Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease
Abstract
:1. Introduction
2. Current Therapeutic Approaches Only Target Symptoms of AD
3. Therapeutic Strategies Based on Targeting Amyloid β and Tau Proteins
4. Prospect of APOE4 as a Drug Target for AD
5. Reactive Oxygen and Reactive Nitrogen Species in AD
6. Single Target Strategies in Management of AD
7. Drug Combinations as a Strategy for AD Therapy
8. Restoring Protein Homeostasis as a Novel Multifactorial Approach
9. Multiple Targets of Polyphenols against AD
9.1. Polyphenols as Antioxidants
9.2. Modulation of Protein Homeostasis and Longevity with Polyphenols
9.3. Polyphenols and Cellular Lipid Balance
9.4. Anti-inflammatory Activity of Polyphenols
9.5. Polyphenols as Anti-amyloid Agents
9.6. Polyphenols in Cognition and Synapsis
10. Future Directions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s Disease |
| Aβ42 | β-amyloid of 42 amino acids |
| Aα | Amyloid α |
| NGF | Nerve Growth Factor |
| FDA | Food and Drug Administration |
| NMDAR | N-Methyl-D-Aspartic Receptor |
| APP | Amyloid Precursor Protein |
| BACE | β-Secretase |
| NFT | Neurofibrillary Tangle |
| NFκB | Nuclear factor kappa B |
| MAPK | Mitogen-Activated Protein Kinase |
| GSK3β | Glycogen Synthase Kinase - 3β |
| CDK | Cyclin dependent kinase |
| APOE | Apolipoprotein E |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| GABA | γ-amino butyric acid |
| cGMP | Cyclic guanosine monophosphate |
| cAMP | Cyclic adenosine monophosphate |
| COX | Cyclooxygenase |
| PPARγ | Peroxisome proliferator-activated receptor γ |
| FAAH | Fatty acid amide hydrolase |
| MAGL | Mono acyl glycerol lipase |
| BDNF | Brain-derived neurotrophic factor |
| NT | Neurotrophin |
| Trk | Tropomyosin receptor kinase |
| PI3K | Phosphatidylinositol-3-kinase |
| Akt | Protein kinase B |
| BBB | Blood Brain Barrier |
| ECB | Encapsulated Cell Bio-delivery |
| AChE | Acetylcholine esterase |
| MAO | Monoamine oxidase |
| UPR | Unfolded protein response |
| IRE | Inositol response element |
| ATF | Activating transcription factor |
| PERK | Protein kinase RNA-like endoplasmic reticulum kinase |
| LAMP | Lysosome associated molecular pattern |
| ATP | Adenosine triphosphate |
| AMPK | Adenosine monophosphate kinase |
| mTOR | Mechanistic Target of Rapamycin |
| NADPH | Dihydronicotinamide-adenine dinucleotide phosphate |
| NOX | NADPH oxidase |
| TFEB | Transcription factor EB |
| SIRT1 | Sirtuin 1 |
| FOXO | Fork head box like protein O |
| Nrf | Nuclear factor erythroid-2 related factor |
| Keap | Kelch-like ECH-associated protein 1 |
| Maf | Masculoaponeurotic fibrosarcoma |
| ARE | Antioxidant response element |
| UDP | Uridine diphosphate |
| PGC1 | PPARγ coactivator-1 |
| TFAM | Transcription factor A, mitochondrial |
| EGCG | Epigallocatechin-3-gallate |
| ULK | Unc-51 like autophagy activating kinase |
| c-JNK | c-Jun N-terminal kinase |
| CLEAR | Coordinated lysosomal expression and regulation |
| HDAC | Histone deacetylase |
| Atg | Autophagy related |
| CAMKK | Calcium/Calmodulin-dependent protein kinase kinase |
| Bcl | Beclin |
| ERK | Extracellular signal-regulated kinases |
| HMGCoA | 3-hydroxy-3-methyl-glutaryl-Coenzyme A |
| DNMT | DNA (cytosine-5)-methyltransferase |
| HO | Heme oxygenase |
| HSP | Heat shock protein |
| TNF | Tumor necrosis factor |
| IL | Interleukin |
| SOD | Superoxide dismutase |
| CREB | cAMP response element-binding protein |
| Bax | Beclin-2- associated X |
References
- Macreadie, I.G.; Arvanitis, C.; Bharadwaj, P. Finding chemopreventatives to reduce amyloid beta in yeast. Neural Regen. Res. 2016, 11, 244–245. [Google Scholar] [CrossRef]
- Sharma, P.; Srivastava, P.; Seth, A.; Tripathi, P.N.; Banerjee, A.G.; Shrivastava, S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019, 174, 53–89. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.A.; Lim, S.; Kim, K.Y. Metal Ion Effects on Aβ and Tau Aggregation. Int. J. Mol. Sci. 2018, 19, 128. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Luo, G.; Tang, Y.; Yao, P. Quercetin and iron metabolism: What we know and what we need to know. Food Chem. Toxicol. 2018, 114, 190–203. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Chen, Y.; Shaw, A.M.; Goldfine, H.; Tian, J.; Cai, J. Enhancing TFEB-mediated cellular degradation pathways by the mTORC1 inhibitor quercetin. Oxidative Med. Cell. Longev. 2018, 2018, 5073420. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Aliaga, K.; Bermejo-Bescos, P.; Benedi, J.; Martin-Aragon, S. Quercetin and rutin exhibit antiamyloidogenic and fibril-disaggregating effects in vitro and potent antioxidant activity in APPswe cells. Life Sci 2011, 89, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Primikyri, A.; Mazzone, G.; Lekka, C.; Tzakos, A.G.; Russo, N.; Gerothanassis, I.P. Understanding zinc(II) chelation with quercetin and luteolin: A combined NMR and theoretical study. J. Phys. Chem. B 2015, 119, 83–95. [Google Scholar] [CrossRef]
- Dosenko, V.E.; Nagibin, V.S.; Tumanovskaya, L.V.; Zagorii, V.Y.; Moibenko, A.A. Effect of quercetin on the activity of purified 20S and 26S proteasome and proteasomal activity in isolated cardiomyocytes. Biomed. Chem. 2007, 1, 40–44. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, G.W.; Liang, Z.M.; Sheng, S.Y.; Shi, Y.S.; Peng, L.; Wang, Y.P.; Wang, F.; Zhang, X.M. Resveratrol improves cognition and decreases amyloid plaque formation in Tg6799 mice. Mol. Med. Rep. 2019, 49, 3783–3790. [Google Scholar] [CrossRef]
- Pallàs, M.; Casadesús, G.; Smith, M.A.; Coto-Montes, A.; Pelegri, C.; Vilaplana, J.; Camins, A. Resveratrol and neurodegenerative diseases: Activation of SIRT1 as the potential pathway towards neuroprotection. Curr. Neurovas. Res. 2009, 6, 70–81. [Google Scholar] [CrossRef]
- Suvorova, I.I.; Knyazeva, A.R.; Pospelov, V.A. Resveratrol-induced p53 activation is associated with autophagy in mouse embryonic stem cells. Biochem. Biophys. Res. Commun. 2018, 503, 2180–2185. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, Y.; Mueller-Steiner, S.; Chen, L.F.; Kwon, H.; Yi, S.; Mucke, L.; Gan, L. SIRT1 protects against microglia-dependent amyloid-β toxicity through inhibiting NF-κB signaling. J. Biol. Chem. 2005, 280, 40364–40374. [Google Scholar] [CrossRef]
- Konings, E.; Timmers, S.; Boekschoten, M.V.; Goossens, G.H.; Jocken, J.W.; Afman, L.A.; Müller, M.; Schrauwen, P.; Mariman, E.C.; Blaak, E.E. The effects of 30 days resveratrol supplementation on adipose tissue morphology and gene expression patterns in obese men. Int. J. Obes. 2014, 38, 470–473. [Google Scholar] [CrossRef]
- Chen, Q.; Ganapathy, S.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.; Yang, H.; Zeng, K.; Zhang, J.; Zhong, N.; Wang, Y.; Ye, J.; Tu, P.; Liu, Z. EGCG Inhibited Lipofuscin Formation Based on Intercepting Amyloidogenic β-Sheet-Rich Structure Conversion. PLoS ONE 2016, 11, e0152064. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Xie, L.P.; Zheng, X.Y.; Wang, Y.B.; Bai, Y.; Shen, H.F.; Li, L.C.; Dahiya, R. A component of green tea, (-)-epigallocatechin-3-gallate, promotes apoptosis in T24 human bladder cancer cells via modulation of the PI3K/Akt pathway and Bcl-2 family proteins. Biochem. Biophys. Res. Commun. 2007, 354, 852–857. [Google Scholar] [CrossRef]
- Hyung, S.J.; Detoma, A.S.; Brender, J.R.; Lee, S.; Vivekanandan, S.; Kochi, A.; Choi, J.S.; Ramamoorthy, A.; Ruotolo, B.T.; Lim, M.H. Insights into antiamyloidogenic properties of the green tea extract (-)-epigallocatechin-3-gallate toward metal-associated amyloid-β species. Proc. Natl. Acad. Sci. USA 2013, 110, 3743–3748. [Google Scholar] [CrossRef]
- Kim, H.S.; Montana, V.; Jang, H.J.; Parpura, V.; Kim, J.A. Epigallocatechin gallate (EGCG) stimulates autophagy in vascular endothelial cells: A potential role for reducing lipid accumulation. J. Biol. Chem. 2013, 288, 22693–22705. [Google Scholar] [CrossRef]
- Zhou, J.; Farah, B.L.; Sinha, R.A.; Wu, Y.; Singh, B.K.; Bay, B.H.; Yang, C.S.; Yen, P.M. Epigallocatechin-3-Gallate (EGCG), a green tea polyphenol, stimulates hepatic autophagy and lipid clearance. PLoS ONE 2014, 9, e87161. [Google Scholar] [CrossRef]
- Pacheco, S.M.; Soares, M.S.P.; Gutierres, J.M.; Gerzson, M.F.B.; Carvalho, F.B.; Azambuja, J.H.; Schetinger, M.R.C.; Stefanello, F.M.; Spanevello, R.M. Anthocyanins as a potential pharmacological agent to manage memory deficit, oxidative stress and alterations in ion pump activity induced by experimental sporadic dementia of Alzheimer’s type. J. Nutr. Biochem. 2018, 56, 193–204. [Google Scholar] [CrossRef]
- Hwang, Y.P.; Choi, J.H.; Yun, H.J.; Han, E.H.; Kim, H.G.; Kim, J.Y.; Park, B.H.; Khanal, T.; Choi, J.M.; Chung, Y.C.; et al. Anthocyanins from purple sweet potato attenuate dimethylnitrosamine-induced liver injury in rats by inducing Nrf2-mediated antioxidant enzymes and reducing COX-2 and iNOS expression. Food Chem. Toxicol. 2011, 49, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Longo, L.; Platini, F.; Scardino, A.; Alabiso, O.; Vasapollo, G.; Tessitore, L. Autophagy inhibition enhances anthocyanin-induced apoptosis in hepatocellular carcinoma. Mol. Cancer Ther. 2008, 7, 2476–2485. [Google Scholar] [CrossRef] [PubMed]
- Ullah, I.; Park, H.Y.; Kim, M.O. Anthocyanins Protect against Kainic Acid-induced Excitotoxicity and Apoptosis via ROS-activated AMPK Pathway in Hippocampal Neurons. Cns Neurosci. Ther. 2014, 20, 327–338. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.W.; Lee, S.Y.; Kim, M.; Cheon, C.; Ko, S.-G. Kaempferol induces autophagic cell death via IRE1-JNK-CHOP pathway and inhibition of G9a in gastric cancer cells. Cell Death Dis. 2018, 9, 875. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-W.; Chen, P.-N.; Chen, M.-K.; Yang, W.-E.; Tang, C.-H.; Yang, S.-F.; Hsieh, Y.-S. Kaempferol Reduces Matrix Metalloproteinase-2 Expression by Down-Regulating ERK1/2 and the Activator Protein-1 Signaling Pathways in Oral Cancer Cells. PLoS ONE 2013, 8, e80883. [Google Scholar] [CrossRef]
- Huang, W.W.; Tsai, S.C.; Peng, S.F.; Lin, M.W.; Chiang, J.H.; Chiu, Y.J.; Fushiya, S.; Tseng, M.T.; Yang, J.S. Kaempferol induces autophagy through AMPK and AKT signaling molecules and causes G 2/M arrest via downregulation of CDK1/cyclin B in SK-HEP-1 human hepatic cancer cells. Int. J. Oncol. 2013, 42, 2069–2077. [Google Scholar] [CrossRef]
- Qiu, W.; Lin, J.; Zhu, Y.; Zhang, J.; Zeng, L.; Su, M.; Tian, Y. Kaempferol modulates DNA methylation and downregulates DNMT3B in bladder cancer. Cell. Physiol. Biochem. 2017, 41, 1325–1335. [Google Scholar] [CrossRef]
- De Pablos, R.M.; Espinosa-Oliva, A.M.; Hornedo-Ortega, R.; Cano, M.; Arguelles, S. Hydroxytyrosol protects from aging process via AMPK and autophagy; a review of its effects on cancer, metabolic syndrome, osteoporosis, immune-mediated and neurodegenerative diseases. Pharmacol. Res. 2019, 143, 58–72. [Google Scholar] [CrossRef]
- Zheng, A.; Li, H.; Xu, J.; Cao, K.; Li, H.; Pu, W.; Yang, Z.; Peng, Y.; Long, J.; Liu, J.; et al. Hydroxytyrosol improves mitochondrial function and reduces oxidative stress in the brain of db/db mice: Role of AMP-activated protein kinase activation. Br. J. Nutr. 2015, 113, 1667–1676. [Google Scholar] [CrossRef]
- Zrelli, H.; Matsuoka, M.; Kitazaki, S.; Zarrouk, M.; Miyazaki, H. Hydroxytyrosol reduces intracellular reactive oxygen species levels in vascular endothelial cells by upregulating catalase expression through the AMPK-FOXO3a pathway. Eur. J. Pharmacol. 2011, 660, 275–282. [Google Scholar] [CrossRef]
- Wang, W.; Jing, T.; Yang, X.; He, Y.; Wang, B.; Xiao, Y.; Shang, C.; Zhang, J.; Lin, R. Hydroxytyrosol regulates the autophagy of vascular adventitial fibroblasts through the SIRT1-mediated signaling pathway. Can. J. Physiol. Pharmacol. 2018, 96, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Ma, Y.; Xu, Z.; Wang, J.; Wang, F.; Wang, D.; Pan, S.; Wu, Y.; Pan, H.; Xu, D.; et al. Hydroxytyrosol, a natural molecule from olive oil, suppresses the growth of human hepatocellular carcinoma cells via inactivating AKT and nuclear factor-kappa B pathways. Cancer Lett. 2014, 347, 79–87. [Google Scholar] [CrossRef] [PubMed]
- Priore, P.; Siculella, L.; Gnoni, G.V. Extra virgin olive oil phenols down-regulate lipid synthesis in primary-cultured rat-hepatocytes. J. Nutr. Biochem. 2014, 25, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Miceli, C.; Santin, Y.; Manzella, N.; Coppini, R.; Berti, A.; Stefani, M.; Parini, A.; Mialet-Perez, J.; Nediani, C. Oleuropein aglycone protects against MAO-a-induced autophagy impairment and cardiomyocyte death through activation of TFEB. Oxidative Med. Cell. Longev. 2018, 2018, 8067592. [Google Scholar] [CrossRef] [PubMed]
