Abstract
Grain size and weight are two important determinants of grain yield in rice. Although overexpression of sucrose synthase (SUS) genes has led to several improvements on cellulose and starch-based traits in transgenic crops, little is reported about SUS enhancement of hull size and grain weight in rice. In this study, we selected transgenic rice plants that overexpressed OsSUS1-6 genes driven with the maize Ubi promoter. Compared to the controls (wild type and empty vector line), all independent OsSUS homozygous transgenic lines exhibited considerably increased grain yield and grain weights. Using the representative OsSUS3 overexpressed transgenic plants, four independent homozygous lines showed much raised cell numbers for larger hull sizes, consistent with their enhanced primary cell wall cellulose biosynthesis and postponed secondary wall synthesis. Accordingly, the OsSUS3 transgenic lines contained much larger endosperm volume and higher starch levels than those of the controls in the mature grains, leading to increased brown grain weights by 15–19%. Hence, the results have demonstrated that OsSUS overexpression could significantly improve hull size and grain weight by dynamically regulating cell division and starch accumulation in the transgenic rice.
1. Introduction
Rice is one of the major staple food crops over the world. As major agronomic traits, grain size and weight are tightly associated with grain yield in rice. In principle, grain length, width and thickness basically determine grain size, while hull size and degree of grain filling are key agronomic traits in determining grain weight []. Over the past years, large number of QTLs for rice size and weight have been identified [], and several major genes are relatedly characterized. For instance, GS3/GL2/GL3.1/qGL3 genes affect grain length [,,,], qSW5/GW5/GSE5/GS2/GS5 determine grain width [,,,,], and GL7/GW8 control both grain length and width [,,]. However, genetic manipulation specific for concurrent improvements of both hull size and grain weight in rice remains to be explored.
In higher plants, sucrose is the major form of photosynthesis to transport from source tissues (leaves) to sink tissues (shoot apex, roots, stems and seeds). Cleavage of sucrose is the first step for utilization of the photo-assimilate in various metabolic pathways. The initial cleavage of sucrose in sink organs is catalyzed by either invertase or sucrose synthase (SUS). Invertase catalyses the irreversible hydrolysis of sucrose to glucose and fructose, while SUS catalyzes the reversible conversion of sucrose and a nucleoside diphosphate into the corresponding nucleoside diphosphate-glucose and fructose [,,,]. Previous studies have showed that invertase can affect seed development in rice [] and maize [,], but whether SUS enzyme has a similar role in crops remains to be explored.
SUS enzyme plays an important role in carbon partitioning by providing UDPG and ADPG substrates for the synthesis of wall polysaccharides and starch, respectively [,,,,,]. Although it has been characterized that SUS is involved in structural and storage carbohydrates biosynthesis, growth processes, biomass accumulation and wood density in different plant species [,,,,,,], much remains unknown about SUS’s impact on seed development in rice. In plants, SUS are coded by multiple genes, and members of them play distinct and diverse roles in development. For instance, among the three genes in maize, ZmSus1 contributes to seed starch biosynthesis, ZmSus2 is responsible for endosperm cell wall integrity, and ZmSus3 may be involved in basal endosperm transfer cell formation []. In Arabidopsis, SUS5 and SUS6, but not SUS1–4, are specifically involved in callose formation in the sieve plate []. Rice has multiple OsSUS genes with distinct and partially overlapping expression patterns, but whether the rice SUS isoforms play different roles in seed development remains to be determined.
In this study, we selected transgenic rice plants that overexpressed each of the six OsSUS genes in the background of “Zhonghua11” (ZH11) cultivar. We then observed significantly enhanced grain yield and weight of the transgenic plants in the field experiments. Notably, this study performed a detailed examination of the representative transgenic rice plants that overexpressed OsSUS3 gene, and attempted to interpret how OsSUSs regulate cell division, cellulose biosynthesis and starch accumulation for enhancements of hull size and grain weight in transgenic rice lines. Thus, this study examined the SUS as an important factor that determines grain size and weight, providing a potential genetic strategy for improved grain yield in rice and beyond.
2. Results
2.1. Phylogenetic and Expression Analyses of OsSUS Family in Rice
Because there are seven sucrose synthase (SUS) isoforms in rice and OsSUS7 exhibits 99% similarity with OsSUS5 [], this study did not perform any investigation of OsSUS7. Among six OsSUSs family genes, OsSUS1, 4 were examined with 11–12 exons, OsSUS3, 4 genes had 14–15 exons, whereas OsSUS5 and 6 had 17 and 12 exons, respectively (Figure 1A). According to the phylogenetic analysis, the SUS family were classified into four distinct clades in plants: Clade I were of dicot members; Clade II contained monocot members including rice OsSUS1, OsSUS2, and OsSUS3; Clade III and IV consisted of both monocot and dicot members with OsSUS4, OsSUS5, and OsSUS6, respectively (Table S1, Figure 1B). Furthermore, we examined six OsSUSs gene expressions based on the microarray analysis (Table S2, Figure 1C). As a result, OsSUS1 had low expression in leaf and sheath, with high expression in seed imbibition/germination, plumule/radicle, seedling, young shoot/root, panicle, and young stem, whereas OsSUS2 expressed highly in almost all tissues especially at early endosperm development. By comparison, OsSUS3 was mainly expressed in the spikelet and endosperm, while OsSUS4 was specific in endosperm. In addition, OsSUS5 and OsSUS6 had a relatively low expression in most tissues.
