Seasonal Variation in the Biological Effects of PM2.5 from Greater Cairo
Abstract
1. Introduction
2. Results
2.1. PM2.5 Physico-Chemical Characterization
2.2. Biological Responses
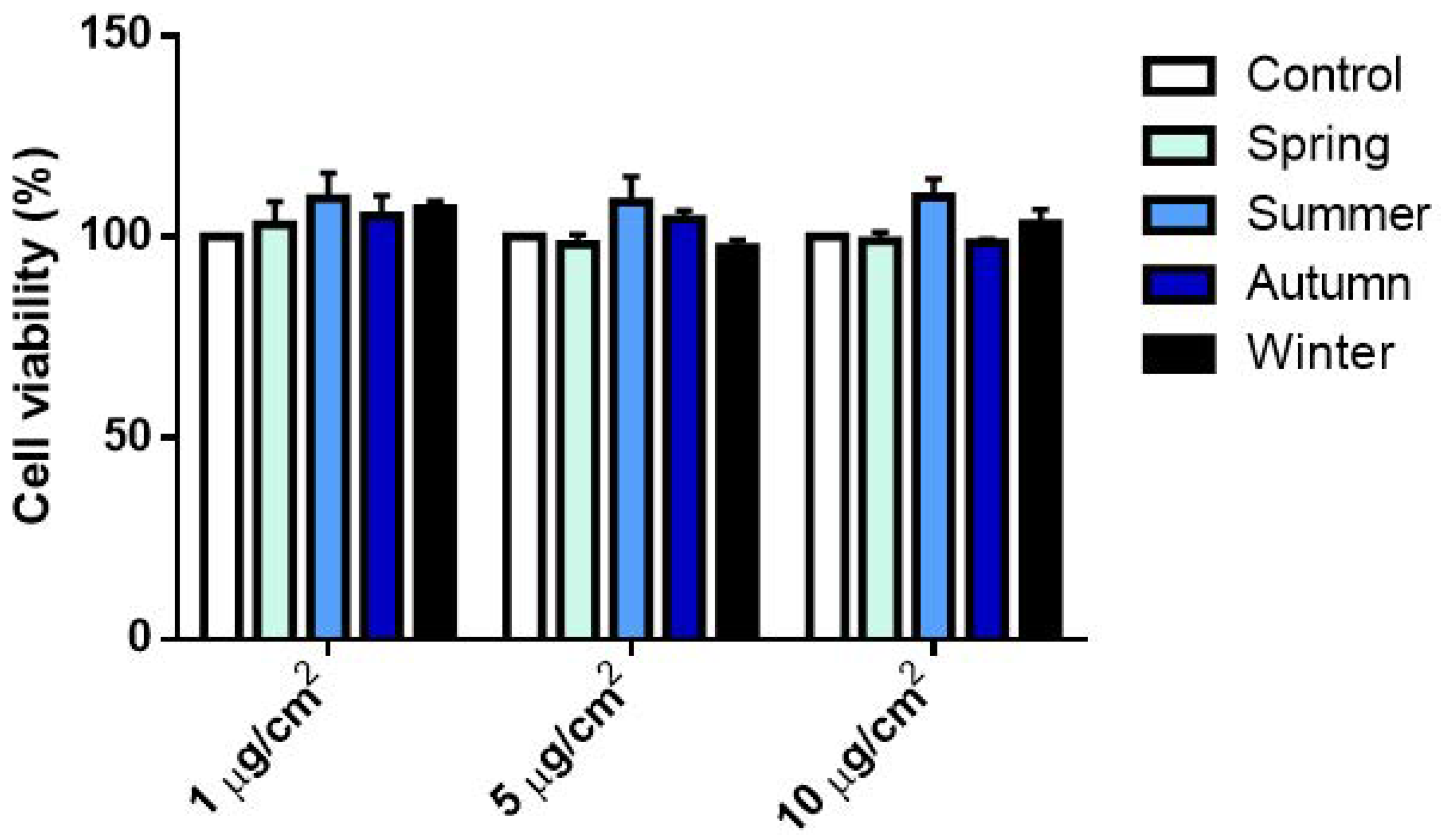
2.2.1. Cell Viability
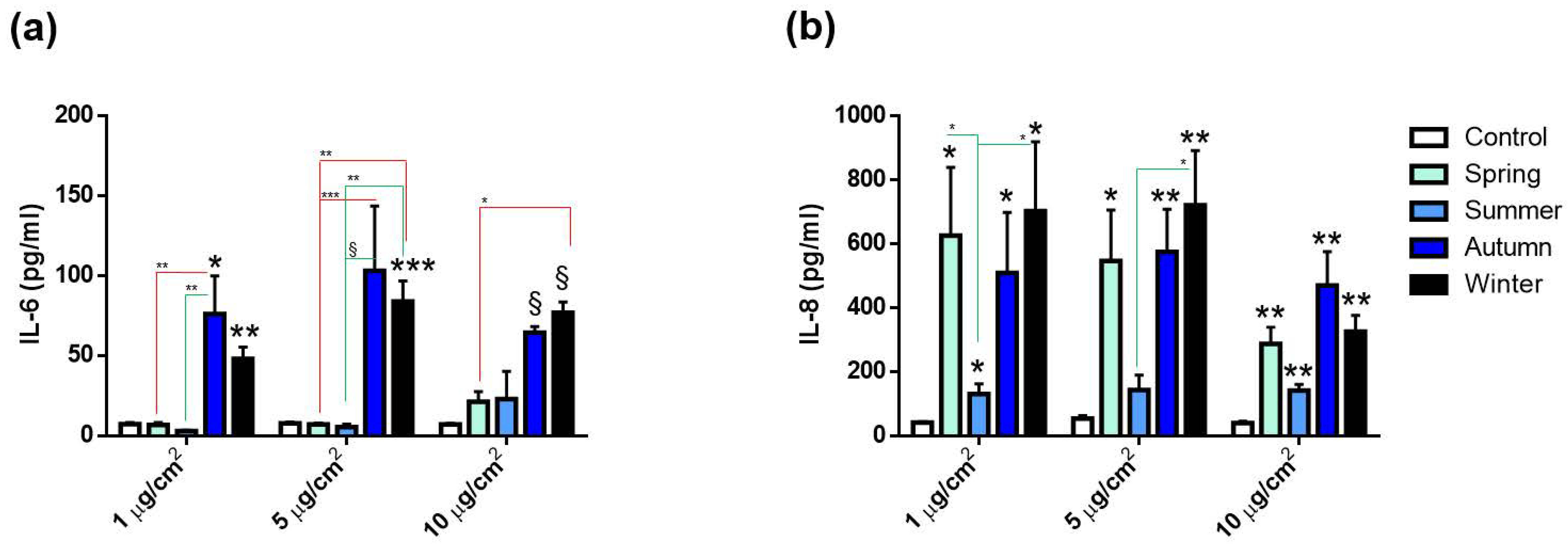
2.2.2. Release of Inflammatory Mediators
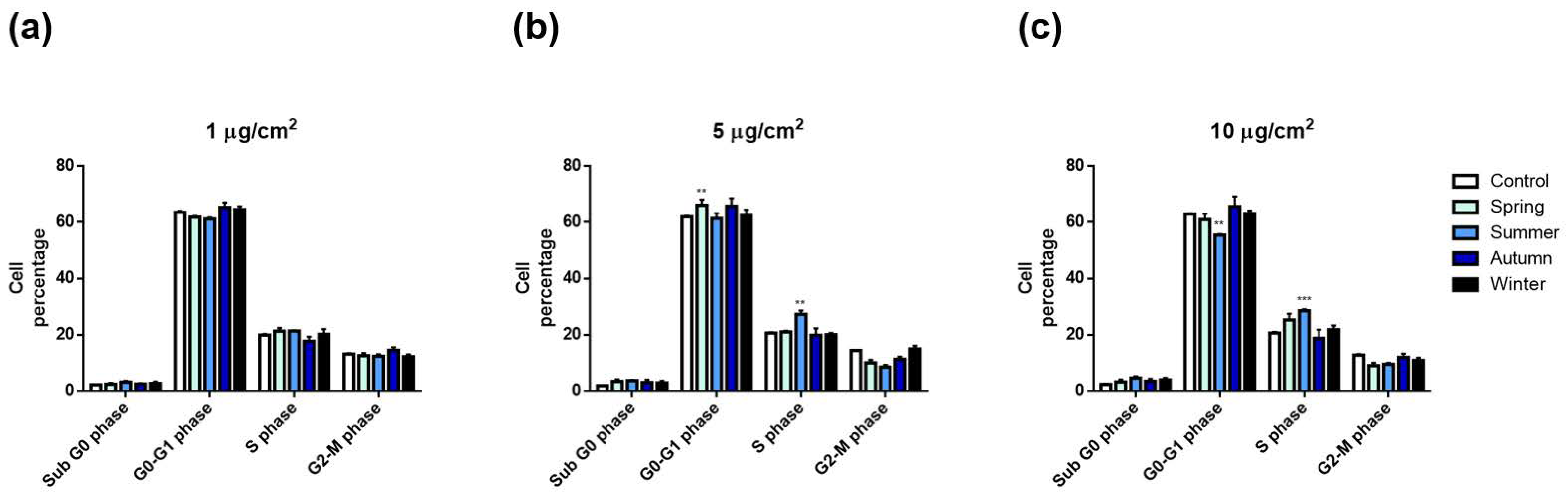
2.2.3. Cell Cycle Alterations
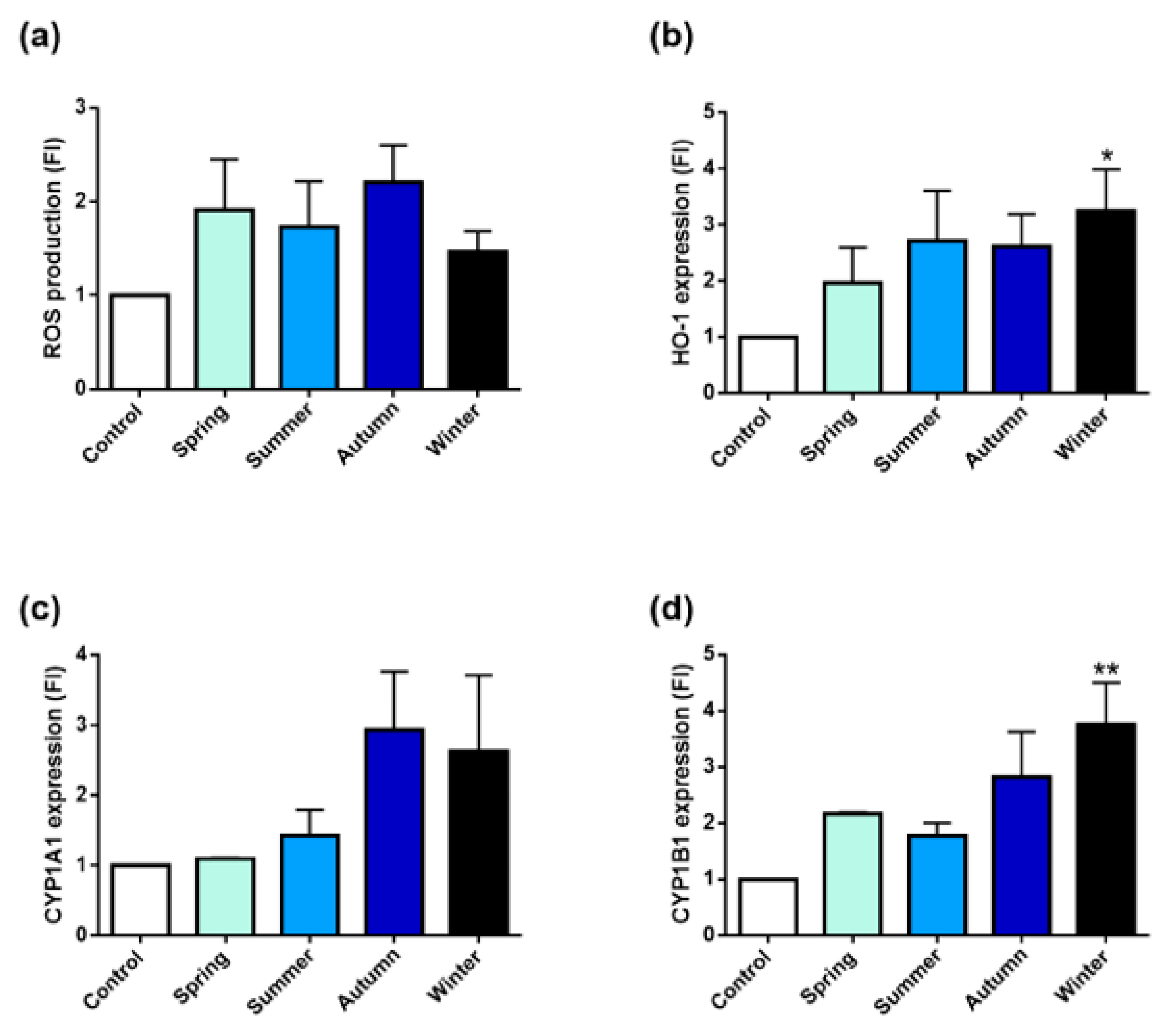
2.2.4. Oxidative Stress and PAH Metabolizing Enzymes’ Activations
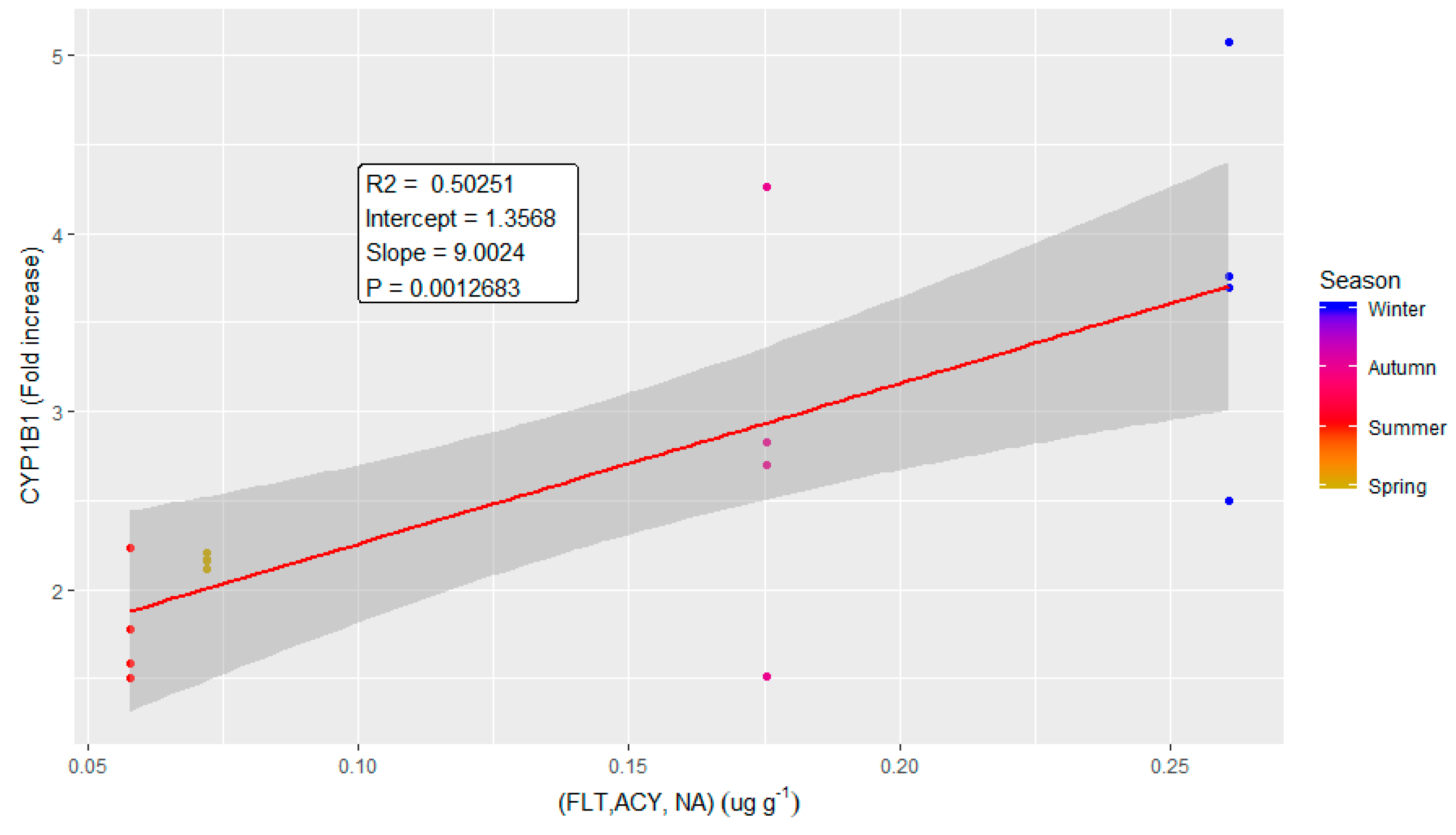
2.3. Correlations between Chemical Parameters and Biological Effects
3. Discussion
4. Materials and Methods
4.1. Site Description and PM2.5 Collection
4.2. PM2.5 Chemical Characterization
4.2.1. Water Soluble Inorganic Ionic Species (WSIs)
4.2.2. Elemental Concentration
4.2.3. Polycyclic Aromatic Hydrocarbons (PAHs)
