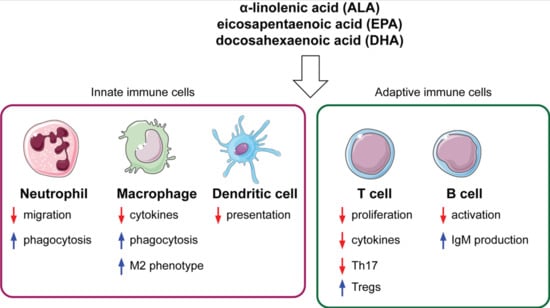
Effects of Omega-3 Fatty Acids on Immune Cells
Abstract
1. Introduction
2. Effects of Omega-3 Fatty Acids on Macrophage Function
2.1. Regulation of the Production and Secretion of Cytokines in Macrophages by Omega-3 Fatty Acids
2.2. Effects on Macrophage Polarization by Omega-3 Fatty Acids
2.3. Effects of Omega-3 Fatty Acids on the Phagocytic Capacity of Macrophages
3. Effects of Omega-3 Fatty Acids on Neutrophil Function
3.1. Effects on Neutrophil Migration and Transmigration by Omega-3 Fatty Acids
3.2. Effects of Omega-3 Fatty Acids on the Phagocytic Capacity of Neutrophils
3.3. Effects of Omega-3 Fatty Acids on the Production of Reactive Oxygen Species
3.4. Other Aspects
4. Effects of Omega-3 Fatty Acids on T Cells
4.1. General Effects of Omega-3 Fatty Acids on T Cells
4.2. Specific Effects of Omega-3 Fatty Acids on the Different Subgroups of T Cells
4.2.1. CD4 T Cells
4.2.2. Th17 Cells
4.2.3. Regulatory T Cells
5. Effects of Omega-3 Fatty Acids on B Cells
5.1. Effects of Omega-3 Fatty Acids on B Cell Populations
5.2. Effects of Omega-3 Fatty Acids on B Cell Activation and Antibody Production
6. Effects of Omega-3 Fatty Acids on Other Immune Cells
6.1. Dendritic Cells
6.2. Natural Killer Cells
6.3. Mast Cells
6.4. Basophils
6.5. Eosinophils
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ALA | Alpha (α)-linolenic acid |
| DHA | docosahexaenoic acid |
| EPA | eicosapentaenoic acid |
| PAMPS | pathogen-associated molecular patterns |
| PUFAs | polyunsaturated fatty acids |
| ROS | reactive oxygen species |
| SPMs | pro-resolving mediators |
References
- Sokol, C.L.; Luster, A.D. The chemokine system in innate immunity. Cold Spring Harb. Perspect Biol. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, A.; Medzhitov, R. Control of adaptive immunity by the innate immune system. Nat. Immunol. 2015, 16, 343–353. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Lewis, E.D.; Pae, M.; Meydani, S.N. Nutritional Modulation of Immune Function: Analysis of Evidence, Mechanisms, and Clinical Relevance. Front. Immunol. 2018, 9, 3160. [Google Scholar] [CrossRef] [PubMed]
- Paschoal, V.A.; Vinolo, M.A.; Crisma, A.R.; Magdalon, J.; Curi, R. Eicosapentaenoic (EPA) and docosahexaenoic (DHA) acid differentially modulate rat neutrophil function in vitro. Lipids 2013, 48, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Metabolic benefits of marine n-3 fatty acids demonstrated in nonhuman primates. J. Nutr. 2014, 144, 1–2. [Google Scholar] [CrossRef]
- Cholewski, M.; Tomczykowa, M.; Tomczyk, M. A Comprehensive Review of Chemistry, Sources and Bioavailability of Omega-3 Fatty Acids. Nutrients 2018, 10, 1662. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Docosahexaenoic Acid. Ann. Nutr. Metab. 2016, 69, 7–21. [Google Scholar] [CrossRef]
- Wiktorowska-Owczarek, A.; Berezinska, M.; Nowak, J.Z. PUFAs: Structures, Metabolism and Functions. Adv. Clin. Exp. Med. 2015, 24, 931–941. [Google Scholar] [CrossRef]
- Metherel, A.H.; Lacombe, R.J.S.; Chouinard-Watkins, R.; Hopperton, K.E.; Bazinet, R.P. Complete assessment of whole-body n-3 and n-6 PUFA synthesis-secretion kinetics and DHA turnover in a rodent model. J. Lipid Res. 2018, 59, 357–367. [Google Scholar] [CrossRef]
- Rodrigues, F.G.; Campos, J.B.; Silva, G.D.; Wexner, S.D. Endoscopic ultrasound in the diagnosis of foreign bodies of the colon and rectum. Rev. Assoc. Med. Bras. 2016, 62, 818–821. [Google Scholar] [CrossRef]
- Calder, P.C. Polyunsaturated fatty acids and inflammation. Biochem Soc. Trans. 2005, 33, 423–427. [Google Scholar] [CrossRef]
- Yessoufou, A.; Ple, A.; Moutairou, K.; Hichami, A.; Khan, N.A. Docosahexaenoic acid reduces suppressive and migratory functions of CD4(+)CD25(+) regulatory T-cells. J. Lipid Res. 2009, 50, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, L.S.; Thorlacius-Ussing, O.; Rasmussen, H.H.; Lundbye-Christensen, S.; Calder, P.C.; Lindorff-Larsen, K.; Schmidt, E.B. Effects of perioperative supplementation with omega-3 fatty acids on leukotriene B(4) and leukotriene B(5) production by stimulated neutrophils in patients with colorectal cancer: A randomized, placebo-controlled intervention trial. Nutrients 2014, 6, 4043–4057. [Google Scholar] [CrossRef] [PubMed]
- Gurzell, E.A.; Teague, H.; Harris, M.; Clinthorne, J.; Shaikh, S.R.; Fenton, J.I. DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J. Leukoc. Biol. 2013, 93, 463–470. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, M.; Hossain, S. Fatty Acids: From Membrane Ingredients to Signaling Molecules. In Biochemistry and Health Benefits of Fatty Acids; Waisundara, V., Ed.; IntechOpen Limited: London, UK, 2018. [Google Scholar] [CrossRef]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef] [PubMed]
- Fritsche, K. Fatty acids as modulators of the immune response. Annu. Rev. Nutr. 2006, 26, 45–73. [Google Scholar] [CrossRef] [PubMed]
- Husson, M.O.; Ley, D.; Portal, C.; Gottrand, M.; Hueso, T.; Desseyn, J.L.; Gottrand, F. Modulation of host defence against bacterial and viral infections by omega-3 polyunsaturated fatty acids. J. Infect. 2016, 73, 523–535. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Li, F.; Liu, S.; Jin, Y.; Zhang, X.; Yang, T.; Dai, Y.; Li, X.; Zhao, A.Z. omega-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J. Clin. Invest. 2017, 127, 1757–1771. [Google Scholar] [CrossRef]
