Probiotics Can Cure Oral Aphthous-Like Ulcers in Inflammatory Bowel Disease Patients: A Review of the Literature and a Working Hypothesis
Abstract
:1. Introduction
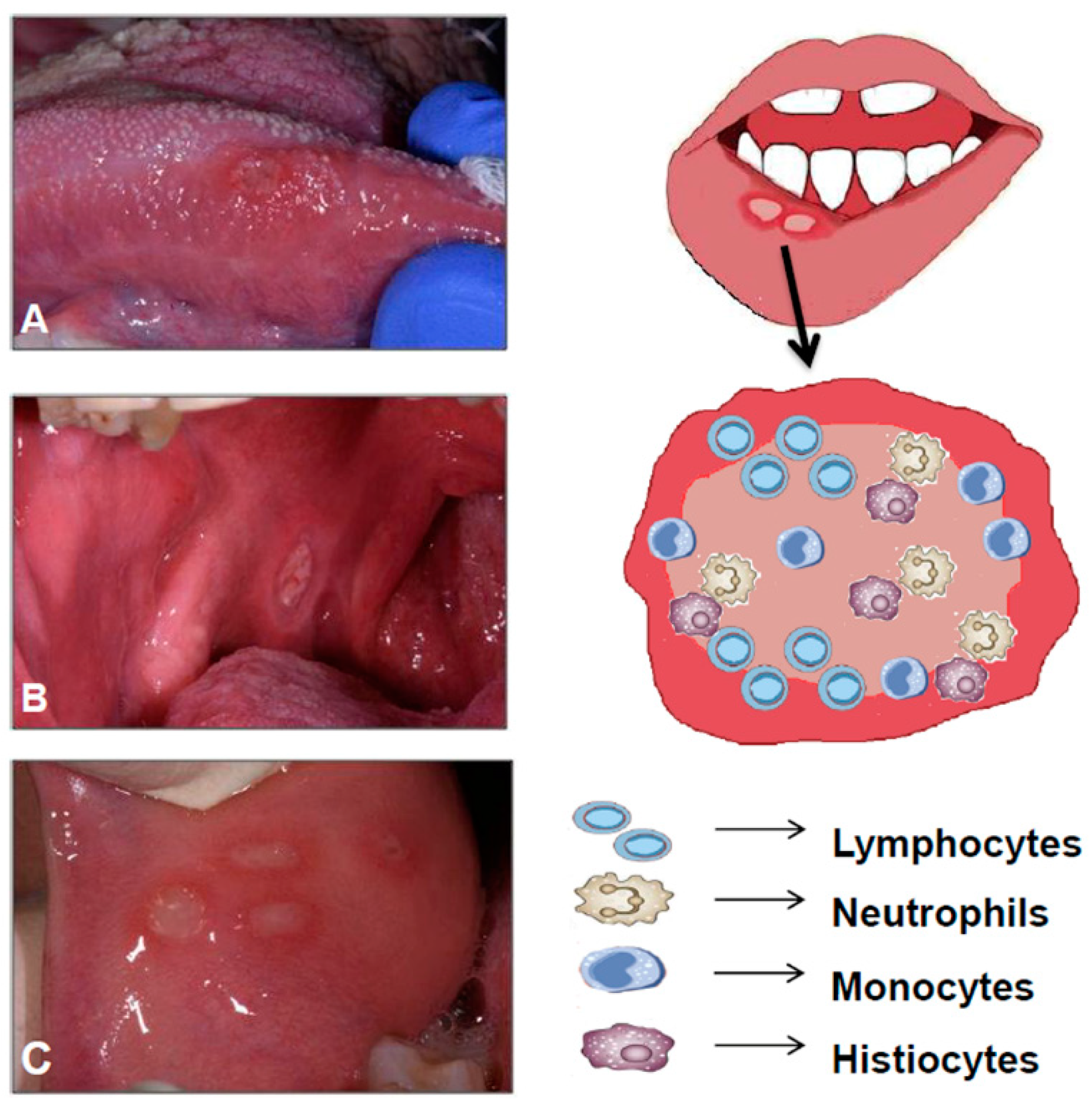
1.1. Oral Manifestations in IBD
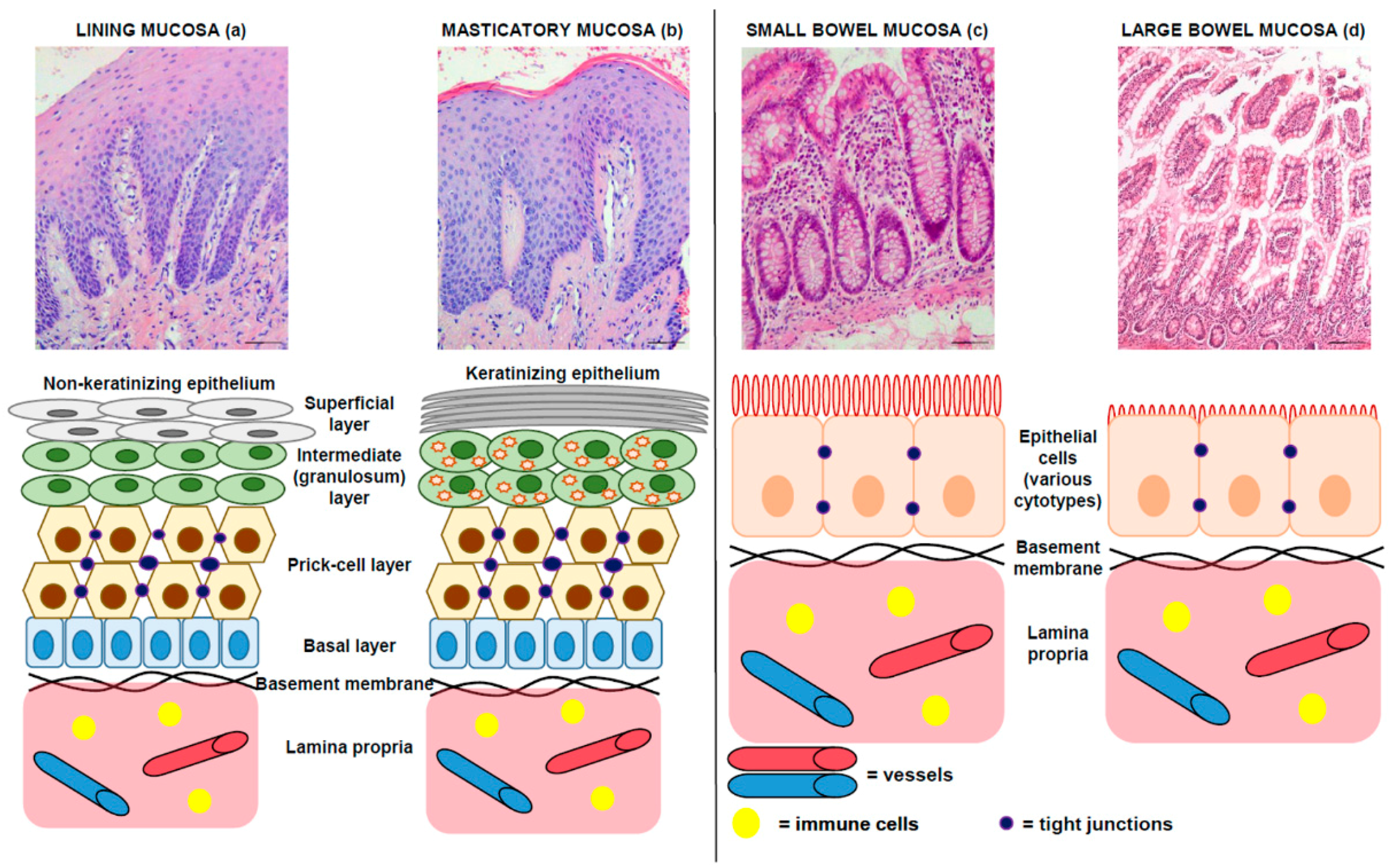
1.2. Morphology: Comparative Microscopic Anatomy of Oral and Intestinal Mucosa
1.3. The Intestinal Microbiota and the Surrounding Mucous: The Fifth Layer of the Bowel Wall
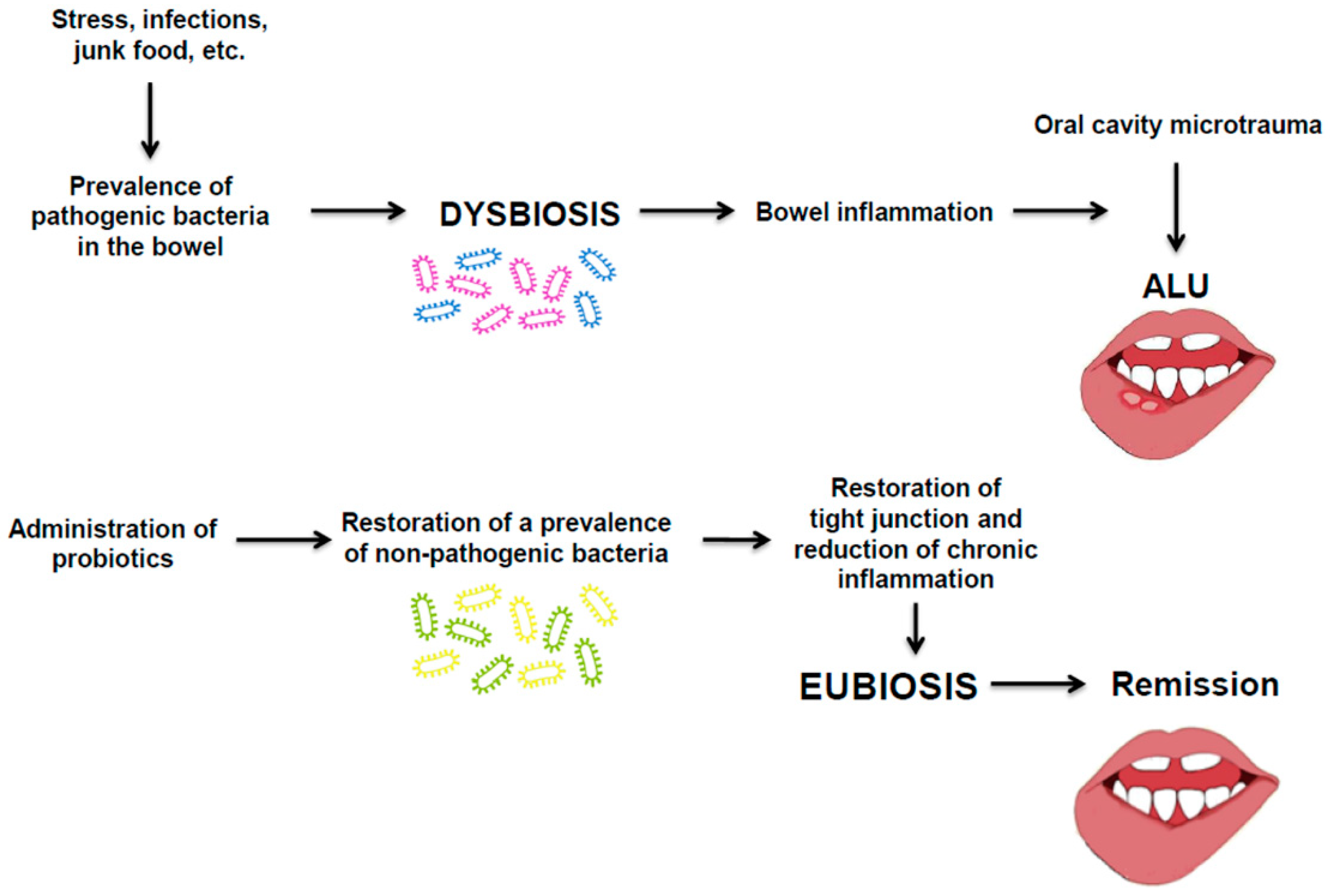
2. Pathobiology of ALU in IBD and Therapeutic Proposal
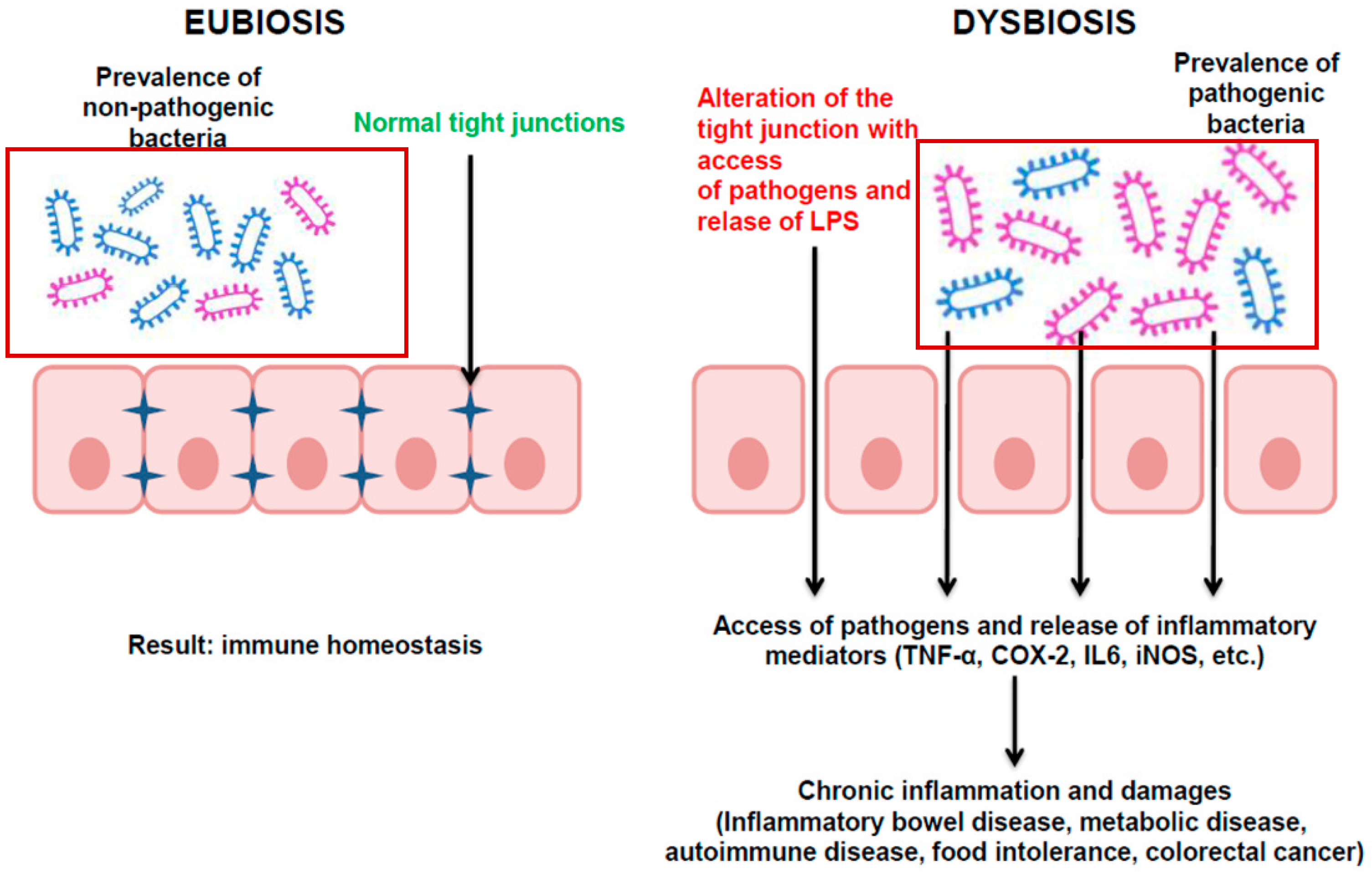
2.1. Pathobiology: A Focus on Dysbiosis
- -
- Alteration of Treg/Th17 due to dysregulated TLRs on antigen-presenting cells [27];
- -
- Resistance to colonization, i.e., ability of the gut microbiota to limit the proliferation of external pathogens. It has been observed that in patients with autoimmune disorders (e.g., systemic lupus erythematosus), resistance to colonization was lower than in healthy controls;
