunol. (Bp). 2012, 2, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Mogensen, T.H. Pathogen Recognition and Inflammatory Signaling in Innate Immune Defenses. Clin. Microbiol. Rev. 2009, 22, 240. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Leading Edge Review Pattern Recognition Receptors and Inflammation. Cell 2010, 140, 805–820. [Google Scholar] [CrossRef]
- Ingersoll, M.A.; Platt, A.M.; Potteaux, S.; Randolph, G.J. Monocyte trafficking in acute and chronic inflammation. Trends Immunol. 2011, 32, 470–477. [Google Scholar] [CrossRef]
- Wherry, E.J.; Masopust, D. Adaptive Immunity: Neutralizing, Eliminating, and Remembering for the Next Time. Viral Pathog. 2016, 57–69. [Google Scholar] [CrossRef]
- Cordeiro, A.S.; Alonso, M.J.; De la Fuente, M. Nanoengineering of vaccines using natural polysaccharides. Biotechnol. Adv. 2015, 33, 1279–1293. [Google Scholar] [CrossRef]
- Lin, K.; Kasko, A.M. Carbohydrate-Based Polymers for Immune Modulation. ACS Macro Lett. 2014, 3, 652–657. [Google Scholar] [CrossRef]
- Yin, M.; Zhang, Y.; Li, H. Advances in Research on Immunoregulation of Macrophages by Plant Polysaccharides. Front. Immunol. 2019, 10, 145. [Google Scholar] [CrossRef]
- Demento, S.L.; Siefert, A.L.; Bandyopadhyay, A.; Sharp, F.A.; Fahmy, T.M. Pathogen-associated molecular patterns on biomaterials: A paradigm for engineering new vaccines. Trends Biotechnol. 2011, 29, 294–306. [Google Scholar] [CrossRef]
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chemie Int. Ed. 2011, 50, 5438–5466. [Google Scholar] [CrossRef] [PubMed]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Dumitriu, S. Polysaccharides: Structural Diversity and Functional Versatility, 2nd ed.; Marcel Dekker: New York, NY, USA, 2005; ISBN 0-8247-5480-8. [Google Scholar]
- Waterschoot, J.; Gomand, S.V.; Fierens, E.; Delcour, J.A. Starch blends and their physicochemical properties. Starch/Staerke 2015, 67, 1–13. [Google Scholar] [CrossRef]
- Kajiwara, K.; Miyamoto, T. Progress in Structural Characterization of Functional Polysaccharides. In Polysaccharides Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- Pérez, S.; Bertoft, E. The molecular structures of starch components and their contribution to the architecture of starch granules: A comprehensive review. Starch Stärke 2010, 62, 389–420. [Google Scholar] [CrossRef]
- Imberty, A.; Buléon, A.; Tran, V.; Péerez, S. Recent Advances in Knowledge of Starch Structure. Starch Stärke 1991, 43, 375–384. [Google Scholar] [CrossRef]
- Jane, J.-L.; Kasemsuwan, T.; Leas, S.; Zobel, H.; Robyt, J.F. Anthology of Starch Granule Morphology by Scanning Electron Microscopy. Starch Stärke 1994, 46, 121–129. [Google Scholar] [CrossRef]
- Liu, Y.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. Gelatinization behavior of starch: Reflecting beyond the endotherm measured by differential scanning calorimetry. Food Chem. 2019, 284, 53–59. [Google Scholar] [CrossRef]
- Carlstedt, J.; Wojtasz, J.; Fyhr, P.; Kocherbitov, V. Understanding starch gelatinization: The phase diagram approach. Carbohydr. Polym. 2015, 129, 62–69. [Google Scholar] [CrossRef]
- Dufresne, A.; Cavaille, J.Y.; Helbert, W. New nanocomposite materials: Microcrystalline starch reinforced thermoplastic. Macromolecules 1996, 29, 7624–7626. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Gamucci, O.; Corvaglia, S.; Brunetti, V.; Bardi, G. Immunological properties of Andean starch films are independent of their nanometric roughness and stiffness. Int. J. Biol. Macromol. 2015, 75, 460–466. [Google Scholar] [CrossRef]
- Putaux, J.; Molina-Boisseau, S.; Momaur, T.; Dufresne, A. Platelet Nanocrystals Resulting from the Disruption of Waxy Maize Starch Granules by Acid Hydrolysis. Biomacromolecules 2003, 4, 1198–1202. [Google Scholar] [CrossRef]
- Chen, Y.; Cao, X.; Chang, P.R.; Huneault, M.A. Comparative study on the films of poly(vinyl alcohol)/pea starch nanocrystals and poly(vinyl alcohol)/native pea starch. Carbohydr. Polym. 2008, 73, 8–17. [Google Scholar] [CrossRef]
- Zheng, H.; Ai, F.; Chang, P.R.; Huang, J.; Dufresne, A. Structure and Properties of Starch Nanocrystal-Reinforced Soy Protein Plastics. Polym. Compos. 2009, 30, 474–480. [Google Scholar] [CrossRef]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.I.; Goyanes, S. Physico-Mechanical Properties of Biodegradable Starch Nanocomposites. Macromol. Mater. Eng. 2009, 294, 169–177. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, L. High-Strength Waterborne Polyurethane Reinforced with Waxy Maize Starch Nanocrystals. J. Nanosci. Nanotechnol. 2008, 8, 5831–5838. [Google Scholar] [CrossRef]