- Rigacci, S.; Miceli, C.; Nediani, C.; Berti, A.; Cascella, R.; Pantano, D.; Nardiello, P.; Luccarini, I.; Casamenti, F.; Stefani, M. Oleuropein aglycone induces autophagy via the AMPK/mTOR signalling pathway: A mechanistic insight. Oncotarget 2015, 6, 35344–35357. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, A.; Sabesan, M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology 2008, 16, 96–99. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Xu, J.; Lu, Y.; Jiang, J.; Wang, L.; Shen, H.M.; Xia, D. Curcumin targets the TFEB-lysosome pathway for induction of autophagy. Oncotarget 2016, 7, 75659–75671. [Google Scholar] [CrossRef] [Green Version]
- Shakeri, A.; Cicero, A.F.G.; Panahi, Y.; Mohajeri, M.; Sahebkar, A. Curcumin: A naturally occurring autophagy modulator. J. Cell. Physiol. 2019, 234, 5643–5654. [Google Scholar] [CrossRef]
- Saljoughian, M. Curcumin: A promising antiamyloidogenic agent. U.S. Pharm. 2011, 36, 27–32. [Google Scholar]
- Zheng, J.; Cheng, J.; Zheng, S.; Feng, Q.; Xiao, X. Curcumin, a polyphenolic curcuminoid with its protective effects and molecular mechanisms in diabetes and diabetic cardiomyopathy. Front. Pharmacol. 2018, 9, 472. [Google Scholar] [CrossRef]
- Yang, F.; Lim, G.P.; Begum, A.N.; Ubeda, O.J.; Simmons, M.R.; Ambegaokar, S.S.; Chen, P.; Kayed, R.; Glabe, C.G.; Frautschy, S.A.; et al. Curcumin inhibits formation of amyloid β oligomers and fibrils, binds plaques, and reduces amyloid in vivo. J. Biol. Chem. 2005, 280, 5892–5901. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Paital, B.; Jena, S.; Swain, S.S.; Kumar, S.; Yadav, M.K.; Chainy, G.B.N.; Samanta, L. Possible activation of NRF2 by Vitamin E/Curcumin against altered thyroid hormone induced oxidative stress via NFĸB/AKT/mTOR/KEAP1 signalling in rat heart. Sci. Rep. 2019, 9, 7408. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Jia, N.; Wang, W.; Jin, H.; Xu, J.; Hu, H. Activation of SIRT1 by curcumin blocks the neurotoxicity of amyloid-β25–35 in rat cortical neurons. Biochem. Biophys. Res. Commun. 2014, 448, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Zhang, X.; Teng, Z.; Zhang, T.; Li, Y. Downregulation of PI3K/Akt/mTOR signaling pathway in curcumin-induced autophagy in APP/PS1 double transgenic mice. Eur. J. Pharmacol. 2014, 740, 312–320. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, C.; Xu, P.; Dang, R.; Cai, H.; Liao, D.; Yang, M.; Feng, Q.; Yan, X.; Jiang, P. Curcumin Activates AMPK Pathway and Regulates Lipid Metabolism in Rats Following Prolonged Clozapine Exposure. Front. Neurosci. 2017, 11, 558. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Chen, H.; Lu, W.; Wu, Y.; Wu, X.; Xia, D.; Zhu, J. Myricetin Induces Protective Autophagy by Inhibiting the Phosphorylation of mTOR in HepG2 Cells. Anat. Rec. 2018, 301, 786–795. [Google Scholar] [CrossRef]
- Akindehin, S.; Jung, Y.S.; Kim, S.N.; Son, Y.H.; Lee, I.; Seong, J.K.; Jeong, H.W.; Lee, Y.H. Myricetin exerts anti-obesity effects through upregulation of SIRT3 in adipose tissue. Nutrients 2018, 10, 1962. [Google Scholar] [CrossRef]
- Jung, H.Y.; Lee, D.; Ryu, H.G.; Choi, B.H.; Go, Y.; Lee, N.; Lee, D.; Son, H.G.; Jeon, J.; Kim, S.H.; et al. Myricetin improves endurance capacity and mitochondrial density by activating SIRT1 and PGC-1α. Sci. Rep. 2017, 7, 6237. [Google Scholar] [CrossRef]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A induces mitophagy and prolongs lifespan in C. elegans and increases muscle function in rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Huang, J.; Xu, B.; Ou, Z.; Zhang, L.; Lin, X.; Ye, X.; Kong, X.; Long, D.; Sun, X.; et al. Urolithin A attenuates memory impairment and neuroinflammation in APP/PS1 mice. J. Neuroinflammation 2019, 16, 62. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Hirohata, M.; Yamada, M. Ferulic acid destabilizes preformed β-amyloid fibrils in vitro. Biochem. Biophys. Res. Commun. 2005, 336, 444–449. [Google Scholar] [CrossRef] [PubMed]
- Maurya, D.K.; Devasagayam, T.P.A. Antioxidant and prooxidant nature of hydroxycinnamic acid derivatives ferulic and caffeic acids. Food Chem. Toxicol. 2010, 48, 3369–3373. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.J.; Jung, J.S.; Kim, T.K.; Hasan, M.A.; Hong, C.W.; Nam, J.S.; Song, D.K. Protective effects of ferulic acid in amyloid precursor protein plus presenilin-1 transgenic mouse model of Alzheimer disease. Biol. Pharm. Bull. 2013, 36, 140–143. [Google Scholar] [CrossRef] [PubMed]
- Bian, Z.; Furuya, N.; Zheng, D.M.; Trejo, J.A.O.; Tada, N.; Ezaki, J.; Ueno, T. Ferulic acid induces mammalian target of rapamycin inactivation in cultured mammalian cells. Biol. Pharm. Bull. 2013, 36, 120–124. [Google Scholar] [CrossRef]
- Chen, Y.; Zheng, R.; Jia, Z.; Ju, Y. Flavonoids as superoxide scavengers and antioxidants. Free Radic. Biol. Med. 1990, 9, 19–21. [Google Scholar] [CrossRef]
- Cho, H.-I.; Park, J.-H.; Choi, H.-S.; Kwak, J.H.; Lee, D.-U.; Lee, S.K.; Lee, S.-M. Protective Mechanisms of Acacetin against d-Galactosamine and Lipopolysaccharide-Induced Fulminant Hepatic Failure in Mice. J. Nat. Prod. 2014, 77, 2497–2503. [Google Scholar] [CrossRef]
- Wang, X.; Perumalsamy, H.; Kwon, H.W.; Na, Y.E.; Ahn, Y.J. Effects and possible mechanisms of action of acacetin on the behavior and eye morphology of Drosophila models of Alzheimer’s disease. Sci. Rep. 2015, 5, 16127. [Google Scholar] [CrossRef]
- Chang, W.; Wu, Q.Q.; Xiao, Y.; Jiang, X.H.; Yuan, Y.; Zeng, X.F.; Tang, Q.Z. Acacetin protects against cardiac remodeling after myocardial infarction by mediating MAPK and PI3K/Akt signal pathway. J. Pharmacol. Sci. 2017, 135, 156–163. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Holscher, C. Therapeutic Potential of Baicalein in Alzheimer’s Disease and Parkinson’s Disease. CNS Drugs 2017, 31, 639–652. [Google Scholar] [CrossRef]
- Lee, H.J.; Noh, Y.H.; Lee, D.Y.; Kim, Y.S.; Kim, K.Y.; Chung, Y.H.; Lee, W.B.; Kim, S.S. Baicalein attenuates 6-hydroxydopamine-induced neurotoxicity in SH-SY5Y cells. Eur. J. Cell Biol. 2005, 84, 897–905. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Obregon, D.; Ehrhart, J.; Deng, J.; Tian, J.; Hou, H.; Giunta, B.; Sawmiller, D.; Tan, J. Baicalein reduces β-amyloid and promotes nonamyloidogenic amyloid precursor protein processing in an Alzheimer’s disease transgenic mouse model. J. Neurosci. Res. 2013, 91, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.H.; Ardah, M.T.; Durairajan, S.S.K.; Liu, L.F.; Xie, L.X.; Fong, W.F.D.; Hasan, M.Y.; Huang, J.D.; El-Agnaf, O.M.A.; Li, M. Baicalein Inhibits Formation of α-Synuclein Oligomers within Living Cells and Prevents Aβ Peptide Fibrillation and Oligomerisation. ChemBioChem 2011, 12, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, J.; Wang, K.S.; Mi, C.; Wang, Z.; Piao, L.X.; Xu, G.H.; Li, X.; Lee, J.J.; Jin, X. Baicalein inhibits TNF-α-induced NF-κB activation and expression of NF-κB-regulated target gene products. Oncol. Rep. 2016, 36, 2771–2776. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Wu, J.; Xu, K.; Cai, F.; Gu, J.; Ma, L.; Chen, J. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. J. Neurochem. 2010, 112, 1500–1512. [Google Scholar] [CrossRef]
- Gu, X.H.; Xu, L.J.; Liu, Z.Q.; Wei, B.; Yang, Y.J.; Xu, G.G.; Yin, X.P.; Wang, W. The flavonoid baicalein rescues synaptic plasticity and memory deficits in a mouse model of Alzheimer’s disease. Behav. Brain Res. 2016, 311, 309–321. [Google Scholar] [CrossRef]
- Angeloni, C.; Barbalace, M.C.; Hrelia, S. Icariin and Its Metabolites as Potential Protective Phytochemicals Against Alzheimer’s Disease. Front. Pharmacol. 2019, 10, 271. [Google Scholar] [CrossRef]
- Luo, Y.; Nie, J.; Gong, Q.H.; Lu, Y.F.; Wu, Q.; Shi, J.S. Protective effects of icariin against learning and memory deficits induced by aluminium in rats. Clin. Exp. Pharmacol. Physiol. 2007, 34, 792–795. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, L.; Chen, Z.B.; Wu, J.Y.; Zhang, X.; Xu, Y. Icariin enhances neuronal survival after oxygen and glucose deprivation by increasing SIRT1. Eur. J. Pharmacol. 2009, 609, 40–44. [Google Scholar] [CrossRef]
- Li, W.W.; Gao, X.M.; Wang, X.M.; Guo, H.; Zhang, B.L. Icariin inhibits hydrogen peroxide-induced toxicity through inhibition of phosphorylation of JNK/p38 MAPK and p53 activity. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2011, 708, 1–10. [Google Scholar] [CrossRef]
- Shi, D.B.; Li, X.X.; Zheng, H.T.; Li, D.W.; Cai, G.X.; Peng, J.J.; Gu, W.L.; Guan, Z.Q.; Xu, Y.; Cai, S.J. Icariin-Mediated Inhibition of NF-κB Activity Enhances the In Vitro and In Vivo Antitumour Effect of 5-Fluorouracil in Colorectal Cancer. Cell Biochem. Biophys. 2014, 69, 523–530. [Google Scholar] [CrossRef]
- Li, F.; Dong, H.X.; Gong, Q.H.; Wu, Q.; Jin, F.; Shi, J.S. Icariin decreases both APP and Aβ levels and increases neurogenesis in the brain of Tg2576 mice. Neuroscience 2015, 304, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.X.; Miao, J.Y.; Qiang, M.; He, R.Q.; Wang, X.M.; Li, W.W. Icariin protects SH-SY5Y cells from formaldehyde-induced injury through suppression of Tau phosphorylation. Chin. J. Integr. Med. 2016, 22, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Xu, P.; Zhou, K.; Deng, D.; Zhang, C.; Wang, Z. Icariin Attenuates Synaptic and Cognitive Deficits in an Aβ1–42-Induced Rat Model of Alzheimer’s Disease. Biomed. Res. Int. 2017, 2017, 7464872. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Ohizumi, Y. Potential benefits of nobiletin, a citrus flavonoid, against Alzheimer’s disease and Parkinson’s disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, H.; Zhang, X.; Chen, L.; Zhao, X.; Bai, X.; Zhang, J. Nobiletin protects against cerebral ischemia via activating the p-Akt, p-CREB, BDNF and Bcl-2 pathway and ameliorating BBB permeability in rat. Brain Res. Bull. 2013, 96, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoi, T.; Ohizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Aβ levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Zhang, C.; Bai, X.; Zhang, J.; Zhao, X.; Chen, L.; Wang, L.; Zhu, C.; Cui, L.; et al. Nobiletin promotes antioxidant and anti-inflammatory responses and elicits protection against ischemic stroke in vivo. Brain Res. 2016, 1636, 130–141. [Google Scholar] [CrossRef]
- Pierzynowska, K.; Podlacha, M.; Gaffke, L.; Majkutewicz, I.; Mantej, J.; Węgrzyn, A.; Osiadły, M.; Myślińska, D.; Węgrzyn, G. Autophagy-dependent mechanism of genistein-mediated elimination of behavioral and biochemical defects in the rat model of sporadic Alzheimer’s disease. Neuropharmacology 2019, 148, 332–346. [Google Scholar] [CrossRef]
- Kazi, A.; Daniel, K.G.; Smith, D.M.; Kumar, N.B.; Dou, Q.P. Inhibition of the proteasome activity, a novel mechanism associated with the tumor cell apoptosis-inducing ability of genistein. Biochem. Pharmacol. 2003, 66, 965–976. [Google Scholar] [CrossRef]
- Borrás, C.; Gambini, J.; Gómez-Cabrera, M.C.; Sastre, J.; Pallardó, F.V.; Mann, G.E.; Viña, J. Genistein, a soy isoflavone, up-regulates expression of antioxidant genes: Involvement of estrogen receptors, ERK1/2, and NFκB. FASEB J. 2006, 20, E1476–E1481. [Google Scholar] [CrossRef]
- Moskot, M.; Montefusco, S.; Jakóbkiewicz-Banecka, J.; Mozolewski, P.; Wȩgrzyn, A.; Di Bernardo, D.; Wȩgrzyn, G.; Medina, D.L.; Ballabio, A.; Gabig-Cimińska, M. The phytoestrogen genistein modulates lysosomal metabolism and Transcription Factor EB (TFEB) activation. J. Biol. Chem. 2014, 289, 17054–17069. [Google Scholar] [CrossRef] [PubMed]
- Kwon, Y. Luteolin as a potential preventive and therapeutic candidate for Alzheimer’s disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Xu, Y.; Cao, M.; Huan, Y.; Zhu, L.; Jiang, Y.; Shen, W.; Zhu, G. Luteolin Induces Apoptosis and Autophagy in Mouse Macrophage ANA-1 Cells via the Bcl-2 Pathway. J. Immunol. Res. 2018, 2018, 4623919. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Li, D.; Pan, H.; Chen, D.; Qi, L.; Zhang, R.; Sun, H. Luteolin inhibits apoptosis and improves cardiomyocyte contractile function through the PI3K/Akt pathway in simulated ischemia/reperfusion. Pharmacology 2011, 88, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Nunes, C.; Almeida, L.; Barbosa, R.M.; Laranjinha, J. Luteolin suppresses the JAK/STAT pathway in a cellular model of intestinal inflammation. Food Funct. 2017, 8, 387–396. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin Promotes Cell Apoptosis by Inducing Autophagy in Hepatocellular Carcinoma. Cell. Physiol. Biochem. 2018, 43, 1803–1812. [Google Scholar] [CrossRef]