- -
- Superantigens, derived from bacteria and viruses that have the ability to activate immune cells by simultaneously binding to the major proteins of the class II histocompatibility complex (MHC II) present in the antigen-presenting cells and to specific receptors present in activated T cells [32];
- -
- -
- Mucosal responses to microbiota, i.e., inflammatory cytokines that activate nearby autoreactive cells [30];
- -
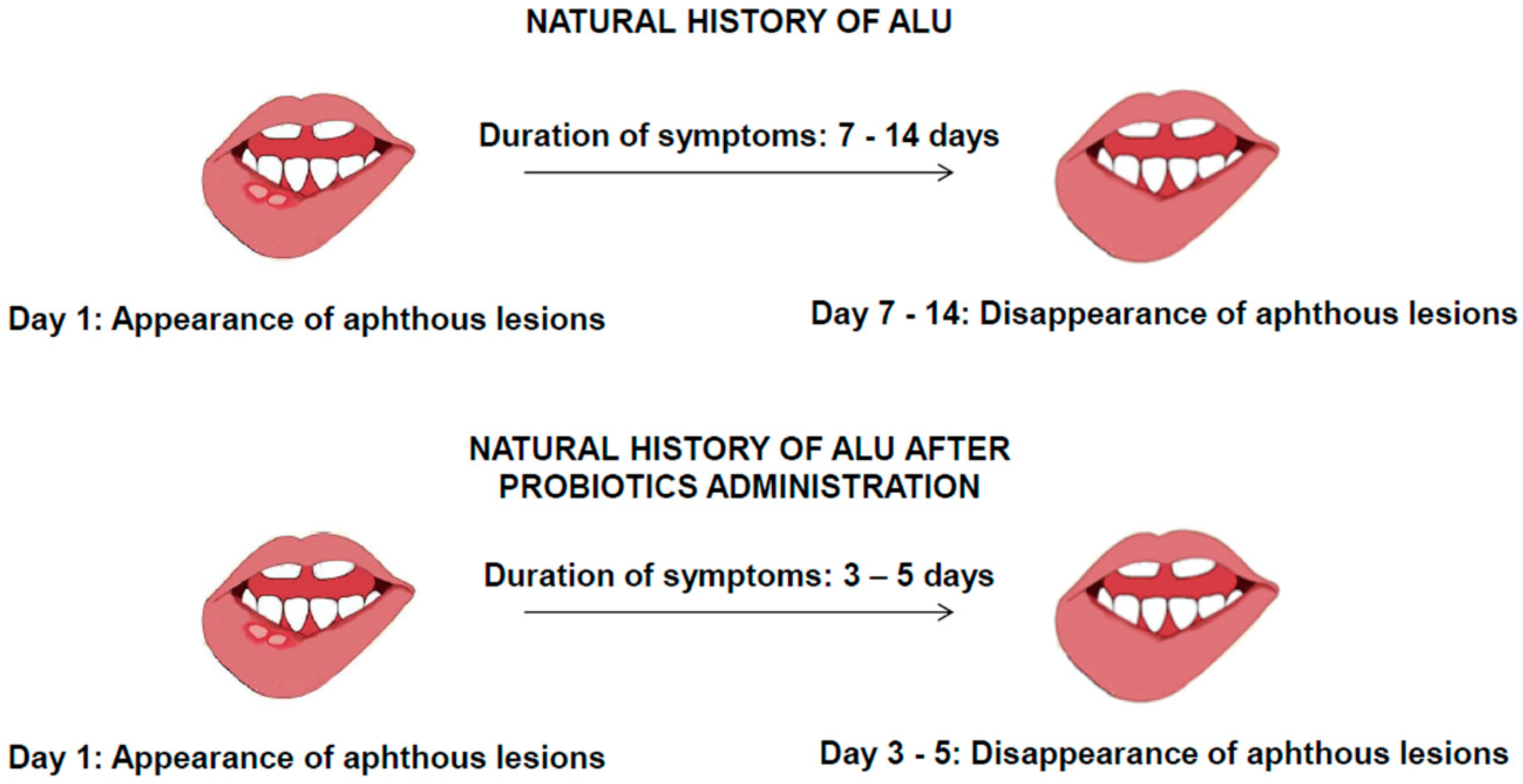
2.2. ALU Treatment: Can Probiotics Be Useful?
- -
- Co-aggregation and growth inhibition;
- -
- Bacteriocin and hydrogen peroxide production;
- -
- Competitive exclusion through antagonistic activities on adhesion and nutrition;
- -
- Immunomodulation.
3. Conclusions
Funding
Conflicts of Interest
References
