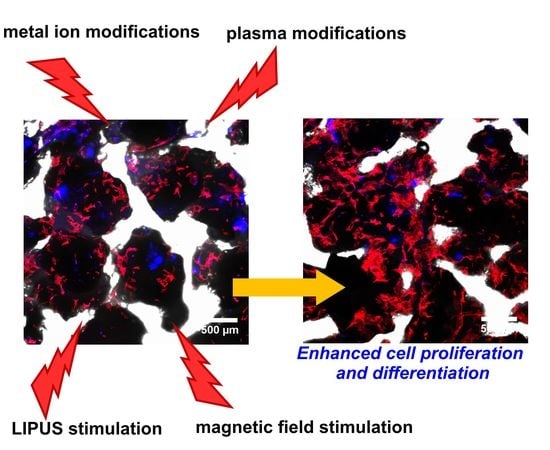
Current Trends in Fabrication of Biomaterials for Bone and Cartilage Regeneration: Materials Modifications and Biophysical Stimulations
Abstract
1. Introduction: Biomaterials for Bone and Cartilage Regeneration
2. Biomaterial Modifications with Metal Ions
2.1. Biomaterials with Osteopromotive Properties
2.2. Biomaterials with Antimicrobial Properties
3. Plasma-Modified Biomaterials
3.1. Biomaterials with Improved Biocompatibility
3.2. Biomaterials with Antibacterial Properties
4. External Biophysical Stimulation of the Biomaterials
4.1. Low-Intensity Pulsed Ultrasound Stimulation
4.2. Magnetic Field Stimulation
5. Piezoelectric Biomaterials
6. Concluding Remarks
Funding
Conflicts of Interest
Abbreviations
| ADSCs | Adipose tissue-derived mesenchymal stem cells |
| bALP | Bone alkaline phosphatase |
| BG | Bioactive glass |
| BMDSCs | Bone marrow-derived mesenchymal stem cells |
| BMPs | Bone Morphogenetic Proteins |
| CPC | Calcium phosphate cement |
| DPSCs | Dental pulp stem cells |
| E | Young’s modulus |
| ECM | Extracellular matrix |
| GAG | Glycosaminoglycan |
| HA | Hydroxyapatite |
| HUVECs | Human umbilical vein endothelial cells |
| KNN | Potassium sodium niobate |
| LIPUS | Low-intensity pulsed ultrasound |
| MNPs | Magnetic nanoparticles |
| MSCs | Mesenchymal stem cells |
| NF-AT | Nuclear Factor of Activated Cells |
| NPs | Nanoparticles |
| PCL | Poly(3-caprolactone) |
| PE | Polyethylene |
| PEG | Poly(ethylene glycol) |
| PIII | Plasma immersion ion implantation |
| PLA | Polylactic acid |
| PLCL | Poly(l-lactide-co-caprolactone) |
| PU | Polyurethane |
| PVDF | Poly (vinylidene fluoride) |
| PVDF-TrFE | Poly (vinylidene fluoride-trifluoroethylene) |
| TCP | Tricalcium phosphate |
References
- Sakai, N.; Hashimoto, C.; Yarimitsu, S.; Sawae, Y.; Komori, M.; Murakami, T. A functional effect of the superficial mechanical properties of articular cartilage as a load bearing system in a sliding condition. Biosurf. Biotribol. 2016, 2, 26–39. [Google Scholar] [CrossRef]
- Töyräs, J.; Lyyra-Laitinen, T.; Niinimäki, M.; Lindgren, R.; Nieminen, M.T.; Kiviranta, I.; Jurvelin, J.S. Estimation of the Young’s modulus of articular cartilage using an arthroscopic indentation instrument and ultrasonic measurement of tissue thickness. J. Biomech. 2001, 34, 251–256. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, B.; Liu, X.S.; Fields, A.J.; Sanyal, A.; Shi, X.; Adams, M.; Keaveny, T.M.; Guo, X.E. Trabecular plates and rods determine elastic modulus and yield strength of human trabecular bone. Bone 2015, 72, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.H.; Rho, J.; Takano, Y.; Tsui, T.Y.; Pharr, G.M. The elastic properties of trabecular and cortical bone tissues are similar: Results from two microscopic measurement techniques. J. Biomech. 1999, 32, 437–441. [Google Scholar] [CrossRef]
- Mirzaali, M.J.; Schwiedrzik, J.J.; Thaiwichai, S.; Best, J.P.; Michler, J.; Zysset, P.K.; Wolfram, U. Mechanical properties of cortical bone and their relationships with age, gender, composition and microindentation properties in the elderly. Bone 2016, 93, 196–211. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Palka, K.; Ginalska, G. Biomedical potential of chitosan/HA and chitosan/β-1,3-glucan/HA biomaterials as scaffolds for bone regeneration—A comparative study. Mater. Sci. Eng. C 2016, 58. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, K.; Nakajima, H. Metallic scaffolds for bone regeneration. Materials 2009, 2, 790–832. [Google Scholar] [CrossRef]
- Przekora, A.; Vandrovcova, M.; Travnickova, M.; Pajorova, J.; Molitor, M.; Ginalska, G.; Bacakova, L. Evaluation of the potential of chitosan/β-1,3-glucan/hydroxyapatite material as a scaffold for living bone graft production in vitro by comparison of ADSC and BMDSC behaviour on its surface. Biomed. Mater. 2017, 12. [Google Scholar] [CrossRef]
- Yang, C.; Huan, Z.; Wang, X.; Wu, C.; Chang, J. 3D printed Fe scaffolds with HA nanocoating for bone regeneration. ACS Biomater. Sci. Eng. 2018, 4, 608–616. [Google Scholar] [CrossRef]
- Dong, Y.; Liang, J.; Cui, Y.; Xu, S.; Zhao, N. Fabrication of novel bioactive hydroxyapatite-chitosan-silica hybrid scaffolds: Combined the sol-gel method with 3D plotting technique. Carbohydr. Polym. 2018, 197, 183–193. [Google Scholar] [CrossRef]