- Feng, S.T.; Wang, Z.Z.; Yuan, Y.H.; Sun, H.M.; Chen, N.H.; Zhang, Y. Mangiferin: A multipotent natural product preventing neurodegeneration in Alzheimer’s and Parkinson’s disease models. Pharm. Res. 2019, 146, 104336. [Google Scholar] [CrossRef]
- Andreu, G.P.; Delgado, R.; Velho, J.A.; Curti, C.; Vercesi, A.E. Iron complexing activity of mangiferin, a naturally occurring glucosylxanthone, inhibits mitochondrial lipid peroxidation induced by Fe2+-citrate. Eur. J. Pharmacol. 2005, 513, 47–55. [Google Scholar] [CrossRef]
- Das, J.; Ghosh, J.; Roy, A.; Sil, P.C. Mangiferin exerts hepatoprotective activity against D-galactosamine induced acute toxicity and oxidative/nitrosative stress via Nrf2-NFκB pathways. Toxicol. Appl. Pharmacol. 2012, 260, 35–47. [Google Scholar] [CrossRef]
- Kasbe, P.; Jangra, A.; Lahkar, M. Mangiferin ameliorates aluminium chloride-induced cognitive dysfunction via alleviation of hippocampal oxido-nitrosative stress, proinflammatory cytokines and acetylcholinesterase level. J. Trace Elem. Med. Biol. 2015, 31, 107–112. [Google Scholar] [CrossRef]
- Jung, J.-S.; Jung, K.; Kim, D.-H.; Kim, H.-S. Selective inhibition of MMP-9 gene expression by mangiferin in PMA-stimulated human astroglioma cells: Involvement of PI3K/Akt and MAPK signaling pathways. Pharmacol. Res. 2012, 66, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Wanka, L.; Iqbal, K.; Schreiner, P.R. The lipophilic bullet hits the targets: Medicinal chemistry of adamantane derivatives. Chem. Rev. 2013, 113, 3516–3604. [Google Scholar] [CrossRef] [PubMed]
- Shrivastava, S.K.; Sinha, S.K.; Srivastava, P.; Tripathi, P.N.; Sharma, P.; Tripathi, M.K.; Tripathi, A.; Choubey, P.K.; Waiker, D.K.; Aggarwal, L.M.; et al. Design and development of novel p-aminobenzoic acid derivatives as potential cholinesterase inhibitors for the treatment of Alzheimer’s disease. Bioorg. Chem. 2019, 82, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Talesa, V.N. Acetylcholinesterase in Alzheimer’s disease. Mech. Ageing Dev. 2001, 122, 1961–1969. [Google Scholar] [CrossRef]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for Alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef]
- O’Brien, R.J.; Wong, P.C. Amyloid precursor protein processing and Alzheimer’s disease. Annu. Rev. Neurosci. 2011, 34, 185–204. [Google Scholar] [CrossRef]
- Thinakaran, G.; Koo, E.H. Amyloid precursor protein trafficking, processing, and function. J. Biol. Chem. 2008, 283, 29615–29619. [Google Scholar] [CrossRef]
- Morgan, C.; Colombres, M.; Nuñez, M.T.; Inestrosa, N.C. Structure and function of amyloid in Alzheimer’s disease. Prog. Neurobiol. 2004, 74, 323–349. [Google Scholar] [CrossRef]
- Kojro, E.; Fahrenholz, F. The non-amyloidogenic pathway: Structure and function of alpha-secretases. Sub-Cell. Biochem. 2005, 38, 105–127. [Google Scholar]
- Menting, K.W.; Claassen, J.A.H.R. β-secretase inhibitor; a promising novel therapeutic drug in Alzheimer’s Disease. Front. Aging Neurosci. 2014, 6, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Cole, S.L.; Vassar, R. The Alzheimer’s disease β-secretase enzyme, BACE1. Mol. Neurodegener. 2007, 2, 22. [Google Scholar] [CrossRef] [PubMed]
- Farzan, M.; Schnitzler, C.E.; Vasilieva, N.; Leung, D.; Choe, H. BACE2, a β-secretase homolog, cleaves at the β site and within the amyloid-β region of the amyloid-β precursor protein. Proc. Natl. Acad. Sci. USA 2000, 97, 9712–9717. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Wang, Y.; McCarthy, D.; Wen, H.; Borchelt, D.R.; Price, D.L.; Wong, P.C. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat. Neurosci. 2001, 4, 233–234. [Google Scholar] [CrossRef]
- Yu, N.; Hayik, S.A.; Wang, B.; Liao, N.; Reynolds, C.H.; Merz, K.M., Jr. Assigning the protonation states of the key aspartates in β-secretase using QM/MM X-ray structure refinement. J. Chem. Theory Comput. 2006, 2, 1057–1069. [Google Scholar] [CrossRef]
- Hernández-Rodríguez, M.; Correa-Basurto, J.; Gutiérrez, A.; Vitorica, J.; Rosales-Hernández, M.C. Asp32 and Asp228 determine the selective inhibition of BACE1 as shown by docking and molecular dynamics simulations. Eur. J. Med. Chem. 2016, 124, 1142–1154. [Google Scholar] [CrossRef]
- Sabbah, D.A.; Zhong, H.A. Modeling the protonation states of β-secretase binding pocket by molecular dynamics simulations and docking studies. J. Mol. Graph. Model. 2016, 68, 206–215. [Google Scholar] [CrossRef]
- Hong, L.; Turner Iii, R.T.; Koelsch, G.; Shin, D.; Ghosh, A.K.; Tang, J. Crystal structure of memapsin 2 (β-secretase) in complex with an inhibitor OM00-3. Biochemistry 2002, 41, 10963–10967. [Google Scholar] [CrossRef]
- Shuto, D.; Kasai, S.; Kimura, T.; Liu, P.; Hidaka, K.; Hamada, T.; Shibakawa, S.; Hayashi, Y.; Hattori, C.; Szabo, B.; et al. KMI-008, a novel β-Secretase inhibitor containing a hydroxymethylcarbonyl isostere as a transition-State mimic: Design and synthesis of substrate-based octapeptides. Bioorg. Med. Chem. Lett. 2003, 13, 4273–4276. [Google Scholar] [CrossRef]
- Dineen, T.A.; Weiss, M.M.; Williamson, T.; Acton, P.; Babu-Khan, S.; Bartberger, M.D.; Brown, J.; Chen, K.; Cheng, Y.; Citron, M.; et al. Design and synthesis of potent, orally efficacious hydroxyethylamine derived β-site amyloid precursor protein cleaving enzyme (BACE1) inhibitors. J. Med. Chem. 2012, 55, 9025–9044. [Google Scholar] [CrossRef]
- Huang, W.H.; Sheng, R.; Hu, Y.Z. Progress in the development of nonpeptidomimetic BACE 1 inhibitors for Alzheimer’s disease. Curr. Med. Chem. 2009, 16, 1806–1820. [Google Scholar] [CrossRef] [PubMed]
- Vega-Hissi, E.G.; Tosso, R.; Enriz, R.D.; Gutierrez, L.J. Molecular insight into the interaction mechanisms of amino-2H-imidazole derivatives with BACE1 protease: A QM/MM and QTAIM study. Int. J. Quantum Chem. 2015, 115, 389–397. [Google Scholar] [CrossRef]
- Hansen, M.M.; Jarmer, D.J.; Arslantas, E.; DeBaillie, A.C.; Frederick, A.L.; Harding, M.; Hoard, D.W.; Hollister, A.; Huber, D.; Kolis, S.P.; et al. Synthesis of BACE Inhibitor LY2886721. Part II. Isoxazolidines as Precursors to Chiral Aminothiazines, Selective Peptide Coupling, and a Controlled Reactive Crystallization. Org. Process Res. Dev. 2015, 19, 1214–1230. [Google Scholar] [CrossRef]
- Stauffer, S.R.; Graham, S.L. Bicyclic Spiropiperidine Beta-Secretase Inhibitors for the Treatment of Alzheimer’s Disease. U.S. Patent No 8,377,954, 2013. [Google Scholar]
- Van Es, J.H.; Van Gijn, M.E.; Riccio, O.; Van Den Born, M.; Vooijs, M.; Begthel, H.; Cozijnsen, M.; Robine, S.; Winton, D.J.; Radtke, F.; et al. Notch/γ-secretase inhibition turns proliferative cells in intestinal crypts and adenomas into goblet cells. Nature 2005, 435, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Grill, J.D.; Cummings, J.L. Current therapeutic targets for the treatment of Alzheimer’s disease. Expert Rev. Neurother. 2010, 10, 711–728. [Google Scholar] [CrossRef] [PubMed]
- Mayer, S.C.; Kreft, A.F.; Harrison, B.; Abou-Gharbia, M.; Antane, M.; Aschmies, S.; Atchison, K.; Chlenov, M.; Cole, D.C.; Comery, T.; et al. Discovery of begacestat, a Notch-1-sparing γ-secretase inhibitor for the treatment of Alzheimer’s disease. J. Med. Chem. 2008, 51, 7348–7351. [Google Scholar] [CrossRef]
- Brahmachari, S.; Paul, A.; Segal, D.; Gazit, E. Inhibition of amyloid oligomerization into different supramolecular architectures by small molecules: Mechanistic insights and design rules. Future Med. Chem. 2017, 9, 797–810. [Google Scholar] [CrossRef]
- Aisen, P.A.; Mehran, M.; Poole, R. Clinical data on Alzhemed after 12 months of treatment in patients with mild to moderate Alzheimer’s disease. Neurobiol Aging 2004, 25, S20. [Google Scholar] [CrossRef]
- Coman, H.; Nemeş, B. New Therapeutic Targets in Alzheimer’s Disease. Int. J. Gerontol. 2017, 11, 2–6. [Google Scholar] [CrossRef]
- Lannfelt, L.; Blennow, K.; Zetterberg, H.; Batsman, S.; Ames, D.; Harrison, J.; Masters, C.L.; Targum, S.; Bush, A.I.; Murdoch, R.; et al. Safety, efficacy, and biomarker findings of PBT2 in targeting Aβ as a modifying therapy for Alzheimer’s disease: A phase IIa, double-blind, randomised, placebo-controlled trial. Lancet Neurol. 2008, 7, 779–786. [Google Scholar] [CrossRef]
- Wang, J.; Ho, L.; Zhao, W.; Ono, K.; Rosensweig, C.; Chen, L.; Humala, N.; Teplow, D.B.; Pasinetti, G.M. Grape-derived polyphenolics prevent Aβ oligomerization and attenuate cognitive deterioration in a mouse model of Alzheimer’s disease. J. Neurosci. 2008, 28, 6388–6392. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Liu, N.; Wang, C.; Qin, B.; Zhou, Y.; Xiao, M.; Chang, L.; Yan, L.J.; Zhao, B. Role of RAGE in Alzheimer’s Disease. Cell. Mol. Neurobiol. 2016, 36, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Orgogozo, J.M.; Gilman, S.; Dartigues, J.F.; Laurent, B.; Puel, M.; Kirby, L.C.; Jouanny, P.; Dubois, B.; Eisner, L.; Flitman, S.; et al. Subacute meningoencephalitis in a subset of patients with AD after Aβ42 immunization. Neurology 2003, 61, 46–54. [Google Scholar] [CrossRef] [PubMed]
- Joseph-Mathurin, N.; Dorieux, O.; Trouche, S.G.; Boutajangout, A.; Kraska, A.; Fontès, P.; Verdier, J.M.; Sigurdsson, E.M.; Mestre-Francés, N.; Dhenain, M. Aβ immunization worsens iron deposits in the choroid plexus and cerebral microbleeds. Neurobiol. Aging 2013, 34, 2613–2622. [Google Scholar] [CrossRef]
- Friedhoff, P.; Schneider, A.; Mandelkow, E.M.; Mandelkow, E. Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry 1998, 37, 10223–10230. [Google Scholar] [CrossRef]
- Kadavath, H.; Hofele, R.V.; Biernat, J.; Kumar, S.; Tepper, K.; Urlaub, H.; Mandelkow, E.; Zweckstetter, M. Tau stabilizes microtubules by binding at the interface between tubulin heterodimers. Proc. Natl. Acad. Sci. USA 2015, 112, 7501–7506. [Google Scholar] [CrossRef] [Green Version]
- Ittner, L.M.; Götz, J. Amyloid-β and tau - a toxic pas de deux in Alzheimer’s disease. Nat. Rev. Neurosci. 2011, 12, 67–72. [Google Scholar] [CrossRef]
- Šimić, G.; Babić Leko, M.; Wray, S.; Harrington, C.; Delalle, I.; Jovanov-Milošević, N.; Bažadona, D.; Buée, L.; de Silva, R.; Giovanni, G.D.; et al. Tau protein hyperphosphorylation and aggregation in Alzheimer’s disease and other tauopathies, and possible neuroprotective strategies. Biomolecules 2016, 6, 6. [Google Scholar] [CrossRef]
- Iqbal, K.; Liu, F.; Gong, C.X. Tau and neurodegenerative disease: The story so far. Nat. Rev. Neurol. 2016, 12, 15–27. [Google Scholar] [CrossRef]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Transl. Med. 2015, 3, 10. [Google Scholar]
- Liu, S.L.; Wang, C.; Jiang, T.; Tan, L.; Xing, A.; Yu, J.T. The Role of Cdk5 in Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 4328–4342. [Google Scholar] [CrossRef] [PubMed]
- Noble, W.; Olm, V.; Takata, K.; Casey, E.; Mary, O.; Meyerson, J.; Gaynor, K.; LaFrancois, J.; Wang, L.; Kondo, T.; et al. Cdk5 is a key factor in tau aggregation and tangle formation in vivo. Neuron 2003, 38, 555–565. [Google Scholar] [CrossRef]
- Shupp, A.; Casimiro, M.C.; Pestell, R.G. Biological functions of CDK5 and potential CDK5 targeted clinical treatments. Oncotarget 2017, 8, 17373–17382. [Google Scholar] [CrossRef] [PubMed]
- Benson, C.; White, J.; De Bono, J.; O’Donnell, A.; Raynaud, F.; Cruickshank, C.; McGrath, H.; Walton, M.; Workman, P.; Kaye, S.; et al. A phase I trial of the selective oral cyclin-dependent kinase inhibitor seliciclib (CYC202; R-Roscovitine), administered twice daily for 7 days every 21 days. Br. J. Cancer 2007, 96, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Sabio, G.; González-Polo, R.A.; Cuenda, A.; Alessi, D.R.; Alonso, J.C.; Fuentes, J.M.; Soler, G.; Centeno, F. Lithium inhibits caspase 3 activation and dephosphorylation of PKB and GSK3 induced by K+ deprivation in cerebellar granule cells. J. Neurochem. 2001, 78, 199–206. [Google Scholar] [CrossRef]
- Martinez, A.; Castro, A.; Dorronsoro, I.; Alonso, M. Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Med. Res. Rev. 2002, 22, 373–384. [Google Scholar] [CrossRef]
- Meijer, L.; Skaltsounis, A.L.; Magiatis, P.; Polychronopoulos, P.; Knockaert, M.; Leost, M.; Ryan, X.P.; Vonica, C.A.; Brivanlou, A.; Dajani, R.; et al. GSK-3-Selective Inhibitors Derived from Tyrian Purple Indirubins. Chem. Biol. 2003, 10, 1255–1266. [Google Scholar] [CrossRef] [Green Version]
- King, M.K.; Pardo, M.; Cheng, Y.; Downey, K.; Jope, R.S.; Beurel, E. Glycogen synthase kinase-3 inhibitors: Rescuers of cognitive impairments. Pharmacol. Ther. 2014, 141, 1–12. [Google Scholar] [CrossRef]
- Wan, Y.; Hur, W.; Cho, C.Y.; Liu, Y.; Adrian, F.J.; Lozach, O.; Bach, S.; Mayer, T.; Fabbro, D.; Meijer, L.; et al. Synthesis and target identification of hymenialdisine analogs. Chem. Biol. 2004, 11, 247–259. [Google Scholar] [CrossRef]