- Yu, J.; Ai, F.; Dufresne, A.; Gao, S.; Huang, J.; Chang, P.R. Structure and Mechanical Properties of Poly(lactic acid) Filled with (Starch nanocrystal)-graft-poly(e-caprolactone). Macromol. Mater. Eng. 2008, 293, 763–770. [Google Scholar] [CrossRef]
- Namazi, H.; Dadkhah, A. Surface Modification of Starch Nanocrystals Through Ring-Opening Polymerization of e-Caprolactone and Investigation of Their Microstructures. Inc. J. Appl. Polym. Sci. 2008, 110, 2405–2412. [Google Scholar] [CrossRef]
- Liu, D.; Wu, Q.; Chen, H.; Chang, P.R. Transitional properties of starch colloid with particle size reduction from micro- to nanometer. J. Colloid Interface Sci. 2009, 339, 117–124. [Google Scholar] [CrossRef]
- Shi, A.; Li, D.; Wang, L.; Li, B.; Adhikari, B. Preparation of starch-based nanoparticles through high-pressure homogenization and miniemulsion cross-linking: Influence of various process parameters on particle size and stability. Carbohydr. Polym. 2011, 83, 1604–1610. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Grande, C.G.; Díaz, D.A. Biocompatibilty of starch-based films from starch of Andean crops for biomedical applications. Mater. Sci. Eng. C 2011, 31, 1737–1740. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Vega, J.; Wong, M. Influence of botanic origin on the morphology and size of starch nanoparticles from andean native starch sources. Polym. Renew. Resour. 2015, 6, 91–104. [Google Scholar] [CrossRef]
- Torres, F.G.; Troncoso, O.P.; Torres, C.; Díaz, D.A.; Amaya, E. Biodegradability and mechanical properties of starch films from Andean crops. Int. J. Biol. Macromol. 2011, 48, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Angellier, H.; Molina-Boisseau, S.; Dole, P.; Dufresne, A. Thermoplastic Starch–Waxy Maize Starch Nanocrystals Nanocomposites. Biomacromolecules 2006, 7, 531–539. [Google Scholar] [CrossRef] [PubMed]
- García, N.L.; Ribba, L.; Dufresne, A.; Aranguren, M.; Goyanes, S. Effect of glycerol on the morphology of nanocomposites made from thermoplastic starch and starch nanocrystals. Carbohydr. Polym. 2011, 84, 203–210. [Google Scholar] [CrossRef]
- Piyada, K.; Waranyou, S.; Thawein, W. Mechanical, thermal and structural properties of rice starch films reinforced with rice starch nanocrystals. Int. Food Res. J. 2013, 20, 439–449. [Google Scholar]
- Viguié, J.; Molina-Boisseau, S.; Dufresne, A. Processing and Characterization of Waxy Maize Starch Films Plasticized by Sorbitol and Reinforced with Starch Nanocrystals. Macromol. Biosci. 2007, 7, 1206–1216. [Google Scholar] [CrossRef]
- Martinez, S.; Rivon, C.; Troncoso, O.P.; Torres, F.G. Botanical origin as a determinant for the mechanical properties of starch films with nanoparticle reinforcements. Starch Stärke 2016, 68, 935–942. [Google Scholar] [CrossRef]
- Torres, F.G.; Commeaux, S.; Troncoso, O.P. Starch-based biomaterials for wound-dressing applications. Starch Stärke 2013, 65, 543–551. [Google Scholar] [CrossRef]
- Gatto, F.; Troncoso, O.P.; Brunetti, V.; Malvindi, M.A.; Pompa, P.P.; Torres, F.G.; Bardi, G. Human monocyte response to Andean-native starch nanoparticles. Starch Stärke 2016, 68, 1016–1023. [Google Scholar] [CrossRef]
- Marques, A.P.; Reis, R.L.; Hunt, J.A. The biocompatibility of novel starch-based polymers and composites: In vitro studies. Biomaterials 2002, 23, 1471–1478. [Google Scholar] [CrossRef]
- Marques, A.P.; Reis, R.L.; Hunt, J.A. An In Vivo Study of the Host Response to Starch-Based Polymers and Composites Subcutaneously Implanted in Rats. Macromol. Biosci. 2005, 5, 775–785. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.P.; Reis, R.L.; Hunt, J.A. Cytokine secretion from mononuclear cells cultured in vitro with starch-based polymers and poly-L-lactide. J. Biomed. Mater. Res. 2004, 71A, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Mendieta, S.; Barrios-Payán, J.; Mata-Espinosa, D.; Sánchez, S.; Hernández-Pando, R.; Rodríguez-Sanoja, R. Raw starch microparticles have immunostimulant activity in mice vaccinated with BCG and challenged with Mycobacterium tuberculosis. Vaccine 2017, 35, 5123–5130. [Google Scholar] [CrossRef] [PubMed]