- Gordon, S.; Pluddemann, A.; Estrada, F.M. Macrophage heterogeneity in tissues: phenotypic diversity and functions. Immunol. Rev. 2014, 262, 36–55. [Google Scholar] [CrossRef]
- Murray, P.J. Macrophage Polarization. Annu Rev. Physiol. 2017, 79, 541–566. [Google Scholar] [CrossRef]
- Magrum, L.J.; Johnston, P.V. Modulation of prostaglandin synthesis in rat peritoneal macrophages with omega-3 fatty acids. Lipids 1983, 18, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Schroit, A.J.; Gallily, R. Macrophage fatty acid composition and phagocytosis: effect of unsaturation on cellular phagocytic activity. Immunology 1979, 36, 199–205. [Google Scholar] [PubMed]
- Allam-Ndoul, B.; Guenard, F.; Barbier, O.; Vohl, M.C. A Study of the Differential Effects of Eicosapentaenoic Acid (EPA) and Docosahexaenoic Acid (DHA) on Gene Expression Profiles of Stimulated Thp-1 Macrophages. Nutrients 2017, 9, 424. [Google Scholar] [CrossRef] [PubMed]
- Roessler, C.; Kuhlmann, K.; Hellwing, C.; Leimert, A.; Schumann, J. Impact of Polyunsaturated Fatty Acids on miRNA Profiles of Monocytes/Macrophages and Endothelial Cells-A Pilot Study. Int. J. Mol. Sci. 2017, 18, 284. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Fatty acids and inflammation: The cutting edge between food and pharma. Eur. J. Pharmacol. 2011, 668, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Oh, D.Y.; Talukdar, S.; Bae, E.J.; Imamura, T.; Morinaga, H.; Fan, W.Q.; Li, P.P.; Lu, W.J.; Watkins, S.M.; Olefsky, J.M. GPR120 Is an Omega-3 Fatty Acid Receptor Mediating Potent Anti-inflammatory and Insulin-Sensitizing Effects. Cell 2010, 142, 687–698. [Google Scholar] [CrossRef] [PubMed]
- Mildenberger, J.; Johansson, I.; Sergin, I.; Kjobli, E.; Damas, J.K.; Razani, B.; Flo, T.H.; Bjorkoy, G. N-3 PUFAs induce inflammatory tolerance by formation of KEAP1-containing SQSTM1/p62-bodies and activation of NFE2L2. Autophagy 2017, 13, 1664–1678. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.Y.; Lee, H.N.; Kim, W.; Surh, Y.J. Docosahexaenoic acid induces M2 macrophage polarization through peroxisome proliferator-activated receptor gamma activation. Life Sci. 2015, 120, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Titos, E.; Rius, B.; Gonzalez-Periz, A.; Lopez-Vicario, C.; Moran-Salvador, E.; Martinez-Clemente, M.; Arroyo, V.; Claria, J. Resolvin D1 and Its Precursor Docosahexaenoic Acid Promote Resolution of Adipose Tissue Inflammation by Eliciting Macrophage Polarization toward an M2-Like Phenotype. J. Immun. 2011, 187, 5408–5418. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Jiang, W.; Spinetti, T.; Tardivel, A.; Castillo, R.; Bourquin, C.; Guarda, G.; Tian, Z.; Tschopp, J.; Zhou, R. Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 2013, 38, 1154–1163. [Google Scholar] [CrossRef]
- Jin, J.F.; Lu, Z.Y.; Li, Y.C.; Cowart, L.A.; Lopes-Virella, M.F.; Yan, H. Docosahexaenoic acid antagonizes the boosting effect of palmitic acid on LPS inflammatory signaling by inhibiting gene transcription and ceramide synthesis. PLoS ONE 2018, 13, e0193343. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Gupta, G.; Anilkumar, K.; Fatima, N.; Karnati, R.; Reddy, G.V.; Giri, P.V.; Reddanna, P. 15-Lipoxygenase metabolites of alpha-linolenic acid, [13-(S)-HPOTrE and 13-(S)-HOTrE], mediate anti-inflammatory effects by inactivating NLRP3 inflammasome. Sci. Rep. 2016, 6, 31649. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.L.; Lamon-Fava, S.; Matthan, N.R.; Wu, D.Y.; Lichtenstein, A.H. Docosahexaenoic acid differentially affects TNF alpha and IL-6 expression in LPS-stimulated RAW 264.7 murine macrophages. Prostaglandins Leukot. Essent. Fat. Acids 2015, 97, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Guenard, F.; Barbier, O.; Vohl, M.C. Effect of different concentrations of omega-3 fatty acids on stimulated THP-1 macrophages. Genes Nutr. 2017, 12, 7. [Google Scholar] [CrossRef] [PubMed]
- Takashima, A.; Fukuda, D.; Tanaka, K.; Higashikuni, Y.; Hirata, Y.; Nishimoto, S.; Yagi, S.; Yamada, H.; Soeki, T.; Wakatsuki, T.; et al. Combination of n-3 polyunsaturated fatty acids reduces atherogenesis in apolipoprotein E-deficient mice by inhibiting macrophage activation. Atherosclerosis 2016, 254, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Chen, L.Y.; Sokolowska, M.; Eberlein, M.; Alsaaty, S.; Martinez-Anton, A.; Logun, C.; Qi, H.Y.; Shelhamer, J.H. The fish oil ingredient, docosahexaenoic acid, activates cytosolic phospholipase A(2) via GPR120 receptor to produce prostaglandin E(2) and plays an anti-inflammatory role in macrophages. Immunology 2014, 143, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.L.; Lamon-Fava, S.; Matthan, N.R.; Wu, D.Y.; Lichtenstein, A.H. EPA and DHA Exposure Alters the Inflammatory Response but not the Surface Expression of Toll-like Receptor 4 in Macrophages. Lipids 2015, 50, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.X.; Xia, H.; Yang, L.G.; Wang, S.K.; Sun, G.J. Lowering the n-6/n-3 PUFAs ratio inhibits the formation of THP-1 macrophage-derived foam cell. Lipids Health Dis. 2018, 17, 125. [Google Scholar] [CrossRef]
- Schoeniger, A.; Adolph, S.; Fuhrmann, H.; Schumann, J. The Impact of Membrane Lipid Composition on Macrophage Activation in the Immune Defense against Rhodococcus equi and Pseudomonas aeruginosa. Int. J. Mol. Sci. 2011, 12, 7510–7528. [Google Scholar] [CrossRef]