- Kim, B.S.; Yang, S.S.; Kim, C.S. Incorporation of BMP-2 nanoparticles on the surface of a 3D-printed hydroxyapatite scaffold using an ε-polycaprolactone polymer emulsion coating method for bone tissue engineering. Colloids Surf. B Biointerfaces 2018, 170, 421–429. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Alibolandi, M.; Abnous, K.; Salmasi, Z.; Jaafari, M.R.; Ramezani, M. Fabrication of hybrid scaffold based on hydroxyapatite-biodegradable nanofibers incorporated with liposomal formulation of BMP-2 peptide for bone tissue engineering. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 1987–1997. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Palka, K.; Ginalska, G. Chitosan/β-1,3-glucan/calcium phosphate ceramics composites-Novel cell scaffolds for bone tissue engineering application. J. Biotechnol. 2014, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhao, R.; Huang, X.; Wang, X.; Tang, S. Fabrication and biocompatibility of agarose acetate nanofibrous membrane by electrospinning. Carbohydr. Polym. 2018, 197, 237–245. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Czechowska, J.; Pijocha, D.; Slosarczyk, A.; Ginalska, G. Do novel cement-type biomaterials reveal ion reactivity that affects cell viability in vitro? Cent. Eur. J. Biol. 2014, 9. [Google Scholar] [CrossRef]
- An, J.; Liao, H.; Kucko, N.W.; Herber, R.P.; Wolke, J.G.C.; Van Den Beucken, J.J.J.P.; Jansen, J.A.; Leeuwenburgh, S.C.G. Long-term evaluation of the degradation behavior of three apatite-forming calcium phosphate cements. J. Biomed. Mater. Res. Part A 2016, 104, 1072–1081. [Google Scholar] [CrossRef]
- Akkineni, A.R.; Luo, Y.; Schumacher, M.; Nies, B.; Lode, A.; Gelinsky, M. 3D plotting of growth factor loaded calcium phosphate cement scaffolds. Acta Biomater. 2015, 27, 264–274. [Google Scholar] [CrossRef]
- Xu, H.H.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Frajkorová, F.; Bodišová, K.; Boháč, M.; Bartoníčková, E.; Sedláček, J. Preparation and characterisation of porous composite biomaterials based on silicon nitride and bioglass. Ceram. Int. 2015, 41, 9770–9778. [Google Scholar] [CrossRef]
- Zhou, Z.; Ruan, J.; Zou, J.; Zhou, Z.; Shen, X. Bioactivity of bioresorbable composite based on bioactive glass and poly-L-lactide. Trans. Nonferrous Met. Soc. China 2007, 17, 394–399. [Google Scholar] [CrossRef]
- Duarte Campos, D.F.; Drescher, W.; Rath, B.; Tingart, M.; Fischer, H. Supporting biomaterials for articular cartilage repair. Cartilage 2012, 3, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Vinatier, C.; Guicheux, J. Cartilage tissue engineering: From biomaterials and stem cells to osteoarthritis treatments. Ann. Phys. Rehabil. Med. 2016, 59, 139–144. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, B.J.; Dormer, N.H.; Ingavle, G.C.; Roatch, C.H.; Lomakin, J.; Detamore, M.S.; Gehrke, S.H. Hierarchically designed agarose and poly(ethylene glycol) interpenetrating network hydrogels for cartilage tissue engineering. Tissue Eng. Part C Methods 2010, 16, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Liu, Y.; Jiang, Z.; Chen, W.; Yu, Y.; Hu, Q. Cell-scaffold interaction within engineered tissue. Exp. Cell Res. 2014, 323, 346–351. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chu, C.R.; Payne, K.; Marra, K.G. Injectable in situ forming biodegradable chitosan-hyaluronic acid based hydrogels for cartilage tissue engineering. Biomaterials 2009, 30, 2499–2506. [Google Scholar] [CrossRef] [PubMed]
- Przekora, A.; Benko, A.; Blazewicz, M.; Ginalska, G. Hybrid chitosan/β-1,3-glucan matrix of bone scaffold enhances osteoblast adhesion, spreading and proliferation via promotion of serum protein adsorption. Biomed. Mater. 2016, 11. [Google Scholar] [CrossRef] [PubMed]
- Ercan, B.; Webster, T. Cell response to nanoscale features and its implications in tissue regeneration: An orthopedic perspective. In Nanotechnology and Tissue Engineering: The Scaffold; Laurencin, C., Nair, L., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 151–155. [Google Scholar]
- Chang, H.-I.; Wang, Y. Cell response to surface and architecture of tissue engineering scaffolds. In Regenerative Medicine and Tissue Engineering—Cells and Biomaterials; Eberli, D., Ed.; InTech Open Access Publisher: Rijeka, Croatia, 2011. [Google Scholar] [CrossRef]
- Le, X.; Poinern, G.E.J.; Ali, N.; Berry, C.M.; Fawcett, D. Engineering a biocompatible scaffold with either micrometre or nanometre scale surface topography for promoting protein adsorption and cellular response. Int. J. Biomater. 2013, 2013, 782549. [Google Scholar] [CrossRef]