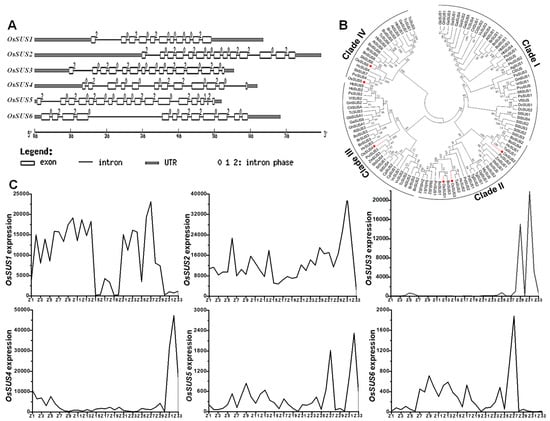
Figure 1.
Structurale, phylogenetic and expression analysis of six OsSUS genes. (A) Structural comparison of OsSUS genes. (B) Phylogenetic tree of plant SUS families. (C) The expression patterns of OsSUSs in ZS97 variety. The x-axis indicates the tissues covering almost all periods of life cycle as shown in Table S2, and the y-axis for the relative gene expression level from the microarray data.
2.2. Increased Grain Weight in All OsSUSs Transgenic Rice Plants
To examine the OsSUS’s roles in rice development, this study generated transgenic rice plants that expressed each OsSUS using the maize Ubi promoter of driving gene constitutive expression []. A dozen independent, single-locus homozygous OsSUS1-6 overexpressed transgenic lines (OE, two lines for each SUS) were selected for use in this study, based on much increased OsSUS1-6 transcription levels, compared to the ZH11 (Table S3 and Figure S1).
In the field experiments, all transgenic rice lines showed a normal growth and development over the life cycles of rice. Compared with the ZH11 and empty vector (EV) controls, the grain yields per plant of OsSUSs transgenic lines were increased by 8–15% (Figure 2A). Statistical analysis showed no significant differences in tiller number per plant, seed number per panicle, and seed setting rate between controls and OsSUSs transgenic lines (Table S4). However, 1000-grain weight were significantly increased by 14–18% than that of controls, from 26.24 g and 26.19 g in the ZH11 and EV to 29.87–31.03 g in the transgenic plants (Figure 2B), respectively.
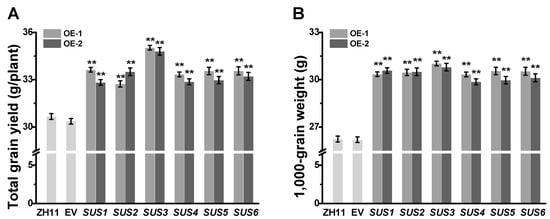
Figure 2.
Characterization of grain yield and 1000-grain weight in OsSUSs-transgenic rice plants. (A) Grain yield of OsSUS1-6 transgenic plants (n = 20). (B) Dry weight of 1000 grains. SUS1-SUS6 are the homozygous transgenic rice plants that respectively overexpressed OsSUS1-6 genes in ZH11 background (1000 grains per replicate, n = 10 replicates). All data are given as means ± SD. * and ** Indicated significant difference between transgenic line and empty vector transgenic line by Student’s t-test at p < 0.05 and p < 0.01, respectively.
2.3. Raised Grain Length in OsSUS3 Transgenic Lines
To understand how grain weight are controlled in the OsSUSs transgenic rice lines, this study focused to investigate OsSUS3 transgenic lines for the following experiments, because the OsSUS3 was of much high expression in the spikelet and early endosperm development (Figure 1C) and the OsSUS3-OE lines had the largest seeds among all the transgenic lines examined (Figure 2). Based on classic genetic screening, four independent single-locus homozygous transgenic OsSUS3 lines were obtained, with greatly increased OsSUS3 expression levels determined by Q-PCR, and significantly elevated SUS enzyme activities by in vitro assay (Figure 3A,B).
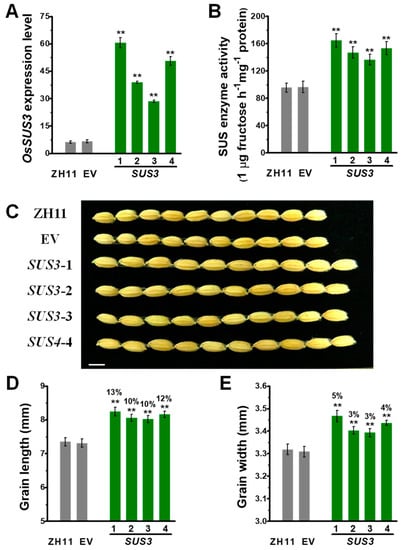
Figure 3.
Characterization of grain weight and related traits in OsSUS3-transgenic plants. (A) Q-PCR analysis of OsSUS3 expression in four transgenic lines. (B) Total OsSUS activity assay. (C) Comparison of ZH11, EV and OsSUS3-transgenic grains, scale bars as 10 mm. (D) Grain length. (E) Grain width. All data are given as means ± SD. A Student’s t-test performed between transgenic plants and EV as ** p < 0.01 and * p < 0.05 (n = 3 in A, B; n = 50 in D, E).
During the three-year field experiments at different locations (Wuhan and Hainan), all transgenic rice lines showed significantly higher grain yield and 1000-grain weight, compared with those of the ZH11 and EV controls (p < 0.05 or p < 0.01) (Table 1). As 1000-grain weights were significantly changed, this study examined the grain length, width and thickness in the transgenic rice plants. We observed a substantial increase (10–13%) of grain length, with a slightly increase in grain width (3–5%), and no obvious differences in grain thickness (Figure 3C–E, Figure S2). Hence, the results indicated that the OsSUS3 overexpression could significantly improve grain weight mainly through increasing grain length and width in the transgenic rice lines.