- Iverson, C.; Bacong, A.; Liu, S.; Baumgartner, S.; Lundstrom, T.; Oscarsson, J.; Miner, J.N. Omega-3-carboxylic acids provide efficacious anti-inflammatory activity in models of crystal-mediated inflammation. Sci. Rep. 2018, 8, 1217. [Google Scholar] [CrossRef]
- Williams-Bey, Y.; Boularan, C.; Vural, A.; Huang, N.N.; Hwang, I.Y.; Shan-Shi, C.; Kehrl, J.H. Omega-3 free fatty acids suppress macrophage inflammasome activation by inhibiting NF-kappaB activation and enhancing autophagy. PLoS ONE 2014, 9, e97957. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.Q.; Hansson, G.K. Innate immunity, macrophage activation, and atherosclerosis. Immunol Rev. 2007, 219, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Schoeniger, A.; Fuhrmann, H.; Schumann, J. LPS- or Pseudomonas aeruginosa-mediated activation of the macrophage TLR4 signaling cascade depends on membrane lipid composition. PeerJ 2016, 4, e1663. [Google Scholar] [CrossRef] [PubMed]
- Hellwing, C.; Schoeniger, A.; Roessler, C.; Leimert, A.; Schumann, J. Lipid raft localization of TLR2 and its co-receptors is independent of membrane lipid composition. PeerJ 2018, 6, 4212. [Google Scholar] [CrossRef]
- Lee, J.Y.; Plakidas, A.; Lee, W.H.; Heikkinen, A.; Chanmugam, P.; Bray, G.; Hwang, D.H. Differential modulation of Toll-like receptors by fatty acids: preferential inhibition by n-3 polyunsaturated fatty acids. J. Lipid Res. 2003, 44, 479–486. [Google Scholar] [CrossRef]
- Sung, J.; Jeon, H.; Kim, I.H.; Jeong, H.S.; Lee, J. Anti-Inflammatory Effects of Stearidonic Acid Mediated by Suppression of NF-kappa B and MAP-Kinase Pathways in Macrophages. Lipids 2017, 52, 781–787. [Google Scholar] [CrossRef]
- Marques-Rocha, J.L.; Garcia-Lacarte, M.; Samblas, M.; Bressan, J.; Martinez, J.A.; Milagro, F.I. Regulatory roles of miR-155 and let-7b on the expression of inflammation-related genes in THP-1 cells: effects of fatty acids. J. Physiol. Biochem. 2018, 74, 579–589. [Google Scholar] [CrossRef]
- Ohue-Kitano, R.; Yasuoka, Y.; Goto, T.; Kitamura, N.; Park, S.B.; Kishino, S.; Kimura, I.; Kasubuchi, M.; Takahashi, H.; Li, Y.J.; et al. alpha-Linolenic acid-derived metabolites from gut lactic acid bacteria induce differentiation of anti-inflammatory M2 macrophages through G protein-coupled receptor 40. Faseb J. 2018, 32, 304–318. [Google Scholar] [CrossRef]
- Cai, W.; Liu, S.X.; Hu, M.Y.; Sun, X.B.; Qiu, W.; Zheng, X.M.; Hu, X.M.; Lu, Z.Q. Post-stroke DHA Treatment Protects Against Acute Ischemic Brain Injury by Skewing Macrophage Polarity Toward the M2 Phenotype. Transl. Stroke Res. 2018, 9, 669–680. [Google Scholar] [CrossRef]
- Haitz, K.A.; Anandasabapathy, N. Docosahexaenoic Acid alleviates atopic dermatitis in mice by generating T regulatory cells and m2 macrophages. J. Invest. Dermatol 2015, 135, 1472–1474. [Google Scholar] [CrossRef]
- Adolph, S.; Fuhrmann, H.; Schumann, J. Unsaturated Fatty Acids Promote the Phagocytosis of P-aeruginosa and R-equi by RAW264.7 Macrophages. Curr. Microbiol. 2012, 65, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Davidson, J.; Kerr, A.; Guy, K.; Rotondo, D. Prostaglandin and fatty acid modulation of Escherichia coli O157 phagocytosis by human monocytic cells. Immunology 1998, 94, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Hellwing, C.; Tigistu-Sahle, F.; Fuhrmann, H.; Kakela, R.; Schumann, J. Lipid composition of membrane microdomains isolated detergent-free from PUFA supplemented RAW264.7 macrophages. J. Cell. Physiol. 2018, 233, 2602–2612. [Google Scholar] [CrossRef] [PubMed]
- Athens, J.W.; Haab, O.P.; Raab, S.O.; Mauer, A.M.; Ashenbrucker, H.; Cartwright, G.E.; Wintrobe, M.M. Leukokinetic studies. IV. The total blood, circulating and marginal granulocyte pools and the granulocyte turnover rate in normal subjects. J. Clin. Invest. 1961, 40, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Athens, J.W.; Raab, S.O.; Haab, O.P.; Mauer, A.M.; Ashenbrucker, H.; Cartwright, G.E.; Wintrobe, M.M. Leukokinetic studies. III. The distribution of granulocytes in the blood of normal subjects. J. Clin. Invest. 1961, 40, 159–164. [Google Scholar] [CrossRef]
- Summers, C.; Rankin, S.M.; Condliffe, A.M.; Singh, N.; Peters, A.M.; Chilvers, E.R. Neutrophil kinetics in health and disease. Trends Immunol. 2010, 31, 318–324. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Puga, I.; Cols, M.; Barra, C.M.; He, B.; Cassis, L.; Gentile, M.; Comerma, L.; Chorny, A.; Shan, M.; Xu, W.; et al. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat. Immunol. 2012, 13, 170–180. [Google Scholar] [CrossRef]
- Veno, S.K.; Nielsen, M.R.; Lundbye-Christensen, S.; Schmidt, E.B.; Handberg, A. The effect of low-dose marine n-3 fatty acids on plasma levels of sCD36 in overweight subjects: a randomized, double-blind, placebo-controlled trial. Mar. Drugs 2013, 11, 3324–3334. [Google Scholar] [CrossRef]
- Serhan, C.N.; Chiang, N.; Dalli, J.; Levy, B.D. Lipid mediators in the resolution of inflammation. Cold Spring Harb Perspect Biol. 2014, 7, a016311. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.N.; Dalli, J.; Dimisko, L.; Wong, E.; Serhan, C.N.; Irimia, D. Microfluidic chambers for monitoring leukocyte trafficking and humanized nano-proresolving medicines interactions. Proc. Natl. Acad. Sci. USA 2012, 109, 20560–20565. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Recchiuti, A.; Chiang, N.; Yacoubian, S.; Lee, C.H.; Yang, R.; Petasis, N.A.; Serhan, C.N. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proc. Natl. Acad. Sci. USA 2010, 107, 1660–1665. [Google Scholar] [CrossRef] [PubMed]