- Chen, S.; Lee, C.Y.; Li, R.W.; Smith, P.N.; Qin, Q.H. Modelling osteoblast adhesion on surface-engineered biomaterials: Optimisation of nanophase grain size. Comput. Methods Biomech. Biomed. Eng. 2017, 20, 905–914. [Google Scholar] [CrossRef]
- Carina, V.; Costa, V.; Raimondi, L.; Pagani, S.; Sartori, M.; Figallo, E.; Setti, S.; Alessandro, R.; Fini, M.; Giavaresi, G. Effect of low-intensity pulsed ultrasound on osteogenic human mesenchymal stem cells commitment in a new bone scaffold. J. Appl. Biomater. Funct. Mater. 2017, 15, e215–e222. [Google Scholar] [CrossRef]
- Zhang, J.; Ding, C.; Ren, L.; Zhou, Y.; Shang, P. The effects of static magnetic fields on bone. Prog. Biophys. Mol. Biol. 2014, 114, 146–152. [Google Scholar] [CrossRef]
- Leppik, L.; Zhihua, H.; Mobini, S.; Thottakkattumana Parameswaran, V.; Eischen-Loges, M.; Slavici, A.; Helbing, J.; Pindur, L.; Oliveira, K.M.C.; Bhavsar, M.B.; et al. Combining electrical stimulation and tissue engineering to treat large bone defects in a rat model. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Griffith, M.; Islam, M.M.; Edin, J.; Papapavlou, G.; Buznyk, O.; Patra, H.K. The quest for anti-inflammatory and anti-infective biomaterials in clinical translation. Front. Bioeng. Biotechnol. 2016, 4, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Borgwardt, L.; Høiby, N.; Wu, H.; Sørensen, T.S.; Borgwardt, A. Prosthesis infections after orthopedic joint replacement: The possible role of bacterial biofilms. Orthop. Rev. 2013, 5, 65–71. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, E.; Awale, G.; Daneshmandi, L.; Umerah, O.; Lo, K.W.H. The roles of ions on bone regeneration. Drug Discov. Today 2018, 23, 879–890. [Google Scholar] [CrossRef]
- Galli, S.; Naito, Y.; Karlsson, J.; He, W.; Miyamoto, I.; Xue, Y.; Andersson, M.; Mustafa, K.; Wennerberg, A.; Jimbo, R. Local release of magnesium from mesoporous TiO2 coatings stimulates the peri-implant expression of osteogenic markers and improves osteoconductivity in vivo. Acta Biomater. 2014, 10, 5193–5201. [Google Scholar] [CrossRef]
- Liu, C.; Fu, X.; Pan, H.; Wan, P.; Wang, L.; Tan, L.; Wang, K.; Zhao, Y.; Yang, K.; Chu, P.K. Biodegradable Mg-Cu alloys with enhanced osteogenesis, angiogenesis, and long-lasting antibacterial effects. Sci. Rep. 2016, 6, 1–17. [Google Scholar] [CrossRef]
- Yusa, K.; Yamamoto, O.; Takano, H.; Fukuda, M.; Iino, M. Zinc-modified titanium surface enhances osteoblast differentiation of dental pulp stem cells in vitro. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef]
- Yusa, K.; Yamamoto, O.; Fukuda, M.; Koyota, S.; Koizumi, Y.; Sugiyama, T. In vitro prominent bone regeneration by release zinc ion from Zn-modified implant. Biochem. Biophys. Res. Commun. 2011, 412, 273–278. [Google Scholar] [CrossRef]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef]
- Andersen, O.Z.; Offermanns, V.; Sillassen, M.; Almtoft, K.P.; Andersen, I.H.; Sørensen, S.; Jeppesen, C.S.; Kraft, D.C.E.; Bøttiger, J.; Rasse, M.; et al. Accelerated bone ingrowth by local delivery of strontium from surface functionalized titanium implants. Biomaterials 2013, 34, 5883–5890. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Arikawa, K.; Nagahama, F. Micromolar levels of sodium fluoride promote osteoblast differentiation through Runx2 signaling. Biol. Trace Elem. Res. 2017, 178, 283–291. [Google Scholar] [CrossRef] [PubMed]
- Uysal, I.; Severcan, F.; Tezcaner, A.; Evis, Z. Co-doping of hydroxyapatite with zinc and fluoride improves mechanical and biological properties of hydroxyapatite. Prog. Nat. Sci. Mater. Int. 2014, 24, 340–349. [Google Scholar] [CrossRef]
- Khorami, M.; Hesaraki, S.; Behnamghader, A.; Nazarian, H.; Shahrabi, S. In vitro bioactivity and biocompatibility of lithium substituted 45S5 bioglass. Mater. Sci. Eng. C 2011, 31, 1584–1592. [Google Scholar] [CrossRef]
- Wu, C.; Zhou, Y.; Fan, W.; Han, P.; Chang, J.; Yuen, J.; Zhang, M.; Xiao, Y. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Xu, M.; Han, P.; Chen, L.; Chang, J.; Xiao, Y. Copper-containing mesoporous bioactive glass scaffolds with multifunctional properties of angiogenesis capacity, osteostimulation and antibacterial activity. Biomaterials 2013, 34, 422–433. [Google Scholar] [CrossRef]