4.3. Filter Extraction and Particle Characterization
4.4. PM2.5 In Vitro Toxicity
4.4.1. Cells Culture and Exposure Conditions
4.4.2. Cell Viability Assay
4.4.3. Pro-Inflammatory Cytokine Release
4.4.4. Flow Cytometry Analysis of the Cell Cycle
4.4.5. ROS Production
4.4.6. Western Blot Analysis
4.5. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| WHO | World Health Organization |
| PM | particulate matter |
| COPD | chronic obstructive pulmonary disease |
| PAHs | polycyclic aromatic hydrocarbons |
| VOCs | volatile organic compounds |
| NA | Naphthalene |
| ACY | Acenaphthylene |
| ACE | Acenaphthene |
| FLU | Fluorene |
| PHE | Phenanthrene |
| ANT | Anthracene |
| FLT | Fluoranthene |
| PYR | Pyrene |
| BaA | Benzo(a)anthracene |
| CRY | Chrysene |
| BbF | Benzo(b)Flouranthene |
| BkF | Benzo(k)Flouranthene |
| BaP | Benzo (a) pyrene |
| IND | Indeno(1,2,3-cd) pyrene |
| DBA | Dibenzo(a,h) anthracene |
| BGP | Benzo(ghi)perylene |
| WSIs | water-soluble ions |
| Ms | metals/metalloids |
| MDLs | method detection limits |
| ICP MS | Inductively Coupled Plasma Optical Emission Spectrometry |
| ICP-QQQ | Triple Quadrupole ICP-MS |
| DCM | dichloromethane |
| GC | gas chromatography |
| LAL | Limulus Amebocyte Lysate |
| PI | Propidium Iodide |
| ROS | reactive oxygen species |
| H2O2 | Hydrogen peroxide |
| TBS | Tris-Buffered Saline |
| TBS-T | TBS with 0.1% Tween20 |
| BSA | bovine serum albumin |
References
- WHO World Health Organization Releases 7 million Premature Deaths Annually Linked to Air Pollution. 2014. Available online: http//www.who.int/mediacentre/news/releases/2014/air-pollution/en/ (accessed on 21 June 2018).