- Tomasello, G.; Mazzola, M.; Leone, A.; Sinagra, E.; Zummo, G.; Farina, F.; Damiani, P.; Cappello, F.; Gerges Geagea, A.; Jurjus, A.; et al. Nutrition, oxidative stress and intestinal dysbiosis: Influence of diet on gut microbiota in inflammatory bowel diseases. Biomed. Pap. Med. Fac. Univ. Palacky Olomouc. Czech Repub 2016, 160, 461–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasello, G.; Mazzola, M.; Jurjus, A.; Cappello, F.; Carini, F.; Damiani, P.; Gerges, A.; Zeenny, M.N.; Leone, A. The fingerprint of the human gastrointestinal tract microbiota: A hypothesis of molecular mapping. J. Biol. Regul. Homeost Agents 2017, 31, 245–249. [Google Scholar] [PubMed]
- Hevia, A.; Milani, C.; López, P.; Cuervo, A.; Arboleya, S.; Duranti, S.; Turroni, F.; González, S.; Suárez, A.; Gueimonde, M.; et al. Intestinal Dysbiosis Associated with Systemic Lupus Erythematosus. MBio 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.J.; Choi, Y.S.; Baek, K.J.; Yoon, S.H.; Park, H.K.; Choi, Y. Mucosal and salivary microbiota associated with recurrent aphthous stomatitis. BMC Microbiol. 2016, 16, 57. [Google Scholar] [CrossRef] [PubMed]