- Kontsekova, E.; Zilka, N.; Kovacech, B.; Novak, P.; Novak, M. First-in-man tau vaccine targeting structural determinants essential for pathological tau-tau interaction reduces tau oligomerisation and neurofibrillary degeneration in an Alzheimer’s disease model. Alzheimer’s Res. Ther. 2014, 6, 44. [Google Scholar] [CrossRef]
- Novak, P.; Schmidt, R.; Kontsekova, E.; Zilka, N.; Kovacech, B.; Skrabana, R.; Vince-Kazmerova, Z.; Katina, S.; Fialova, L.; Prcina, M.; et al. Safety and immunogenicity of the tau vaccine AADvac1 in patients with Alzheimer’s disease: A randomised, double-blind, placebo-controlled, phase 1 trial. Lancet Neurol. 2017, 16, 123–134. [Google Scholar] [CrossRef]
- Boyles, J.K.; Zoellner, C.D.; Anderson, L.J.; Kosik, L.M.; Pitas, R.E.; Weisgraber, K.H.; Hui, D.Y.; Mahley, R.W.; Gebicke-Haerter, P.J.; Ignatius, M.J.; et al. A role for apolipoprotein E, apolipoprotein A-I, and low density lipoprotein receptors in cholesterol transport during regeneration and remyelination of the rat sciatic nerve. J. Clin. Investig. 1989, 83, 1015–1031. [Google Scholar] [CrossRef] [PubMed]
- Michaelson, D.M. APOE ε4: The most prevalent yet understudied risk factor for Alzheimer’s disease. Alzheimer Dement. 2014, 10, 861–868. [Google Scholar] [CrossRef]
- Wisniewski, T.; Golabek, A.; Matsubara, E.; Ghiso, J.; Frangione, B. Apolipoprotein E: Binding to Soluble Alzheimer′s β-Amyloid. Biochem. Biophys. Res. Commun. 1993, 192, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Galpern, W.R.; Lang, A.E. Interface between tauopathies and synucleinopathies: A tale of two proteins. Ann. Neurol. 2006, 59, 449–458. [Google Scholar] [CrossRef]
- Hashimoto, M.; Yasuda, M.; Tanimukai, S.; Matsui, M.; Hirono, N.; Kazui, H.; Mori, E. Apolipoprotein E ε4 and the pattern of regional brain atrophy in Alzheimer’s disease. Neurology 2001, 57, 1461–1466. [Google Scholar] [CrossRef]
- Giau, V.V.; Bagyinszky, E.; An, S.S.A.; Kim, S.Y. Role of apolipoprotein E in neurodegenerative diseases. Neuropsychiatr. Dis. Treat. 2015, 11, 1723–1737. [Google Scholar] [CrossRef]
- Mahley, R.W.; Weisgraber, K.H.; Huang, Y. Apolipoprotein E4: A causative factor and therapeutic target in neuropathology, including Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2006, 103, 5644–5651. [Google Scholar] [CrossRef]
- Ungar, L.; Altmann, A.; Greicius, M.D. Apolipoprotein E, gender, and Alzheimer’s disease: An overlooked, but potent and promising interaction. Brain Imaging Behav. 2014, 8, 262–273. [Google Scholar] [CrossRef]
- Praticò, D. Oxidative stress hypothesis in Alzheimer’s disease: A reappraisal. Trends Pharmacol. Sci. 2008, 29, 609–615. [Google Scholar] [CrossRef]
- Lin, M.T.; Beal, M.F. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 2006, 443, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Kurz, T.; Terman, A.; Brunk, U.T. Autophagy, ageing and apoptosis: The role of oxidative stress and lysosomal iron. Arch. Biochem. Biophys. 2007, 462, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Petranovic, D. Amyloid-ß peptide-induced cytotoxicity and mitochondrial dysfunction in yeast. FEMS Yeast Res. 2015, 15, 6. [Google Scholar] [CrossRef] [PubMed]
- Rizzi, F.; Trougakos, I.P.; Pintus, G.; Sykiotis, G.P. Redox Status and Proteostasis in Ageing and Disease. Oxidative Med. Cell. Longev. 2016, 2016. [Google Scholar] [CrossRef] [PubMed]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal 2014, 20, 1126–1167. [Google Scholar] [CrossRef] [PubMed]
- Kandola, K.; Bowman, A.; Birch-Machin, M.A. Oxidative stress—A key emerging impact factor in health, ageing, lifestyle and aesthetics. Int. J. Cosmet. Sci. 2015, 37, 1–8. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, A. A review on mitochondrial restorative mechanism of antioxidants in Alzheimer’s disease and other neurological conditions. Front. Pharmacol. 2015, 6, 206. [Google Scholar] [CrossRef]
- Caine, J.; Sankovich, S.; Antony, H.; Waddington, L.; Macreadie, P.; Varghese, J.; Macreadie, I. Alzheimer’s Aβ fused to green fluorescent protein induces growth stress and a heat shock response. FEMS Yeast Res. 2007, 7, 1230–1236. [Google Scholar] [CrossRef]
- Deibel, M.A.; Ehmann, W.D.; Markesbery, W.R. Copper, iron, and zinc imbalances in severely degenerated brain regions in Alzheimer’s disease: Possible relation to oxidative stress. J. Neurol. Sci. 1996, 143, 137–142. [Google Scholar] [CrossRef]
- Tomljenovic, L. Aluminum and Alzheimer’s disease: After a century of controversy, is there a plausible link? J. Alzheimer Dis. 2011, 23, 567–598. [Google Scholar] [CrossRef]
- Karlsson, M.; Frennesson, C.; Gustafsson, T.; Brunk, U.T.; Nilsson, S.E.G.; Kurz, T. Autophagy of iron-binding proteins may contribute to the oxidative stress resistance of ARPE-19 cells. Exp. Eye Res. 2013, 116, 359–365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhao, B. Oxidative stress and the pathogenesis of Alzheimer’s disease. Oxidative Med. Cell. Longev. 2013. [Google Scholar] [CrossRef]
- Reddy, P.H.; Beal, M.F. Amyloid beta, mitochondrial dysfunction and synaptic damage: Implications for cognitive decline in aging and Alzheimer’s disease. Trends Mol. Med. 2008, 14, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Anekonda, T.S. Resveratrol-a boon for treating Alzheimer’s disease? Brain Res. Rev. 2006, 52, 316–326. [Google Scholar] [CrossRef]
- Khan, H.; Marya; Amin, S.; Kamal, M.A.; Patel, S. Flavonoids as acetylcholinesterase inhibitors: Current therapeutic standing and future prospects. Biomed. Pharmacother. 2018, 101, 860–870. [Google Scholar] [CrossRef]
- Ding, X.; Ouyang, M.A.; Liu, X.; Wang, R.Z. Acetylcholinesterase inhibitory activities of flavonoids from the leaves of Ginkgo biloba against brown planthopper. J. Chem. 2013, 2013, 645086. [Google Scholar] [CrossRef]
- Cox, C.J.; Choudhry, F.; Peacey, E.; Perkinton, M.S.; Richardson, J.C.; Howlett, D.R.; Lichtenthaler, S.F.; Francis, P.T.; Williams, R.J. Dietary (-)-epicatechin as a potent inhibitor of βγ-secretase amyloid precursor protein processing. Neurobiol. Aging 2015, 36, 178–187. [Google Scholar] [CrossRef]
- Kook, S.Y.; Lee, K.M.; Kim, Y.; Cha, M.Y.; Kang, S.; Baik, S.H.; Lee, H.; Park, R.; Mook-Jung, I. High-dose of vitamin C supplementation reduces amyloid plaque burden and ameliorates pathological changes in the brain of 5XFAD mice. Cell Death Dis. 2014, 5, e1083. [Google Scholar] [CrossRef]
- Zhang, L.F.; Zhou, Z.W.; Wang, Z.H.; Du, Y.H.; He, Z.X.; Cao, C.; Zhou, S.F. Coffee and caffeine potentiate the antiamyloidogenic activity of melatonin via inhibition of aβ oligomerization and modulation of the Tau-mediated pathway in N2a/APP cells. Drug Des. Dev. Ther. 2015, 9, 241–272. [Google Scholar]
- Vacek, J.C.; Behera, J.; George, A.K.; Kamat, P.K.; Kalani, A.; Tyagi, N. Tetrahydrocurcumin ameliorates homocysteine-mediated mitochondrial remodeling in brain endothelial cells. J. Cell. Physiol. 2018, 233, 3080–3092. [Google Scholar] [CrossRef] [PubMed]
- Mancino, A.M.; Hindo, S.S.; Kochi, A.; Lim, M.H. Effects of Clioquinol on Metal-Triggered Amyloid-β Aggregation Revisited. Inorg. Chem. 2009, 48, 9596–9598. [Google Scholar] [CrossRef] [PubMed]
- Gomes, L.M.F.; Vieira, R.P.; Jones, M.R.; Wang, M.C.P.; Dyrager, C.; Souza-Fagundes, E.M.; Da Silva, J.G.; Storr, T.; Beraldo, H. 8-Hydroxyquinoline Schiff-base compounds as antioxidants and modulators of copper-mediated Aβ peptide aggregation. J. Inorg. Biochem. 2014, 139, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Liang, S.H.; Southon, A.G.; Fraser, B.H.; Krause-Heuer, A.M.; Zhang, B.; Shoup, T.M.; Lewis, R.; Volitakis, I.; Han, Y.; Greguric, I.; et al. Novel Fluorinated 8-Hydroxyquinoline Based Metal Ionophores for Exploring the Metal Hypothesis of Alzheimer’s Disease. ACS Med. Chem. Lett. 2015, 6, 1025–1029. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.R.; Fillingame, R.H. Regulation of amino acid decarboxylation. Annu. Rev. Biochem. 1974, 43, 303–325. [Google Scholar] [CrossRef]
- Bai, X.; Edden, R.A.E.; Gao, F.; Wang, G.; Wu, L.; Zhao, B.; Wang, M.; Chan, Q.; Chen, W.; Barker, P.B. Decreased γ-aminobutyric acid levels in the parietal region of patients with Alzheimer’s disease. J. Magn. Reson. Imaging 2015, 41, 1326–1331. [Google Scholar] [CrossRef]
- Sternfeld, F.; Carling, R.W.; Jelley, R.A.; Ladduwahetty, T.; Merchant, K.J.; Moore, K.W.; Reeve, A.J.; Street, L.J.; O’Connor, D.; Sohal, B.; et al. Selective, Orally Active γ-Aminobutyric AcidA α5 Receptor Inverse Agonists as Cognition Enhancers. J. Med. Chem. 2004, 47, 2176–2179. [Google Scholar] [CrossRef]
- Lee, J.Y.; Friedman, J.E.; Angel, I.; Kozak, A.; Koh, J.Y. The lipophilic metal chelator DP-109 reduces amyloid pathology in brains of human β-amyloid precursor protein transgenic mice. Neurobiol. Aging 2004, 25, 1315–1321. [Google Scholar] [CrossRef]
- Rose, G.M.; Hopper, A.; De Vivo, M.; Tehim, A. Phosphodiesterase inhibitors for cognitive enhancement. Curr. Pharm. Des. 2005, 11, 3329–3334. [Google Scholar] [CrossRef]
- Li, J.; Liu, C.N.; Wei, N.; Li, X.D.; Liu, Y.Y.; Yang, R.; Jia, Y.J. Protective effects of BAY 73–6691, a selective inhibitor of phosphodiesterase 9, on amyloid-β peptides-induced oxidative stress in in-vivo and in-vitro models of Alzheimer’s disease. Brain Res. 2016, 1642, 327–335. [Google Scholar] [CrossRef]
- Hagen, T.J.; Mo, X.; Burgin, A.B.; Fox Iii, D.; Zhang, Z.; Gurney, M.E. Discovery of triazines as selective PDE4B versus PDE4D inhibitors. Bioorg. Med. Chem. Lett. 2014, 24, 4031–4034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blokland, A.; Schreiber, R.; Prickaerts, J. Improving memory: A role for phosphodiesterases. Curr. Pharm. Des. 2006, 12, 2511–2523. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Ho, L.; Pompl, P.N.; Peng, Y.; Zhao, Z.; Xiang, Z.; Robakis, N.K.; Shioi, J.; Suh, J.; Pasinetti, G.M. Cyclooxygenase (COX)-2 and COX-1 Potentiate β-Amyloid Peptide Generation through Mechanisms That Involve γ-Secretase Activity. J. Biol. Chem. 2003, 278, 50970–50977. [Google Scholar] [CrossRef] [PubMed]
- Hewett, S.J.; Uliasz, T.F.; Vidwans, A.S.; Hewett, J.A. Cyclooxygenase-2 contributes to N-methyl-D-aspartate-mediated neuronal cell death in primary cortical cell culture. J. Pharmacol. Exp. Ther. 2000, 293, 417–425. [Google Scholar] [PubMed]
- Park, S.A.; Chevallier, N.; Tejwani, K.; Hung, M.M.; Maruyama, H.; Golde, T.E.; Koo, E.H. Deficiency in either COX-1 or COX-2 genes does not affect amyloid beta protein burden in amyloid precursor protein transgenic mice. Biochem. Biophys. Res. Commun. 2016, 478, 286–292. [Google Scholar] [CrossRef]
- Brioni, J.D.; Esbenshade, T.A.; Garrison, T.R.; Bitner, S.R.; Cowart, M.D. Discovery of histamine H3 antagonists for the treatment of cognitive disorders and Alzheimer’s disease. J. Pharmacol. Exp. Ther. 2011, 336, 38–46. [Google Scholar] [CrossRef]
- Delay-Goyet, P.; Blanchard, V.; Schussler, N.; Lopez-Grancha, M.; Ménager, J.; Mary, V.; Sultan, E.; Buzy, A.; Guillemot, J.C.; Stemmelin, J.; et al. SAR110894, a potent histamine H3-receptor antagonist, displays disease-modifying activity in a transgenic mouse model of tauopathy. Alzheimer Dement. Transl. Res. Clin. Interv. 2016, 2, 267–280. [Google Scholar] [CrossRef] [Green Version]
- Yun, H.M.; Park, K.R.; Kim, E.C.; Kim, S.; Hong, J.T. Serotonin 6 receptor controls Alzheimer’s disease and depression. Oncotarget 2015, 6, 26716–26728. [Google Scholar] [CrossRef]
- Claeysen, S.; Bockaert, J.; Giannoni, P. Serotonin: A New Hope in Alzheimer’s Disease? Acs Chem. Neurosci. 2015, 6, 940–943. [Google Scholar] [CrossRef]
- Chinetti, G.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors (PPARs): Nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm. Res. 2000, 49, 497–505. [Google Scholar] [CrossRef]
- Braissant, O.; Foufelle, F.; Scotto, C.; Dauça, M.; Wahli, W. Differential expression of peroxisome proliferator-activated receptors (PPARs): Tissue distribution of PPAR-α, -β, and -γ in the adult rat. Endocrinology 1996, 137, 354–366. [Google Scholar] [CrossRef] [PubMed]
- Bordet, R.; Ouk, T.; Petrault, O.; Gele, P.; Gautier, S.; Laprais, M.; Deplanque, D.; Duriez, P.; Staels, B.; Fruchart, J. PPAR: A New Pharmacological Target for Neuroprotection in Stroke and Neurodegenerative Diseases. Biochem Soc Trans. 2006, 34, 1341–1346. [Google Scholar] [CrossRef] [PubMed]