- Anderson, J.O.; Thundiyil, J.G.; Stolbach, A. Clearing the Air: A Review of the Effects of Particulate Matter Air Pollution on Human Health. J. Med. Toxicol. 2012, 8, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Burnett, R.; Chen, H.; Szyszkowicz, M.; Fann, N.; Hubbell, B.; Pope, C.A.; Apte, J.S.; Brauer, M.; Cohen, A.; Weichenthal, S.; et al. Global estimates of mortality associated with long-term exposure to outdoor fine particulate matter. Proc. Natl. Acad. Sci. USA 2018. [Google Scholar] [CrossRef] [PubMed]
- Cesari, D.; De Benedetto, G.E.; Bonasoni, P.; Busetto, M.; Dinoi, A.; Merico, E.; Chirizzi, D.; Cristofanelli, P.; Donateo, A.; Grasso, F.M.; et al. Seasonal variability of PM2.5 and PM10 composition and sources in an urban background site in Southern Italy. Sci. Total Environ. 2018, 612, 202–213. [Google Scholar] [CrossRef] [PubMed]
- Yanagi, Y.; de Assunção, J.V.; Barrozo, L.V. The impact of atmospheric particulate matter on cancer incidence and mortality in the city of São Paulo, Brazil. Cad. Saúde Pública 2012, 28, 1737–1748. [Google Scholar] [CrossRef] [PubMed]
- Zauli-Sajani, S.; Rovelli, S.; Trentini, A.; Bacco, D.; Marchesi, S.; Scotto, F.; Zigola, C.; Lauriola, P.; Cavallo, D.M.; Poluzzi, V.; et al. Higher health effects of ambient particles during the warm season: The role of infiltration factors. Sci. Total Environ. 2018, 627, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Karakatsani, A.; Analitis, A.; Perifanou, D.; Ayres, J.G.; Harrison, R.M.; Kotronarou, A.; Kavouras, I.G.; Pekkanen, J.; Hämeri, K.; Kos, G.P.; et al. Particulate matter air pollution and respiratory symptoms in individuals having either asthma or chronic obstructive pulmonary disease: A European multicentre panel study. Environ. Heal. A Glob. Access Sci. Source 2012, 11, 1–16. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, K.; Loridas, S. Pulmonary oxidative stress, inflammation and cancer: Respirable particulate matter, fibrous dusts and ozone as major causes of lung carcinogenesis through reactive oxygen species mechanisms. Int. J. Environ. Res. Public Health 2013, 10, 3886–3907. [Google Scholar] [CrossRef]
- Im, U.; Markakis, K.; Koçak, M.; Gerasopoulos, E.; Daskalakis, N.; Mihalopoulos, N.; Poupkou, A.; Kindap, T.; Unal, A.; Kanakidou, M. Summertime aerosol chemical composition in the Eastern Mediterranean and its sensitivity to temperature. Atmos. Environ. 2012, 50, 164–173. [Google Scholar] [CrossRef]
- Zhang, Y.; Wen, X.-Y.; Wang, K.; Vijayaraghavan, K.; Jacobson, M.Z. Probing into regional O 3 and particulate matter pollution in the United States: 2. An examination of formation mechanisms through a process analysis technique and sensitivity study. J. Geophys. Res. 2009, 114, D22305. [Google Scholar] [CrossRef]
- Tai, A.P.K.; Mickley, L.J.; Jacob, D.J. Correlations between fine particulate matter (PM2.5) and meteorological variables in the United States: Implications for the sensitivity of PM2.5 to climate change. Atmos. Environ. 2010, 44, 3976–3984. [Google Scholar] [CrossRef]
- Hassan, S.K.; Khoder, M.I. Chemical characteristics of atmospheric PM2.5 loads during air pollution episodes in Giza, Egypt. Atmos. Environ. 2017, 150, 346–355. [Google Scholar] [CrossRef]
- Khoder, M.I. Diurnal, seasonal and weekdays-weekends variations of ground level ozone concentrations in an urban area in greater Cairo. Environ. Monit. Assess. 2009, 149, 349–362. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, D.H.; Gertler, A.W.; Labib, M.W. Particulate matter source apportionment in Cairo: Recent measurements and comparison with previous studies. Int. J. Environ. Sci. Technol. 2014, 11, 657–670. [Google Scholar] [CrossRef]
- Boman, J.; Shaltout, A.A.; Abozied, A.M.; Hassan, S.K. On the elemental composition of PM2.5 in central Cairo, Egypt. X-Ray Spectrom. 2013, 42, 276–283. [Google Scholar] [CrossRef]