- Heinze, T. Cellulose: Structure and properties. Adv. Polym. Sci. 2015, 271, 1–52. [Google Scholar] [CrossRef]
- Perez, S.; Mazeau, K. Conformations, structures, and morphologies of celluloses. In Polysaccharides Structural Diversity and Functional Versatility; Dumitriu, S., Ed.; CRC Press: Boca Raton, FL, USA, 2004; pp. 41–68. [Google Scholar]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994. [Google Scholar] [CrossRef]
- Saxena, I.M.; Brown, R.M., Jr. Cellulose biosynthesis: Current views and evolving concepts. Ann. Bot. 2005, 96, 9–21. [Google Scholar] [CrossRef]
- Habibi, Y.; Lucia, L.A.; Rojas, O.J. Cellulose Nanocrystals: Chemistry, Self-Assembly, and Applications. Chem. Rev. 2010, 110, 3479–3500. [Google Scholar] [CrossRef]
- Van Hai, L.; Son, H.N.; Seo, Y.B. Physical and bio-composite properties of nanocrystalline cellulose from wood, cotton linters, cattail, and red algae. Cellulose 2015, 22, 1789–1798. [Google Scholar] [CrossRef]
- Torres, F.; Commeaux, S.; Troncoso, O. Biocompatibility of Bacterial Cellulose Based Biomaterials. J. Funct. Biomater. 2012, 3, 864–878. [Google Scholar] [CrossRef]
- Chakraborty, A.; Sain, M.; Kortschot, M. Cellulose microfibrils: A novel method of preparation using high shear refining and cryocrushing. Holzforschung 2005, 59, 102–107. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665. [Google Scholar] [CrossRef] [PubMed]
- Gindl, W.; Keckes, J. All-cellulose nanocomposite. Polymer (Guildf). 2005, 46, 10221–10225. [Google Scholar] [CrossRef]
- Duchemin, B.J.C.; Newman, R.H.; Staiger, M.P. Structure–property relationship of all-cellulose composites. Compos. Sci. Technol. 2009, 69, 1225–1230. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Troncoso, O.P.; Canet-Ferrer, J.; Martínez-Pastor, J. Development of self-assembled bacterial cellulose-starch nanocomposites. Mater. Sci. Eng. C 2009, 29, 1098–1104. [Google Scholar] [CrossRef]
- Grande, C.J.; Torres, F.G.; Gomez, C.M.; Carmen Bañó, M. Nanocomposites of bacterial cellulose/hydroxyapatite for biomedical applications. Acta Biomater. 2009, 5, 1605–1615. [Google Scholar] [CrossRef]
- Siró, I.; Plackett, D. Microfibrillated cellulose and new nanocomposite materials: A review. Cellulose 2010, 17, 459–494. [Google Scholar] [CrossRef]
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose – Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764. [Google Scholar] [CrossRef]
- Kumar, V.; Bollström, R.; Yang, A.; Chen, Q.; Chen, G.; Salminen, P.; Bousfield, D.; Toivakka, M. Comparison of nano- and microfibrillated cellulose films. Cellulose 2014, 21, 3443–3456. [Google Scholar] [CrossRef]
- Pan, M.; Zhou, X.; Chen, M. Cellulose Nanowhiskers Isolation and Properties from Acid Hydrolysis Combined with High Pressure Homogenization. BioResources 2013, 8, 933–943. [Google Scholar] [CrossRef]
- Mao, J.; Osorio-Madrazo, A.; Laborie, M.-P. Preparation of cellulose I nanowhiskers with a mildly acidic aqueous ionic liquid: Reaction efficiency and whiskers attributes. Cellulose 2013, 20, 1829–1840. [Google Scholar] [CrossRef]
- Geissler, A.; Biesalski, M.; Heinze, T.; Zhang, K. Formation of nanostructured cellulose stearoyl esters via nanoprecipitation. J. Mater. Chem. A 2014, 2, 1107–1116. [Google Scholar] [CrossRef]
- Hornig, S.; Heinze, T. Efficient Approach To Design Stable Water-Dispersible Nanoparticles of Hydrophobic Cellulose Esters. Biomacromolecules 2008, 9, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
- Dugan, J.M.; Gough, J.E.; Eichhorn, S.J. Bacterial cellulose scaffolds and cellulose nanowhiskers for tissue engineering. Nanomedicine 2013, 8, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Kalia, S.; Boufi, S.; Celli, A.; Kango, S. Nanofibrillated cellulose: Surface modification and potential applications. Colloid Polym. Sci. 2014, 292, 5–31. [Google Scholar] [CrossRef]
- Lin, N.; Dufresne, A. Nanocellulose in biomedicine: Current status and future prospect. Eur. Polym. J. 2014, 59, 302–325. [Google Scholar] [CrossRef]
- Dong, S.; Hirani, A.A.; Colacino, K.R.; Lee, Y.W.; Roman, M. Cytotoxicity and cellular uptake of cellulose nanocrystals. Nano Life 2012, 2, 1241006. [Google Scholar] [CrossRef]
- Catalán, J.; Ilves, M.; Järventaus, H.; Hannukainen, K.-S.; Kontturi, E.; Vanhala, E.; Alenius, H.; Savolainen, K.M.; Norppa, H. Genotoxic and immunotoxic effects of cellulose nanocrystals in vitro. Environ. Mol. Mutagen. 2015, 56, 171–182. [Google Scholar] [CrossRef]