- Tull, S.P.; Yates, C.M.; Maskrey, B.H.; O’Donnell, V.B.; Madden, J.; Grimble, R.F.; Calder, P.C.; Nash, G.B.; Rainger, G.E. Omega-3 Fatty acids and inflammation: novel interactions reveal a new step in neutrophil recruitment. PLoS Biol. 2009, 7, e1000177. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Winkler, J.W.; Colas, R.A.; Arnardottir, H.; Cheng, C.Y.; Chiang, N.; Petasis, N.A.; Serhan, C.N. Resolvin D3 and aspirin-triggered resolvin D3 are potent immunoresolvents. Chem. Biol. 2013, 20, 188–201. [Google Scholar] [CrossRef]
- Serhan, C.N.; Yang, R.; Martinod, K.; Kasuga, K.; Pillai, P.S.; Porter, T.F.; Oh, S.F.; Spite, M. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. J. Exp. Med. 2009, 206, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Wang, C.W.; Arnardottir, H.H.; Li, Y.; Cheng, C.Y.; Dalli, J.; Serhan, C.N. Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS ONE 2014, 9, e102362. [Google Scholar] [CrossRef]
- Serhan, C.N.; Fredman, G.; Yang, R.; Karamnov, S.; Belayev, L.S.; Bazan, N.G.; Zhu, M.; Winkler, J.W.; Petasis, N.A. Novel proresolving aspirin-triggered DHA pathway. Chem. Biol. 2011, 18, 976–987. [Google Scholar] [CrossRef]
- Gorjao, R.; Verlengia, R.; Lima, T.M.; Soriano, F.G.; Boaventura, M.F.; Kanunfre, C.C.; Peres, C.M.; Sampaio, S.C.; Otton, R.; Folador, A.; et al. Effect of docosahexaenoic acid-rich fish oil supplementation on human leukocyte function. Clin. Nutr. 2006, 25, 923–938. [Google Scholar] [CrossRef]
- Arnardottir, H.H.; Freysdottir, J.; Hardardottir, I. Dietary fish oil increases the proportion of a specific neutrophil subpopulation in blood and total neutrophils in peritoneum of mice following endotoxin-induced inflammation. J. Nutr. Biochem. 2013, 24, 248–255. [Google Scholar] [CrossRef]
- Svahn, S.L.; Ulleryd, M.A.; Grahnemo, L.; Stahlman, M.; Boren, J.; Nilsson, S.; Jansson, J.O.; Johansson, M.E. Dietary Omega-3 Fatty Acids Increase Survival and Decrease Bacterial Load in Mice Subjected to Staphylococcus aureus-Induced Sepsis. Infect Immun. 2016, 84, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Pisani, L.F.; Lecchi, C.; Invernizzi, G.; Sartorelli, P.; Savoini, G.; Ceciliani, F. In vitro modulatory effect of omega-3 polyunsaturated fatty acid (EPA and DHA) on phagocytosis and ROS production of goat neutrophils. Vet. Immunol. Immunopathol. 2009, 131, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Rees, D.; Miles, E.A.; Banerjee, T.; Wells, S.J.; Roynette, C.E.; Wahle, K.W.; Calder, P.C. Dose-related effects of eicosapentaenoic acid on innate immune function in healthy humans: a comparison of young and older men. Am. J. Clin. Nutr. 2006, 83, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Svahn, S.L.; Gutierrez, S.; Ulleryd, M.A.; Nookaew, I.; Osla, V.; Beckman, F.; Nilsson, S.; Karlsson, A.; Jansson, J.O.; Johansson, M.E. Dietary polyunsaturated fatty acids promote neutrophil accumulation in spleen by altering chemotaxis and delaying cell death. Infect. Immun. 2019, 87, 270. [Google Scholar] [CrossRef] [PubMed]
- Duriancik, D.M.; Comstock, S.S.; Langohr, I.M.; Fenton, J.I. High levels of fish oil enhance neutrophil development and activation and influence colon mucus barrier function in a genetically susceptible mouse model. J. Nutr. Biochem. 2015, 26, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Mukaro, V.R.; Costabile, M.; Murphy, K.J.; Hii, C.S.; Howe, P.R.; Ferrante, A. Leukocyte numbers and function in subjects eating n-3 enriched foods: selective depression of natural killer cell levels. Arthritis Res. Ther. 2008, 10. [Google Scholar] [CrossRef]
- Rosales, C. Neutrophil: A Cell with Many Roles in Inflammation or Several Cell Types? Front. Physiol. 2018, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Capo, X.; Martorell, M.; Sureda, A.; Tur, J.A.; Pons, A. Effects of docosahexaenoic supplementation and in vitro vitamin C on the oxidative and inflammatory neutrophil response to activation. Oxid Med. Cell Longev. 2015, 2015, 187849. [Google Scholar] [CrossRef]
- Spinosa, M.; Su, G.; Salmon, M.D.; Lu, G.; Cullen, J.M.; Fashandi, A.Z.; Hawkins, R.B.; Montgomery, W.; Meher, A.K.; Conte, M.S.; et al. Resolvin D1 decreases abdominal aortic aneurysm formation by inhibiting NETosis in a mouse model. J. Vasc. Surg. 2018, 68, 93S–103S. [Google Scholar] [CrossRef]
- Xu, R. Important Bioactive Properties of Omega-3 Fatty Acids. Ital. J. Food Sci. 2015, 27, 129–135. [Google Scholar]
- Gagliani, N.; Huber, S. Basic Aspects of T Helper Cell Differentiation. Methods Mol. Biol. 2017, 1514, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Bettelli, E.; Oukka, M.; Kuchroo, V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009, 27, 485–517. [Google Scholar] [CrossRef] [PubMed]