Table 1.
Grain-yield related traits of OsSUS3-transgenic rice plants in the three-year field experiment.
2.4. Increased Cell Number in OsSUS3-OE Spikelet Hulls
As the spikelet hulls of rice have been proposed to control grain growth for grain size [], we firstly examined the spikelet hull growth and development. The representative OsSUS3-OE line exhibited a consistently increased hull length during the hull development from stage 1 to 5 (Figure 4A,B). Cell proliferation and expansion processes have been known to coordinately regulate spikelet hull growth. To further clarify the causes of the larger grains in OsSUS3-OE lines, we examined both cell size and cell number of spikelet hulls. Using scanning electron microscope (SEM), we further observed the outer epidermal cells of spikelet hulls (Figure 4C). As a result, the average length and width of the epidermal cells were not significantly changed in OsSUS3-OE line compared with the control (Figure 4D), while the cell numbers both in grain- length and grain- width directions were significantly increased (Figure 4E), suggesting that the larger hull sizes may mainly result from the increased cell numbers.
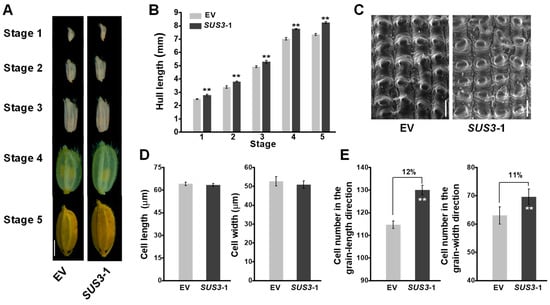
Figure 4.
Characterization of hull length and related traits in OsSUS3-transgenic plants. (A) Light images of hulls at five stages of hull development; scale bars as 2 mm. (B) Hull length at five stages of hull development. (C) Scanning electron microscope images of glume outer surfaces at mature stage of hull, scale bars as 50 μm (D) Cell length of hulls observed in C. (E) Cell number of glume outer surfaces. All data are given as means ± SD. A Student’s t-test performed between transgenic plants and EV as ** p < 0.01 and * p < 0.05 (n = 50 in B, D, E).
2.5. Altered Gene Expressions Associated with Cell Division and Cellulose Biosynthesis
As plant organ size and cell number is determined by cell division, this study examined the expression of genes related to cell division. Compared to the controls, the relative expression levels of genes involved in cell cycle (E2F2, CDKA1, CAK1, H1, MCM3, CYCB2.2) [] were considerably up-regulated in OsSUS3-OE line during hull development from stages 2 to 4 (Figure 5A), consistent with the increased cell number. Thus, the results indicated that the larger hull sizes in the OsSUS3 transgenic lines may mainly result from the increased cell numbers in the spikelet hulls by promoting cell division. In addition, the OsSUS3 transgenic hulls exhibited much higher expressions of OsCESA1, A3 and lower expressions of OsCESA4, A9 genes, which are respectively involved in cellulose biosynthesis of primary and secondary cell walls (Figure 5B).
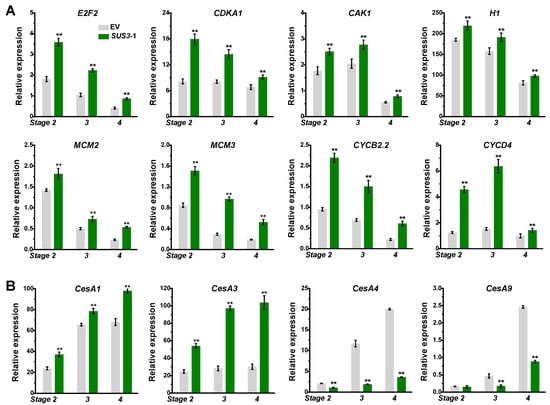
Figure 5.
The expression profiling of cell division related genes and cellulose synthase genes. (A) Cell division related genes. (B) Cellulose synthase genes. All data are given as means ± SD. A Student’s t-test performed between transgenic plants and EV as ** p < 0.01 and * p < 0.05 (n = 3).
2.6. Augmented Starch Accumulation in the OsSUS3 Transgenic Grains
We further compared the sizes of brown grains (grain without hull) between the representative OsSUS3 transgenic line and the EV control (Figure 6). During grain development, the OsSUS3 transgenic line showed consistently larger brown grain sizes, resulting in significantly increased 1000 brown grains than those of the EV (Figure 6A,B). In particular, the transgenic lines had increased dry weight of 1000 brown grains by 17–21% at mature stage, compared with the ZH11 and EV (Figure 6C). Because starch is the major component of brown rice grain, we measured that the four OsSUS3 transgenic lines exhibited higher starch levels by 5–9% than those in the ZH11 (Figure 6D). In terms of the increased starch levels in the transgenic lines, we analyzed the transcript levels of OsSSI, OsSSIII-2, OsGBSS1, OsAGPS2 and OsAGPL1 genes, which are preferentially expressed in the endosperm for starch synthesis []. In comparison, those genes were much higher expressed in the OsSUS3 transgenic grains than those in the EV (Figure 6E), consistent with the increased starch contents (Figure 6D) and the enhanced OsSUS3 expression in the endosperm (Figure S3) of OsSUS3 transgenic plants. Taken together, the results suggested that the OsSUS3 overexpression might increase the endosperm volume and up-regulate OsSSs and OsAGPs expression for starch synthesis, leading to a relatively increased grain weight during grain-filling in the OsSUS3 transgenic rice plants.