- Vartiainen, J.; Pöhler, T.; Sirola, K.; Pylkkänen, L.; Alenius, H.; Hokkinen, J.; Tapper, U.; Lahtinen, P.; Kapanen, A.; Putkisto, K.; et al. Health and environmental safety aspects of friction grinding and spray drying of microfibrillated cellulose. Cellulose 2011, 18, 775–786. [Google Scholar] [CrossRef]
- Väänänen, V.; Rydman, E.; Ilves, M.; Hannukainen, K.; Norppa, H.; Von Wright, H.; Honkalampi, U.; Tsitko, I.; Rouhiainen, J. Evaluation of the suitability of the developed methodology for nanoparticle health and safety studies. In Proceedings of the Scale-up Nanoparticles in Modern Papermaking (SUNPAP 2012), Milan, Italy, 27 August 2012. [Google Scholar]
- Pereira, M.M.; Raposo, N.R.B.; Brayner, R.; Teixeira, E.M.; Oliveira, V.; Quintão, C.C.R.; Camargo, L.S.A.; Mattoso, L.H.C.; Brandão, H.M. Cytotoxicity and expression of genes involved in the cellular stress response and apoptosis in mammalian fibroblast exposed to cotton cellulose nanofibers. Nanotechnology 2013, 24, 075103. [Google Scholar] [CrossRef]
- Čolić, M.; Mihajlović, D.; Mathew, A.; Naseri, N.; Kokol, V. Cytocompatibility and immunomodulatory properties of wood based nanofibrillated cellulose. Cellulose 2015, 22, 763–778. [Google Scholar] [CrossRef]
- Tomić, S.; Kokol, V.; Mihajlović, D.; Mirčić, A.; Čolić, M. Native cellulose nanofibrills induce immune tolerance in vitro by acting on dendritic cells. Sci. Rep. 2016, 6, 31618. [Google Scholar] [CrossRef] [PubMed]
- Lopes, V.R.; Sanchez-Martinez, C.; Strømme, M.; Ferraz, N. In vitro biological responses to nanofibrillated cellulose by human dermal, lung and immune cells: Surface chemistry aspect. Part. Fibre Toxicol. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Alexandrescu, L.; Syverud, K.; Gatti, A.; Chinga-Carrasco, G. Cytotoxicity tests of cellulose nanofibril-based structures. Cellulose 2013, 20, 1765–1775. [Google Scholar] [CrossRef]
- Saska, S.; Scarel-Caminaga, R.M.; Teixeira, L.N.; Franchi, L.P.; Dos Santos, R.A.; Gaspar, A.M.M.; De Oliveira, P.T.; Rosa, A.L.; Takahashi, C.S.; Messaddeq, Y.; et al. Characterization and in vitro evaluation of bacterial cellulose membranes functionalized with osteogenic growth peptide for bone tissue engineering. J. Mater. Sci. Mater. Med. 2012, 23, 2253–2266. [Google Scholar] [CrossRef]
- Jeong, S.I.; Lee, S.E.; Yang, H.; Jin, Y.-H.; Park, C.-S.; Park, Y.S. Toxicologic evaluation of bacterial synthesized cellulose in endothelial cells and animals. Mol. Cell. Toxicol. 2010, 6, 370–377. [Google Scholar] [CrossRef]
- Kim, G.-D.; Yang, H.; Park, H.R.; Park, C.-S.; Park, Y.S.; Lee, S.E. Evaluation of immunoreactivity of in vitro and in vivo models against bacterial synthesized cellulose to be used as a prosthetic biomaterial. BioChip J. 2013, 7, 201–209. [Google Scholar] [CrossRef]
- Helenius, G.; Bäckdahl, H.; Bodin, A.; Nannmark, U.; Gatenholm, P.; Risberg, B. In vivo biocompatibility of bacterial cellulose. J. Biomed. Mater. Res. Part A 2006, 76A, 431–438. [Google Scholar] [CrossRef]
- Olatunji, O. Classification of Natural Polymers. In Natural Polymers; Springer International Publishing: Cham, Switzerland, 2016; pp. 1–17. [Google Scholar]
- Dhillon, G.S.; Kaur, S.; Brar, S.K.; Verma, M. Green synthesis approach: Extraction of chitosan from fungus mycelia. Crit. Rev. Biotechnol. 2013, 33, 379–403. [Google Scholar] [CrossRef]
- Merzendorfer, H. Chitin. In The sugar code: Fundamentals of Glycosciences; Gabius, H.-J., Ed.; Wiley-VCH Verlag: Weinheim, Germany, 2011; p. 597. ISBN 978-3-527-32089-9. [Google Scholar]
- Raabe, D.; Romano, P.; Sachs, C.; Fabritius, H.; Al-Sawalmih, A.; Yi, S.-B.; Servos, G.; Hartwig, H.G. Microstructure and crystallographic texture of the chitin–protein network in the biological composite material of the exoskeleton of the lobster Homarus americanus. Mater. Sci. Eng. A 2006, 421, 143–153. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Lin, A.Y.-M.; McKittrick, J.; Meyers, M.A. Structure and mechanical properties of crab exoskeletons. Acta Biomater. 2008, 4, 587–596. [Google Scholar] [CrossRef]
- Faria, R.R.; Guerra, R.F.; De Sousa Neto, L.R.; Motta, L.F.; De Franca, E.F. Computational study of polymorphic structures of α- and β- chitin and chitosan in aqueous solution. J. Mol. Graph. Model. 2016, 63, 78–84. [Google Scholar] [CrossRef] [PubMed]