- Sharabi, A.; Tsokos, M.G.; Ding, Y.; Malek, T.R.; Klatzmann, D.; Tsokos, G.C. Regulatory T cells in the treatment of disease. Nat. Rev. Drug Discov. 2018, 17, 823–844. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Bettelli, E.; Carrier, Y.J.; Gao, W.D.; Korn, T.; Strom, T.B.; Oukka, M.; Weiner, H.L.; Kuchroo, V.K. Reciprocal developmental pathways for the generation of pathogenic effector T(H)17 and regulatory T cells. Nature 2006, 441, 235–238. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Fukuhara, A.; Shin, J.; Hayakawa, T.; Otsuki, M.; Shimomura, I. Eicosapentaenoic acid and 5-HEPE enhance macrophage-mediated Treg induction in mice. Sci. Rep. 2017, 7, 4560. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, J.A.; Wold, A.E.; Sandberg, A.S.; Ostman, S.M. The Polyunsaturated Fatty Acids Arachidonic Acid and Docosahexaenoic Acid Induce Mouse Dendritic Cells Maturation but Reduce T-Cell Responses In Vitro. PLoS ONE 2015, 10, 0143741. [Google Scholar] [CrossRef]
- Endres, S.; Meydani, S.N.; Ghorbani, R.; Schindler, R.; Dinarello, C.A. Dietary supplementation with n-3 fatty acids suppresses interleukin-2 production and mononuclear cell proliferation. J. Leukoc. Biol. 1993, 54, 599–603. [Google Scholar] [CrossRef]
- Yaqoob, P.; Newsholme, E.A.; Calder, P.C. The effect of dietary lipid manipulation on rat lymphocyte subsets and proliferation. Immunology 1994, 82, 603–610. [Google Scholar]
- Li, Y.L.; Tang, Y.; Wang, S.J.; Zhou, J.; Zhou, J.; Lu, X.; Bai, X.C.; Wang, X.Y.; Chen, Z.L.; Zuo, D.M. Endogenous n-3 Polyunsaturated Fatty Acids Attenuate T Cell-Mediated Hepatitis via Autophagy Activation. Front. Immunol. 2016, 7, 350. [Google Scholar] [CrossRef]
- Farjadian, S.; Moghtaderi, M.; Kalani, M.; Gholami, T.; Teshnizi, S.H. Effects of omega-3 fatty acids on serum levels of T-helper cytokines in children with asthma. Cytokine 2016, 85, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.H.; Hou, Y.C.; Pai, M.H.; Yeh, C.L.; Yeh, S.L. Dietary omega-6/omega-3 Polyunsaturated Fatty Acid Ratios Affect the Homeostasis of Th/Treg Cells in Mice With Dextran Sulfate Sodium-Induced Colitis. JPEN J. Parenter. Enter. Nutr. 2017, 41, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Banerjee, T.; Wells, S.J.; Calder, P.C. Limited effect of eicosapentaenoic acid on T-lymphocyte and natural killer cell numbers and functions in healthy young males. Nutrition 2006, 22, 512–519. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; McMurray, D.N.; Chapkin, R.S. n3 PUFAs Reduce Mouse CD4(+) T-Cell Ex Vivo Polarization into Th17 Cells. J. Nutr. 2013, 143, 1501–1508. [Google Scholar] [CrossRef] [PubMed]
- Monk, J.M.; Hou, T.Y.; Turk, H.F.; Weeks, B.; Wu, C.D.; McMurray, D.N.; Chapkin, R.S. Dietary n-3 Polyunsaturated Fatty Acids (PUFA) Decrease Obesity-Associated Th17 Cell-Mediated Inflammation during Colitis. PLoS ONE 2012, 7, 49739. [Google Scholar] [CrossRef] [PubMed]
- Chiurchiu, V.; Leuti, A.; Dalli, J.; Jacobsson, A.; Battistini, L.; Maccarrone, M.; Serhan, C.N. Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Sci. Transl. Med. 2016, 8, 353ra111. [Google Scholar] [CrossRef]
- Kim, W.; Fan, Y.Y.; Barhoumi, R.; Smith, R.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress the localization and activation of signaling proteins at the immunological synapse in murine CD4+ T cells by affecting lipid raft formation. J. Immunol. 2008, 181, 6236–6243. [Google Scholar] [CrossRef]
- Yog, R.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress mitochondrial translocation to the immunologic synapse and modulate calcium signaling in T cells. J. Immunol. 2010, 184, 5865–5873. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Ly, L.H.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. Dietary docosahexaenoic acid suppresses T cell protein kinase C theta lipid raft recruitment and IL-2 production. J. Immunol. 2004, 173, 6151–6160. [Google Scholar] [CrossRef]
- Schieffer, D.; Naware, S.; Bakun, W.; Bamezai, A.K. Lipid raft-based membrane order is important for antigen-specific clonal expansion of CD4(+) T lymphocytes. BMC Immunol. 2014, 15, 58. [Google Scholar] [CrossRef]
- Hou, T.Y.; Barhoumi, R.; Fan, Y.Y.; Rivera, G.M.; Hannoush, R.N.; McMurray, D.N.; Chapkin, R.S. n-3 polyunsaturated fatty acids suppress CD4(+) T cell proliferation by altering phosphatidylinositol-(4,5)-bisphosphate [PI(4,5)P-2] organization. Biochim. Et. Biophys. Acta-Biomembr. 2016, 1858, 85–96. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.Y.; Fuentes, N.R.; Hou, T.Y.; Barhoumi, R.; Li, X.C.; Deutz, N.E.P.; Engelen, M.P.K.J.; McMurray, D.N.; Chapkin, R.S. Remodelling of primary human CD4(+) T cell plasma membrane order by n-3 PUFA. Br. J. Nutr. 2018, 119, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, L.; Fisk, H.L.; Calder, P.C.; Filer, A.; Raza, K.; Buckley, C.D.; McInnes, I.; Taylor, P.C.; Fisher, B.A. Plasma Levels of Eicosapentaenoic Acid Are Associated with Anti-TNF Responsiveness in Rheumatoid Arthritis and Inhibit the Etanercept-driven Rise in Th17 Cell Differentiation in Vitro. J. Rheumatol. 2017, 44, 748–756. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Fan, Y.Y.; Monk, J.M.; Hou, T.Y.; Barhoumi, R.; McMurray, D.N.; Chapkin, R.S. n-3 PUFAs Reduce T-Helper 17 Cell Differentiation by Decreasing Responsiveness to Interleukin-6 in Isolated Mouse Splenic CD4(+) T Cells. J. Nutr. 2014, 144, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Shoda, H.; Yanai, R.; Yoshimura, T.; Nagai, T.; Kimura, K.; Sobrin, L.; Connor, K.M.; Sakoda, Y.; Tamada, K.; Ikeda, T.; et al. Dietary Omega-3 Fatty Acids Suppress Experimental Autoimmune Uveitis in Association with Inhibition of Th1 and Th17 Cell Function. PLoS ONE 2015, 10, 0138241. [Google Scholar] [CrossRef] [PubMed]