- EEAA State of the Environment Report in Egypt. Issued 2014 2012. [CrossRef]
- Perrone, M.G.; Gualtieri, M.; Consonni, V.; Ferrero, L.; Sangiorgi, G.; Longhin, E.; Ballabio, D.; Bolzacchini, E.; Camatini, M. Particle size, chemical composition, seasons of the year and urban, rural or remote site origins as determinants of biological effects of particulate matter on pulmonary cells. Environ. Pollut. 2013, 176, 215–227. [Google Scholar] [CrossRef]
- Michael, S.; Montag, M.; Dott, W. Pro-inflammatory effects and oxidative stress in lung macrophages and epithelial cells induced by ambient particulate matter. Environ. Pollut. 2013. [Google Scholar] [CrossRef]
- Gualtieri, M.; Øvrevik, J.; Mollerup, S.; Asare, N.; Longhin, E.; Dahlman, H.J.; Camatini, M.; Holme, J.A. Airborne urban particles (Milan winter-PM2.5) cause mitotic arrest and cell death: Effects on DNA, mitochondria, AhR binding and spindle organization. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2011, 713, 18–31. [Google Scholar] [CrossRef]
- Líbalová, H.; Krčková, S.; Uhlířová, K.; Milcová, A.; Schmuczerová, J.; Ciganek, M.; Kléma, J.; Machala, M.; Šrám, R.J.; Topinka, J. Genotoxicity but not the AhR-mediated activity of PAHs is inhibited by other components of complex mixtures of ambient air pollutants. Toxicol. Lett. 2014, 225, 350–357. [Google Scholar] [CrossRef]
- Longhin, E.; Holme, J.A.; Gutzkow, K.B.; Arlt, V.M.; Kucab, J.E.; Camatini, M.; Gualtieri, M. Cell cycle alterations induced by urban PM2.5 in bronchial epithelial cells: Characterization of the process and possible mechanisms involved. Part. Fibre Toxicol. 2013, 10, 63. [Google Scholar] [CrossRef]
- Longhin, E.; Pezzolato, E.; Mantecca, P.; Holme, J.A.; Franzetti, A.; Camatini, M.; Gualtieri, M. Season linked responses to fine and quasi-ultrafine Milan PM in cultured cells. Toxicol. Vitr. 2013, 27, 551–559. [Google Scholar] [CrossRef]
- Nemmar, A.; Al-Maskari, S.; Ali, B.H.; Al-Amri, I.S. Cardiovascular and lung inflammatory effects induced by systemically administered diesel exhaust particles in rats. Am. J. Physiol. Cell. Mol. Physiol. 2007, 292, L664–L670. [Google Scholar] [CrossRef] [PubMed]
- Sancini, G.; Farina, F.; Battaglia, C.; Cifola, I.; Mangano, E.; Mantecca, P.; Camatini, M.; Palestini, P. Health risk assessment for air pollutants: Alterations in lung and cardiac gene expression in mice exposed to milano winter fine particulate matter (PM2.5). PLoS One 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Hu, T.; Cao, J.; Shen, Z.; Wang, G.; Lee, S.; Ho, K. Size Differentiation of individual atmospheric aerosol during winter in Xi’an, China. Aerosol Air Qual. Res. 2012, 12, 951–960. [Google Scholar] [CrossRef]
- Krzyzanowski, M.; Apte, J.S.; Bonjour, S.P.; Brauer, M.; Cohen, A.J.; Prüss-Ustun, A.M. Air Pollution in the Mega-cities. Curr. Environ. Heal. Rep. 2014, 1, 185–191. [Google Scholar] [CrossRef]
- Shaltout, A.A.; Hassan, S.K.; Karydas, A.G.; Zaki, Z.I.; Mostafa, N.Y.; Kregsamer, P.; Wobrauschek, P.; Streli, C. Comparative elemental analysis of fine particulate matter (PM2.5) from industrial and residential areas in Greater Cairo-Egypt by means of a multi-secondary target energy dispersive X-ray fluorescence spectrometer. Spectrochim. Acta Part B At. Spectrosc. 2018, 145, 29–35. [Google Scholar] [CrossRef]
- Liu, W.J.; Xu, Y.S.; Liu, W.X.; Liu, Q.Y.; Yu, S.Y.; Liu, Y.; Wang, X.; Tao, S. Oxidative potential of ambient PM2.5 in the coastal cities of the Bohai Sea, northern China: Seasonal variation and source apportionment. Environ. Pollut. 2018, 236, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Øvrevik, J.; Holme, J.A.; Perrone, M.G.; Bolzacchini, E.; Schwarze, P.E.; Camatini, M. Differences in cytotoxicity versus pro-inflammatory potency of different PM fractions in human epithelial lung cells. Toxicol. Vitr. 2010, 24, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Jalava, P.I.; Happo, M.S.; Huttunen, K.; Sillanpää, M.; Hillamo, R.; Salonen, R.O.; Hirvonen, M.R. Chemical and microbial components of urban air PM cause seasonal variation of toxicological activity. Environ. Toxicol. Pharmacol. 2016, 40, 375–387. [Google Scholar] [CrossRef]