- Bankvall, M.; Sjöberg, F.; Gale, G.; Wold, A.; Jontell, M.; Östman, S. The oral microbiota of patients with recurrent aphthous stomatitis. J. Oral Microbiol. 2014, 6, 25739. [Google Scholar] [CrossRef] [PubMed]
- Hijazi, K.; Lowe, T.; Meharg, C.; Berry, S.H.; Foley, J.; Hold, G.L. Mucosal microbiome in patients with recurrent aphthous stomatitis. J. Dent. Res. 2015, 94, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Honda, K.; Littman, D.R. The microbiota in adaptive immune homeostasis and disease. Nature 2016, 7, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Ribaldone, D.G.; Pellicano, R.; Actis, G.C. The gut and the Inflammatory Bowel Diseases inside-out: The extra-intestinal manifestations. Minerva Gastroenterol. Dietol. 2019, in press. [Google Scholar] [CrossRef]
- Yu, Y.R.; Rodriguez, J.R. Clinical presentation of Crohn’s, ulcerative colitis, and indeterminate colitis: Symptoms, extraintestinal manifestations, and disease phenotypes. Semin. Pediatr. Surg. 2017, 26, 349–355. [Google Scholar] [CrossRef]
- Alawi, F. Granulomatous diseases of the oral tissues: Differential diagnosis and update. Dent. Clin. 2005, 49, 203–221. [Google Scholar] [CrossRef]
- Rothfuss, K.S.; Stange, E.F.; Herrlinger, K.R. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J. Gastroenterol. 2006, 12, 4819–4831. [Google Scholar] [CrossRef] [PubMed]
- Muhvić-Urek, M.; Tomac-Stojmenović, M.; Mijandrušić-Sinčić, B. Oral pathology in inflammatory bowel disease. World J. Gastroenterol. 2016, 22, 5655–5667. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J. Oral Aphtous Ulcers. BMJ Best Pract. 2018. Available online: https://bestpractice.bmj.com/topics/en-us/564/pdf/564.pdf (accessed on 16 September 2019).