- Lian, M.; Luo, W.J.; Sui, Y.H.; Li, Z.P.; Hua, J. Dietary n-3 PUFA Protects Mice from Con A Induced Liver Injury by Modulating Regulatory T Cells and PPAR-gamma Expression. PLoS ONE 2015, 10, 0132741. [Google Scholar] [CrossRef] [PubMed]
- Woodworth, H.L.; McCaskey, S.J.; Duriancik, D.M.; Clinthorne, J.F.; Langohr, I.M.; Gardner, E.M.; Fenton, J.I. Dietary fish oil alters T lymphocyte cell populations and exacerbates disease in a mouse model of inflammatory colitis. Cancer Res. 2010, 70, 7960–7969. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lim, K.; Kim, K.H.; Kim, J.H.; Choi, J.S.; Shim, S.C. N-3 polyunsaturated fatty acids restore Th17 and Treg balance in collagen antibody-induced arthritis. PLoS ONE 2018, 13, e0194331. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Koo, D.H.; Kang, N.J.; Yoon, W.J.; Kang, G.J.; Kang, H.K.; Yoo, E.S. Docosahexaenoic Acid Alleviates Atopic Dermatitis by Generating Tregs and IL-10/TGF-beta-Modified Macrophages via a TGF-beta-Dependent Mechanism. J. Invest. Derm. 2015, 135, 1556–1564. [Google Scholar] [CrossRef] [PubMed]
- Hardy, R.R.; Hayakawa, K. B cell development pathways. Annu. Rev. Immunol. 2001, 19, 595–621. [Google Scholar] [CrossRef] [PubMed]
- Baumgarth, N. The double life of a B-1 cell: Self-reactivity selects for protective effector functions. Nat. Rev. Immunol. 2011, 11, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Prieto, J.M.B.; Felippe, M.J.B. Development, phenotype, and function of non-conventional B cells. Comp. Immunol. Microbiol. Infect. Dis. 2017, 54, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Teague, H.; Fhaner, C.J.; Harris, M.; Duriancik, D.M.; Reid, G.E.; Shaikh, S.R. n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. J. Lipid Res. 2013, 54, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Teague, H.; Harris, M.; Whelan, J.; Comstock, S.S.; Fenton, J.I.; Shaikh, S.R. Short-term consumption of n-3 PUFAs increases murine IL-5 levels, but IL-5 is not the mechanistic link between n-3 fatty acids and changes in B-cell populations. J. Nutr. Biochem. 2016, 28, 30–36. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tomasdottir, V.; Thorleifsdottir, S.; Vikingsson, A.; Hardardottir, I.; Freysdottir, J. Dietary omega-3 fatty acids enhance the B1 but not the B2 cell immune response in mice with antigen-induced peritonitis. J. Nutr. Biochem. 2014, 25, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Teague, H.; Harris, M.; Fenton, J.; Lallemand, P.; Shewchuk, B.M.; Shaikh, S.R. Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. J. Lipid Res. 2014, 55, 1420–1433. [Google Scholar] [CrossRef] [PubMed]
- Rockett, B.D.; Harris, M.; Shaikh, S.R. High dose of an n-3 polyunsaturated fatty acid diet lowers activity of C57BL/6 mice. Prostaglandins Leukot. Essent. Fat. Acids 2012, 86, 137–140. [Google Scholar] [CrossRef][Green Version]
- Tarlinton, D. B cells still front and centre in immunology. Nat. Rev. Immunol. 2019, 19, 85–86. [Google Scholar] [CrossRef]
- Harwood, N.E.; Batista, F.D. Early events in B cell activation. Annu. Rev. Immunol. 2010, 28, 185–210. [Google Scholar] [CrossRef]
- Weise, C.; Hilt, K.; Milovanovic, M.; Ernst, D.; Ruhl, R.; Worm, M. Inhibition of IgE production by docosahexaenoic acid is mediated by direct interference with STAT6 and NFkappaB pathway in human B cells. J. Nutr. Biochem. 2011, 22, 269–275. [Google Scholar] [CrossRef]
- Ramon, S.; Gao, F.; Serhan, C.N.; Phipps, R.P. Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J. Immunol. 2012, 189, 1036–1042. [Google Scholar] [CrossRef] [PubMed]
- Rockett, B.D.; Salameh, M.; Carraway, K.; Morrison, K.; Shaikh, S.R. n-3 PUFA improves fatty acid composition, prevents palmitate-induced apoptosis, and differentially modifies B cell cytokine secretion in vitro and ex vivo. J. Lipid Res. 2010, 51, 1284–1297. [Google Scholar] [CrossRef] [PubMed]
- Rockett, B.D.; Teague, H.; Harris, M.; Melton, M.; Williams, J.; Wassall, S.R.; Shaikh, S.R. Fish oil increases raft size and membrane order of B cells accompanied by differential effects on function. J. Lipid Res. 2012, 53, 674–685. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.R.; Edidin, M. Immunosuppressive effects of polyunsaturated fatty acids on antigen presentation by human leukocyte antigen class I molecules. J. Lipid Res. 2007, 48, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Verlengia, R.; Gorjao, R.; Kanunfre, C.C.; Bordin, S.; de Lima, T.M.; Martins, E.F.; Newsholme, P.; Curi, R. Effects of EPA and DHA on proliferation, cytokine production, and gene expression in Raji cells. Lipids 2004, 39, 857–864. [Google Scholar] [CrossRef] [PubMed]
- Gurzell, E.A.; Teague, H.; Duriancik, D.; Clinthorne, J.; Harris, M.; Shaikh, S.R.; Fenton, J.I. Marine fish oils are not equivalent with respect to B-cell membrane organization and activation. J. Nutr. Biochem. 2015, 26, 369–377. [Google Scholar] [CrossRef] [PubMed]
- Schraml, B.U.; Sousa, C.R.E. Defining dendritic cells. Curr. Opin. Immunol. 2015, 32, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hao, Q.; Li, Q.R.; Yan, X.W.; Ye, S.; Li, Y.S.; Li, N.; Li, J.S. omega-3 Polyunsaturated fatty acids affect lipopolysaccharide-induced maturation of dendritic cells through mitogen-activated protein kinases p38. Nutrition 2007, 23, 474–482. [Google Scholar] [CrossRef]