- Combs, C.K.; Johnson, D.E.; Karlo, J.C.; Cannady, S.B.; Landreth, G.E. Inflammatory mechanisms in Alzheimer’s disease: Inhibition of β- amyloid-stimulated proinflammatory responses and neurotoxicity by PPARγ agonists. J. Neurosci. 2000, 20, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Sastre, M.; Dumitrescu-Ozimek, L.; Hanke, A.; Dewachter, I.; Kuiperi, C.; O’Banion, K.; Klockgether, T.; Van Leuven, F.; Landreth, G.E. Acute treatment with the PPARγ agonist pioglitazone and ibuprofen reduces glial inflammation and Aβ1-42 levels in APPV717I transgenic mice. Brain 2005, 128, 1442–1453. [Google Scholar] [CrossRef]
- Cramer, P.E.; Cirrito, J.R.; Wesson, D.W.; Lee, C.Y.D.; Karlo, J.C.; Zinn, A.E.; Casali, B.T.; Restivo, J.L.; Goebel, W.D.; James, M.J.; et al. ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science 2012, 335, 1503–1506. [Google Scholar] [CrossRef]
- Skerrett, R.; Pellegrino, M.P.; Casali, B.T.; Taraboanta, L.; Landreth, G.E. Combined liver X receptor/peroxisome proliferator-activated receptor γ agonist treatment reduces amyloid β levels and improves behavior in amyloid precursor protein/presenilin 1 mice. J. Biol. Chem. 2015, 290, 21591–21602. [Google Scholar] [CrossRef]
- Koster, K.P.; Smith, C.; Valencia-Olvera, A.C.; Thatcher, G.R.J.; LaDu, M.J.; Tai, L.M. Rexinoids as therapeutics for Alzheimer disease: Role of APOE. Curr. Top. Med. Chem. 2016, 16, 708–720. [Google Scholar]
- Tong, M.; Dominguez, C.; Didsbury, J.; de la Monte, S.M. Targeting Alzheimer’s disease neuro-metabolic dysfunction with a small molecule nuclear receptor agonist (T3D-959) reverses disease pathologies. J. Alzheimers Dis. Parkinsonism 2016, 6, 238–244. [Google Scholar] [CrossRef]
- Watson, G.S.; Cholerton, B.A.; Reger, M.A.; Baker, L.D.; Plymate, S.R.; Asthana, S.; Fishel, M.A.; Kulstad, J.J.; Green, P.S.; Cook, D.G.; et al. Preserved cognition in patients with early Alzheimer disease and amnestic mild cognitive impairment during treatment with rosiglitazone: A preliminary study. J. Am. Assoc. Geriatr. Psychiatry 2005, 13, 950–958. [Google Scholar] [CrossRef]
- Janefjord, E.; Mååg, J.L.V.; Harvey, B.S.; Smid, S.D. Cannabinoid effects on β amyloid fibril and aggregate formation, neuronal and microglial-activated neurotoxicity in vitro. Cell. Mol. Neurobiol. 2014, 34, 31–42. [Google Scholar] [CrossRef]
- Rampa, A.; Gobbi, S.; Belluti, F.; Bisi, A. Emerging targets in neurodegeneration: New opportunities for Alzheimer’s disease treatment? Curr. Top. Med. Chem. 2013, 13, 1879–1904. [Google Scholar] [CrossRef] [PubMed]
- Altamura, C.; Ventriglia, M.; Martini, M.G.; Montesano, D.; Errante, Y.; Piscitelli, F.; Scrascia, F.; Quattrocchi, C.; Palazzo, P.; Seccia, S.; et al. Elevation of plasma 2-arachidonoylglycerol levels in alzheimer’s disease patients as a potential protective mechanism against neurodegenerative decline. J. Alzheimer Dis. 2015, 46, 497–506. [Google Scholar]
- Watabiki, T.; Tsuji, N.; Kiso, T.; Ozawa, T.; Narazaki, F.; Kakimoto, S. In vitro and in vivo pharmacological characterization of ASP8477: A novel highly selective fatty acid amide hydrolase inhibitor. Eur. J. Pharmacol. 2017, 815, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Bedse, G.; Romano, A.; Lavecchia, A.M.; Cassano, T.; Gaetani, S. The role of endocannabinoid signaling in the molecular mechanisms of neurodegeneration in Alzheimer’s disease. J. Alzheimer Dis. 2014, 43, 1115–1136. [Google Scholar] [CrossRef] [PubMed]
- Dias, I.H.K.; Mistry, J.; Fell, S.; Reis, A.; Spickett, C.M.; Polidori, M.C.; Lip, G.Y.H.; Griffiths, H.R. Oxidized LDL lipids increase β-amyloid production by SH-SY5Y cells through glutathione depletion and lipid raft formation. Free Radic. Biol. Med. 2014, 75, 48–59. [Google Scholar] [CrossRef]
- Wolozin, B.; Wang, S.W.; Li, N.C.; Lee, A.; Lee, T.A.; Kazis, L.E. Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med. 2007, 5, 20. [Google Scholar] [CrossRef]
- Atta, M. Exploring the relationship between statins and Alzheimer’s disease: Can statins really prevent Alzheimer’s disease? Adv. Alzheimer. Dis. 2015, 4, 10–14. [Google Scholar] [CrossRef]
- Geifman, N.; Brinton, R.D.; Kennedy, R.E.; Schneider, L.S.; Butte, A.J. Evidence for benefit of statins to modify cognitive decline and risk in Alzheimer’s disease. Alzheimer Res. Ther. 2017, 9, 10. [Google Scholar] [CrossRef]
- Dhakal, S.; Subhan, M.; Fraser, M.J.; Gardiner, K.; Macreadie, I. Simvastatin Efficiently Reduces Levels of Alzheimer’s Amyloid Beta in Yeast. Int. J. Mol. Sci. 2019, 20, 3531. [Google Scholar] [CrossRef]
- Terenghi, G. Peripheral nerve regeneration and neurotrophic factors. J Anat 1999, 194, 1–14. [Google Scholar] [CrossRef]
- Levy, Y.S.; Gilgun-Sherki, Y.; Melamed, E.; Offen, D. Therapeutic Potential of Neurotrophic Factors in Neurodegenerative Diseases. BioDrugs 2005, 19, 97–127. [Google Scholar] [CrossRef] [PubMed]
- Maisonpierre, P.C.; Belluscio, L.; Friedman, B.; Alderson, R.F.; Wiegand, S.J.; Furth, M.E.; Lindsay, R.M.; Yancopoulos, G.D. NT-3, BDNF, and NGF in the developing rat nervous system: Parallel as well as reciprocal patterns of expression. Neuron 1990, 5, 501–509. [Google Scholar] [CrossRef]
- Huang, E.J.; Reichardt, L.F. Trk receptors: Roles in neuronal signal transduction. Annu. Rev. Biochem. 2003, 72, 609–642. [Google Scholar] [CrossRef] [PubMed]
- Longo, F.M.; Massa, S.M. Small-molecule modulation of neurotrophin receptors: A strategy for the treatment of neurological disease. Nat. Rev. Drug Discov. 2013, 12, 507. [Google Scholar] [CrossRef] [PubMed]
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef]
- Levi-Montalcini, R. The nerve growth factor: Thirty-five years later. EMBO J 1987, 6, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Korsching, S.; Auburger, G.; Heumann, R.; Scott, J.; Thoenen, H. Levels of nerve growth factor and its mRNA in the central nervous system of the rat correlate with cholinergic innervation. EMBO J 1985, 4, 1389–1393. [Google Scholar] [CrossRef] [PubMed]
- Olson, L. NGF and the Treatment of Alzheimer’s Disease. Exp. Neurol. 1993, 124, 5–15. [Google Scholar] [CrossRef]
- Salehi, A.; Delcroix, J.D.; Swaab, D. Alzheimer’s disease and NGF signaling. J. Neural Transm. 2004, 111, 323–345. [Google Scholar] [CrossRef]
- Capsoni, S.; Cattaneo, A. On the Molecular Basis Linking Nerve Growth Factor (NGF) to Alzheimer’s Disease. Cell. Mol. Neurobiol. 2006, 26, 617–631. [Google Scholar] [CrossRef]
- Jakob-Roetne, R.; Jacobsen, H. Alzheimer’s disease: From pathology to therapeutic approaches. Angew. Chem. Int. Ed. 2009, 48, 3030–3059. [Google Scholar] [CrossRef]
- Nisticò, R.; Pignatelli, M.; Piccinin, S.; Mercuri, N.B.; Collingridge, G. Targeting synaptic dysfunction in Alzheimer’s disease therapy. Mol. Neurobiol. 2012, 46, 572–587. [Google Scholar] [CrossRef]
- Hefti, F. Nerve growth factor promotes survival of septal cholinergic neurons after fimbrial transections. J. Neurosci. 1986, 6, 2155–2162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fischer, W.; Wictorin, K.; Björklund, A.; Williams, L.; Varon, S.; Gage, F. Amelioration of cholinergic neuron atrophy and spatial memory impairment in aged rats by nerve growth factor. Nature 1987, 329, 65. [Google Scholar] [CrossRef]
- Hagg, T.; Manthorpe, M.; Vahlsing, H.L.; Varon, S. Delayed treatment with nerve growth factor reverses the apparent loss of cholinergic neurons after acute brain damage. Exp. Neurol. 1988, 101, 303–312. [Google Scholar] [CrossRef]
- Hagg, T.; Vahlsing, H.L.; Manthorpe, M.; Varon, S. Nerve growth factor infusion into the denervated adult rat hippocampal formation promotes its cholinergic reinnervation. J. Neurosci. 1990, 10, 3087–3092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagg, T.; Hagg, F.; Vahlsing, H.; Manthorpe, M.; Varon, S. Nerve growth factor effects on cholinergic neurons of neostriatum and nucleus accumbens in the adult rat. Neuroscience 1989, 30, 95–103. [Google Scholar] [CrossRef]
- Friden, P.M.; Walus, L.R.; Watson, P.; Doctrow; Kozarich, J.W.; Backman, C.; Bergman, H.; Hoffer, B.; Bloom, F.; Granholm, A.C. Blood-brain barrier penetration and in vivo activity of an NGF conjugate. Science 1993, 259, 373. [Google Scholar] [CrossRef] [PubMed]
- Poduslo, J.F.; Curran, G.L. Permeability at the blood-brain and blood-nerve barriers of the neurotrophic factors: NGF, CNTF, NT-3, BDNF. Mol. Brain Res. 1996, 36, 280–286. [Google Scholar] [CrossRef]
- Pan, W.; Banks, W.A.; Kastin, A.J. Permeability of the blood–brain barrier to neurotrophins. Brain Res. 1998, 788, 87–94. [Google Scholar] [CrossRef]
- Thoenen, H.; Sendtner, M. Neurotrophins: From enthusiastic expectations through sobering experiences to rational therapeutic approaches. Nat. Neurosci. 2002, 5, 1046. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Behbahani, H.; Eriksdotter, M. Innovative therapy for Alzheimer’s disease-with focus on biodelivery of NGF. Front. Neurosci. 2019, 13, 38. [Google Scholar] [CrossRef]
- Zhu, Y.; Xiao, K.; Ma, L.; Xiong, B.; Fu, Y.; Yu, H.; Wang, W.; Wang, X.; Hu, D.; Peng, H.; et al. Design, synthesis and biological evaluation of novel dual inhibitors of acetylcholinesterase and β-secretase. Bioorg. Med. Chem. 2009, 17, 1600–1613. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, T.; Yeung, J.C.K.; Vasefi, M.S.; Beazely, M.A.; Rao, P.P.N. Development and evaluation of multifunctional agents for potential treatment of Alzheimer’s disease: Application to a pyrimidine-2,4-diamine template. Bioorg. Med. Chem. Lett. 2012, 22, 4707–4712. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Bachiller, M.I.; Pérez, C.; Monjas, L.; Rademann, J.; Rodríguez-Franco, M.I. New tacrine-4-oxo-4H-chromene hybrids as multifunctional agents for the treatment of Alzheimer’s disease, with cholinergic, antioxidant, and β-amyloid-reducing properties. J. Med. Chem. 2012, 55, 1303–1317. [Google Scholar] [CrossRef] [PubMed]
- Camps, P.; El Achab, R.; Morral, J.; Munoz-Torrero, D.; Badia, A.; Eladi Banos, J.; Vivas, N.M.; Barril, X.; Orozco, M.; Javier Luque, F. New tacrine-huperzine A hybrids (huprines): Highly potent tight-binding acetylcholinesterase inhibitors of interest for the treatment of Alzheimer’s Disease. J. Med. Chem. 2000, 43, 4657–4666. [Google Scholar] [CrossRef] [PubMed]
- Zha, X.; Lamba, D.; Zhang, L.; Lou, Y.; Xu, C.; Kang, D.; Chen, L.; Xu, Y.; Zhang, L.; De Simone, A.; et al. Novel Tacrine-Benzofuran Hybrids as Potent Multitarget-Directed Ligands for the Treatment of Alzheimers Disease: Design, Synthesis, Biological Evaluation, and X-ray Crystallography. J. Med. Chem. 2016, 59, 114–131. [Google Scholar] [CrossRef] [PubMed]
- Gabr, M.T.; Abdel-Raziq, M.S. Design and synthesis of donepezil analogues as dual AChE and BACE-1 inhibitors. Bioorg. Chem. 2018, 80, 245–252. [Google Scholar] [CrossRef]
- Zhang, P.; Xu, S.; Zhu, Z.; Xu, J. Multi-target design strategies for the improved treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2019, 228–247. [Google Scholar] [CrossRef]
- Prati, F.; De Simone, A.; Bisignano, P.; Armirotti, A.; Summa, M.; Pizzirani, D.; Scarpelli, R.; Perez, D.I.; Andrisano, V.; Perez-Castillo, A.; et al. Multitarget drug discovery for Alzheimer’s disease: Triazinones as BACE-1 and GSK-3β inhibitors. Angew. Chem. Int. Ed. 2015, 54, 1578–1582. [Google Scholar] [CrossRef]
- Prasad, S.; Gupta, S.C.; Tyagi, A.K.; Aggarwal, B.B. Curcumin, a component of golden spice: From bedside to bench and back. Biotechnol. Adv. 2014, 32, 1053–1064. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Chen, J.; Li, X.; Su, T.; Wang, Y.; Wang, Z.; Huang, L.; Li, X. Synthesis and evaluation of selegiline derivatives as monoamine oxidase inhibitor, antioxidant and metal chelator against Alzheimer’s disease. Bioorg. Med. Chem. 2015, 23, 3722–3729. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, Y.; Wang, B.; Li, W.; Huang, L.; Li, X. Design, synthesis, and evaluation of orally available clioquinol-moracin M hybrids as multitarget-directed ligands for cognitive improvement in a rat model of neurodegeneration in Alzheimer’s disease. J. Med. Chem. 2015, 58, 8616–8637. [Google Scholar] [CrossRef] [PubMed]
- Daniele, S.; Giacomelli, C.; Martini, C. Brain ageing and neurodegenerative disease: The role of cellular waste management. Biochem. Pharmacol. 2018, 158, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Sands, W.A.; Page, M.M.; Selman, C. Proteostasis and ageing: Insights from long-lived mutant mice. J. Physiol. 2017, 595, 6383–6390. [Google Scholar] [CrossRef] [PubMed]
- He, L.Q.; Lu, J.H.; Yue, Z.Y. Autophagy in ageing and ageing-associated diseases. Acta Pharmacol. Sin. 2013, 34, 605–611. [Google Scholar] [CrossRef] [Green Version]
- Labbadia, J.; Morimoto, R.I. The biology of proteostasis in aging and disease. Annu. Rev. Biochem. 2015, 84, 435–464. [Google Scholar] [CrossRef]
- Jung, T.; Grune, T. Structure of the proteasome. Prog. Mol. Biol. Transl. Sci. 2012, 109, 1–39. [Google Scholar]