- Okuzu, Y.; Fujibayashi, S.; Yamaguchi, S.; Yamamoto, K.; Shimizu, T.; Sono, T.; Goto, K.; Otsuki, B.; Matsushita, T.; Kokubo, T.; et al. Strontium and magnesium ions released from bioactive titanium metal promote early bone bonding in a rabbit implant model. Acta Biomater. 2017, 63, 383–392. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.S.; Zhou, W.-S.; He, X.W.; Liu, W.; Bai, B.L.; Zhou, Q.; Li, H.; Huang, Z.L.; Tu, K.; Hang, L.; et al. A comparative study of zinc, magnesium, strontium-incorporated hydroxyapatite-coated titanium implants for osseointegration of osteopenic rats. Mater. Sci. Eng. C 2016, 62, 226–232. [Google Scholar] [CrossRef]
- Yusa, K.; Yamamoto, O.; Iino, M.; Takano, H.; Fukuda, M.; Qiao, Z.; Sugiyama, T. Eluted zinc ions stimulate osteoblast differentiation and mineralization in human dental pulp stem cells for bone tissue engineering. Arch. Oral Biol. 2016, 71, 162–169. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, W.; Tian, P.; Meng, F.; Zhu, H.; Jiang, X.; Liu, X.; Chu, P.K. Stimulation of bone growth following zinc incorporation into biomaterials. Biomaterials 2014, 35, 6882–6897. [Google Scholar] [CrossRef]
- Offermanns, V.; Andersen, O.Z.; Riede, G.; Sillassen, M.; Jeppesen, C.S.; Almtoft, K.P.; Talasz, H.; Öhman-Mägi, C.; Lethaus, B.; Tolba, R.; et al. Effect of strontium surface-functionalized implants on early and late osseointegration: A histological, spectrometric and tomographic evaluation. Acta Biomater. 2018, 69, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Perez, R.A.; Kim, J.H.; Buitrago, J.O.; Wall, I.B.; Kim, H.W. Novel therapeutic core-shell hydrogel scaffolds with sequential delivery of cobalt and bone morphogenetic protein-2 for synergistic bone regeneration. Acta Biomater. 2015, 23, 295–308. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Lotfibakhshaiesh, N.; Ai, J.; Mozafari, M.; Brouki Milan, P.; Hamzehlou, S.; Barati, M.; Baino, F.; Hill, R.G.; Joghataei, M.T. Strontium- and cobalt-substituted bioactive glasses seeded with human umbilical cord perivascular cells to promote bone regeneration via enhanced osteogenic and angiogenic activities. Acta Biomater. 2017, 58, 502–514. [Google Scholar] [CrossRef]
- Zhu, H.; Hu, C.; Zhang, F.; Feng, X.; Li, J.; Liu, T.; Chen, J.; Zhang, J. Preparation and antibacterial property of silver-containing mesoporous 58S bioactive glass. Mater. Sci. Eng. C 2014, 42, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.J.; Hsieh, M.F.; Huang, C.W.; Perng, L.H.; Wen, H.W.; Chin, T.S. Antimicrobial effects and human gingival biocompatibility of hydroxyapatite sol-gel coatings. J. Biomed. Mater. Res. Part B Appl. Biomater. 2006, 76, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Radovanović, Ž.; Jokić, B.; Veljović, D.; Dimitrijević, S.; Kojić, V.; Petrović, R.; Janaćković, D. Antimicrobial activity and biocompatibility of Ag+- and Cu2+-doped biphasic hydroxyapatite/α-tricalcium phosphate obtained from hydrothermally synthesized Ag+- and Cu2+-doped hydroxyapatite. Appl. Surf. Sci. 2014, 307, 513–519. [Google Scholar] [CrossRef]
- Stanić, V.; Dimitrijević, S.; Antić-Stanković, J.; Mitrić, M.; Jokić, B.; Plećaš, I.B.; Raičević, S. Synthesis, characterization and antimicrobial activity of copper and zinc-doped hydroxyapatite nanopowders. Appl. Surf. Sci. 2010, 256, 6083–6089. [Google Scholar] [CrossRef]
- Gritsch, L.; Lovell, C.; Goldmann, W.H.; Boccaccini, A.R. Fabrication and characterization of copper(II)-chitosan complexes as antibiotic-free antibacterial biomaterial. Carbohydr. Polym. 2018, 179, 370–378. [Google Scholar] [CrossRef]
- Bari, A.; Bloise, N.; Fiorilli, S.; Novajra, G.; Vallet-Regí, M.; Bruni, G.; Torres-Pardo, A.; González-Calbet, J.M.; Visai, L.; Vitale-Brovarone, C. Copper-containing mesoporous bioactive glass nanoparticles as multifunctional agent for bone regeneration. Acta Biomater. 2017, 55, 493–504. [Google Scholar] [CrossRef]
- Lin, Y.; Yang, Z.; Cheng, J. Preparation, characterization and antibacterial property of cerium substituted hydroxyapatite nanoparticles. J. Rare Earths 2007, 25, 452–456. [Google Scholar] [CrossRef]
- Moghanian, A.; Firoozi, S.; Tahriri, M. Synthesis and in vitro studies of sol-gel derived lithium substituted 58S bioactive glass. Ceram. Int. 2017, 43, 12835–12843. [Google Scholar] [CrossRef]
- Rau, J.V.; Fosca, M.; Graziani, V.; Egorov, A.A.; Zobkov, Y.V.; Fedotov, A.Y.; Ortenzi, M.; Caminiti, R.; Baranchikov, A.E.; Komlev, V.S. Silver-doped calcium phosphate bone cements with antibacterial properties. J. Funct. Biomater. 2016, 7, 10. [Google Scholar] [CrossRef] [PubMed]