- Thrash, B.; Patel, M.; Shah, K.R.; Boland, C.R.; Menter, A. Cutaneous manifestations of gastrointestinal disease: Part II. J. Am. Acad Dermatol. 2013, 68, 189. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.X.; Brand, H.S.; de Boer, N.K.; Forouzanfar, T. Gastrointestinal diseases and their oro-dental manifestations: Part 1: Crohn’s disease. Br. Dent. J. 2016, 221, 794–799. [Google Scholar] [CrossRef] [PubMed]
- Trost, L.B.; McDonnell, J.K. Important cutaneous manifestations of inflammatory bowel disease. Postgrad. Med. J. 2005, 81, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Best, W.R.; Becktel, J.M.; Singleton, J.W.; Kern, F. Development of a Crohn’s disease activity index. National Cooperative Crohn’s Disease Study. Gastroenterology 1976, 70, 439–444. [Google Scholar] [CrossRef]
- Veloso, F.T.; Carvalho, J.; Magro, F. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J. Clin. Gastroenterol. 1996, 23, 29–34. [Google Scholar] [CrossRef]
- Compilato, D.; Carroccio, A.; Calvino, F.; Di Fede, G.; Campisi, G. Haematological deficiencies in patients with recurrent aphthosis. J. Eur. Acad Dermatol. Venereol. 2010, 24, 667–673. [Google Scholar] [CrossRef]
- Nielsen, O.H.; Soendergaard, C.; Vikner, M.E.; Weiss, G. Rational Management of Iron-Deficiency Anaemia in Inflammatory Bowel Disease. Nutrients 2018, 10, 82. [Google Scholar] [CrossRef]
- Pellicer, Z.; Santiago, J.M.; Rodriguez, A.; Alonso, V.; Antón, R.; Bosca, M.M. Management of cutaneous disorders related to inflammatory bowel disease. Ann. Gastroenterol. 2012, 25, 21–26. [Google Scholar]
- Mackenzie, I.C.; Binnie, W.H. Recent advances in oral mucosal research. J. Oral Pathol. 1983, 12, 389–415. [Google Scholar] [CrossRef] [PubMed]
- Standring, S. Gray’s Anatomy. In The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone: London, UK, 2005. [Google Scholar]
- Sternberg, S. Histology for Pathologist; Lippincott-Raven: Philadelphia, PA, USA, 1992; p. 199. [Google Scholar]
- Bucchieri, F.; Fucarino, A.; Marino Gammazza, A.; Pitruzzella, A.; Marciano, V.; Paderni, C.; De Caro, V.; Siragusa, M.G.; Lo Muzio, L.; Holgate, S.T.; et al. Medium-term culture of normal human oral mucosa: A novel three-dimensional model to study the effectiveness of drugs administration. Curr. Pharm. Des. 2012, 18, 5421–5430. [Google Scholar] [CrossRef] [PubMed]
- Campisi, G.; Giannola, L.I.; Fucarino, A.; Marino Gammazza, A.; Pitruzzella, A.; Marciano, V.; De Caro, V.; Siragusa, M.G.; Giandalia, G.; Compilato, D.; et al. Medium-term culture of primary oral squamous cell carcinoma in a three-dimensional model: Effects on cell survival following topical 5-fluororacile delivery by drug-loaded matrix tablets. Curr. Pharm. Des. 2012, 18, 5411–5420. [Google Scholar] [CrossRef] [PubMed]
- Schmehl, K.; Florian, S.; Jacobasch, G.; Körber, J. Deficiency of epithelial basement membrane laminin in ulcerative colitis affected human colonic mucosa. Int. J. Colorectal. Dis. 2000, 15, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Caruso Bavisotto, C.; Marino Gammazza, A.; Rappa, F.; Fucarino, A.; Pitruzzella, A.; David, S.; Campanella, C. Exosomes: Can doctors still ignore their existence? Euro. Mediterr. Biomed. J. 2013, 8, 136–139. [Google Scholar]
- Zocco, D.; Ferruzzi, P.; Cappello, F.; Kuo, W.P.; Fais, S. Extracellular vesicles as shuttles of tumor biomarkers and anti-tumor drugs. Front Oncol. 2014, 4, 267. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Mazzola, M.; Jurjus, A.; Zeenny, M.N.; Jurjus, R.; Carini, F.; Leone, A.; Bonaventura, G.; Tomasello, G.; Bucchieri, F.; et al. Hsp60 as a novel target in IBD management: A prospect. Front. Pharmacol. 2019, 10, 26. [Google Scholar] [CrossRef]