- Wilhelm, T.; Richly, H. Autophagy during ageing – from Dr Jekyll to Mr Hyde. FEBS J. 2018, 285, 2367–2376. [Google Scholar] [CrossRef]
- Li, W.W.; Li, J.; Bao, J.K. Microautophagy: Lesser-known self-eating. Cell. Mol. Life Sci. Cmls 2012, 69, 1125–1136. [Google Scholar] [CrossRef]
- Dice, J.F. Chaperone-Mediated Autophagy. Autophagy 2007, 3, 295–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Y.; He, D.; Yao, Z.; Klionsky, D.J. The machinery of macroautophagy. Cell Res. 2013, 24, 24. [Google Scholar] [CrossRef] [PubMed]
- Kamada, Y.; Sekito, T.; Ohsumi, Y. Autophagy in Yeast: ATOR-Mediated Response to Nutrient Starvation. In TOR: Target of Rapamycin; Thomas, G., Sabatini, D.M., Hall, M.N., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 73–84. [Google Scholar]
- Settembre, C.; Ballabio, A. TFEB regulates autophagy: An integrated coordination of cellular degradation and recycling processes. Autophagy 2011, 7, 1379–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciarretta, S.; Yee, D.; Ammann, P.; Nagarajan, N.; Volpe, M.; Frati, G.; Sadoshima, J. Role of NADPH oxidase in the regulation of autophagy in cardiomyocytes. Clin. Sci. 2015, 128, 387–403. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Ma, X.; Ouyang, T.; Chen, H.; Lin, J.; Liu, J.; Xiao, Y.; Yu, J.; Huang, Y. SIRT1 reverses senescence via enhancing autophagy and attenuates oxidative stress-induced apoptosis through promoting p53 degradation. Int. J. Biol. Macromol. 2018, 117, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Liao, W.; Yang, J.; Ma, K.; Li, X.; Wang, Y.; Wang, D.; Wang, L.; Zhang, Y.; Yin, Y.; et al. FOXO3 induces FOXO1-dependent autophagy by activating the AKT1 signaling pathway. Autophagy 2012, 8, 1712–1723. [Google Scholar] [CrossRef] [Green Version]
- Höhn, A.; Jung, T.; Grimm, S.; Catalgol, B.; Weber, D.; Grune, T. Lipofuscin inhibits the proteasome by binding to surface motifs. Free Radic. Biol. Med. 2011, 50, 585–591. [Google Scholar] [CrossRef]
- Singh, R.; Kaushik, S.; Wang, Y.; Xiang, Y.; Novak, I.; Komatsu, M.; Tanaka, K.; Cuervo, A.M.; Czaja, M.J. Autophagy regulates lipid metabolism. Nature 2009, 458, 1131. [Google Scholar] [CrossRef]
- Kaushik, S.; Cuervo, A.M. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat. Cell Biol. 2015, 17, 759. [Google Scholar] [CrossRef]
- Koseoglu, M.M.; Norambuena, A.; Sharlow, E.R.; Lazo, J.S.; Bloom, G.S. Aberrant Neuronal Cell Cycle Re-Entry: The Pathological Confluence of Alzheimer’s Disease and Brain Insulin Resistance, and Its Relation to Cancer. J. Alzheimer Dis. 2019, 67, 1–11. [Google Scholar] [CrossRef]
- Birdsall, V.; Waites, C.L. Autophagy at the synapse. Neurosci. Lett. 2019, 697, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Hansen, M.; Rubinsztein, D.C.; Walker, D.W. Autophagy as a promoter of longevity: Insights from model organisms. Nat. Rev. Mol. Cell Biol. 2018, 19, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Luna-Guevara, M.L.; Luna-Guevara, J.J.; Hernández-Carranza, P.; Ruíz-Espinosa, H.; Ochoa-Velasco, C.E. Chapter 3 - Phenolic Compounds: A Good Choice Against Chronic Degenerative Diseases. In Studies in Natural Products Chemistry; Attaur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Volume 59, pp. 79–108. [Google Scholar]
- Manach, C.; Scalbert, A.; Morand, C.; Rémésy, C.; Jiménez, L. Polyphenols: Food sources and bioavailability. Am. J. Clin. Nutr. 2004, 79, 727–747. [Google Scholar] [CrossRef]
- Ferreira, I.C.; Barros, L.; Abreu, R. Antioxidants in wild mushrooms. Curr. Med. Chem. 2009, 16, 1543–1560. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ramírez, A.; Pavo-Caballero, C.; Baeza, E.; Baenas, N.; Garcia-Viguera, C.; Marín, F.R.; Soler-Rivas, C. Mushrooms do not contain flavonoids. J. Funct. Foods 2016, 25, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Surguchov, A.; Emamzadeh, F.N.; Surguchev, A.A. Amyloidosis and longevity: A lesson from plants. Biology 2019, 8, 43. [Google Scholar] [CrossRef]
- Sandoval-Acuña, C.; Ferreira, J.; Speisky, H. Polyphenols and mitochondria: An update on their increasingly emerging ROS-scavenging independent actions. Arch. Biochem. Biophys. 2014, 559, 75–90. [Google Scholar] [CrossRef]
- Kim, M.Y.; Seguin, P.; Ahn, J.K.; Kim, J.J.; Chun, S.C.; Kim, E.H.; Seo, S.H.; Kang, E.Y.; Kim, S.L.; Park, Y.J.; et al. Phenolic compound concentration and antioxidant activities of edible and medicinal mushrooms from Korea. J. Agric. Food Chem. 2008, 56, 7265–7270. [Google Scholar] [CrossRef]
- Heleno, S.A.; Barros, L.; Martins, A.; Queiroz, M.J.R.P.; Santos-Buelga, C.; Ferreira, I.C.F.R. Phenolic, polysaccharidic, and lipidic fractions of mushrooms from northeastern portugal: Chemical compounds with antioxidant properties. J. Agric. Food Chem. 2012, 60, 4634–4640. [Google Scholar] [CrossRef]
- Zhang, D.D.; Hannink, M. Distinct Cysteine Residues in Keap1 Are Required for Keap1-Dependent Ubiquitination of Nrf2 and for Stabilization of Nrf2 by Chemopreventive Agents and Oxidative Stress. Mol. Cell. Biol. 2003, 23, 8137. [Google Scholar] [CrossRef]
- Matzinger, M.; Fischhuber, K.; Heiss, E.H. Activation of Nrf2 signaling by natural products-can it alleviate diabetes? Biotechnol. Adv. 2018, 36, 1738–1767. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Huélamo, M.; Rodríguez-Morató, J.; Boronat, A.; de la Torre, R. Modulation of Nrf2 by Olive Oil and Wine Polyphenols and Neuroprotection. Antioxid 2017, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Vasto, S.; Abraham, N.G.; Caruso, C.; Zella, D.; Fabio, G. Modulation of Nrf2/ARE pathway by food polyphenols: A nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol Neurobiol 2011, 44, 192–201. [Google Scholar] [CrossRef] [PubMed]
- Yahfoufi, N.; Alsadi, N.; Jambi, M.; Matar, C. The Immunomodulatory and Anti-Inflammatory Role of Polyphenols. Nutrients 2018, 10, 1618. [Google Scholar] [CrossRef] [PubMed]
- Nayernia, Z.; Jaquet, V.; Krause, K.-H. New insights on NOX enzymes in the central nervous system. Antioxid Redox Signal 2014, 20, 2815–2837. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, S.; Abramov, A.Y. Mechanism of Oxidative Stress in Neurodegeneration. Oxidative Med. Cell. Longev. 2012, 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Cohen, G.; Kesler, N. Monoamine Oxidase and Mitochondrial Respiration. J. Neurochem. 1999, 73, 2310–2315. [Google Scholar] [CrossRef]
- Dos Santos, T.W.; Pereira, Q.C.; Teixeira, L.; Gambero, A.; Villena, J.A.; Ribeiro, M.L. Effects of polyphenols on thermogenesis and mitochondrial biogenesis. Int. J. Mol. Sci. 2018, 19, 5757. [Google Scholar] [CrossRef]
- Dong, W.; Wang, F.; Guo, W.; Zheng, X.; Chen, Y.; Zhang, W.; Shi, H. Aβ25–35 Suppresses Mitochondrial Biogenesis in Primary Hippocampal Neurons. Cell. Mol. Neurobiol. 2016, 36, 83–91. [Google Scholar] [CrossRef]
- Xu, W.; Barrientos, T.; Andrews, N.C. Iron and copper in mitochondrial diseases. Cell Metab. 2013, 17, 319–328. [Google Scholar] [CrossRef]
- Perez, C.A.; Wei, Y.; Guo, M. Iron-binding and anti-Fenton properties of baicalein and baicalin. J. Inorg. Biochem. 2009, 103, 326–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Refat, M.S. Synthesis and characterization of ligational behavior of curcumin drug towards some transition metal ions: Chelation effect on their thermal stability and biological activity. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2013, 105, 326–337. [Google Scholar] [CrossRef] [PubMed]
- Valdés, A.; Sullini, G.; Ibáñez, E.; Cifuentes, A.; García-Cañas, V. Rosemary polyphenols induce unfolded protein response and changes in cholesterol metabolism in colon cancer cells. J. Funct. Foods 2015, 15, 429–439. [Google Scholar] [CrossRef]
- Shen, M.; Chan, T.H.; Dou, Q.P. Targeting tumor ubiquitin-proteasome pathway with polyphenols for chemosensitization. Anti-Cancer Agents Med. Chem. 2012, 12, 891–901. [Google Scholar] [CrossRef]
- Pallauf, K.; Rimbach, G. Autophagy, polyphenols and healthy ageing. Ageing Res. Rev. 2013, 12, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Rastogi, M.; Ojha, R.P.; Sagar, C.; Agrawal, A.; Dubey, G.P. Protective effect of curcuminoids on age-related mitochondrial impairment in female Wistar rat brain. Biogerontology 2014, 15, 21–31. [Google Scholar] [CrossRef]
- Chung, S.; Yao, H.; Caito, S.; Hwang, J.W.; Arunachalam, G.; Rahman, I. Regulation of SIRT1 in cellular functions: Role of polyphenols. Arch. Biochem. Biophys. 2010, 501, 79–90. [Google Scholar] [CrossRef] [Green Version]
- Horio, Y. Elucidation of the roles of protein deacetylase SIRT1 in health and diseases. Sapporo Med. J. 2018, 87, 1–8. [Google Scholar]
- Ren, Z.; He, H.; Zuo, Z.; Xu, Z.; Wei, Z.; Deng, J. The role of different SIRT1-mediated signaling pathways in toxic injury. Cell. Mol. Biol. Lett. 2019, 24, 36. [Google Scholar] [CrossRef]
- Maiese, K. The mechanistic target of rapamycin (mTOR) and the silent mating-type information regulation 2 homolog 1 (SIRT1): Oversight for neurodegenerative disorders. Biochem. Soc. Trans. 2018, 46, 351–360. [Google Scholar] [CrossRef]
- Bao, J.; Zheng, L.; Zhang, Q.; Li, X.; Zhang, X.; Li, Z.; Bai, X.; Zhang, Z.; Huo, W.; Zhao, X.; et al. Deacetylation of TFEB promotes fibrillar Aβ degradation by upregulating lysosomal biogenesis in microglia. Protein Cell 2016, 7, 417–433. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Impey, S.; Kang, H.; di Ronza, A.; Pelz, C.; Sardiello, M.; Ballabio, A. Characterization of the CLEAR network reveals an integrated control of cellular clearance pathways. Hum. Mol. Genet. 2011, 20, 3852–3866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brunet, A.; Sweeney, L.B.; Sturgill, J.F.; Chua, K.F.; Greer, P.L.; Lin, Y.; Tran, H.; Ross, S.E.; Mostoslavsky, R.; Cohen, H.Y.; et al. Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 2004, 303, 2011–2015. [Google Scholar] [CrossRef] [PubMed]
- Pillarisetti, S. A Review of Sirt1 and Sirt1 Modulators in Cardiovascular and Metabolic Diseases. Recent Pat. Cardiovasc. Drug Discov. 2008, 3, 156–164. [Google Scholar] [CrossRef]
- Allard, J.S.; Perez, E.; Zou, S.; de Cabo, R. Dietary activators of Sirt1. Mol. Cell. Endocrinol. 2009, 299, 58–63. [Google Scholar] [CrossRef] [Green Version]
- Nardiello, P.; Pantano, D.; Lapucci, A.; Stefani, M.; Casamenti, F. Diet Supplementation with Hydroxytyrosol Ameliorates Brain Pathology and Restores Cognitive Functions in a Mouse Model of Amyloid-β Deposition. J. Alzheimer Dis. 2018, 63, 1161–1172. [Google Scholar] [CrossRef]
- Pantano, D.; Luccarini, I.; Nardiello, P.; Servili, M.; Stefani, M.; Casamenti, F. Oleuropein aglycone and polyphenols from olive mill waste water ameliorate cognitive deficits and neuropathology. Br. J. Clin. Pharmacol. 2017, 83, 54–62. [Google Scholar] [CrossRef]
- Cordero, J.G.; García-Escudero, R.; Avila, J.; Gargini, R.; García-Escudero, V. Benefit of Oleuropein Aglycone for Alzheimer’s Disease by Promoting Autophagy. Oxidative Med. Cell. Longev. 2018, 2018, 5010741. [Google Scholar] [CrossRef]
- Kim, H.S.; Quon, M.J.; Kim, J.A. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol 2014, 2, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Ulakcsai, Z.; Bagaméry, F.; Szökő, É.; Tábi, T. The role of autophagy induction in the mechanism of cytoprotective effect of resveratrol. Eur. J. Pharm. Sci. 2018, 123, 135–142. [Google Scholar] [CrossRef]
- Song, J.; Huang, Y.; Zheng, W.; Yan, J.; Cheng, M.; Zhao, R.; Chen, L.; Hu, C.; Jia, W. Resveratrol reduces intracellular reactive oxygen species levels by inducing autophagy through the AMPK-mTOR pathway. Front. Med. 2018, 12, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Velagapudi, R.; Lepiarz, I.; El-Bakoush, A.; Katola, F.O.; Bhatia, H.; Fiebich, B.L.; Olajide, O.A. Induction of Autophagy and Activation of SIRT-1 Deacetylation Mechanisms Mediate Neuroprotection by the Pomegranate Metabolite Urolithin A in BV2 Microglia and Differentiated 3D Human Neural Progenitor Cells. Mol. Nutr. Food Res. 2019, 63, 1801237. [Google Scholar] [CrossRef] [PubMed]
- Sarubbo, F.; Ramis, M.R.; Kienzer, C.; Aparicio, S.; Esteban, S.; Miralles, A.; Moranta, D. Chronic Silymarin, Quercetin and Naringenin Treatments Increase Monoamines Synthesis and Hippocampal Sirt1 Levels Improving Cognition in Aged Rats. J. Neuroimmune Pharmacol. 2018, 13, 24–38. [Google Scholar] [CrossRef] [PubMed]
- Putteeraj, M.; Lim, W.L.; Teoh, S.L.; Yahaya, M.F. Flavonoids and its neuroprotective effects on brain ischemia and neurodegenerative diseases. Curr. Drug Targets 2018, 19, 1710–1720. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.M.; Tsai, S.Y.; Lin, J.A.; Wu, C.H.; Yen, G.C. Cytoprotective effects of hesperetin and hesperidin against amyloid ss-induced impairment of glucose transport through downregulation of neuronal autophagy. Mol. Nutr. Food Res. 2012, 56, 601–609. [Google Scholar] [CrossRef] [PubMed]