- Guan, T.; Yao, M.; Wang, J.; Fang, Y.; Hu, S.; Wang, Y.; Dutta, A.; Yang, J.; Wu, Y.; Hu, M.; et al. Airborne endotoxin in fine particulate matter in Beijing. Atmos. Environ. 2014, 97, 35–42. [Google Scholar] [CrossRef]
- Heinrich, J.; Pitz, M.; Bischof, W.; Krug, N.; Borm, P.J.A. Endotoxin in fine (PM2.5) and coarse (PM2.5-10) particle mass of ambient aerosols. A temporo-spatial analysis. Atmos. Environ. 2003, 37, 3659–3667. [Google Scholar] [CrossRef]
- Li, N.; Hao, M.; Phalen, R.F.; Hinds, W.C.; Nel, A.E. Particulate air pollutants and asthma: A paradigm for the role of oxidative stress in PM-induced adverse health effects. Clin. Immunol. 2003, 109, 250–265. [Google Scholar] [CrossRef] [PubMed]
- Xing, Y.F.; Xu, Y.H.; Shi, M.H.; Lian, Y.X. The impact of PM2.5 on the human respiratory system. J. Thorac. Dis. 2016, 8, E69–E74. [Google Scholar] [CrossRef] [PubMed]
- Longhin, E.; Holme, J.A.; Gualtieri, M.; Camatini, M.; Øvrevik, J. Milan winter fine particulate matter (wPM2.5) induces IL-6 and IL-8 synthesis in human bronchial BEAS-2B cells, but specifically impairs IL-8 release. Toxicol. Vitr. 2018, 52, 365–373. [Google Scholar] [CrossRef] [PubMed]
- Hetland, R.B.; Cassee, F.R.; Refsnes, M.; Schwarze, P.E.; Låg, M.; Boere, A.J.F.; Dybing, E. Release of inflammatory cytokines, cell toxicity and apoptosis in epithelial lung cells after exposure to ambient air particles of different size fractions. Toxicol. Vitr. 2004, 18. [Google Scholar] [CrossRef]
- Steenhof, M.; Gosens, I.; Strak, M.; Godri, K.J.; Hoek, G.; Cassee, F.R.; Mudway, I.S.; Kelly, F.J.; Harrison, R.M.; Lebret, E.; et al. In vitro toxicity of particulate matter (PM) collected at different sites in the Netherlands is associated with PM composition, size fraction and oxidative potential—The RAPTES project. Part. Fibre Toxicol. 2011, 8, 26. [Google Scholar] [CrossRef]
- Genies, C.; Jullien, A.; Lefebvre, E.; Revol, M.; Maitre, A.; Douki, T. Inhibition of the formation of benzo[a]pyrene adducts to DNA in A549 lung cells exposed to mixtures of polycyclic aromatic hydrocarbons. Toxicol. Vitr. 2016, 35, 1–10. [Google Scholar] [CrossRef]
- Dergham, M.; Lepers, C.; Verdin, A.; Billet, S.; Cazier, F.; Courcot, D.; Shirali, P.; Garçon, G. Prooxidant and proinflammatory potency of air pollution particulate matter (PM2.5-0.3) produced in rural, urban, or industrial surroundings in human bronchial epithelial cells (BEAS-2B). Chem. Res. Toxicol. 2012, 25, 904–919. [Google Scholar] [CrossRef]
- Líbalová, H.; Krčková, S.; Uhlířová, K.; Kléma, J.; Ciganek, M.; Rössner, P.; Šrám, R.J.; Vondráček, J.; Machala, M.; Topinka, J. Analysis of gene expression changes in A549 cells induced by organic compounds from respirable air particles. Mutat. Res. Fundam. Mol. Mech. Mutagen. 2014, 770, 94–105. [Google Scholar] [CrossRef]
- Cachon, B.F.; Firmin, S.; Verdin, A.; Ayi-Fanou, L.; Billet, S.; Cazier, F.; Martin, P.J.; Aissi, F.; Courcot, D.; Sanni, A.; et al. Proinflammatory effects and oxidative stress within human bronchial epithelial cells exposed to atmospheric particulate matter (PM2.5 and PM>2.5) collected from Cotonou, Benin. Environ. Pollut. 2014, 185, 340–351. [Google Scholar] [CrossRef]