- Kong, W.; Yen, J.H.; Vassiliou, E.; Adhikary, S.; Toscano, M.G.; Ganea, D. Docosahexaenoic acid prevents dendritic cell maturation and in vitro and in vivo expression of the IL-12 cytokine family. Lipids Health Dis. 2010, 9, 12. [Google Scholar] [CrossRef] [PubMed]
- Zapata-Gonzalez, F.; Rueda, F.; Petriz, J.; Domingo, P.; Villarroya, F.; Diaz-Delfin, J.; de Madariaga, M.A.; Domingo, J.C. Human dendritic cell activities are modulated by the omega-3 fatty acid, docosahexaenoic acid, mainly through PPAR(gamma):RXR heterodimers: comparison with other polyunsaturated fatty acids. J. Leukoc. Biol. 2008, 84, 1172–1182. [Google Scholar] [CrossRef]
- Zeyda, M.; Kirsch, B.M.; Geyeregger, R.; Stuhlmeier, K.M.; Zlabinger, G.J.; Horl, W.H.; Saemann, M.D.; Stulnig, T.M. Inhibition of human dendritic cell maturation and function by the novel immunosuppressant FK778. Transplantation 2005, 80, 1105–1111. [Google Scholar] [CrossRef]
- Sanderson, P.; MacPherson, G.G.; Jenkins, C.H.; Calder, P.C. Dietary fish oil diminishes the antigen presentation activity of rat dendritic cells. J. Leukoc. Biol. 1997, 62, 771–777. [Google Scholar] [CrossRef] [PubMed]
- Zeyda, M.; Saemann, M.D.; Stuhlmeier, K.M.; Mascher, D.G.; Nowotny, P.N.; Zlabinger, G.J.; Waldhausl, W.; Stulnig, T.M. Polyunsaturated fatty acids block dendritic cell activation and function independently of NF-kappaB activation. J. Biol. Chem. 2005, 280, 14293–14301. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Liu, J.; Wu, J.S.; Sun, Z.M.; Huang, S.A. Effects of soluble secreted by acute myeloid leukemia cells on differentiation, maturation, apoptosis, and functions of dendritic cells. Ai. Zheng 2007, 26, 142–147. [Google Scholar] [PubMed]
- Abel, A.M.; Yang, C.; Thakar, M.S.; Malarkannan, S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front. Immunol. 2018, 9, 1869. [Google Scholar] [CrossRef] [PubMed]
- Han, L.R.; Lei, H.N.; Tian, Z.W.; Wang, X.; Cheng, D.; Wang, C.L. The immunomodulatory activity and mechanism of docosahexenoic acid (DHA) on immunosuppressive mice models. Food Funct. 2018, 9, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Schwerbrock, N.M.J.; Karlsson, E.A.; Shi, Q.; Sheridan, P.A.; Beck, M.A. Fish Oil-Fed Mice Have Impaired Resistance to Influenza Infection. J. Nutr. 2009, 139, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Thies, F.; Nebe-von-Caron, G.; Powell, J.R.; Yaqoob, P.; Newsholme, E.A.; Calder, P.C. Dietary supplementation with eicosapentaenoic acid, but not with other long-chain n-3 or n-6 polyunsaturated fatty acids, decreases natural killer cell activity in healthy subjects aged >55 y. Am. J. Clin. Nutr. 2001, 73, 539–548. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2015, 6, 620. [Google Scholar] [CrossRef]
- Latif, M.A.; Abdul-Hamid, M.; Galaly, S.R. Effect of diethylcarbamazine citrate and omega-3 fatty acids on trimellitic anhydride-induced rat skin allergy. Asian Pac. J. Allergy Immunol. 2015, 33, 33–41. [Google Scholar] [CrossRef]
- Van den Elsen, L.W.; Nusse, Y.; Balvers, M.; Redegeld, F.A.; Knol, E.F.; Garssen, J.; Willemsen, L.E. n-3 Long-chain PUFA reduce allergy-related mediator release by human mast cells in vitro via inhibition of reactive oxygen species. Br. J. Nutr. 2013, 109, 1821–1831. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, D.W.; Kang, J.X.; Kulka, M. n-3 Polyunsaturated fatty acids inhibit Fc epsilon receptor I-mediated mast cell activation. J. Nutr. Biochem. 2015, 26, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Koo, J.; Park, B. Atopic dermatitis-like skin lesions are suppressed in fat-1 transgenic mice through the inhibition of inflammasomes. Allergy 2018, 73, 829. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.H.; Kim, G.D.; Jin, Y.H.; Park, Y.S.; Park, C.S. Omega-3 fatty acid-derived mediator, Resolvin E1, ameliorates 2,4-dinitrofluorobenzene-induced atopic dermatitis in NC/Nga mice. Int. Immunopharmacol. 2012, 14, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Park, B.K.; Park, S.; Park, J.B.; Park, M.C.; Min, T.S.; Jin, M. Omega-3 fatty acids suppress Th2-associated cytokine gene expressions and GATA transcription factors in mast cells. J. Nutr. Biochem. 2013, 24, 868–876. [Google Scholar] [CrossRef] [PubMed]
- Brannan, J.D.; Bood, J.; Alkhabaz, A.; Balgoma, D.; Otis, J.; Delin, I.; Dahlen, B.; Wheelock, C.E.; Nair, P.; Dahlen, S.E.; et al. The Effect of Omega-3 Fatty Acids on Bronchial Hyperresponsiveness, Sputum Eosinophilia, and Mast Cell Mediators in Asthma. Chest 2015, 147, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Yamanishi, Y.; Miyake, K.; Iki, M.; Tsutsui, H.; Karasuyama, H. Recent advances in understanding basophil-mediated Th2 immune responses. Immunol. Rev. 2017, 278, 237–245. [Google Scholar] [CrossRef]
- Jin, M.; Park, S.; Park, B.K.; Choi, J.J.; Yoon, S.J.; Yang, M.; Pyo, M.Y. Eicosapentaenoic Acid and Docosahexaenoic Acid Suppress Th2 Cytokine Expression in RBL-2H3 Basophilic Leukemia Cells. J. Med. Food 2014, 17, 198–205. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, M.; Toyoda, M.; Teshima, R.; Sawada, J.; Saito, Y. Effect of Alpha-Linolenic Acid on the Metabolism of Omega-3 and Omega-6 Polyunsaturated Fatty-Acids and Histamine-Release in Rbl-2h3 Cells. Biol. Pharm. Bull. 1994, 17, 1321–1325. [Google Scholar] [CrossRef][Green Version]
- Cho, E.; Park, Y. Association between serum fatty acid composition and innate immune markers in healthy adults. Nutr. Res. Pract. 2016, 10, 182–187. [Google Scholar] [CrossRef]