- Xiao, J.B.; Högger, P. Dietary polyphenols and type 2 diabetes: Current insights and future perspectives. Curr. Med. Chem. 2015, 22, 23–38. [Google Scholar] [CrossRef]
- Rodríguez-García, C.; Sánchez-Quesada, C.; Gaforio, J.J.; Gaforio, J.J. Dietary flavonoids as cancer chemopreventive agents: An updated review of human studies. Antioxidants 2019, 8, 137. [Google Scholar] [CrossRef]
- Carrasco-Pozo, C.; Cires, M.J.; Gotteland, M. Quercetin and Epigallocatechin Gallate in the Prevention and Treatment of Obesity: From Molecular to Clinical Studies. J. Med. Food 2019, 22, 753–770. [Google Scholar] [CrossRef]
- Zang, M.; Xu, S.; Maitland-Toolan, K.A.; Zuccollo, A.; Hou, X.; Jiang, B.; Wierzbicki, M.; Verbeuren, T.J.; Cohen, R.A. Polyphenols Stimulate AMP-Activated Protein Kinase, Lower Lipids, and Inhibit Accelerated Atherosclerosis in Diabetic LDL Receptor–Deficient Mice. Diabetes 2006, 55, 2180. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, Y.; Zhou, Y.; Zhang, Z.; Xie, Z.; Zhang, J.; Wan, X. Green Tea Polyphenols Alleviate Obesity in Broiler Chickens through the Regulation of Lipid-Metabolism-Related Genes and Transcription Factor Expression. J. Agric. Food Chem. 2013, 61, 8565–8572. [Google Scholar] [CrossRef]
- Bigagli, E.; Toti, S.; Lodovici, M.; Giovannelli, L.; Cinci, L.; D’Ambrosio, M.; Luceri, C. Dietary extra-virgin olive oil polyphenols do not attenuate colon inflammation in transgenic HLAB-27 rats but exert hypocholesterolemic effects through the modulation of HMGCR and PPAR-α gene expression in the liver. Lifestyle Genom. 2019, 11, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Barquissau, V.; Ghandour, R.A.; Ailhaud, G.; Klingenspor, M.; Langin, D.; Amri, E.Z.; Pisani, D.F. Control of adipogenesis by oxylipins, GPCRs and PPARs. Biochimie 2017, 136, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Q.; Zhang, Y.; Shen, C.; Wang, Z.; Wu, Q.; Zhang, Y.; Li, S.; Qiao, Y. Agrimol B suppresses adipogenesis through modulation of SIRT1-PPAR gamma signal pathway. Biochem. Biophys. Res. Commun. 2016, 477, 454–460. [Google Scholar] [CrossRef] [PubMed]
- Simons, K.; Ehehalt, R. Cholesterol, lipid rafts, and disease. J. Clin. Investig. 2002, 110, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kauppinen, A.; Suuronen, T.; Kaarniranta, K. SIRT1 longevity factor suppresses NF-κB-driven immune responses: Regulation of aging via NF-κB acetylation? BioEssays 2008, 30, 939–942. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215S–217S. [Google Scholar] [CrossRef]
- Rahman, I.; Chung, S. Dietary Polyphenols, Deacetylases and Chromatin Remodeling in Inflammation. World Rev. Nutr. Diet. 2010, 101, 84–94. [Google Scholar]
- Kostomoiri, M.; Fragkouli, A.; Sagnou, M.; Skaltsounis, L.A.; Pelecanou, M.; Tsilibary, E.C.; Τzinia, A.K. Oleuropein, an Anti-oxidant Polyphenol Constituent of Olive Promotes α-Secretase Cleavage of the Amyloid Precursor Protein (AβPP). Cell. Mol. Neurobiol. 2013, 33, 147–154. [Google Scholar] [CrossRef]
- Porzoor, A.; Alford, B.; Hügel, H.M.; Grando, D.; Caine, J.; Macreadie, I. Anti-amyloidogenic properties of some phenolic compounds. Biomolecules 2015, 5, 505–527. [Google Scholar] [CrossRef]
- Wobst, H.J.; Sharma, A.; Diamond, M.I.; Wanker, E.E.; Bieschke, J. The green tea polyphenol (-)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Lett. 2015, 589, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Lee, D.H.; Lee, J.S. Characterization of a new antidementia β-secretase inhibitory peptide from Saccharomyces cerevisiae. Enzym. Microb. Technol. 2007, 42, 83–88. [Google Scholar] [CrossRef]
- Park, I.H.; Jeon, S.Y.; Lee, H.J.; Kim, S.I.; Song, K.S. A β-secretase (BACE1) inhibitor hispidin from the mycelial cultures of Phellinus linteus. Planta Med. 2004, 70, 143–146. [Google Scholar] [PubMed]
- Bennett, L.; Sheean, P.; Zabaras, D.; Head, R. Heat-stable components of wood ear mushroom, Auricularia polytricha (higher basidiomycetes), inhibit in vitro activity of beta secretase (BACE1). Int. J. Med. Mushrooms 2013, 15, 233–249. [Google Scholar] [CrossRef]
- Song, S.H.; Choi, S.M.; Kim, J.E.; Sung, J.E.; Lee, H.A.; Choi, Y.H.; Bae, C.J.; Choi, Y.W.; Hwang, D.Y. α-Isocubebenol alleviates scopolamine-induced cognitive impairment by repressing acetylcholinesterase activity. Neurosci. Lett. 2017, 638, 121–128. [Google Scholar] [CrossRef]
- Sameem, B.; Saeedi, M.; Mahdavi, M.; Shafiee, A. A review on tacrine-based scaffolds as multi-target drugs (MTDLs) for Alzheimer’s disease. Eur. J. Med. Chem. 2017, 128, 332–345. [Google Scholar] [CrossRef]
- Orhan, I.; Kartal, M.; Tosun, F.; Şener, B. Screening of various phenolic acids and flavonoid derivatives for their anticholinesterase potential. Z. Fur Nat. Sect. C J. Biosci. 2007, 62, 829–832. [Google Scholar] [CrossRef]
- Dgachi, Y.; Sokolov, O.; Luzet, V.; Godyń, J.; Panek, D.; Bonet, A.; Martin, H.; Iriepa, I.; Moraleda, I.; García-Iriepa, C.; et al. Tetrahydropyranodiquinolin-8-amines as new, non-hepatotoxic, antioxidant, and acetylcholinesterase inhibitors for Alzheimer’s disease therapy. Eur. J. Med. Chem. 2017, 126, 576–589. [Google Scholar] [CrossRef]
- Kuppusamy, A.; Arumugam, M.; George, S. Combining in silico and in vitro approaches to evaluate the acetylcholinesterase inhibitory profile of some commercially available flavonoids in the management of Alzheimer’s disease. Int. J. Biol. Macromol. 2017, 95, 199–203. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Renaud, J.; Martinoli, M.-G. Considerations for the Use of Polyphenols as Therapies in Neurodegenerative Diseases. Int J Mol Sci 2019, 20, 1883. [Google Scholar] [CrossRef] [PubMed]
- Margină, D.; Ilie, M.; Grădinaru, D.; Androutsopoulos, V.P.; Kouretas, D.; Tsatsakis, A.M. Natural products—friends or foes? Toxicol. Lett. 2015, 236, 154–167. [Google Scholar] [CrossRef]
- Hodek, P.; Trefil, P.; Stiborová, M. Flavonoids-potent and versatile biologically active compounds interacting with cytochromes P450. Chem. Biol. Interact. 2002, 139, 1–21. [Google Scholar] [CrossRef]
- Bailey, D.G.; Malcolm, J.; Arnold, O.; Spence, J.D. Grapefruit juice–drug interactions. Br. J. Clin. Pharmacol. 1998, 46, 101–110. [Google Scholar] [CrossRef] [PubMed]
- Doostdar, H.; Burke, M.D.; Mayer, R.T. Bioflavonoids: Selective substrates and inhibitors for cytochrome P450 CYP1A and CYP1B1. Toxicology 2000, 144, 31–38. [Google Scholar] [CrossRef]
- Boocock, D.J.; Faust, G.E.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Prev. Biomark. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Vaz-da-Silva, M.; Loureiro, A.; Falcao, A.; Nunes, T.; Rocha, J.; Fernandes-Lopes, C.; Soares, E.; Wright, L.; Almeida, L.; Soares-da-Silva, P. Effect of food on the pharmacokinetic profile of trans-resveratrol. Int. J. Clin. Pharm. 2008, 46, 564–570. [Google Scholar] [CrossRef]
- Almeida, L.; Vaz-da-Silva, M.; Falcão, A.; Soares, E.; Costa, R.; Loureiro, A.I.; Fernandes-Lopes, C.; Rocha, J.F.; Nunes, T.; Wright, L. Pharmacokinetic and safety profile of trans-resveratrol in a rising multiple-dose study in healthy volunteers. Mol. Nutr. Food Res. 2009, 53, S7–S15. [Google Scholar] [CrossRef]
- Chow, H.S.; Cai, Y.; Hakim, I.A.; Crowell, J.A.; Shahi, F.; Brooks, C.A.; Dorr, R.T.; Hara, Y.; Alberts, D.S. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin. Cancer Res. 2003, 9, 3312–3319. [Google Scholar]
- Herrschaft, H.; Nacu, A.; Likhachev, S.; Sholomov, I.; Hoerr, R.; Schlaefke, S. Ginkgo biloba extract EGb 761® in dementia with neuropsychiatric features: A randomised, placebo-controlled trial to confirm the efficacy and safety of a daily dose of 240 mg. J. Psychiatr. Res. 2012, 46, 716–723. [Google Scholar] [CrossRef]
- Ringman, J.M.; Frautschy, S.A.; Teng, E.; Begum, A.N.; Bardens, J.; Beigi, M.; Gylys, K.H.; Badmaev, V.; Heath, D.D.; Apostolova, L.G. Oral curcumin for Alzheimer’s disease: Tolerability and efficacy in a 24-week randomized, double blind, placebo-controlled study. Alzheimer Res. Ther. 2012, 4, 43. [Google Scholar] [CrossRef] [PubMed]
- Ihl, R.; Tribanek, M.; Bachinskaya, N.; Group, G.S. Efficacy and tolerability of a once daily formulation of Ginkgo biloba extract EGb 761® in Alzheimer’s disease and vascular dementia: Results from a randomised controlled trial. Pharmacopsychiatry 2012, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Menezes, R.; Macedo, D.; Costa, I.; Nunes dos Santos, C. Polyphenols beyond barriers: A glimpse into the brain. Curr. Neuropharmacol 2017, 15, 562–594. [Google Scholar] [CrossRef] [PubMed]
- Moussa, C.; Hebron, M.; Huang, X.; Ahn, J.; Rissman, R.A.; Aisen, P.S.; Turner, R.S. Resveratrol regulates neuro-inflammation and induces adaptive immunity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 1. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.S.; Thomas, R.G.; Craft, S.; van Dyck, C.H.; Mintzer, J.; Reynolds, B.A.; Brewer, J.B.; Rissman, R.A.; Raman, R.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of resveratrol for Alzheimer disease. Neurology 2015, 85, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Beck, S.M.; Ruge, H.; Schindler, C.; Burkart, M.; Miller, R.; Kirschbaum, C.; Goschke, T. Effects of Ginkgo biloba extract EGb 761® on cognitive control functions, mental activity of the prefrontal cortex and stress reactivity in elderly adults with subjective memory impairment–a randomized double-blind placebo-controlled trial. Hum. Psychopharmacol. Clin. Exp. 2016, 31, 227–242. [Google Scholar] [CrossRef] [PubMed]
- Levin, J.; Maaß, S.; Schuberth, M.; Respondek, G.; Paul, F.; Mansmann, U.; Oertel, W.H.; Lorenzl, S.; Krismer, F.; Seppi, K. The PROMESA-protocol: Progression rate of multiple system atrophy under EGCG supplementation as anti-aggregation-approach. J. Neural Transm. 2016, 123, 439–445. [Google Scholar] [CrossRef]
- Pandareesh, M.; Mythri, R.; Bharath, M.S. Bioavailability of dietary polyphenols: Factors contributing to their clinical application in CNS diseases. Neurochem. Int. 2015, 89, 198–208. [Google Scholar] [CrossRef]
- Hu, M.; Wu, B.; Liu, Z. Bioavailability of polyphenols and flavonoids in the era of precision medicine. Mol. Pharm. 2017, 14, 2861–2863. [Google Scholar] [CrossRef]
- D Archivio, M.; Filesi, C.; Di Benedetto, R.; Gargiulo, R.; Giovannini, C.; Masella, R. Polyphenols, dietary sources and bioavailability. Ann. Ist. Super. Sanita 2007, 43, 348. [Google Scholar]
- Bravo, L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998, 56, 317–333. [Google Scholar] [CrossRef] [PubMed]
- Scalbert, A.; Morand, C.; Manach, C.; Rémésy, C. Absorption and metabolism of polyphenols in the gut and impact on health. Biomed. Pharmacother. 2002, 56, 276–282. [Google Scholar] [CrossRef]
- Cermak, R.; Wolffram, S. The potential of flavonoids to influence drug metabolism and pharmacokinetics by local gastrointestinal mechanisms. Curr. Drug Metab. 2006, 7, 729–744. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood–brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Figueira, I.; Garcia, G.; Pimpão, R.C.; Terrasso, A.; Costa, I.; Almeida, A.; Tavares, L.; Pais, T.; Pinto, P.; Ventura, M. Polyphenols journey through blood-brain barrier towards neuronal protection. Sci. Rep. 2017, 7, 11456. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Cimini, A.; Peluso, G.; Giordano, A.; Melone, M.A.B. Nano-delivery systems for encapsulation of dietary polyphenols: An experimental approach for neurodegenerative diseases and brain tumors. Biochem. Pharmacol. 2018, 154, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Prado-Audelo, D.; María, L.; Caballero-Florán, I.H.; Meza-Toledo, J.A.; Mendoza-Muñoz, N.; González-Torres, M.; Florán, B.; Cortés, H.; Leyva-Gómez, G. Formulations of curcumin nanoparticles for brain diseases. Biomolecules 2019, 9, 56. [Google Scholar] [CrossRef]
- Yavarpour-Bali, H.; Ghasemi-Kasman, M.; Pirzadeh, M. Curcumin-loaded nanoparticles: A novel therapeutic strategy in treatment of central nervous system disorders. Int. J. Nanomed. 2019, 14, 4449. [Google Scholar] [CrossRef]
- Davidov-Pardo, G.; McClements, D.J. Resveratrol encapsulation: Designing delivery systems to overcome solubility, stability and bioavailability issues. Trends Food Sci. Technol. 2014, 38, 88–103. [Google Scholar] [CrossRef]
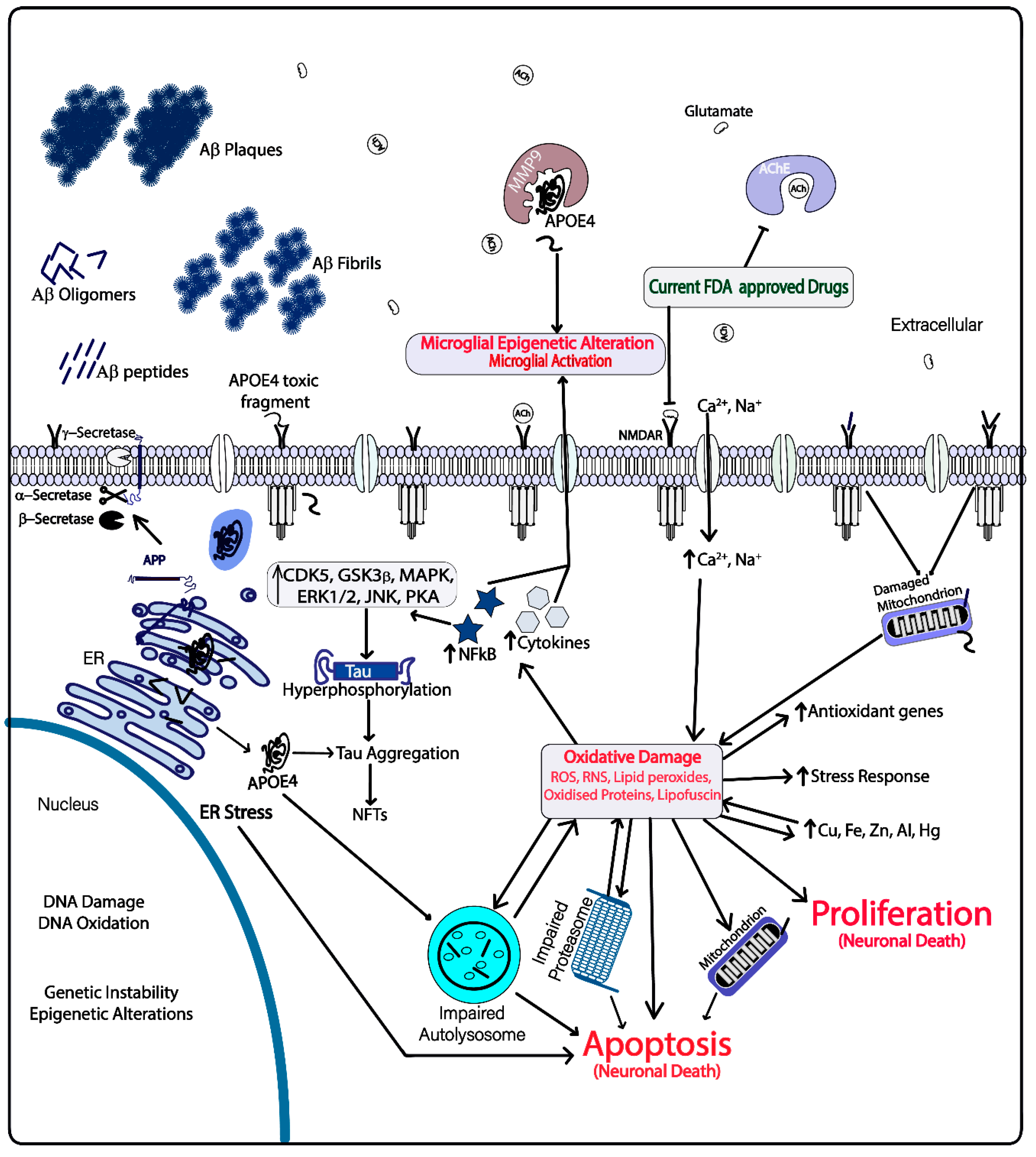
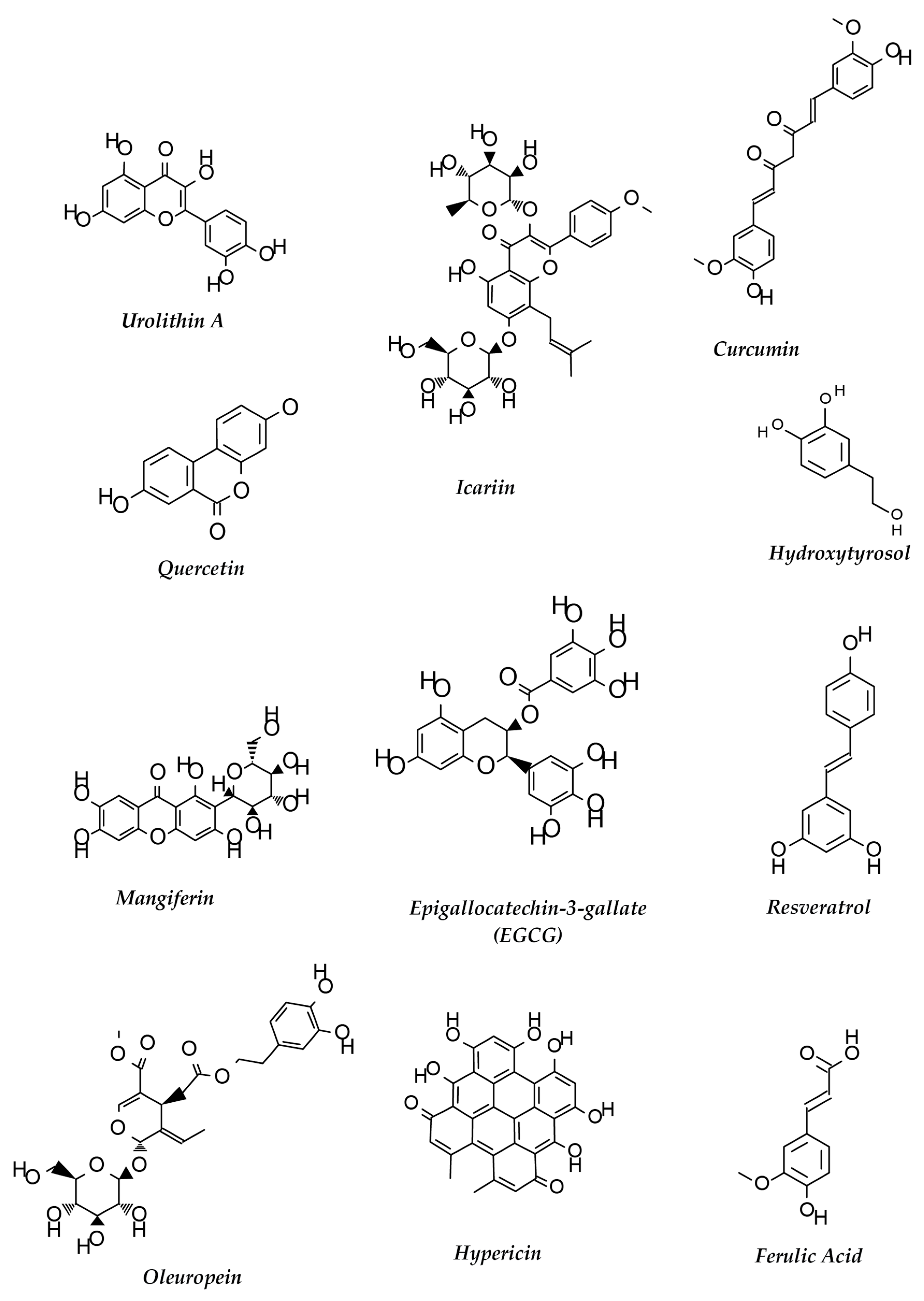
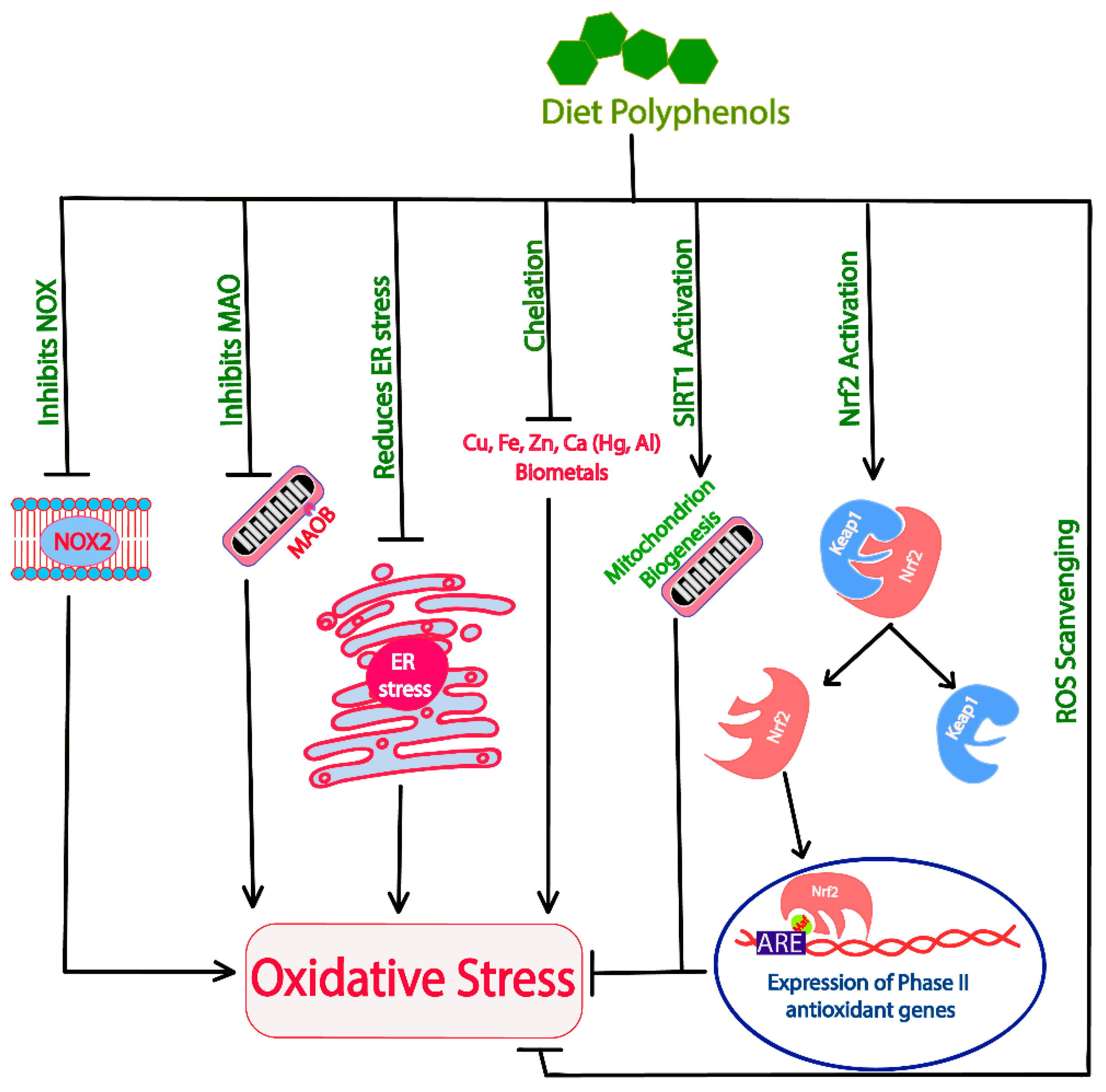