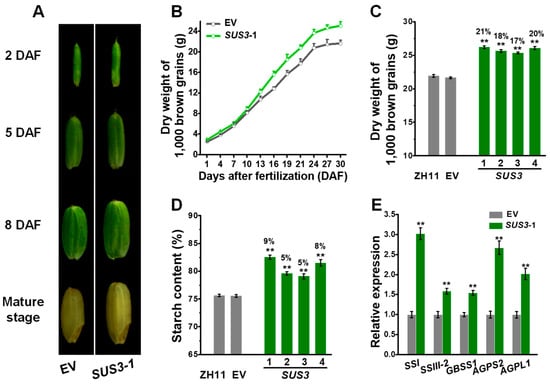
Figure 6.
Assessments of grain filling in OsSUS3-transgenic plants. (A) Images of developing brown grains at four stages (DAF, days after fertilization). (B) Time-course of dry weights of 1000 brown grains. (C) Dry weight of 1000 brown grains at mature stage. (D) Starch content of brown grains at mature stage. (E) Q-PCR analysis of starch synthase genes in endosperm. All data are given as means ± SD (n = 10 in B, C; n = 3 in D, E). A Student’s t-test performed between transgenic plants and ZH11 as ** p < 0.01 and * p < 0.05.
3. Discussion
Grain yield in rice is determined by tillers number per plant, grain number per panicle, and grain weight. It is important to increase grain weight for further improvement in grain yield, when tillers number per plant and grain number per panicle reach optimum levels. Grain weight is mainly determined by grain size. In rice, spikelet hulls are proposed to restrict seed growth, thereby determining the final grain size [,]. In principle, the spikelet hull growth is determined by cell division, expansion and differentiation []. In this study, despite that all OsSUS-overexpressed transgenic rice plants showed consistently increased grain yield and 1000-grain weight (Figure 2), an examination from large-scale field experiments is needed in the future. Further analysis in OsSUS3-OE plants indicated that the hull size, especially hull length was enhanced (Figure 3). Notably, the OsSUS3-OE transgenic rice plants showed consistently increased cell number for large hull size, supported by the enhanced transcript levels of cell division-related genes during hull growth and development (Figure 4 and Figure 5). It has been shown that sucrose metabolism is tightly coupled with sugar signaling by the generation of sugar signaling molecules such as sucrose, glucose, fructose, and trehalose-6-phosphate, and the sugar signaling modulates plant development either directly or through interactions with other signaling pathways, including hormone- and redox-mediated processes []. As SUS catalyzes the conversion of sucrose and a nucleoside diphosphate into the nucleoside diphosphate-glucose and fructose, the contents of sugar signaling molecules should be altered in OsSUSs overexpressed plants. This may modulates rice hull development by regulating cell division.
In addition, cell size and shape are basically decided by plant cell walls [,,]. In plants, primary cell wall synthesis is intimately associated with cell division and elongation processes that determine an organ/tissue size, whereas secondary wall synthesis is initiated for the process of cell differentiation and biomass deposition [,,]. As SUS enzymes are characterized to provide UDPG substrates for the biosynthesis of cellulose [,,,,,,,], the OsSUS-overexpressed transgenic rice plants showed an increased expression of primary cell wall cellulose synthesis genes (OsCESA1, A3) and the reduced expression of secondary wall cellulose biosynthesis genes (OsCESA4, A9) during hull growth (Figure 5B). Thus, OsSUS overexpression may prolong primary wall cellulose synthesis and consequently delay secondary wall synthesis for enhanced cell division in the hull tissues, resulting in relatively increased cell numbers for larger hull sizes in the transgenic rice plants.
Grain weight is also determined by the degree of grain filling in crops []. Generally, photo-assimilate supply is an important limiting factor for grain filling in the spikelets. The poor grain filling of the spikelets is mainly due to a weak competence for the photo-assimilate, as a result of low grain sink capacity. As SUS has been regarded as a biochemical marker for sink strength [], the OsSUS overexpression may accordingly enhance the sink strength in grain, and increase ADPG substrate for starch biosynthesis, result in increased grain fillings in the transgenic rice plants in this study. In addition, because it has been reported that OsSUS3 provides high-temperature tolerance during the ripening stage in rice [], it remains interesting to examine whether the abiotic stresses would be enhanced in the OsSUS-overexpressed transgenic rice plants in the future studies.
Although six OsSUSs family genes exhibit different genomic structures and distinct expression patterns in the life cycle of rice, all selected OsSUS1-6 overexpressed plants appeared to show increased 1000-grain weight and grain yield in this study. In addition, our preliminary data also showed that the co-overexpression between OsSUS3 and other five OsSUSs genes could not further enhance grain weight (data not shown), suggesting that each OsSUS enzyme may have an identical role in improvements of hull size and grain weight when overexpressed in rice.
It has been characterized that grain weight is affected by the GIF1 gene, a cell wall invertase required for carbon partitioning during early grain filling, because the gif1 mutant shows a slowing down of grain filling for a reduced grain weight [], whereas the GIF1 overexpressed transgenic maize plants produce larger cobs and kernels for increased grain yield []. Since both OsSUS and invertase enzymes could catalyze sucrose version for carbon partitioning regulation, it is assumed that those two enzymes should play a similar role for increased grain weight in the transgenic crops. However, it remains to test which enzyme could be more effective for enhanced grain weight, in particular on grain yield in the field. In addition, it is interesting to explore whether co-overexpression of both SUS and invertase genes could lead to an accumulative enhancement of grain weight in rice and other crops in the future.