- Capasso, L.; Longhin, E.; Caloni, F.; Camatini, M.; Gualtieri, M. Synergistic inflammatory effect of PM10 with mycotoxin deoxynivalenol on human lung epithelial cells. Toxicon 2015, 104, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Manzano-León, N.; Serrano-Lomelin, J.; Sánchez, B.N.; Quintana-Belmares, R.; Vega, E.; Vázquez-López, I.; Rojas-Bracho, L.; López-Villegas, M.T.; Vadillo-Ortega, F.; de Vizcaya-Ruiz, A.; et al. TNFα and IL-6 responses to particulate matter in vitro: Variation according to PM size, season, and polycyclic aromatic hydrocarbon and soil content. Environ. Health Perspect. 2016, 124, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Deng, F.; Guo, X.; Liu, H.; Fang, X.; Yang, M.; Chen, W. Effects of dust storm PM2.5on cell proliferation and cell cycle in human lung fibroblasts. Toxicol. Vitr. 2007, 21, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Øvrevik, J.; Arlt, V.M.; Øya, E.; Nagy, E.; Mollerup, S.; Phillips, D.H.; Låg, M.; Holme, J.A. Differential effects of nitro-PAHs and amino-PAHs on cytokine and chemokine responses in human bronchial epithelial BEAS-2B cells. Toxicol. Appl. Pharmacol. 2010, 242, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Hockley, S.L.; Arlt, V.M.; Brewer, D.; Giddings, I.; Phillips, D.H. Time- and concentration-dependent changes in gene expression induced by benzo(a)pyrene in two human cell lines, MCF-7 and HepG2. BMC Genomics 2006, 7, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Mantecca, P.; Farina, F.; Moschini, E.; Gallinotti, D.; Gualtieri, M.; Rohr, A.; Sancini, G.; Palestini, P.; Camatini, M. Comparative acute lung inflammation induced by atmospheric PM and size-fractionated tire particles. Toxicol. Lett. 2010, 198, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Loxham, M.; Morgan-Walsh, R.J.; Cooper, M.J.; Blume, C.; Swindle, E.J.; Dennison, P.W.; Howarth, P.H.; Cassee, F.R.; Teagle, D.A.H.; Palmer, M.R.; et al. The effects on bronchial epithelial mucociliary cultures of coarse, fine, and ultrafine particulate matter from an underground railway station. Toxicol. Sci. 2015, 145, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, M.; Longhin, E.; Mattioli, M.; Mantecca, P.; Tinaglia, V.; Mangano, E.; Carla, M.; Bestetti, G.; Camatini, M.; Battaglia, C. Gene expression profiling of A549 cells exposed to Milan PM2.5. Toxicol. Lett. 2012, 209, 136–145. [Google Scholar] [CrossRef]
- Shetaya, W.H.; Osterwalder, S.; Bigalke, M.; Mestrot, A.; Huang, J.H.; Alewell, C. An Isotopic Dilution Approach for Quantifying Mercury Lability in Soils. Environ. Sci. Technol. Lett. 2017, 4, 556–561. [Google Scholar] [CrossRef]
- Fang, G.C.; Chang, C.N.; Wu, Y.S.; Fu, P.P.C.; Yang, I.L.; Chen, M.H. Characterization, identification of ambient air and road dust polycyclic aromatic hydrocarbons in central Taiwan, Taichung. Sci. Total Environ. 2004, 327, 135–146. [Google Scholar] [CrossRef]
- Park, J.-S.; Wade, T.L.; Sweet, S. Atmospheric distribution of polycyclic aromatic hydrocarbons and deposition to Galveston Bay, Texas, USA. Atmos. Environ. 2001, 35, 3241–3249. [Google Scholar] [CrossRef]
- Bein, K.J.; Wexler, A.S. Compositional variance in extracted particulate matter using different filter extraction techniques. Atmos. Environ. 2015, 107, 24–34. [Google Scholar] [CrossRef]
- Robertson, S.; Gray, G.A.; Duffin, R.; McLean, S.G.; Shaw, C.A.; Hadoke, P.W.F.; Newby, D.E.; Miller, M.R. Diesel exhaust particulate induces pulmonary and systemic inflammation in rats without impairing endothelial function ex vivo or in vivo. Part. Fibre Toxicol. 2012, 9, 9. [Google Scholar] [CrossRef] [PubMed]
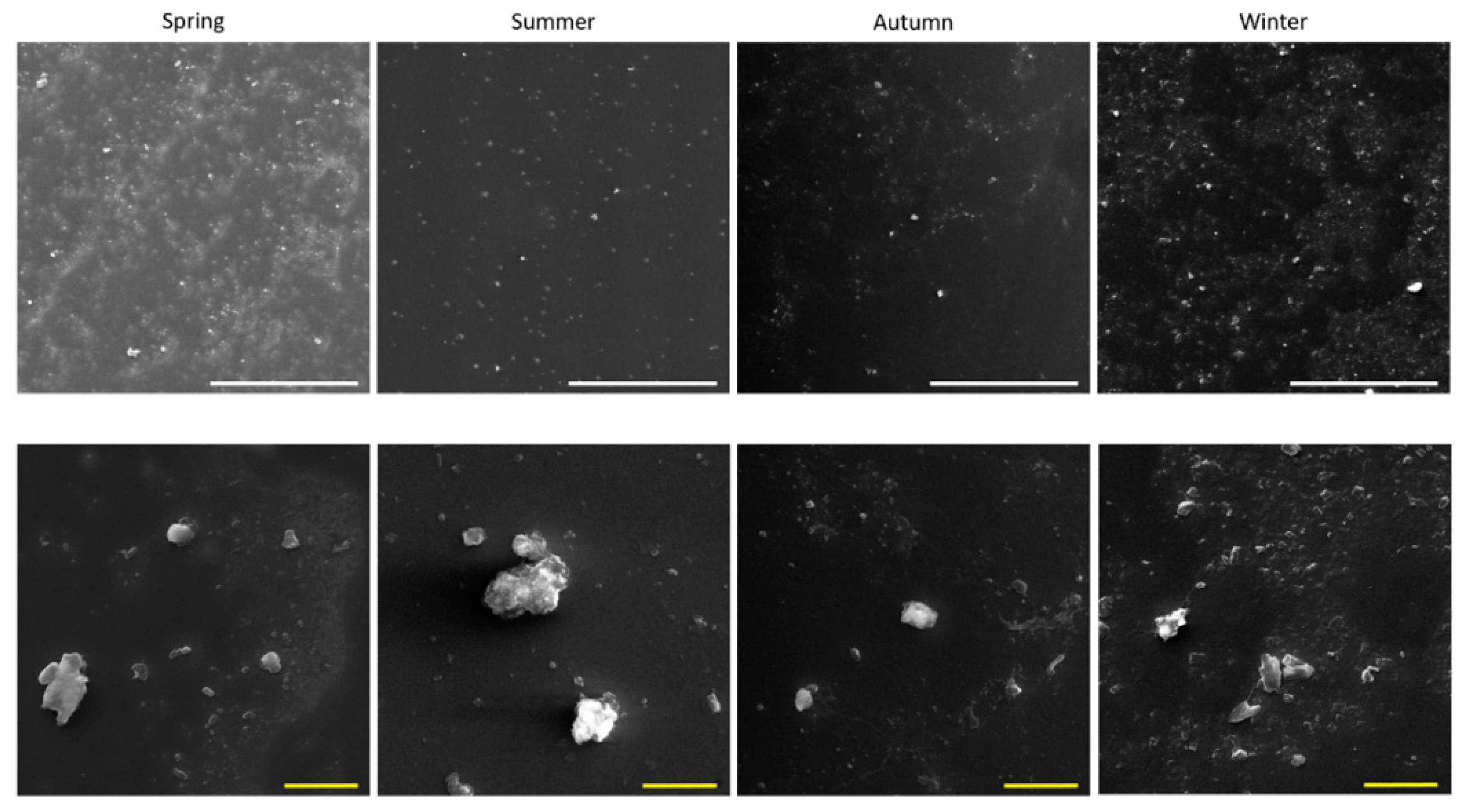

| Water-Soluble Inorganic Ions Title | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Cl− | 22.66 ± 4.57 | 33.79 ± 1.54 | 42.67 ± 25.99 | 33.71 ± 10.94 |
| NO3− | 50.81 ± 11.24 | 65.42 ± 15.14 | 55.58 ± 3.64 | 38.35 ± 14.02 |
| SO42− | 106.74 ± 21.52 | 204.33 ± 24.44 | 107.02 ± 8.10 | 86.13 ± 20.29 |
| Na+ | 23.94 ± 10.52 | 19.83 ± 1.88 | 30.73 ± 19.39 | 32.93 ± 11.01 |
| NH4+ | 28.63 ± 1.97 | 53.33 ± 2.01 | 37.86 ± 0.79 | 25.60 ± 10.33 |