- Peter, M.G. Chitin and Chitosan in Fungi. In Biopolymers Online; Vandamme, E.J., De Baets, S., Steinbüchel, A., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005. [Google Scholar]
- Harish Prashanth, K.V.; Tharanathan, R.N. Chitin/chitosan: Modifications and their unlimited application potential-an overview. Trends Food Sci. Technol. 2007, 18, 117–131. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Noishiki, Y.; Wada, M. X-ray structure of anhydrous β-chitin at 1 Å resolution. Macromolecules 2011, 44, 950–957. [Google Scholar] [CrossRef]
- Weiner, S.; Addadi, L. Design strategies in mineralized biological materials. J. Mater. Chem. 1997, 7, 689–702. [Google Scholar] [CrossRef]
- Zargar, V.; Asghari, M.; Dashti, A. A Review on Chitin and Chitosan Polymers: Structure, Chemistry, Solubility, Derivatives, and Applications. ChemBioEng Rev. 2015, 2, 204–226. [Google Scholar] [CrossRef]
- Zeng, J.-B.; He, Y.-S.; Li, S.-L.; Wang, Y.-Z. Chitin Whiskers: An Overview. Biomacromolecules 2012, 13, 1–11. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Different routes to turn chitin into stunning nano-objects. Eur. Polym. J. 2015, 68, 503–515. [Google Scholar] [CrossRef]
- Chatelet, C.; Damour, O.; Domard, A. Influence of the degree of acetylation on some biological properties of chitosan films. Biomaterials 2001, 22, 261–268. [Google Scholar] [CrossRef]
- Karim, Z.; Mathew, A.P.; Grahn, M.; Mouzon, J.; Oksman, K. Nanoporous membranes with cellulose nanocrystals as functional entity in chitosan: Removal of dyes from water. Carbohydr. Polym. 2014, 112, 668–676. [Google Scholar] [CrossRef]
- Uragami, T.; Saito, T.; Miyata, T. Pervaporative dehydration characteristics of an ethanol/water azeotrope through various chitosan membranes. Carbohydr. Polym. 2015, 120, 1–6. [Google Scholar] [CrossRef]
- Chen, D.; Hu, B.; Huang, C. Chitosan modified ordered mesoporous silica as micro-column packing materials for on-line flow injection-inductively coupled plasma optical emission spectrometry determination of trace heavy metals in environmental water samples. Talanta 2009, 78, 491–497. [Google Scholar] [CrossRef] [PubMed]
- Chantarasataporn, P.; Tepkasikul, P.; Kingcha, Y.; Yoksan, R.; Pichyangkura, R.; Visessanguan, W.; Chirachanchai, S. Water-based oligochitosan and nanowhisker chitosan as potential food preservatives for shelf-life extension of minced pork. Food Chem. 2014, 159, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Minet, E.P.; O’Carroll, C.; Rooney, D.; Breslin, C.; McCarthy, C.P.; Gallagher, L.; Richards, K.G. Slow delivery of a nitrification inhibitor (dicyandiamide) to soil using a biodegradable hydrogel of chitosan. Chemosphere 2013, 93, 2854–2858. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, Y.A.; El-Khordagui, L.K.; Boraei, N.A.; Darwish, I.A. Chitosan microparticles incorporating a hydrophilic sunscreen agent. Carbohydr. Polym. 2010, 81, 234–242. [Google Scholar] [CrossRef]
- Sonia, T.A.; Sharma, C.P. Chitosan and its derivatives for drug delivery perspective. Adv. Polym. Sci. 2011, 243, 23–54. [Google Scholar] [CrossRef]
- Sriupayo, J.; Supaphol, P.; Blackwell, J.; Rujiravanit, R. Preparation and characterization of α-chitin whisker-reinforced chitosan nanocomposite films with or without heat treatment. Carbohydr. Polym. 2005, 62, 130–136. [Google Scholar] [CrossRef]
- Mathew, A.P.; Laborie, M.-P.G.; Oksman, K. Cross-Linked Chitosan/Chitin Crystal Nanocomposites with Improved Permeation Selectivity and pH Stability. Biomacromolecules 2009, 10, 1627–1632. [Google Scholar] [CrossRef]
- Araki, J.; Yamanaka, Y.; Ohkawa, K. Chitin-chitosan nanocomposite gels: Reinforcement of chitosan hydrogels with rod-like chitin nanowhiskers. Polym. J. 2012, 44, 713–717. [Google Scholar] [CrossRef]
- Corsello, F.A.; Bolla, P.A.; Anbinder, P.S.; Serradell, M.A.; Amalvy, J.I.; Peruzzo, P.J. Morphology and properties of neutralized chitosan-cellulose nanocrystals biocomposite films. Carbohydr. Polym. 2017, 156, 452–459. [Google Scholar] [CrossRef]
- Celebi, H.; Kurt, A. Effects of processing on the properties of chitosan/cellulose nanocrystal films. Carbohydr. Polym. 2015, 133, 284–293. [Google Scholar] [CrossRef]
- Cuesta, A.; Esteban, M.A.; Meseguer, J. In vitro effect of chitin particles on the innate cellular immune system of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2003, 15, 1–11. [Google Scholar] [CrossRef]