- Arm, J.P.; Boyce, J.A.; Wang, L.; Chhay, H.; Zahid, M.; Patil, V.; Govindarajulu, U.; Ivester, P.; Weaver, K.L.; Sergeant, S.; et al. Impact of botanical oils on polyunsaturated fatty acid metabolism and leukotriene generation in mild asthmatics. Lipids Health Dis. 2013, 12, 141. [Google Scholar] [CrossRef]
- Wen, T.; Rothenberg, M.E. The Regulatory Function of Eosinophils. Microbiol. Spectr. 2016, 4, MCHD-0020-2015. [Google Scholar] [CrossRef]
- De Matos, O.G.; Amaral, S.S.; da Silva, P.E.M.P.; Perez, D.A.; Alvarenga, D.M.; Ferreira, A.V.M.; Alvarez-Leite, J.; Menezes, G.B.; Cara, D.C. Dietary Supplementation with Omega-3-PUFA-Rich Fish Oil Reduces Signs of Food Allergy in Ovalbumin-Sensitized Mice. Clin. Dev. Immunol. 2012, 2012, 236564. [Google Scholar] [CrossRef]
- Mochimaru, T.; Fukunaga, K.; Miyata, J.; Matsusaka, M.; Masaki, K.; Kabata, H.; Ueda, S.; Suzuki, Y.; Goto, T.; Urabe, D.; et al. 12-OH-17,18-Epoxyeicosatetraenoic acid alleviates eosinophilic airway inflammation in murine lungs. Allergy 2018, 73, 369–378. [Google Scholar] [CrossRef]
- Yoshida, S.; Yasutomo, K.; Watanabe, T. Treatment with DHA/EPA ameliorates atopic dermatitis-like skin disease by blocking LTB4 production. J. Med. Investig. 2016, 63, 187–191. [Google Scholar] [CrossRef] [PubMed]
- Hirakata, T.; Lee, H.C.; Ohba, M.; Saeki, K.; Okuno, T.; Murakami, A.; Matsuda, A.; Yokomizo, T. Dietary omega-3 fatty acids alter the lipid mediator profile and alleviate allergic conjunctivitis without modulating Th2 immune responses. FASEB J. 2019, 33, 3392–3403. [Google Scholar] [CrossRef]
- Moustaka, K.; Maleskou, E.; Lambrianidou, A.; Papadopoulos, S.; Lekka, M.E.; Trangas, T.; Kitsiouli, E. Docosahexaenoic Acid Inhibits Proliferation of EoL-1 Leukemia Cells and Induces Cell Cycle Arrest and Cell Differentiation. Nutrients 2019, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Tanigai, T.; Ueki, S.; Kihara, J.; Kamada, R.; Yamauchi, Y.; Sokal, A.; Takeda, M.; Ito, W.; Kayaba, H.; Adachi, T.; et al. Docosahexaenoic Acid Exerts Anti-Inflammatory Action on Human Eosinophils through Peroxisome Proliferator-Activated Receptor-Independent Mechanisms. Int. Arch. Allergy Immunol. 2012, 158, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Ostermann, A.I.; Waindok, P.; Schmidt, M.J.; Chiu, C.Y.; Smyl, C.; Rohwer, N.; Weylandt, K.H.; Schebb, N.H. Modulation of the endogenous omega-3 fatty acid and oxylipin profile in vivo-A comparison of the fat-1 transgenic mouse with C57BL/6 wildtype mice on an omega-3 fatty acid enriched diet. PLoS ONE 2017, 12, e0184470. [Google Scholar] [CrossRef] [PubMed]
- Abbott, S.K.; Else, P.L.; Atkins, T.A.; Hulbert, A.J. Fatty acid composition of membrane bilayers: importance of diet polyunsaturated fat balance. Biochim. Biophys. Acta 2012, 1818, 1309–1317. [Google Scholar] [CrossRef] [PubMed]
- Ferreri, C.; Masi, A.; Sansone, A.; Giacometti, G.; Larocca, A.V.; Menounou, G.; Scanferlato, R.; Tortorella, S.; Rota, D.; Conti, M.; et al. Fatty Acids in Membranes as Homeostatic, Metabolic and Nutritional Biomarkers: Recent Advancements in Analytics and Diagnostics. Diagnostics 2016, 7, 1. [Google Scholar] [CrossRef] [PubMed]
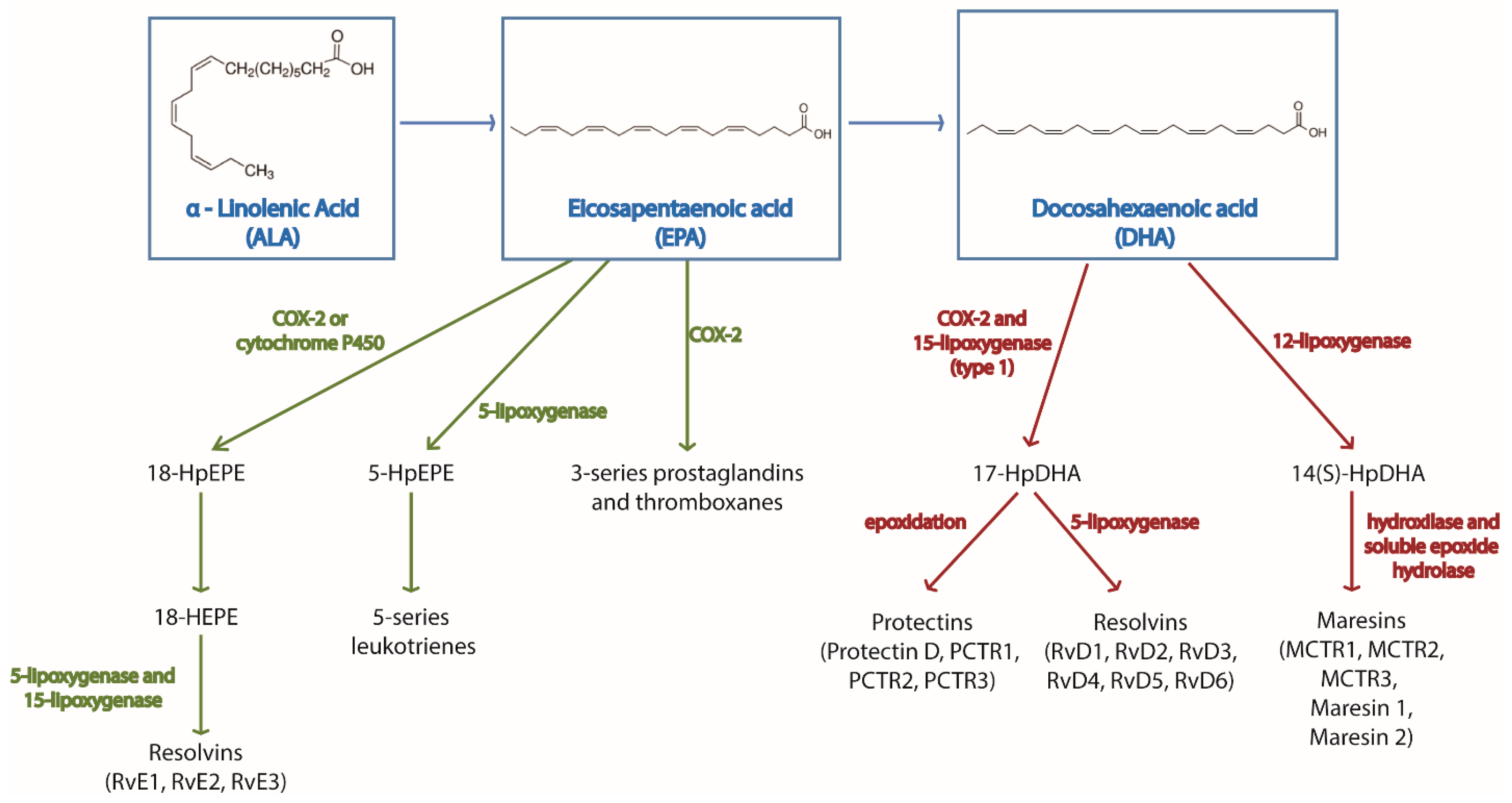
| Cell Type | Effect | References |
|---|---|---|
| Macrophages | ↓ cytokines | Cytokine production and secretion [29,30,31,32,33,34,35,36,37,39,40,41,42] Signaling [28,38,44,45,46,47] |
| ↑ polarization towards M2 phenotype | [29,30,49] Stroke [50] Atopic dermatitis [51] | |
| ↑ phagocytosis | Zymosan [29] R.equi, P.aeruginosa [52] E.coli [53] Apoptotic cells [29] | |
| Neutrophils | ↑ production of pro-resolving mediators | [61,62] |
| ↓ migration | [63,64,65,66,67,68,71] | |
| ↑ phagocytosis | Particles [72] C. albicans [4] E.coli [73,74] Zymosan [70] | |
| ↔ ROS production | Rat [4] Goat [73] Human [70,74] | |
| ↑ frequency | [72,75,76] | |
| Eosinophils | ↓ infiltration | Airway inflammation [155] Skin [141,156] Allergy [154,157] |
| Basophils | ↓ activation | [149,150,152] |
| Dendritic cells | ↓ antigen presentation | [88,129,130,131,133,134] |
| NK cells | ↔ activation | [137,138,139] |
| Mast cells | ↓ activation | [141,142,143,144,145,146] |
| T cells | ↓ activation | General effects [87,88,89,90,91,92] CD4+ T cells [95,97,98,99] Th17 T cells [92,95,97,104,105,106] |
| ↑ Treg differentiation | [19,87,107,108,109,110] | |
| B cells | ↔ activation | Human [121,122,125,126] Mouse [14,123,124] |
| ↑ IgM production | [114,117,121,122] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gutiérrez, S.; Svahn, S.L.; Johansson, M.E. Effects of Omega-3 Fatty Acids on Immune Cells. Int. J. Mol. Sci. 2019, 20, 5028. https://doi.org/10.3390/ijms20205028
Gutiérrez S, Svahn SL, Johansson ME. Effects of Omega-3 Fatty Acids on Immune Cells. International Journal of Molecular Sciences. 2019; 20(20):5028. https://doi.org/10.3390/ijms20205028
Chicago/Turabian StyleGutiérrez, Saray, Sara L Svahn, and Maria E Johansson. 2019. "Effects of Omega-3 Fatty Acids on Immune Cells" International Journal of Molecular Sciences 20, no. 20: 5028. https://doi.org/10.3390/ijms20205028
APA StyleGutiérrez, S., Svahn, S. L., & Johansson, M. E. (2019). Effects of Omega-3 Fatty Acids on Immune Cells. International Journal of Molecular Sciences, 20(20), 5028. https://doi.org/10.3390/ijms20205028