- Siek, D.; Ślósarczyk, A.; Przekora, A.; Belcarz, A.; Zima, A.; Ginalska, G.; Czechowska, J. Evaluation of antibacterial activity and cytocompatibility of α-TCP based bone cements with silver-doped hydroxyapatite and CaCO3. Ceram. Int. 2017, 43. [Google Scholar] [CrossRef]
- Lim, P.N.; Teo, E.Y.; Ho, B.; Tay, B.Y.; Thian, E.S. Effect of silver content on the antibacterial and bioactive properties of silver-substituted hydroxyapatite. J. Biomed. Mater. Res. Part A 2013, 101 A, 2456–2464. [Google Scholar] [CrossRef]
- Pelletier, D.A.; Suresh, A.K.; Holton, G.A.; McKeown, C.K.; Wang, W.; Gu, B.; Mortensen, N.P.; Allison, D.P.; Joy, D.C.; Allison, M.R.; et al. Effects of engineered cerium oxide nanoparticles on bacterial growth and viability. Appl. Environ. Microbiol. 2010, 76, 7981–7989. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Lee, B.L.-P.; Komvopoulos, K.; Yan, Z.; Li, S. Plasma surface chemical treatment of electrospun poly(l-Lactide) microfibrous scaffolds for enhanced cell adhesion, growth, and infiltration. Tissue Eng. Part A 2013, 19, 1188–1198. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.F.; Siow, K.S.; Ng, P.Y.; Majlis, B.Y. Enhancing the biocompatibility of the polyurethane methacrylate and off-stoichiometry thiol-ene polymers by argon and nitrogen plasma treatment. Mater. Sci. Eng. C 2017, 79, 613–621. [Google Scholar] [CrossRef]
- Griffin, M.; Palgrave, R.; Baldovino-, V.G.; Butler, P.E.; Kalaskar, D.M. Argon plasma improves the tissue integration and angiogenesis of subcutaneous implants by modifying surface chemistry and topography. Int. J. Nanomed. 2018, 13, 6123–6141. [Google Scholar] [CrossRef]
- Sagbas, B. Argon/oxygen plasma surface modification of biopolymers for improvement of wettability and wear resistance. Int. J. Chem. Mol. Nucl. Mater. Metall. Eng. 2016, 10, 889–894. [Google Scholar]
- Kostov, K.G.; Nishime, T.M.C.; Castro, A.H.R.; Toth, A.; Hein, L.R.O. Surface modification of polymeric materials by cold atmospheric plasma jet. Appl. Surf. Sci. 2014, 314, 367–375. [Google Scholar] [CrossRef]
- Jordá-Vilaplana, A.; Fombuena, V.; García-García, D.; Samper, M.D.; Sánchez-Nácher, L. Surface modification of polylactic acid (PLA) by air atmospheric plasma treatment. Eur. Polym. J. 2014, 58, 23–33. [Google Scholar] [CrossRef]
- Griffin, M.F.; Ibrahim, A.; Seifalian, A.M.; Butler, P.E.M.; Kalaskar, D.M.; Ferretti, P. Chemical group-dependent plasma polymerisation preferentially directs adipose stem cell differentiation towards osteogenic or chondrogenic lineages. Acta Biomater. 2017, 50, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Favi, P.; Cheng, X.; Golshan, N.H.; Ziemer, K.S.; Keidar, M.; Webster, T.J. Cold atmospheric plasma (CAP) surface nanomodified 3D printed polylactic acid (PLA) scaffolds for bone regeneration. Acta Biomater. 2016, 46, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, A.; Yeung, K.W.K.; Kao, R.Y.T.; Wu, G.; Hu, T.; Xu, Z.; Chu, P.K. Plasma-modified biomaterials for self-antimicrobial applications. ACS Appl. Mater. Interfaces 2011, 3, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Bazaka, K.; Jacob, M.V.; Crawford, R.J.; Ivanova, E.P. Plasma-assisted surface modification of organic biopolymers to prevent bacterial attachment. Acta Biomater. 2011, 7, 2015–2028. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Luo, Y.; Wang, H.; Jiang, J.; Pu, S.; Chu, P.K. Ag and Ag/N2 plasma modification of polyethylene for the enhancement of antibacterial properties and cell growth/proliferation. Acta Biomater. 2008, 4, 2028–2036. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chu, P.K.; Ji, J.; Zhang, Y.; Ng, S.C.; Yan, Q. Surface antibacterial characteristics of plasma-modified polyethylene. Biopolymers 2006, 83, 62–68. [Google Scholar] [CrossRef]
- Zhang, W.; Chu, P.K.; Ji, J.; Zhang, Y.; Liu, X.; Fu, R.K.Y.; Ha, P.C.T.; Yan, Q. Plasma surface modification of poly vinyl chloride for improvement of antibacterial properties. Biomaterials 2006, 27, 44–51. [Google Scholar] [CrossRef]
- Wang, H.; Ji, J.; Zhang, W.; Wang, W.; Zhang, Y.; Wu, Z.; Zhang, Y.; Chu, P.K. Rat calvaria osteoblast behavior and antibacterial properties of O2 and N2 plasma-implanted biodegradable poly(butylene succinate). Acta Biomater. 2010, 6, 154–159. [Google Scholar] [CrossRef]