- Esteban, M.; Mulero, V.; Cuesta, A.; Ortuño, J.; Meseguer, J. Effects of injecting chitin particles on the innate immune response of gilthead seabream (Sparus aurata L.). Fish Shellfish Immunol. 2000, 10, 543–554. [Google Scholar] [CrossRef] [PubMed]
- Sum Chow, K.; Khor, E.; Chwee Aun Wan, A. Porous chitin matrices for tissue engineering: Fabrication and in vitro cytotoxic assessment. J. Polym. Res. 2001, 8, 27–35. [Google Scholar] [CrossRef]
- Dev, A.; Mohan, J.C.; Sreeja, V.; Tamura, H.; Patzke, G.R.; Hussain, F.; Weyeneth, S.; Nair, S.V.; Jayakumar, R. Novel carboxymethyl chitin nanoparticles for cancer drug delivery applications. Carbohydr. Polym. 2010, 79, 1073–1079. [Google Scholar] [CrossRef]
- Alvarez, F. The Effect of Chitin Size, Shape, Source and Purification Method on Immune Recognition. Molecules 2014, 19, 4433–4451. [Google Scholar] [CrossRef] [PubMed]
- Amarsaikhan, N.; Templeton, S.P. Co-recognition of β-glucan and chitin and programming of adaptive immunity to Aspergillus fumigatus. Front. Microbiol. 2015, 6, 344. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.; Okawa, Y.; Hashimoto, K.; Suzuki, S.; Suzuki, M. Protecting Effect of Chitin and Chitosan on Experimentally Induced Murine Candidiasis. Microbiol. Immunol. 1984, 28, 903–912. [Google Scholar] [CrossRef]
- Da Silva, C.A.; Hartl, D.; Liu, W.; Lee, C.G.; Elias, J.A. TLR-2 and IL-17A in chitin-induced macrophage activation and acute inflammation. J. Immunol. 2008, 181, 4279–4286. [Google Scholar] [CrossRef]
- Van Dyken, S.J.; Mohapatra, A.; Nussbaum, J.C.; Molofsky, A.B.; Thornton, E.E.; Ziegler, S.F.; McKenzie, A.N.J.; Krummel, M.F.; Liang, H.-E.; Locksley, R.M. Chitin activates parallel immune modules that direct distinct inflammatory responses via innate lymphoid type 2 and γδ T cells. Immunity 2014, 40, 414–424. [Google Scholar] [CrossRef]
- Reese, T.A.; Liang, H.-E.; Tager, A.M.; Luster, A.D.; Van Rooijen, N.; Voehringer, D.; Locksley, R.M. Chitin induces accumulation in tissue of innate immune cells associated with allergy. Nature 2007, 447, 92–96. [Google Scholar] [CrossRef]
- Nagatani, K.; Wang, S.; Llado, V.; Lau, C.W.; Li, Z.; Mizoguchi, A.; Nagler, C.R.; Shibata, Y.; Reinecker, H.-C.; Mora, R.J.; et al. Chitin microparticles for the control of intestinal inflammation. Inflamm. Bowel Dis. 2012, 18, 1698–1710. [Google Scholar] [CrossRef]
- Thanou, M.; Verhoef, J.C.; Junginger, H.E. Oral drug absorption enhancement by chitosan and its derivatives. Adv. Drug Deliv. Rev. 2001, 52, 117–126. [Google Scholar] [CrossRef]
- Schipper, N.G.; Vårum, K.M.; Artursson, P. Chitosans as absorption enhancers for poorly absorbable drugs. 1: Influence of molecular weight and degree of acetylation on drug transport across human intestinal epithelial (Caco-2) cells. Pharm. Res. 1996, 13, 1686–1692. [Google Scholar] [CrossRef]
- Schipper, N.G.; Vârum, K.M.; Stenberg, P.; Ocklind, G.; Lennernäs, H.; Artursson, P. Chitosans as absorption enhancers of poorly absorbable drugs. 3: Influence of mucus on absorption enhancement. Eur. J. Pharm. Sci. 1999, 8, 335–343. [Google Scholar] [CrossRef]
- Rao, S.B.; Sharma, C.P. Use of chitosan as a biomaterial: Studies on its safety and hemostatic potential. J. Biomed. Mater. Res. 1997, 34, 21–28. [Google Scholar] [CrossRef]
- Arai, L.; Kinumaki , Y.; Fujita , T. Toxicity of chitosan. Bull. Tokai Reg. Fish Res. Lab. 1968, 43, 56–89. [Google Scholar]
- Illum, L.; Farraj, N.F.; Davis, S.S. Chitosan as a Novel Nasal Delivery System for Peptide Drugs. Pharm. Res. 1994, 11, 1186–1189. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Choi, I.-S.; Han, J.-H.; Yoo, H.-S. Chitosan and D-glucosamine induce expression of Th1 cytokine genes in porcine spleen cells. J. Vet. Med. Sci. 2002, 64, 645–648. [Google Scholar] [CrossRef][Green Version]
- Wen, Z.-S.; Xu, Y.-L.; Zou, X.-T.; Xu, Z.-R.; Wen, Z.-S.; Xu, Y.-L.; Zou, X.-T.; Xu, Z.-R. Chitosan Nanoparticles Act as an Adjuvant to Promote both Th1 and Th2 Immune Responses Induced by Ovalbumin in Mice. Mar. Drugs 2011, 9, 1038–1055. [Google Scholar] [CrossRef]
- Zhang, P.; Liu, W.; Peng, Y.; Han, B.; Yang, Y. Toll like receptor 4 (TLR4) mediates the stimulating activities of chitosan oligosaccharide on macrophages. Int. Immunopharmacol. 2014, 23, 254–261. [Google Scholar] [CrossRef]