4. Materials and Methods
4.1. Sequence and Phylogenetic Analysis
DNA sequences of OsSUS genes were obtained from the rice genome annotation project (RGAP) (http://rice.plantbiology.msu.edu). Exon-intron structure analysis was performed using GSDS (http://gsds. cbi.pku.edu.cn/), and the sequences were aligned using ClustalW program implemented in MEGA7 (https://www.megasoftware.net/). The protein-coding sequences were downloaded from the National Center for Biotechnology Information (NCBI) web site (http://www.ncbi.nlm.nih.gov). One hundred and twelve protein sequences from thirty-seven plant species were collected (Table S1). The phylogenetic tree was constructed by MEGA7 with neighbor-joining (NJ) method.
4.2. Expression Analysis of OsSUS Gene Family
Expression profile data of 33 rice tissue samples (Table S2) in Zhenshan97 (ZS97) were obtained from CREP database (http://crep.ncpgr.cn) and rice transcriptome project using Affymetrix Rice GeneChip microarray [].
4.3. Vectors Construction and Gene Transformation
The full-length cDNA clones of six OsSUS were amplified from rice cultivar “Nipponbare” (a japonica variety), and inserted into the plant binary vector pCAMBIA1300 (Cambia) driven with maize polyubiquitin (Ubi) promoter. The constructs were introduced into Agrobacterium tumefaciens strain EHA105 and transferred to rice cultivar “Zhonghua11” (ZH11) by Agrobacterium-mediated transformation. The transgenic plants were selected by the PCR analysis using hygromycin gene sequence as primers. The single-locus homozygous OsSUS1-6 transgenic lines were then identified by the genetic analyses of segregation at 3:1 in the T1 generation and no separations in the T2 and T3 generations (n > 30). All primers used for gene cloning were listed in Table S3.
4.4. Field Experiments and Plant Sample Collection
Transgenic rice plants were respectively grown in the Experimental Stations of Huazhong Agricultural University, Wuhan and Hainan, China. Conventional rice cropping practices, including irrigation, fertilizer application, and pest control, were applied to the field experiments in this study. The spikelet hulls at five developing stages (stage 1, 2 mm; stage 2, 3 mm; stage 3, 5 mm; stage 4, 7 mm; stage 5, maturity) were collected, frozen in liquid nitrogen and stored at −80 °C until use. The grains of various stages from 1 day after fertilization (DAF) to 30 DAF were collected, dried to constant weight and calculated for 1000-grain weight. Mature grains were used for measuring 1000-grain weight, grain length, width and thickness as previously described by Li et al. []. Leica stereomicroscope (Leica S6 D, Leica DFC295 digital camera, Mannheim, Baden-Württemberg, Germany) was used for hull and grain observations. The lengths of hulls at five developing stages were measured using Image J (https://imagej.nih.gov/ij/). For all measurements, grains were obtained from 20 plants grown with 16.7 × 23 cm spacing in paddies under normal cultivation conditions, 1000 seeds per replicate for 1000-grain weight and more than three independent replicates were used for measurements.
4.5. RNA Preparation and Quantitative PCR
Total RNA of plant tissues was extracted using Trizol reagent (Invitrogen, Carlsbad, CA, USA), and reverse-transcribed into cDNA with the GoScript™ Reverse Transcription System (Promega, Madison, WI,, USA). The RT-PCR reaction was performed as described previously []. Quantitative PCR reactions were carried out on a Bio-rad MyCycler thermal cycler (Hampton, NH, USA) with the 2 × SYBR Green qPCR Mix (TransGen Co., Ltd., Beijing, China) according to the manufacturer’s instruction. The rice polyubiquitin gene (OsUBQ1) was used as the internal control. Each measurement was performed using at least two biological samples and each test of sample was conducted with three replicates. The relative quantification of the transcript levels was performed using the comparative Ct method []. All primers used for qPCR were listed in Table S3.
4.6. SUS Enzyme Activity Assay
Total proteins were extracted with the buffer (50 mM Hepes-KOH, 10 mM MgCl2, 1 mM EDTA, 2 mM DTT, 1 mM PMSF, 5 mM Amino-n-caproic acid, 0.1% v/v Triton X-100, 10% v/v glycerol, pH 7.5) [], and SUS activity was assayed as described [] with minor modification. Each reaction contained 200 μL enzyme extract and 400 μL reaction buffer with 50 mM Hepes-KOH (pH 6.5), 100 mM sucrose and 2 mM UDP. The control reactions were performed without UDP in the reaction buffer and the produced fructose levels were subtracted. The fructose contents were tested by GC-MS as previously described []. Protein concentration was determined by the Bradford method with Bovine Serum Albumin (BSA) as standard, and one unit of enzyme activity is defined as 1 mg enzyme releasing 1 μg fructose h−1. Three independent biological replicates were performed for all samples.
4.7. Microscope Observation
The glumes outer surfaces of rice spikelet hulls were observed under a scanning electron microscope (SEM) (JSM-6390LV, JEOL, Tokyo, Japan). The spikelet hulls at developing stages were collected, subsequently fixed with 2.5% (v/v) glutaraldehyde, vacuumed three times, and fixed for at least 24 h. Samples were air-dried, sputter-coated with gold particles, observed and photographed using a scanning electron microscope (JSM-6390LV; JEOL, Tokyo, Japan). The cell density of the glume was calculated as cell number mm−1 in longitude from at least 20 replicates [,].