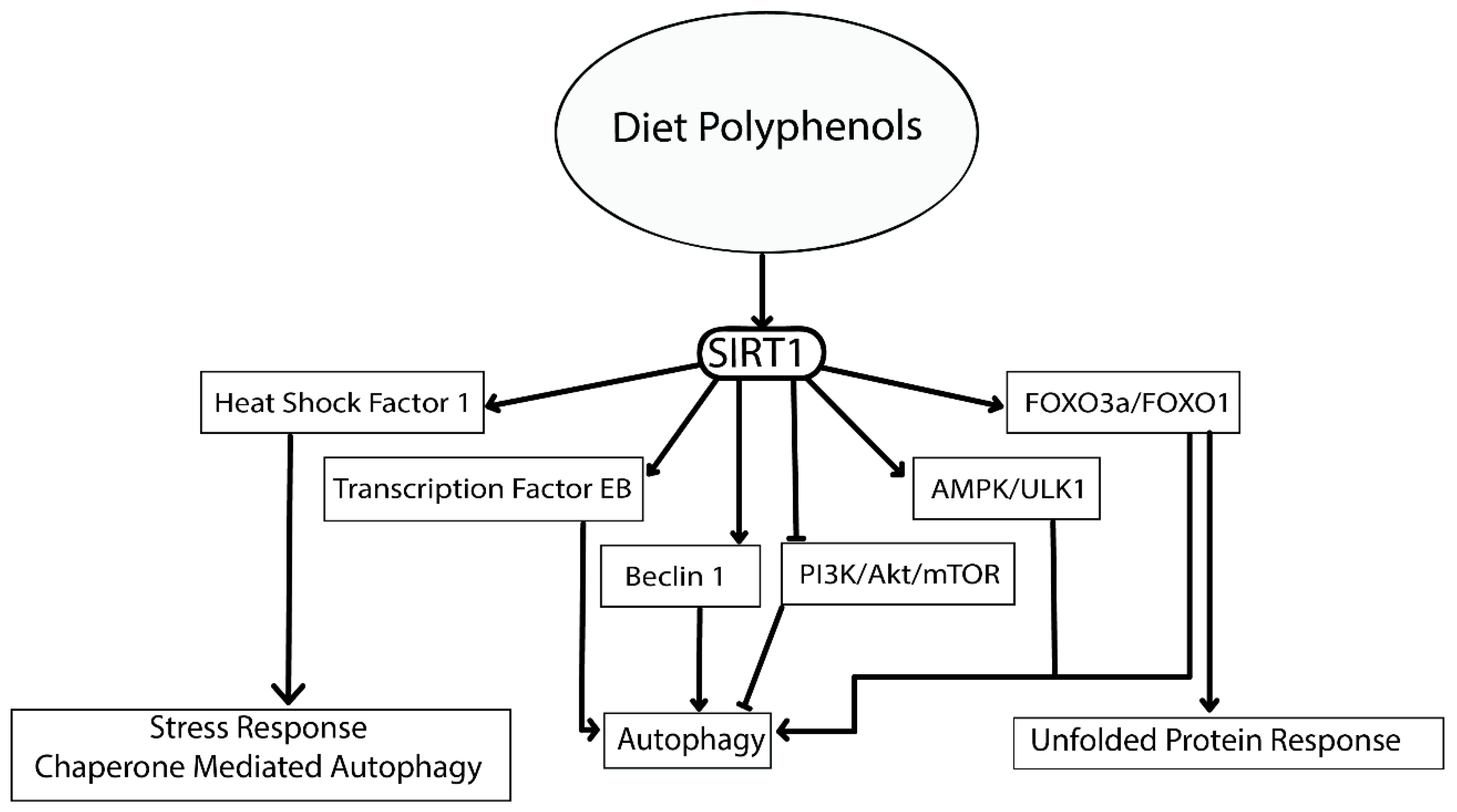

| Polyphenol | Analytical System | EPCa/ROAb | Effects of Polyphenols at Cellular Level | Effects in Relation to AD | Reference |
|---|---|---|---|---|---|
| Quercetin | In vitro | NA | mTORC inhibitor | Induces autophagy, anti-amyloidogenic, inhibits proteasomal degradation, antioxidant, restores biometal distribution, antiproliferative and enhances neuronal synapsis | [4,5,6,7,8] |
| ARPE 19 cells | 2 μM | TFEB activation | |||
| APPswe cells | 10 μM | Inhibits Aβ fibril formation | |||
| Rat neonatal cardiomyocytes | 5 μM | Inhibits all the catalytic subunits of proteasome | |||
| In vitro | NA | Chelates iron | |||
| In vitro | NA | Reduces ROS and RNS | |||
| In silico and in vitro | NA | Inhibits acetyl choline esterase | |||
| Resveratrol | Tg6799 mice | 60 mg/kg/d for 60 d/oral administration | Reduces amyloid plaque formation | Induces autophagy, increases lysosomal biogenesis, restores lipid homeostasis, increases stress resistance, regulates cell cycle, antiproliferative, anti-apoptotic, increases longevity and anti-inflammatory | [9,10,11,12,13,14] |
| Primary neuronal culture | 30 μM | SIRT1 activation and NFκB inhibition | |||
| Obese healthy men clinical trial | 150 mg/d for 30 d/oral administration | TFEB activation | |||
| Human aortic endothelial cells | 50 μM | AMPK mediated LC3II activation | |||
| Human aortic endothelial cells | 10 μM | Decreases ROS and RNS, increases SOD | |||
| LNCaP cells | 20 μM | p53 regulation, PI3K/Akt/mTOR inhibition, induces FOXO transcriptional activity including cell cycle regulation and stress resistance | |||
| Epigallocatechin gallate (EGCG) | Human bladder cancer cell line T24 | 20 μg/ml | Inhibits Beclin1 suppressors and PI3K/Akt/mTOR | Induces autophagy, restores lipid homeostasis, anti-amyloidogenic, increases antioxidant capacity, restores impaired autophagosomes and biometal distribution, increases cell survival | [15,16,17,18,19] |
| Bovine aortic endothelial cells | 10 μM | Increases LC3II formation and activates AMPK/ULK1 | |||
| HepG2 cells | 40 μM | Degrades lipid droplets through Ca2+/CAMKKB AMPK dependent mechanism | |||
| In vitro | NA | Chelates zinc and copper | |||
| PC12 cells (rat pheochromocytoma | 100 μg/mL | Interacts with Aβ40 and changes its conformation, inhibits lipofuscin formation | |||
| Anthocyanin | Sprague–Dawley rats | 100 mg/kg/d for 28 d/oral administration | Restores calcium homeostasis and activates Nrf2 subsequently activating phase II detoxifying genes | Activates autophagy, increases expression of anti-oxidant genes, reduces ROS and increases cell survival | [20,21,22,23] |
| HT22 cells and primary cultures of hippocampal neurons | 0.1 mg/mL | Induces AMPK | |||
| In vitro | 0.005 mg/mL | ROS scavenging | |||
| HCC cell lines PLC/PRF/5 and HepG2 cells | 0.2 mg/mL | Increase expression of Beclin1, LC3 II | |||
| Kaempferol | SK-HEP-1 human hepatic cancer cell | 75 μM | Increases the levels of p-AMPK, LC3-II, Atg 5, Atg 7, Atg 12 and beclin 1, inhibits PI3K/Akt/mTOR | Reduces mitochondrial dysfunction, anti-proliferative, increases autophagy, increases unfolded protein response, reduces APOE4 fragmentation and associated toxicity | [24,25,26,27] |
| BALB/c nude mice | 150 mg/kg/d for 31 d/intraperitoneal injection | Activates DNMT methyltransferase ubiquitination | |||
| SCC-4, human tongue squamous cell carcinoma cell | 50 µM | Activates IRE1-JNK-CHOP signaling, downregulates ERK1/2 signaling which reduces MMP2 | |||
| Hydroxytyrosol | Male db/db (C57BL/6J) mice | 10 mg/kg/d for 8 weeks/oral administration | Activates Nrf2 and SIRT1/AMPK/PGC-1, reduces protein oxidation, increases NMDAR1 and NGF mRNA expression | Enhances autophagy, increases stress resistance and longevity, antioxidant, anti-inflammatory, restores lipid homeostasis and improves cognition | [28,29,30,31,32,33] |
| VECs cells | 50 μM | Activates AMPK/FOXO3a | |||
| VECs cells | 10 μM | Reduces ROS | |||
| VAFs from Sprague–Dawley rats | 25 μM | Increases LC3II/LC3I, Bcl1 and SIRT1 expression | |||
| HepG2 and Huh7 cells | 100 μM | Inhibits PI3K/Akt/mTOR, expression of IL1β & IL6, and NFκB DNA binding | |||
| Rat hepatocytes | 25 μM | Inhibits Acetyl CoA carboxylase, HMG CoA reductase, diacylglycerol acyl transferase | |||
| Oleuropein aglycone | Rat ventricular myocyte | 100 μM | Increases Bcl1 and LC3II expression, TFEB nuclear localization, LAMP1 and p62 expression | Induces autophagy, increases lysosomal biogenesis and reduces oxidative damage | [33,34,35] |
| Human SH-SY5Y neuroblastoma cells and rat RIN5F insulinoma cells | 50 μM | Inhibits MAOA, induces AMPK/ULK1, inhibits mTOR | |||
| Rat hepatocytes | 25 μM | Inhibits acetyl CoA carboxylase, HMG CoA reductase and diacylglycerol acyl transferase | |||
| Curcumin | Male Sprague–Dawley rats | 15 mg/kg/d for 4 weeks/subcutaneous injection | Activates AMPK and regulates lipid metabolism | Induces autophagy, restores lipid homeostasis, antioxidant, anti-amyloidogenic, anti-inflammatory, anti-apoptotic antiproliferative, increases lysosomal biogenesis and longevity | [36,37,38,39,40,41,42,43,44,45] |
| Adult male Wistar rats | 30 mg/kg for 30 d/oral administration | Activates Nrf2, inhibits NFκB and mTOR | |||
| Adult Swiss male albino mice | 80 mg/kg/d for 7 d/intraperitoneal injection | Inhibits MaoB and reduces ROS | |||
| APPswe Tg2576 transgenic mice (chronic 500 ppm curcumin diet) | Blood curcumin level ~2 μM for 1 h/injection in right carotid artery | Inhibits formation of Aβ, oligomers, fibrils and plaques | |||
| Tsc2+/+, Tsc2−/− MEFs and HCT116 cells | 10 μM | Activates TFEB, increases levels of LC3 and inhibits pAkt | |||
| Sprague–Dawley rats’ primary cortical neurons | 10 μM | Upregulates SIRT1 and inhibits Bax | |||
| APP/PS1 double transgenic mice | 160 ppm for 6 months/oral administration | Inhibits PI3K/Akt/mTOR signaling, increases LC3I/II and Beclin1 expression | |||
| Myricetin | HepG2 Cells | 50 μM | Inhibits mTOR and increases LC3II expression | Induces autophagy, antiproliferative, increases stress resistance, longevity, antioxidant capacity and mitochondrial regeneration | [46,47,48] |
| Adipocytes differentiated from C3H10T1/2 cells | 10 μM | Activates SIRT1/SIRT3/SIRT5 | |||
| Male ICR mice | 50 mg/kg/d for 21 d/oral administration | Increases mitochondrial mass and increases PGC1α, SIRT1, TFAM, Nrf1 & FOXO1 | |||
| Urolithin A | C2C12 myoblasts | 50 μM | Induces mitophagy, increases LC3I/LC3II and activates AMPK signaling | Increases mitophagy, and autophagy, antioxidant, increases lysosomal biogenesis, anti-inflammatory, anti-amyloidogenic, improves cognition and longevity | [49,50] |
| Female APP/PS1 transgenic mice B6C3-Tg (APPswe, PS1dE9) 85Dbo/J and age-matched wild type mice | 300 mg/kg/d for 14 d/oral administration | Activates AMPK, decreases NFκB/MAPK/BACE1 activities and APP levels | |||
| Ferulic Acid | HeLa cells and mouse primary hepatocytes | 1 mM | Increases LC3 II and inhibits mTOR | Anti-apoptotic, anti-amyloidogenic, antioxidant, anti-inflammatory and induces autophagy | [51,52,53,54] |
| In vitro | NA | Inhibits Aβ aggregation and reduces ROS | |||
| (APP)swe/presenilin 1(PS1)dE9 (APP/PS1) mouse model | 5.3 mg/kg/d for 6 months/oral administration | Reduces amyloid deposition and interleukin-1 beta (IL-1β) levels | |||
| Acacetin | Drosophila melanogaster | 100 μM | Inhibits BACE1 | Anti-amyloidogenic, antioxidant, anti-inflammatory and induces autophagy | [55,56,57,58] |
| C57BL/6J mice | ∼10 mg/kg/d for 14 d/oral administration (gavage) | Inhibits MAPK and PI3K/Akt pathways | |||
| ICR mice | 100 mg/kg for 7 h/intraperitoneal injection | Increases LC3II, Atg5 and Atg7 expression, modulates TNF-α/IL-6 expression and suppresses TLR4 signaling | |||
| Baicalein | SH-SY5Y human neuroblastoma cells | 12.5 μM | Increases ROS scavenging and activates Nrf2 | Anti-amyloidogenic, anti-apoptotic, antioxidant, anti-inflammatory, inhibits excitotoxicity, stimulates neurogenesis and neuronal differentiation | [59,60,61,62,63,64,65] |
| In vitro | NA | Chelates iron | |||
| CHO/APPwt cells | 5 μM | Induces α-secretase and inhibits Aβ formation | |||
| In vitro | 30 μM | Dissociates amyloid aggregates, Aβ oligomerization and fibrillation | |||
| HeLa cells | 100 μM | Inhibits NFκB activation | |||
| C57BL/6J APP/PS1 mice | 80 mg/kg/d for 60 d/oral administration (drinking water) | Inhibits GSK3β mediated tau phosphorylation | |||
| Sprague-Dawley male rats | 20 mg/kg 30 min before and 2/4 h after onset of ischemia/intraperitoneal injection | Induces Bcl-2/Bcl-xL associated phosphorylation | |||
| Icariin | Primary cortical neurons prepared from E16-17 mouse embryos | 1.2 μM | Activates SIRT1 | Antioxidant, anti-amyloidogenic, reduces ER stress, increases synapsis and neuronal plasticity, inhibits tau hyperphosphorylation, increases cell viability, antiapoptotic and anti-inflammatory | [66,67,68,69,70,71,72,73] |
| Wistar rats | 60 mg/kg/d for 3 months/oral administration | Increases SOD activity | |||
| Tg2576 mouse model | 60 mg/kg/d for 3 months/oral administration | Reduces expression of BACE1 and APP | |||
| Sprague-Dawley rats | 120 mg/kg/d for 28 d/oral administration | Induces PSD95, BDNF, pTrkB, pAkt, and pCREB expression | |||
| SH-SY5Y cells | 1 μM | Inhibits GSK3β activation | |||
| PC12 cells | 10 μM | Inhibits JNK/p38, MAPK and p53 activity | |||
| HT29 and HCT116 | 20 μM | Inhibits NFκB signaling | |||
| Nobiletin | Male 3XTg-AD mice | 30 mg/kg/d for 3 months/intraperitoneal injection | Reduces Aβ levels and plaque formation in brain | Anti-amyloidogenic, increases stress resistance, neuronal synapsis and plasticity, antioxidant and anti-inflammatory | [74,75,76,77] |
| Male Sprague-Dawley rats | 25 mg/kg/d for 3d/intraperitoneal injection | Increases activity of Akt, CREB, BDNF and Bcl2, increases Nrf2, HO-1, SOD1 and GSH expression, reduces NFκB, MMP-9 and MDA expression | |||
| Genistein | In silico and in vitro | NA | Inhibits chymotrypsin-like activity of proteasomes | Antioxidant, increases degradation of Aβ, increases apoptosis, enhances autophagy and inhibits proteasomal protein degradation | [78,79,80,81] |
| LNCaP cells | 100 μM | Increases Kip1 and reduces IκBα/Bax | |||
| Human dermal fibroblasts (HDFa) | 30 μM | Increases TFEB expression | |||
| Human mammary gland tumor cells (MCF-7) | 0.5 μM | Enhances antioxidant gene expression | |||
| Luteolin | HT-29 cells | 50 μM | Reduces ROS, NFκB signaling, Cox2 expression, blocks JAK/STAT signaling | Anti-inflammatory, antioxidant, modulates autophagy and apoptosis, increases survival | [82,83,84,85,86] |
| Male Sprague-Dawley rat myocytes | 8 μM | Downregulates Bax expression, upregulates PI3k/Akt signaling and Bcl-2 expression | |||
| Human HCC cell line SMMC-772 | 100 μM | Increases expression of LC3B-II, Bcl1 and caspase 8 | |||
| Mangiferin | Swiss albino male rats | 15 mg/kg/d for 14 d/intraperitoneal injection | Increases ROS scavenging, activates Nrf2, inhibits NFκB signaling, increases GSH levels, decreases lipid peroxidation | Antioxidant, anti-apoptotic, chelates metals, increases stress resistance, autophagy, longevity, neuronal synapsis and plasticity | [87,88,89,90,91] |
| In vitro | NA | Rescues mitochondrial respiration, chelates iron | |||
| Male Swiss albino mice | 40 mg/kg/d for 21 d/oral administration | Reduces lipid peroxides and ROS/RNS induced by aluminum and restores regulation of BDNF and NGF | |||
| Human astroglioma U87MG, U373MG and CRT-MG cells | 100 μM | Inhibits PI3K/Akt signaling, MAPK pathway, MMP9 gene expression |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhakal, S.; Kushairi, N.; Phan, C.W.; Adhikari, B.; Sabaratnam, V.; Macreadie, I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 5090. https://doi.org/10.3390/ijms20205090
Dhakal S, Kushairi N, Phan CW, Adhikari B, Sabaratnam V, Macreadie I. Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. International Journal of Molecular Sciences. 2019; 20(20):5090. https://doi.org/10.3390/ijms20205090
Chicago/Turabian StyleDhakal, Sudip, Naufal Kushairi, Chia Wei Phan, Benu Adhikari, Vikineswary Sabaratnam, and Ian Macreadie. 2019. "Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease" International Journal of Molecular Sciences 20, no. 20: 5090. https://doi.org/10.3390/ijms20205090
APA StyleDhakal, S., Kushairi, N., Phan, C. W., Adhikari, B., Sabaratnam, V., & Macreadie, I. (2019). Dietary Polyphenols: A Multifactorial Strategy to Target Alzheimer’s Disease. International Journal of Molecular Sciences, 20(20), 5090. https://doi.org/10.3390/ijms20205090