- Costa, V.; Carina, V.; Fontana, S.; De Luca, A.; Monteleone, F.; Pagani, S.; Sartori, M.; Setti, S.; Faldini, C.; Alessandro, R.; et al. Osteogenic commitment and differentiation of human mesenchymal stem cells by low-intensity pulsed ultrasound stimulation. J. Cell. Physiol. 2018, 233, 1558–1573. [Google Scholar] [CrossRef]
- Uddin, S.M.Z.; Qin, Y.X. Enhancement of osteogenic differentiation and proliferation in human mesenchymal stem cells by a modified low intensity ultrasound stimulation under simulated microgravity. PLoS ONE 2013, 8, e73914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Chow, S.K.H.; Leung, K.S.; Cheung, W.H. Ultrasound as a stimulus for musculoskeletal disorders. J. Orthop. Transl. 2017, 9, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Kusuyama, J.; Bandow, K.; Shamoto, M.; Kakimoto, K.; Ohnishi, T.; Matsuguchi, T. Low intensity pulsed ultrasound (LIPUS) influences the multilineage differentiation of mesenchymal stem and progenitor cell lines through ROCK-Cot/Tpl2-MEK-ERK signaling pathway. J. Biol. Chem. 2014, 289, 10330–10344. [Google Scholar] [CrossRef] [PubMed]
- Moonga, S.S.; Qin, Y.X. MC3T3 infiltration and proliferation in bovine trabecular scaffold regulated by dynamic flow bioreactor and augmented by low-intensity pulsed ultrasound. J. Orthop. Transl. 2018, 14, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Castro, N.J.; Zhu, W.; Cui, H.; Aliabouzar, M.; Sarkar, K.; Zhang, L.G. Improved human bone marrow mesenchymal stem cell osteogenesis in 3D bioprinted tissue scaffolds with low intensity pulsed ultrasound stimulation. Sci. Rep. 2016, 6, 32876. [Google Scholar] [CrossRef] [PubMed]
- Aliabouzar, M.; Lee, S.J.; Zhou, X.; Zhang, G.L.; Sarkar, K. Effects of scaffold microstructure and low intensity pulsed ultrasound on chondrogenic differentiation of human mesenchymal stem cells. Biotechnol. Bioeng. 2018, 115, 495–506. [Google Scholar] [CrossRef] [PubMed]
- Aliabouzar, M.; Zhang, L.G.; Sarkar, K. Lipid coated microbubbles and low intensity pulsed ultrasound enhance chondrogenesis of human mesenchymal stem cells in 3D printed scaffolds. Sci. Rep. 2016, 6, 37728. [Google Scholar] [CrossRef]
- Gujjalapudi, M.; Anam, C.; Mamidi, P.; Chiluka, R.; Kumar, A.; Bibinagar, R. Effect of magnetic field on bone healing around endosseous implants – An in-vivo study. J. Clin. Diagn. Res. 2016, 10, ZF01–ZF04. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, J.; Zhao, L.; Zhang, F.; Liang, X.-J.; Guo, Y.; Weir, M.D.; Reynolds, M.A.; Gu, N.; Xu, H.H.K. Magnetic field and nano-scaffolds with stem cells to enhance bone regeneration. Biomaterials 2018, 183, 151–170. [Google Scholar] [CrossRef]
- Galli, C.; Pedrazzi, G.; Mattioli-Belmonte, M.; Guizzardi, S. The Use of Pulsed Electromagnetic fields to promote bone responses to biomaterials in vitro and in vivo. Int. J. Biomater. 2018, 2018, 8935750. [Google Scholar] [CrossRef]
- Samal, S.K.; Dash, M.; Shelyakova, T.; Declercq, H.A.; Uhlarz, M.; Bañobre-López, M.; Dubruel, P.; Cornelissen, M.; Herrmannsdörfer, T.; Rivas, J.; et al. Biomimetic magnetic silk scaffolds. ACS Appl. Mater. Interfaces 2015, 7, 6282–6292. [Google Scholar] [CrossRef] [PubMed]
- Cai, Q.; Shi, Y.; Shan, D.; Jia, W.; Duan, S.; Deng, X.; Yang, X. Osteogenic differentiation of MC3T3-E1 cells on poly(l-lactide)/Fe3O4 nanofibers with static magnetic field exposure. Mater. Sci. Eng. C 2015, 55, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Yun, H.M.; Ahn, S.J.; Park, K.R.; Kim, M.J.; Kim, J.J.; Jin, G.Z.; Kim, H.W.; Kim, E.C. Magnetic nanocomposite scaffolds combined with static magnetic field in the stimulation of osteoblastic differentiation and bone formation. Biomaterials 2016, 85, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, Y.; Zuo, Y.; Zou, Q.; Li, J.; Li, Y. Development of Fe3O4–HA/PU superparamagnetic composite porous scaffolds for bone repair application. Mater. Lett. 2018, 212, 303–306. [Google Scholar] [CrossRef]
- Heidari, F.; Razavi, M.; Bahrololoom, M.E.; Yazdimamaghani, M.; Tahriri, M.; Kotturi, H.; Tayebi, L. Evaluation of the mechanical properties, in vitro biodegradability and cytocompatibility of natural chitosan/hydroxyapatite/nano-Fe3O4 composite. Ceram. Int. 2018, 44, 275–281. [Google Scholar] [CrossRef]
- D’Amora, U.; Russo, T.; Gloria, A.; Rivieccio, V.; D’Antò, V.; Negri, G.; Ambrosio, L.; De Santis, R. 3D additive-manufactured nanocomposite magnetic scaffolds: Effect of the application mode of a time-dependent magnetic field on hMSCs behavior. Bioact. Mater. 2017, 2, 138–145. [Google Scholar] [CrossRef]