4.8. Starch Content Assay
The endosperm at different development of OsSUS3-OE and wild-type plants were used to measure the content of starch. The starch were collected and assayed by total starch assay kit (Megazyme, Bray, Co. Wicklow, Ireland).
5. Conclusions
Individual overexpression of six OsSUS genes leads to significantly increased grain weights in all OsSUS1-6 transgenic rice lines, compared to ZH11 and EV controls. The representative OsSUS3 transgenic hulls show raised cell numbers for large hull size during hull growth and development by enhancing cell division and related cellulose biosynthesis. Meanwhile, the OsSUS3 transgenic grains exhibit much starch accumulation for improved grain filling. Hence, this study has demonstrated that OsSUS could enhance cell division in hulls and starch accumulation in grains for increased grain weight in transgenic rice plants.
Supplementary Materials
The following are available online at https://www.mdpi.com/1422-0067/20/20/4971/s1.
Author Contributions
C.F. completed major experiments and wrote the manuscript; G.W. participated in the rice transformation; Y.W. (Youmei Wang) and R.Z. participated in transgenic plants collection; S.F. and Y.W. (Yanting Wang) participated in starch determination and qRT-PCR analysis; K.L. participated in the microscope observation; L.P. supervised experiments and finalized the manuscript.
Funding
This work was in part supported by grants from the National Science Foundation of China (31670296), the National 111 Project (B08032) and the National Transgenic Project (2009ZX08009-119B).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fan, C.; Xing, Y.; Mao, H.; Lu, T.; Han, B.; Xu, C.; Li, X.H.; Zhang, Q. GS3, a major QTL for grain length and weight and minor QTL for grain width and thickness in rice, encodes a putative transmembrane protein. Theor. Appl. Genet. 2006, 112, 1164–1171. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Li, J. Molecular genetic dissection of quantitative trait loci regulating rice grain size. Annu. Rev. Genet. 2014, 48, 99–118. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Sun, S.; Yao, J.; Wang, C.; Yu, S.; Xu, C.; Li, X.; Zhang, Q. Linking differential domain functions of the GS3 protein to natural variation of grain size in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 19579–19584. [Google Scholar] [CrossRef]
- Qi, P.; Lin, Y.S.; Song, X.J.; Shen, J.B.; Huang, W.; Shan, J.X.; Zhu, M.Z.; Jiang, L.; Gao, J.P.; Lin, H.X. The novel quantitative trait locus GL3.1 controls rice grain size and yield by regulating Cyclin-T1; 3. Cell Res. 2012, 22, 1666–1680. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, J.; Huang, J.; Lan, H.; Wang, C.; Yin, C.; Wu, Y.; Tang, H.; Qian, Q.; Li, J.; et al. Rare allele of OsPPKL1 associated with grain length causes extra-large grain and a significant yield increase in rice. Proc. Natl. Acad. Sci. USA 2012, 109, 21534–21539. [Google Scholar] [CrossRef] [PubMed]
- Che, R.; Tong, H.; Shi, B.; Liu, Y.; Fang, S.; Liu, D.; Xiao, Y.; Hu, B.; Liu, L.; Wang, H.; et al. Control of grain size and rice yield by GL2-mediated brassinosteroid responses. Nat. Plants 2015, 2, 15195. [Google Scholar] [CrossRef] [PubMed]
- Shomura, A.; Izawa, T.; Ebana, K.; Ebitani, T.; Kanegae, H.; Konishi, S.; Yano, M. Deletion in a gene associated with grain size increased yields during rice domestication. Nat. Genet. 2008, 40, 1023–1028. [Google Scholar] [CrossRef]
- Li, Y.B.; Fan, C.C.; Xing, Y.Z.; Jiang, Y.H.; Luo, L.J.; Sun, L.; Shao, D.; Xu, C.J.; Li, X.H.; Xiao, J.H.; et al. Natural variation in GS5 plays an important role in regulating grain size and yield in rice. Nat. Genet. 2011, 43, 1266–1269. [Google Scholar] [CrossRef] [PubMed]
- Duan, P.; Ni, S.; Wang, J.; Zhang, B.; Xu, R.; Wang, Y.; Chen, H.; Zhu, X.; Li, Y. Regulation of OsGRF4 by OsmiR396 controls grain size and yield in rice. Nat. Plants 2015, 2, 15203. [Google Scholar] [CrossRef]
- Duan, P.; Xu, J.; Zeng, D.; Zhang, B.; Geng, M.; Zhang, G.; Huang, K.; Huang, L.; Xu, R.; Ge, S.; et al. Natural variation in the promoter of GSE5 contributes to grain size diversity in rice. Mol. Plant 2017, 10, 685–694. [Google Scholar] [CrossRef]