- Feng, X.; Lu, X.; Huang, D.; Xing, J.; Feng, G.; Jin, G.; Yi, X.; Li, L.; Lu, Y.; Nie, D.; et al. 3D Porous Chitosan Scaffolds Suit Survival and Neural Differentiation of Dental Pulp Stem Cells. Cell. Mol. Neurobiol. 2014, 34, 859–870. [Google Scholar] [CrossRef]
- Chakrabarti, A.; Talukdar, D.; Pal, A.; Ray, M. Immunomodulation of macrophages by methylglyoxal conjugated with chitosan nanoparticles against Sarcoma-180 tumor in mice. Cell. Immunol. 2014, 287, 27–35. [Google Scholar] [CrossRef]
- Coviello, T.; Matricardi, P.; Marianecci, C.; Alhaique, F. Polysaccharide hydrogels for modified release formulations. J. Control. Release 2007, 119, 5–24. [Google Scholar] [CrossRef]
- Jiao, G.; Yu, G.; Zhang, J.; Ewart, H.S. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar. Drugs 2011, 9, 196–233. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Nasif, M.; Sultan, N.; Aslam, N.; Noreen, A.; Zuber, M. A review on synthesis, properties and applications of natural polymer based carrageenan blends and composites. Int. J. Biol. Macromol. 2017, 96, 282–301. [Google Scholar] [CrossRef]
- Yuan, H.; Song, J.; Li, X.; Li, N.; Dai, J. Immunomodulation and antitumor activity of κ-carrageenan oligosaccharides. Cancer Lett. 2006, 243, 228–234. [Google Scholar] [CrossRef]
- Zhou, G.; Sun, Y.P.; Xin, H.; Zhang, Y.; Li, Z.; Xu, Z. In vivo antitumor and immunomodulation activities of different molecular weight lambda-carrageenans from Chondrus ocellatus. Pharmacol. Res. 2004, 50, 47–53. [Google Scholar] [CrossRef]
- Cáceres, P.J.; Carlucci, M.J.; Damonte, E.B.; Matsuhiro, B.; Zúñiga, E.A. Carrageenans from chilean samples of Stenogramme interrupta (Phyllophoraceae): Structural analysis and biological activity. Phytochemistry 2000, 53, 81–86. [Google Scholar] [CrossRef]
- Panlasigui, L.N.; Baello, O.Q.; Dimatangal, J.M.; Dumelod, B.D. Blood cholesterol and lipid-lowering effects of carrageenan on human volunteers. Asia Pac. J. Clin. Nutr. 2003, 12, 209–214. [Google Scholar]
- Nilson, H.W.; Wagner, J.A. Feeding Test With Carrageenin. J. Food Sci. 1959, 24, 235–239. [Google Scholar] [CrossRef]
- Rustia, M.; Shubik, P.; Patil, K. Lifespan carcinogenicity tests with native carrageenan in rats and hamsters. Cancer Lett. 1980, 11, 1–10. [Google Scholar] [CrossRef]
- Weiner, M.L. Toxicological properties of carrageenan. Agents Actions 1991, 32, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Catanzaro, P.J.; Schwartz, H.J.; Graham, R.C., Jr. Spectrum and Possible Mechanism of Carrageenan Cytotoxicity. Am. J. Pathol. 1971, 64, 387. [Google Scholar] [PubMed]
- Grenha, A.; Gomes, M.E.; Rodrigues, M.; Santo, V.E.; Mano, J.F.; Neves, N.M.; Reis, R.L. Development of new chitosan/carrageenan nanoparticles for drug delivery applications. J. Biomed. Mater. Res. Part A 2009, 92, 1265–1272. [Google Scholar] [CrossRef]
- Popa, E.G.; Gomes, M.E.; Reis, R.L. Cell Delivery Systems Using Alginate–Carrageenan Hydrogel Beads and Fibers for Regenerative Medicine Applications. Biomacromolecules 2011, 12, 3952–3961. [Google Scholar] [CrossRef]
- Liang, W.; Mao, X.; Peng, X.; Tang, S. Effects of sulfate group in red seaweed polysaccharides on anticoagulant activity and cytotoxicity. Carbohydr. Polym. 2014, 101, 776–785. [Google Scholar] [CrossRef]
- Benard, C.; Cultrone, A.; Michel, C.; Rosales, C.; Segain, J.-P.; Lahaye, M.; Galmiche, J.-P.; Cherbut, C.; Blottière, H.M. Degraded Carrageenan Causing Colitis in Rats Induces TNF Secretion and ICAM-1 Upregulation in Monocytes through NF-κB Activation. PLoS ONE 2010, 5, e8666. [Google Scholar] [CrossRef]
- Stephanie, B.; Eric, D.; Sophie, F.M.; Christian, B.; Yu, G. Carrageenan from Solieria chordalis (Gigartinales): Structural analysis and immunological activities of the low molecular weight fractions. Carbohydr. Polym. 2010, 81, 448–460. [Google Scholar] [CrossRef]
- Yermak, I.M.; Barabanova, A.O.; Aminin, D.L.; Davydova, V.N.; Sokolova, E.V.; Solov’eva, T.F.; Kim, Y.H.; Shin, K.S. Effects of structural peculiarities of carrageenans on their immunomodulatory and anticoagulant activities. Carbohydr. Polym. 2012, 87, 713–720. [Google Scholar] [CrossRef]
- Remminghorst, U.; Rehm, B.H.A. Bacterial alginates: From biosynthesis to applications. Biotechnol. Lett. 2006, 28, 1701–1712. [Google Scholar] [CrossRef]
- Szekalska, M.; Puciłowska, A.; Szymańska, E.; Ciosek, P.; Winnicka, K. Alginate: Current Use and Future Perspectives in Pharmaceutical and Biomedical Applications. Int. J. Polym. Sci. 2016, 2016, 1–17. [Google Scholar] [CrossRef]