| K+ | 20.67 ± 1.16 | 18.41 ± 6.09 | 19.83 ± 2.45 | 13.02 ± 6.44 |
| Mg2+ | 19.16 ± 5.48 | 18.55 ± 2.41 | 21.40 ± 9.22 | 13.59 ± 8.49 |
| Ca2+ | 33.96 ± 3.18 | 35.75 ± 5.28 | 37.99 ± 19.59 | 32.47 ± 10.86 |
| Ʃ WSIs | 306.58 § ± 59.65 | 449.42 ± 58.79 | 353.08 *** ± 89.17 | 275.79 § ‡ ± 92.37 |
| Metals | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| Al | 32.41 ± 8.26 | 23.11 ± 5.08 | 20.93 ± 0.15 | 27.98 ± 7.86 |
| Fe | 14.4 ± 7.34 | 8.58 ± 2.32 | 9.96 ± 0.13 | 14.54 ± 4.5 |
| Mn | 0.50 ± 0.08 | 0.44 ± 0.10 | 0.39 ± 0.02 | 0.52 ± 0.09 |
| V | 0.31 ± 0.11 | 0.23 ± 0.07 | 0.20 ± 0.01 | 0.18 ± 0.04 |
| Zn | 0.99 ± 0.61 | 0.68 ± 0.15 | 0.69 ± 0.01 | 0.86 ± 0.17 |
| Ni | 0.31 ± 0.10 | 0.19 ± 0.03 | 0.20 ± 0.02 | 0.17 ± 0.02 |
| Co | 0.011 ± 0.00 | 0.010 ± 0.00 | 0.053 ± 0.01 | 0.033 ± 0.03 |
| Cd | 0.09 ± 0.08 | 0.02 ± 0.00 | 0.18 ± 0.01 | 0.11 ± 0.09 |
| Cu | 1.27 ± 0.06 | 0.99 ± 0.30 | 0.75 ± 0.01 | 0.88 ± 0.14 |
| Pb | 2.93 ± 0.61 | 2.08 ± 1.13 | 2.60 ± 0.23 | 2.5 ± 0.78 |
| As | 0.16 ± 0.03 | 0.18 ± 0.05 | 0.15 ± 0.01 | 0.2 ± 0.06 |
| Hg | 0.008 ± 0.00 | 0.010 ± 0.00 | 0.008 ± 0.00 | 0.010 ± 0.00 |
| Ʃ Ms | 53.39 ± 15.68 | 40.51 *** ± 6.24 | 36.12 § ± 0.41 | 47.96 ‡ ± 12.40 |
| PAHs | Spring | Summer | Autumn | Winter |
|---|---|---|---|---|
| NA | 0.17 ± 0.09 | 0.13 ± 0.03 | 0.28 ± 0.04 | 0.33 ± 0.01 |
| ACY | 0.25 ± 0.02 | 0.23 ± 0.06 | 0.26 ± 0.05 | 0.31 ± 0.01 |
| ACE | 0.32 ± 0.02 | 0.42 ± 0.10 | 0.38 ± 0.06 | 0.53 ± 0.01 |
| FLU | 0.29 ± 0.02 | 0.38 ± 0.09 | 0.29 ± 0.04 | 0.47 ± 0.01 |
| PHE | 0.46 ± 0.03 | 0.60 ± 0.14 | 0.52 ± 0.11 | 0.61 ± 0.01 |
| ANT | 1.84 ± 0.10 | 2.31 ± 0.38 | 0.52 ± 0.10 | 0.63 ± 0.02 |
| FLT | 1.69 ± 0.08 | 1.93 ± 0.38 | 2.41 ± 0.42 | 2.55 ± 0.04 |
| PYR | 1.85 ± 0.10 | 2.17 ± 0.48 | 1.99 ± 0.37 | 2.05 ± 0.03 |
| BaA | 2.10 ± 0.13 | 2.29 ± 0.50 | 2.38 ± 0.44 | 2.49 ± 0.02 |
| CRY | 3.42 ± 0.15 | 4.21 ± 0.89 | 3.87 ± 0.59 | 4.10 ± 0.04 |
| BbF | 4.27 ± 0.25 | 4.95 ± 1.01 | 4.41 ± 0.62 | 5.06 ± 0.09 |
| BkF | 3.71 ± 0.17 | 5.13 ± 1.04 | 3.74 ± 0.57 | 4.48 ± 0.04 |
| BaP | 3.21 ± 0.19 | 4.12 ± 0.71 | 2.78 ± 0.36 | 3.31 ± 0.06 |
| IND | 2.33 ± 0.13 | 2.79 ± 0.54 | 3.49 ± 0.56 | 3.50 ± 0.05 |
| DBA | 3.25 ± 0.23 | 3.84 ± 0.77 | 3.94 ± 0.77 | 4.12 ± 0.06 |
| BGP | 3.64 ± 0.20 | 4.35 ± 0.78 | 4.78 ± 0.70 | 4.62 ± 0.06 |
| ƩPAHs | 32.82 ± 1.90 | 39.85 § ± 7.20 | 36.05 ‡ ± 5.78 | 39.14 § ± 0.55 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marchetti, S.; Hassan, S.K.; Shetaya, W.H.; El-Mekawy, A.; Mohamed, E.F.; Mohammed, A.M.F.; El-Abssawy, A.A.; Bengalli, R.; Colombo, A.; Gualtieri, M.; et al. Seasonal Variation in the Biological Effects of PM2.5 from Greater Cairo. Int. J. Mol. Sci. 2019, 20, 4970. https://doi.org/10.3390/ijms20204970
Marchetti S, Hassan SK, Shetaya WH, El-Mekawy A, Mohamed EF, Mohammed AMF, El-Abssawy AA, Bengalli R, Colombo A, Gualtieri M, et al. Seasonal Variation in the Biological Effects of PM2.5 from Greater Cairo. International Journal of Molecular Sciences. 2019; 20(20):4970. https://doi.org/10.3390/ijms20204970
Chicago/Turabian StyleMarchetti, Sara, Salwa K. Hassan, Waleed H. Shetaya, Asmaa El-Mekawy, Elham F. Mohamed, Atef M. F. Mohammed, Ahmed A. El-Abssawy, Rossella Bengalli, Anita Colombo, Maurizio Gualtieri, and et al. 2019. "Seasonal Variation in the Biological Effects of PM2.5 from Greater Cairo" International Journal of Molecular Sciences 20, no. 20: 4970. https://doi.org/10.3390/ijms20204970
APA StyleMarchetti, S., Hassan, S. K., Shetaya, W. H., El-Mekawy, A., Mohamed, E. F., Mohammed, A. M. F., El-Abssawy, A. A., Bengalli, R., Colombo, A., Gualtieri, M., & Mantecca, P. (2019). Seasonal Variation in the Biological Effects of PM2.5 from Greater Cairo. International Journal of Molecular Sciences, 20(20), 4970. https://doi.org/10.3390/ijms20204970