- Aliramaji, S.; Zamanian, A.; Mozafari, M. Super-paramagnetic responsive silk fibroin/chitosan/magnetite scaffolds with tunable pore structures for bone tissue engineering applications. Mater. Sci. Eng. C 2017, 70, 736–744. [Google Scholar] [CrossRef]
- Anjaneyulu, U.; Vijayalakshmi, U. Preparation and characterization of novel sol-gel derived hydroxyapatite/Fe3O4 composites coatings on Ti-6Al-4V for biomedical applications. Mater. Lett. 2017, 189, 118–121. [Google Scholar] [CrossRef]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A.; et al. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef]
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric biomaterials for sensors and actuators. Adv. Mater. 2018, 1802084, 1802084. [Google Scholar] [CrossRef]
- Jacob, J.; More, N.; Kalia, K.; Kapusetti, G. Piezoelectric smart biomaterials for bone and cartilage tissue engineering. Inflamm. Regen. 2018, 38, 2. [Google Scholar] [CrossRef] [PubMed]
- Tandon, B.; Blaker, J.J.; Cartmell, S.H. Piezoelectric materials as stimulatory biomedical materials and scaffolds for bone repair. Acta Biomater. 2018, 73, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, Z.; Pang, J.; Yu, P.; Tan, G.; Ning, C. Fabrication of biocompatible potassium sodium niobate piezoelectric ceramic as an electroactive implant. Materials 2017, 10, 345. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Li, J.; Men, T.-L.; Wu, J.; Zhang, X.; Wang, K.; Li, J.-F.; Xiao, D.; Zhu, J. High-performance 0-3 type niobate-based lead-free piezoelectric composite ceramics with ZnO inclusions. ACS Appl. Mater. Interfaces 2018, 10, 30566–30573. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Wu, C.; Wu, Z.; Hu, L.; Zhang, W.; Zhao, K. Fabrication and in vitro biological properties of piezoelectric bioceramics for bone regeneration. Sci. Rep. 2017, 7, 43360. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Dan, P.; Sosnik, A.; Kalarikkal, N.; Tran, N.; Vincent, B.; Thomas, S.; Menu, P.; Rouxel, D. Electrospun poly(vinylidene fluoride-trifluoroethylene)/zinc oxide nanocomposite tissue engineering scaffolds with enhanced cell adhesion and blood vessel formation. Nano Res. 2017, 10, 3358–3376. [Google Scholar] [CrossRef]
- Damaraju, S.M.; Shen, Y.; Elele, E.; Khusid, B.; Eshghinejad, A.; Li, J.; Jaffe, M.; Arinzeh, T.L. Three-dimensional piezoelectric fibrous scaffolds selectively promote mesenchymal stem cell differentiation. Biomaterials 2017, 149, 51–62. [Google Scholar] [CrossRef]
- Nunes-Pereira, J.; Ribeiro, S.; Ribeiro, C.; Gombek, C.J.; Gama, F.M.; Gomes, A.C.; Patterson, D.A.; Lanceros-Méndez, S. Poly(vinylidene fluoride) and copolymers as porous membranes for tissue engineering applications. Polym. Test. 2015, 44, 234–241. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, W.; He, T.; Qian, L.; Tan, G.; Ning, C. Polarization of an electroactive functional film on titanium for inducing osteogenic differentiation. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Ribeiro, C.; Moreira, S.; Correia, V.; Sencadas, V.; Rocha, J.G.; Gama, F.M.; Gómez Ribelles, J.L.; Lanceros-Méndez, S. Enhanced proliferation of pre-osteoblastic cells by dynamic piezoelectric stimulation. RSC Adv. 2012, 2, 11504–11509. [Google Scholar] [CrossRef]
- Ribeiro, C.; Parssinen, J.; Sencadas, V.V.; Correia, V.V.; Miettinen, S.; Hytonen, V.P.; Lanceros-Mendez, S.; Pärssinen, J.; Sencadas, V.V.; Correia, V.V.; et al. Dynamic piezoelectric stimulation enhances osteogenic differentiation of human adipose stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 2172–2175. [Google Scholar] [CrossRef] [PubMed]
- Cartmell, S.H.; Thurstan, S.; Gittings, J.P.; Griffiths, S.; Bowen, C.R.; Turner, I.G. Polarization of porous hydroxyapatite scaffolds: Influence on osteoblast cell proliferation and extracellular matrix production. J. Biomed. Mater. Res. Part A 2014, 102, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Kumar, D.; Gittings, J.P.; Turner, I.G.; Bowen, C.R.; Bastida-Hidalgo, A.; Cartmell, S.H. Polarization of hydroxyapatite: Influence on osteoblast cell proliferation. Acta Biomater. 2010, 6, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.K.; Basu, B. Pulsed electrical stimulation and surface charge induced cell growth on multistage spark plasma sintered hydroxyapatite-barium titanate piezobiocomposite. J. Am. Ceram. Soc. 2014, 97, 481–489. [Google Scholar] [CrossRef]