- Wright, B.; De Bank, P.A.; Luetchford, K.A.; Acosta, F.R.; Connon, C.J. Oxidized alginate hydrogels as niche environments for corneal epithelial cells. J. Biomed. Mater. Res. Part A 2014, 102, 3393–3400. [Google Scholar] [CrossRef]
- Maiti, S.; Singha, K.; Ray, S.; Dey, P.; Sa, B. Adipic acid dihydrazide treated partially oxidized alginate beads for sustained oral delivery of flurbiprofen. Pharm. Dev. Technol. 2009, 14, 461–470. [Google Scholar] [CrossRef]
- Izawa, H.; Kawakami, K.; Sumita, M.; Tateyama, Y.; Hill, J.P.; Ariga, K. β-Cyclodextrin-crosslinked alginate gel for patient-controlled drug delivery systems: Regulation of host–guest interactions with mechanical stimuli. J. Mater. Chem. B 2013, 1, 2155. [Google Scholar] [CrossRef]
- Hurteaux, R.; Edwards-Lévy, F.; Laurent-Maquin, D.; Lévy, M.-C. Coating alginate microspheres with a serum albumin-alginate membrane: Application to the encapsulation of a peptide. Eur. J. Pharm. Sci. 2005, 24, 187–197. [Google Scholar] [CrossRef]
- Mahou, R.; Meier, R.; Bühler, L.; Wandrey, C. Alginate-Poly(ethylene glycol) Hybrid Microspheres for Primary Cell Microencapsulation. Materials (Basel). 2014, 7, 275–286. [Google Scholar] [CrossRef]
- Coleman, R.J.; Lawrie, G.; Lambert, L.K.; Whittaker, M.; Jack, K.S.; Grøndahl, L. Phosphorylation of Alginate: Synthesis, Characterization, and Evaluation of in Vitro Mineralization Capacity. Biomacromolecules 2011, 12, 889–897. [Google Scholar] [CrossRef]
- Wang, X.; Hao, T.; Qu, J.; Wang, C.; Chen, H. Synthesis of Thermal Polymerizable Alginate-GMA Hydrogel for Cell Encapsulation. J. Nanomater. 2015, 2015, 1–8. [Google Scholar] [CrossRef]
- Yang, D.; Jones, K.S. Effect of alginate on innate immune activation of macrophages. J. Biomed. Mater. Res. Part A 2009, 90A, 411–418. [Google Scholar] [CrossRef]
- Ge, F.; Zhu, L.; Yang, L.; Li, W.; Wei, S.; Tao, Y.; Du, G. The Soluble and Particulate Form of Alginates Positively Regulate Immune Response. Iran. J. Immunol. 2018, 15, 228–238. [Google Scholar] [CrossRef]
- Iwamoto, Y.; Xu, X.; Tamura, T.; Oda, T.; Muramatsu, T. Enzymatically Depolymerized Alginate Oligomers That Cause Cytotoxic Cytokine Production in Human Mononuclear Cells. Biosci. Biotechnol. Biochem. 2003, 67, 258–263. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Kurachi, M.; Yamaguchi, K.; Oda, T. Induction of Multiple Cytokine Secretion from RAW264.7 Cells by Alginate Oligosaccharides. Biosci. Biotechnol. Biochem. 2007, 71, 238–241. [Google Scholar] [CrossRef]



| Origin | Polysaccharide |
|---|---|
| Plants | Starch, cellulose, glucomannan, pectin, hemicellulose, gums, mucilage |
| Algae | Agar, galactans, alginates, carrageenans |
| Animals | Chitin, chitosan, hyaluronic acid, glycosaminoglycans, cellulose |
| Bacteria | Dextran, levan, polygalactosamine, gellan, xanthan, cellulose |
| Fungal | Elsinan, chitin, chitosan, pollulan, yeast glucans |
| Cellulose Nano-Object | Diameter (nm) | Length (nm) | Aspect Ratio | References |
|---|---|---|---|---|
| Cellulose microfibers | 10–40 | >1000 | 100–150 | [69,70] |
| Cellulose nanofibers | 4–10 | ~200 | 50–20 | [71] |
| Cellulose nanowhiskers | 2–20 | 100–600 | 10–100 | [21,72,73] |
| Bacterial cellulose nanofibers | 100 | - | - | [62] |
| Cellulose nanoparticles | 50–300 | - | - | [74,75] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres, F.G.; Troncoso, O.P.; Pisani, A.; Gatto, F.; Bardi, G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. Int. J. Mol. Sci. 2019, 20, 5092. https://doi.org/10.3390/ijms20205092
Torres FG, Troncoso OP, Pisani A, Gatto F, Bardi G. Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. International Journal of Molecular Sciences. 2019; 20(20):5092. https://doi.org/10.3390/ijms20205092
Chicago/Turabian StyleTorres, Fernando G., Omar P. Troncoso, Anissa Pisani, Francesca Gatto, and Giuseppe Bardi. 2019. "Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties" International Journal of Molecular Sciences 20, no. 20: 5092. https://doi.org/10.3390/ijms20205092
APA StyleTorres, F. G., Troncoso, O. P., Pisani, A., Gatto, F., & Bardi, G. (2019). Natural Polysaccharide Nanomaterials: An Overview of Their Immunological Properties. International Journal of Molecular Sciences, 20(20), 5092. https://doi.org/10.3390/ijms20205092