- Chen, B.; Sun, L.; Zhang, X. Integration of microbiome and epigenome to decipher the pathogenesis of autoimmune diseases. J. Autoimmun. 2017, 83, 31–42. [Google Scholar] [CrossRef]
- Slebioda, Z.; Szponar, E.; Kowalska, A. Recurrent aphthous stomatitis: Genetic aspects of etiology. Postepy Dermatol. Alergol. 2013, 30, 96–102. [Google Scholar] [CrossRef]
- Kuhn, K.A.; Pedraza, I.; Demoruelle, M.K. Mucosal immune responses to microbiota in the development of autoimmune disease. Rheum. Dis. Clin. 2014, 40, 711–725. [Google Scholar] [CrossRef]
- Sun, A.; Chen, H.M.; Cheng, S.J.; Wang, Y.P.; Chang, J.Y.; Wu, Y.C.; Chiang, C.P. Significant association of deficiencies of hemoglobin, iron, vitamin B12, and folic acid and high homocysteine level with recurrent aphthous stomatitis. J. Oral Pathol. Med. 2015, 44, 300–305. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, L.; Wang, X.; Xian, C.J.; Lu, H. A Possible Role of Intestinal Microbiota in the Pathogenesis of Ankylosing Spondylitis. Int. J. Mol. Sci. 2016, 17, 2126. [Google Scholar] [CrossRef] [PubMed]
- Di Felice, V.; David, S.; Cappello, F.; Farina, F.; Zummo, G. Is chlamydial heat shock protein 60 a risk factor for oncogenesis? Cell. Mol. Life Sci. 2005, 62, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Conway de Macario, E.; Marasà, L.; Zummo, G.; Macario, A.J. Hsp60 expression, new locations, functions and perspectives for cancer diagnosis and therapy. Cancer Biol. Ther. 2008, 7, 801–809. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Conway de Macario, E.; Di Felice, V.; Zummo, G.; Macario, A.J. Chlamydia trachomatis infection and anti-Hsp60 immunity: The two sides of the coin. PLoS Pathog. 2009, 5, e1000552. [Google Scholar] [CrossRef] [PubMed]
- Bellavia, M.; Tomasello, G.; Romeo, M.; Damiani, P.; Lo Monte, A.I.; Lozio, L.; Campanella, C.; Marino Gammazza, A.; Rappa, F.; Zummo, G.; et al. Gut microbiota imbalance and chaperoning system malfunction are central to ulcerative colitis pathogenesis and can be counteracted with specifically designed probiotics: A working hypothesis. Med. Microbiol. Immunol. 2013, 202, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.A.; Zenobia, C.; Darveau, R.P. The relationship of the oral microbiotia to periodontal health and disease. Cell Host Microbe 2011, 10, 302–306. [Google Scholar] [CrossRef] [PubMed]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar] [CrossRef]
- Said, H.S.; Suda, W.; Nakagome, S.; Chinen, H.; Oshima, K.; Kim, S.; Kimura, R.; Iraha, A.; Ishida, H.; Fujita, J.; et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res. 2014, 21, 15–25. [Google Scholar] [CrossRef]
- Caglar, E.; Kargul, B.; Tanboga, I. Bacteriotherapy and pro-biotics’ role on oral health. Oral Dis. 2005, 11, 131–137. [Google Scholar] [CrossRef]
- Reid, G.; Younes, J.A.; Van der Mei, H.C.; Gloor, G.B.; Knight, R.; Busscher, H.J. Microbiota restoration: Natural and supplemented recovery of human microbial communities. Nat. Rev. Microbiol. 2011, 9, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Round, J.L. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol. 2014, 16, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Round, J.L.; Mazmanian, S.K. The gut microbiota shapes intestinal immune responses during health and disease. Nat. Rev. Immunol. 2009, 9, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Schoultz, I.; Keita, Å.V. Cellular and Molecular Therapeutic Targets in Inflammatory Bowel Disease-Focusing on Intestinal Barrier Function. Cells 2019, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, R.; Boivin, M.; Ma, T. Mechanism of cytokine modulation of epithelial tight junction barrier. Front. Biosci. 2009, 14, 2765–2778. [Google Scholar] [CrossRef]