- Liu, J.; Chen, J.; Zheng, X.; Wu, F.; Lin, Q.; Heng, Y.; Tian, P.; Cheng, Z.; Yu, X.; Zhou, K.; et al. GW5 acts in the brassinosteroid signalling pathway to regulate grain width and weight in rice. Nat. Plants 2017, 3, 17043. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wu, K.; Yuan, Q.; Liu, X.; Liu, Z.; Lin, X.; Zeng, R.; Zhu, H.; Dong, G.; Qian, Q.; et al. Control of grain size, shape and quality by OsSPL16 in rice. Nat. Genet. 2012, 44, 950–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, S.; Liu, Q.; Wu, K.; Zhang, J.; Wang, S.; Wang, Y.; Chen, X.; Zhang, Y.; Gao, C.; et al. The OsSPL16-GW7 regulatory module determines grain shape and simultaneously improves rice yield and grain quality. Nat. Genet. 2015, 47, 949–954. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiong, G.; Hu, J.; Jiang, L.; Yu, H.; Xu, J.; Fang, Y.; Zeng, L.; Xu, E.; Xu, J.; et al. Copy number variation at the GL7 locus contributes to grain size diversity in rice. Nat. Genet. 2015, 47, 944–948. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Loboda, T.; Sung, S.J.; Black, C.C. Sucrose synthase in wild tomato, Lycopersicon chmielewskii, and tomato fruit sink strength. Plant Physiol. 1992, 98, 1163–1169. [Google Scholar] [CrossRef] [PubMed]
- Amor, Y.; Haigler, C.H.; Johnson, S.; Wainscott, M.; Delmer, D.P. A membrane-associated form of sucrose synthase and its potential role in synthesis of cellulose and callose in plants. Proc. Natl. Acad. Sci. USA 1995, 92, 9353–9357. [Google Scholar] [CrossRef] [PubMed]
- Zrenner, R.; Salanoubat, M.; Willmitzer, L.; Sonnewald, U. Evidence of the crucial role of sucrose synthase for sink strength using transgenic potato plants (Solanum tuberosum L.). Plant J. 1995, 7, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Sonnewald, U.; Hajirezaei, M.R.; Kossmann, J.; Heyer, A.; Trethewey, R.N.; Willmitzer, L. Increased potato tuber size resulting from apoplastic expression of a yeast invertase. Nat. Biotechnol. 1997, 15, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Wang, J.; Zhu, X.; Hao, W.; Wang, L.; Li, Q.; Zhang, L.; He, W.; Lu, B.; Lin, H.; et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008, 40, 1370–1374. [Google Scholar] [CrossRef]
- Cheng, W.H.; Taliercio, E.W.; Chourey, P.S. The miniature1 seed locus of maize encodes a cell wall invertase required for normal development of endosperm and maternal cells in the pedicel. Plant Cell 1996, 8, 971–983. [Google Scholar] [CrossRef]
- Liu, J.; Huang, J.; Guo, H.; Lan, L.; Wang, H.; Xu, Y.; Yang, X.; Li, W.; Tong, H.; Xiao, Y.; et al. The conserved and unique genetic architecture of kernel size and weight in maize and rice. Plant Physiol. 2017, 175, 774–785. [Google Scholar] [CrossRef] [PubMed]
- Arioli, T.; Peng, L.; Betzner, A.S.; Burn, J.; Wittke, W.; Herth, W.; Camilleri, C.; Höfte, H.; Plazinski, J.; Birch, R.; et al. Molecular analysis of cellulose biosynthesis in Arabidopsis. Science 1998, 279, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Subbaiah, C.C.; Sachs, M.M. Altered patterns of sucrose synthase phosphorylation and localization precede callose induction and root tip death in anoxic maize seedling. Plant Physiol. 2001, 125, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Baroja-Fernández, E.; Muñoz, F.J.; Montero, M.; Etxeberria, E.; Sesma, M.T.; Ovecka, M.; Bahaji, A.; Ezquer, I.; Li, J.; Prat, S.; et al. Enhancing sucrose synthase activity in transgenic potato (Solanum tuberosum L.) tubers results in increased levels of starch, ADPglucose and UDPglucose and total yield. Plant Cell Physiol. 2009, 50, 1651–1662. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Liu, H.; Zhang, Y.; Kang, T.; Zhang, L.; Tong, J.; Xiao, L.; Zhang, H. Constitutive expression of cell wall invertase genes increases grain yield and starch content in maize. Plant Biotechnol. J. 2013, 11, 1080–1091. [Google Scholar] [CrossRef]
- Bahaji, A.; Li, J.; Sánchez-López, Á.M.; Baroja-Fernándeza, E.; Francisco Muñoz, F.J.; Ovecka, M.; Almagro, G.; Montero, M.; Ezquer, I.; Etxeberria, E.; et al. Starch biosynthesis, its regulation and biotechnological approaches to improve crop yields. Biotechnol. Adv. 2014, 32, 87–106. [Google Scholar] [CrossRef] [PubMed]
- Cakir, B.; Shiraishi, S.; Tuncel, A.; Matsusaka, H.; Satoh, R.; Singh, S.; Crofts, N.; Hosaka, Y.; Fujita, N.; Hwang, S.; et al. Analysis of the rice ADP-glucose transporter (OsBT1) indicates the presence of regulatory processes in the amyloplast stroma that control ADP-glucose flux into starch. Plant Physiol. 2016, 170, 1271–1283. [Google Scholar] [PubMed]
- Konishi, T.; Ohmiya, Y.; Hayashi, T. Evidence that sucrose loaded into the phloem of a poplar leaf is used directly by sucrose synthase associated with various β-glucan synthases in the stem. Plant Physiol. 2004, 134, 1146–1152. [Google Scholar] [CrossRef][Green Version]
- Coleman, H.D.; Ellis, D.D.; Gilbert, M.; Mansfield, S.D. Up-regulation of sucrose synthase and UDP-glucose pyrophosphorylase impacts plant growth and metabolism. Plant Biotechnol. J. 2006, 4, 87–101. [Google Scholar] [CrossRef]
- Coleman, H.D.; Yan, J.; Mansfield, S.D. Sucrose synthase affects carbon partitioning to increase cellulose production and altered cell wall ultrastructure. Proc. Natl. Acad. Sci. USA 2009, 106, 13118–13123. [Google Scholar] [CrossRef]