- Roselli, M.; Finamore, A.; Nuccitelli, S.; Carnevali, P.; Brigidi, P.; Vitali, B.; Nobili, F.; Rami, R.; Garaguso, I.; Mengheri, E. Prevention of TNBS-induced colitis by different Lactobacillus and Bifidobacterium strains is associated with an expansion of gamma delta T and regulatory T cells of intestinal intraepithelial lymphocytes. Inflamm. Bowel. Dis. 2009, 15, 1526–1536. [Google Scholar] [CrossRef]
- Singh, R.; Chandrashekharappa, S.; Bodduluri, S.R.; Baby, B.V.; Hegde, B.; Kotla, N.G.; Hiwale, A.A.; Saiyed, T.; Patel, P.; Vijay-Kumar, M.; et al. Enhancement of the gut barrier integrity by a microbial metabolite through the Nrf2 pathway. Nat. Commun. 2019, 10, 89. [Google Scholar] [CrossRef]
- Jurge, S.; Kuffer, R.; Scully, C.; Porter, S.R. Mucosal disease series: Number VI. Recurrent aphthous stomatitis. Oral Dis. 2006, 12, 1–21. [Google Scholar] [CrossRef]
| Nonspecific Oral Lesions | Clinical Presentation |
|---|---|
| Aphtous stomatitis | Shallow, round ulcers surrounded by an erythematous halo with a central fibrin membrane |
| Angular cheilitis | Erythema with/without painful fissures and sores at the corners of the mouth |
| Glossitis | Painful atrophy of the tongue |
| Pyostomatitis vegetans | Small exophytic lesions covered with a vulnerable membrane, their cracking and confluence results in the characteristicsign of a “snail track” |
| Oral Lichen/Oral Lichenoid reactions | Associated to taste disturbances |
| Gingivitis/Periodontitis | Associated to a vitamin D deficiency |
| Specific Oral Lesions | Clinical Presentation |
|---|---|
| Indurated tag-like lesions (mucosal tags) | White reticular tags (labial and buccal vestibules, retromolar region) |
| Cobblestoning | Fissured and corrugated swollen mucosa with hyperplastic appearance (posterior buccal mucosa) |
| Mucogingivitis | Edematous, hyperplastic and granular gingiva (whole gingiva up to the mucogingival line) |
| Lip swelling | Associated to vertical fissures |
| Deep linear ulcerations | Associated to hyperplastic margins (vestibule) |
| Tongue and midline lip fissuring | Lip and tongue fissures |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cappello, F.; Rappa, F.; Canepa, F.; Carini, F.; Mazzola, M.; Tomasello, G.; Bonaventura, G.; Giuliana, G.; Leone, A.; Saguto, D.; et al. Probiotics Can Cure Oral Aphthous-Like Ulcers in Inflammatory Bowel Disease Patients: A Review of the Literature and a Working Hypothesis. Int. J. Mol. Sci. 2019, 20, 5026. https://doi.org/10.3390/ijms20205026
Cappello F, Rappa F, Canepa F, Carini F, Mazzola M, Tomasello G, Bonaventura G, Giuliana G, Leone A, Saguto D, et al. Probiotics Can Cure Oral Aphthous-Like Ulcers in Inflammatory Bowel Disease Patients: A Review of the Literature and a Working Hypothesis. International Journal of Molecular Sciences. 2019; 20(20):5026. https://doi.org/10.3390/ijms20205026
Chicago/Turabian StyleCappello, Francesco, Francesca Rappa, Federica Canepa, Francesco Carini, Margherita Mazzola, Giovanni Tomasello, Giuseppe Bonaventura, Giovanna Giuliana, Angelo Leone, Dario Saguto, and et al. 2019. "Probiotics Can Cure Oral Aphthous-Like Ulcers in Inflammatory Bowel Disease Patients: A Review of the Literature and a Working Hypothesis" International Journal of Molecular Sciences 20, no. 20: 5026. https://doi.org/10.3390/ijms20205026
APA StyleCappello, F., Rappa, F., Canepa, F., Carini, F., Mazzola, M., Tomasello, G., Bonaventura, G., Giuliana, G., Leone, A., Saguto, D., Scalia, F., Bucchieri, F., Fucarino, A., & Campisi, G. (2019). Probiotics Can Cure Oral Aphthous-Like Ulcers in Inflammatory Bowel Disease Patients: A Review of the Literature and a Working Hypothesis. International Journal of Molecular Sciences, 20(20), 5026. https://doi.org/10.3390/ijms20205026