- Jiang, Y.; Guo, W.; Zhu, H.; Ruan, Y.L.; Zhang, T. Overexpression of GhSusA1 increases plant biomass and improves cotton fiber yield and quality. Plant Biotechnol. J. 2012, 10, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.M.; Brill, E.; Llewellyn, D.J.; Furbank, R.T.; Ruan, Y.L. Overexpression of a potato sucrose synthase gene in cotton accelerates leaf expansion, reduces seed abortion, and enhances fiber production. Mol. Plant 2012, 5, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Poovaiah, C.R.; Mazarei, M.; Decker, S.R.; Turner, G.B.; Sykes, R.W.; Davis, M.F.; Stewart, C.N. Transgenic switchgrass (Panicum virgatum L.) biomass is increased by overexpression of switchgrass sucrose synthase (PvSUS1). Biotechnol. J. 2015, 10, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Feng, S.; Huang, J.; Wang, Y.; Wu, L.; Li, X.; Wang, L.; Tu, Y.; Xia, T.; Li, J.; et al. AtCesA8-driven OsSUS3 expression leads to largely enhanced biomass saccharification and lodging resistance by distinctively altering lignocellulose features in rice. Biotechnol. Biofuels 2017, 10, 221. [Google Scholar] [CrossRef] [PubMed]
- Chourey, P.S.; Taliercio, E.W.; Carlson, S.J.; Ruan, Y.L. Genetic evidence that the two isozymes of sucrose synthase present in developing maize endosperm are critical, one for cell wall integrity and the other for starch biosynthesis. Mol. Gen. Genet. 1998, 259, 88–96. [Google Scholar] [PubMed]
- Barratt, D.H.; Derbyshire, P.; Findlay, K.; Pike, M.; Wellner, N.; Lunn, J.; Feil, R.; Simpson, C.; Maule, A.J.; Smith, A.M. Normal growth of Arabidopsis requires cytosolic invertase but not sucrose synthase. Proc. Natl. Acad. Sci. USA 2009, 106, 13124–13129. [Google Scholar] [CrossRef]
- Cho, J.I.; Kim, H.B.; Kim, C.Y.; Hahn, T.R.; Jeon, J.S. Identification and characterization of the duplicate rice sucrose synthase genes OsSUS5 and OsSUS7 which are associated with the plasma membrane. Mol. Cells. 2011, 31, 553–561. [Google Scholar] [CrossRef]
- Cornejo, M.J.; Luth, D.; Blankenship, K.M.; Anderson, O.D.; Blechl, A.E. Activity of a maize ubiquitin promoter in transgenic rice. Plant Mol. Biol. 1993, 23, 567–581. [Google Scholar] [CrossRef]
- Li, N.; Li, Y. Signaling pathways of seed size control in plants. Curr. Opin. Plant Biol. 2016, 33, 23–32. [Google Scholar] [CrossRef]
- Dian, W.; Jiang, H.; Wu, P. Evolution and expression analysis of starch synthase III and IV in rice. J. Exp. Bot. 2005, 56, 623–632. [Google Scholar] [CrossRef]
- Dupuy, L.; Mackenzie, J.; Haseloff, J. Coordination of plant cell division and expansion in a simple morphogenetic system. Proc. Natl. Acad. Sci. USA 2010, 107, 2711–2716. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Annu. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Szymanski, D.B.; Cosgrove, D.J. Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr. Biol. 2009, 19, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G. The role of the cell wall in plant immunity. Front. Plant Sci. 2014, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- Fan, C.; Li, Y.; Hu, Z.; Hu, H.; Wang, G.; Li, A.; Wang, Y.; Tu, Y.; Xia, T.; Peng, L.; et al. Ectopic expression of a novel OsExtensin-like gene consistently enhances plant lodging resistance by regulating cell elongation and cell wall thickening in rice. Plant Biotechnol. J. 2018, 16, 254–263. [Google Scholar] [CrossRef] [PubMed]
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. Toward a systems approach to understanding plant-cell walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef]
- Keegstra, K. Plant Cell Walls. Plant Physiol. 2010, 154, 483–486. [Google Scholar] [CrossRef]
- Schuetz, M.; Smith, R.; Ellis, B. Xylem tissue specification, patterning, and differentiation mechanisms. J. Exp. Bot. 2013, 64, 11–31. [Google Scholar] [CrossRef]
- Takehara, K.; Murata, K.; Yamaguchi, T.; Yamaguchi, K.; Chaya, G.; Kido, S.; Iwasaki, Y.; Ogiwara, H.; Ebitani, T.; Miura, K. Thermo-responsive allele of sucrose synthase 3 (Sus3) provides high-temperature tolerance during the ripening stage in rice (Oryza sativa L.). Breed. Sci. 2018, 18007. [Google Scholar] [CrossRef]
- Wang, L.; Xie, W.; Chen, Y.; Tang, W.; Yang, J.; Ye, R.; Liu, L.; Lin, Y.; Xu, C.; Xiao, J.; et al. A dynamic gene expression atlas covering the entire life cycle of rice. Plant J. 2010, 61, 752–766. [Google Scholar] [CrossRef]
- Bieniawska, Z.; Paul Barratt, D.H.; Garlick, A.P.; Thole, V.; Kruger, N.J.; Martin, C.; Zrenner, R.; Smith, A.M. Analysis of the sucrose synthase gene family in Arabidopsis. Plant J. 2007, 49, 810–828. [Google Scholar] [CrossRef] [PubMed]
- Matamoros Fernández, L.E.; Obel, N.; Scheller, H.V.; Roepstorff, P. Differentiation of isomeric oligosaccharide structures by ESI tandem MS and GC-MS. Carbohyd. Res. 2004, 339, 655–664. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).