- Liu, B.; Chen, L.; Shao, C.; Zhang, F.; Zhou, K.; Cao, J.; Zhang, D. Improved osteoblasts growth on osteomimetic hydroxyapatite/BaTiO3composites with aligned lamellar porous structure. Mater. Sci. Eng. C 2016, 61, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Dubey, A.; Kakimoto, K.; Obata, A.; Kasuga, T. Enhanced polarization of hydroxyapatite using the design concept of functionally graded materials with sodium potassium niobate. RSC Adv. 2014, 4, 24601–24611. [Google Scholar] [CrossRef]
- Kou, P.M.; Babensee, J.E. Macrophage and dendritic cell phenotypic diversity in the context of biomaterials. J. Biomed. Mater. Res. Part A 2011, 96 A, 239–260. [Google Scholar] [CrossRef]
- Pajarinen, J.; Lin, T.; Gibon, E.; Kohno, Y.; Maruyama, M.; Nathan, K.; Lu, L.; Yao, Z.; Goodman, S.B. Mesenchymal stem cell-macrophage crosstalk and bone healing. Biomaterials 2018. [Google Scholar] [CrossRef]
- Selders, G.S.; Fetz, A.E.; Radic, M.Z.; Bowlin, G.L. An overview of the role of neutrophils in innate immunity, inflammation and host-biomaterial integration. Regen. Biomater. 2017, 4, 55–68. [Google Scholar] [CrossRef]
- El-Jawhari, J.J.; Jones, E.; Giannoudis, P.V. The roles of immune cells in bone healing; what we know, do not know and future perspectives. Injury 2016, 47, 2399–2406. [Google Scholar] [CrossRef]
- Crop, M.J.; Korevaar, S.S.; De Kuiper, R.; Ijzermans, J.N.M.; Van Besouw, N.M.; Baan, C.C.; Weimar, W.; Hoogduijn, M.J. Human mesenchymal stem cells are susceptible to lysis by CD8 + T Cells and NK cells. Cell Transplant. 2011, 20, 1547–1559. [Google Scholar] [CrossRef] [PubMed]
- Ho-Shui-Ling, A.; Bolander, J.; Rustom, L.E.; Johnson, A.W.; Luyten, F.P.; Picart, C. Bone regeneration strategies: Engineered scaffolds, bioactive molecules and stem cells current stage and future perspectives. Biomaterials 2018, 180, 143–162. [Google Scholar] [CrossRef] [PubMed]
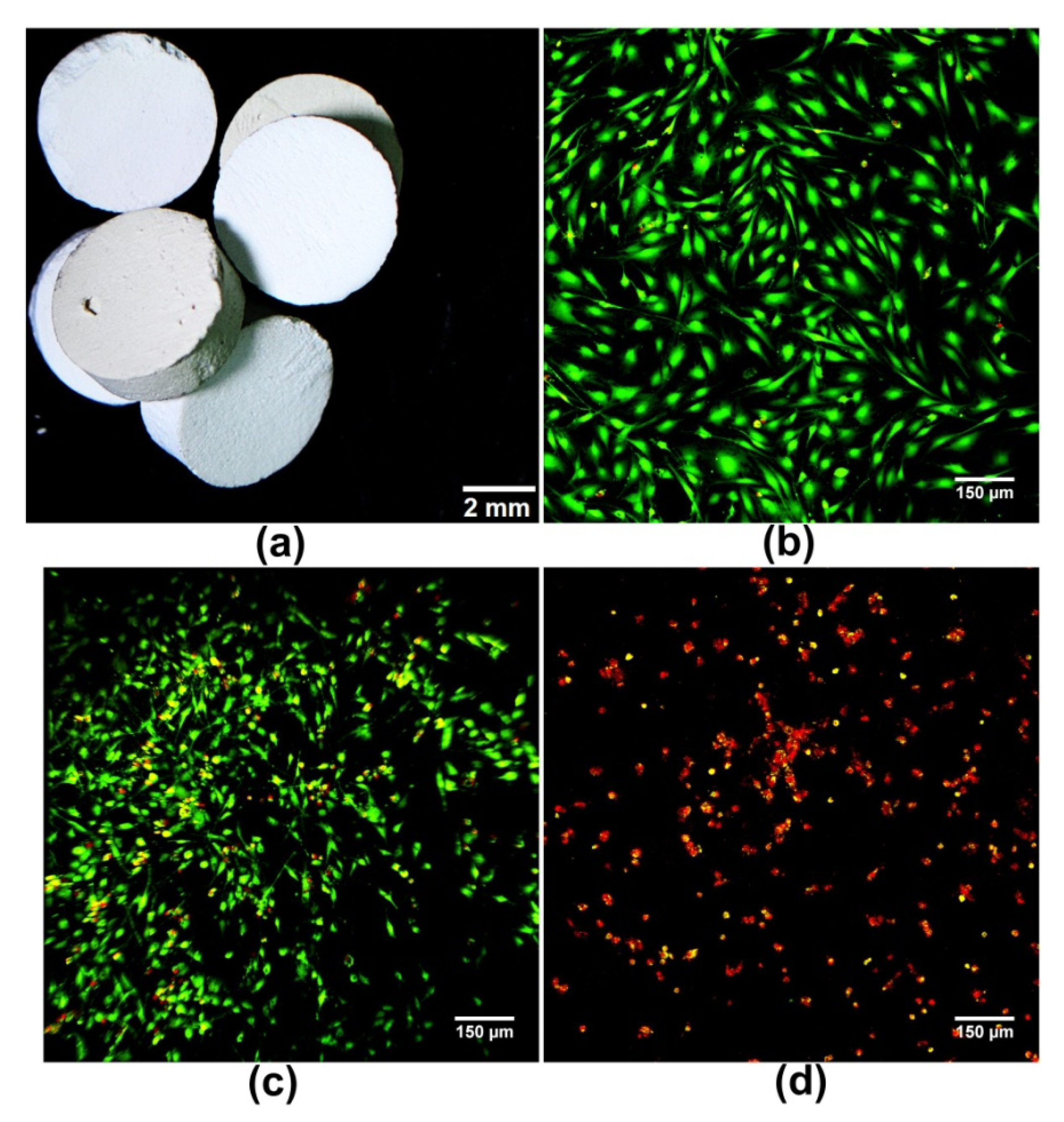
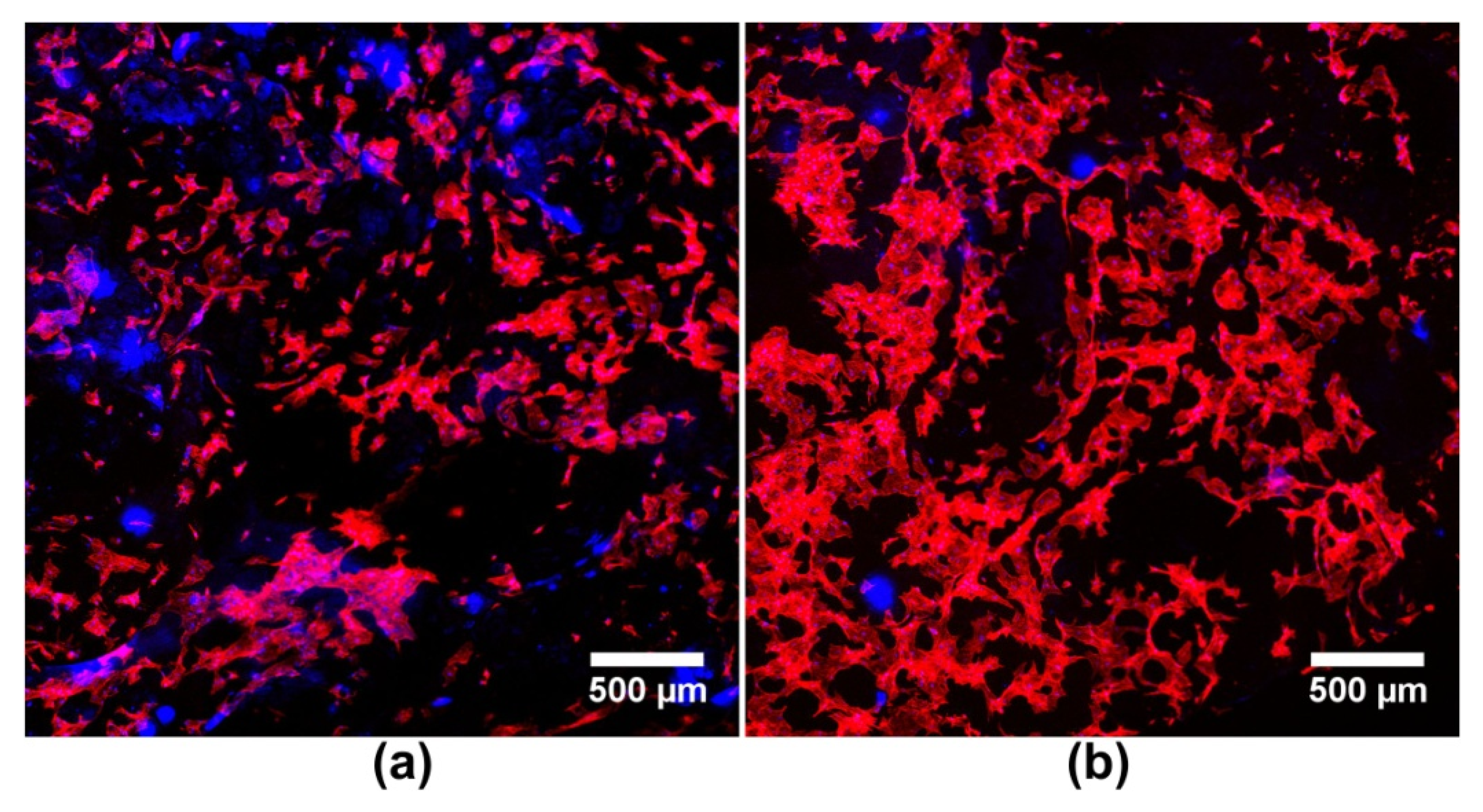
| Metal Ion | Type of Biomaterial | Experimental Model | Biomaterial Effect on Bone Regeneration | Ref. |
|---|---|---|---|---|
| Mg2+ | Ti threaded screws with Mg-incorporated mesoporous TiO2 coating | In vivo rabbit model | Improved biomaterial osteoconductivity, enhanced expression of genes related to bone regeneration process | [38] |
| Mg2+ Sr2+ | Pure Ti samples with Mg or Sr ions deposited on the surface | In vivo rabbit model; In vitro model: MC3T3-E1 cell line | Enhanced proliferation and osteogenic differentiation in vitro, improved biomechanical strength and osseointegration in vivo | [49] |
| Mg2+ Zn2+ Sr2+ | Ti implants with Mg- or Zn- or Sr-doped HA coating | In vivo rat model | Increased new bone formation, improved implant osseointegration | [50] |
| Zn2+ | Zn-modified Ti sponge | In vitro model: human DPSCs | Increased osteogenic differentiation and mineralization | [51] |
| Zn2+ | Ti rods/plates with Zn-incorporated TiO2 coating | In vivo rat model; In vitro model: rat BMDSCs | Enhanced expression of genes related to bone regeneration process, improved bone formation process in vitro and in vivo | [52] |
| Sr2+ | Ti implant with Ti-Sr-O coating | In vivo rabbit model | Improved early implant osseointegration | [53] |
| Co2+ | Alginate/collagen/α-TCP scaffold with Co incorporated | In vitro model: rat BMDSCs | Enhanced angiogenic properties of cells and osteogenic differentiation | [54] |
| Sr2+ Co2+ | BG co-substituted with Sr and Co | In vivo rabbit model; In vitro model: human umbilical cord perivascular cells (HUCPVCs), Saos-2 cell line | Increased expression of genes related to osteogenesis and angiogenesis processes in Saos-2 and HUCPVC cells, respectively, improved bone healing process in vivo | [55] |
| Metal Ion | Type of Biomaterial | Demonstrated Antimicrobial Activity (Microbial Strain) | Effect on Eukaryotic Cells | Ref. |
|---|---|---|---|---|
| Ag+ | Mesoporous 58S BG containing Ag | Escherichia coli; Staphylococcus aureus | Biocompatible (studies on primary rat calvarial osteoblasts) | [56] |
| Ag+ Zn2+ | Ti-6Al-4V alloy with Ag- or Zn-doped HA coating | Streptococcus mutans | Reduced cell attachment (studies on human gingival fibroblast cell line—HGF-1) | [57] |
| Ag+ Cu2+ | Ag- or Cu-doped HA/α-TCP Ag- or Cu-doped HA | Staphylococcus aureus; Escherichia coli; Pseudomonas aeruginosa; Candida albicans | Non-toxic, slightly reduced cell proliferation rate (studies on human lung fibroblast cell line—MRC-5) | [58] |
| Zn2+ Cu2+ | Zn- or Cu-doped nanoHA | Staphylococcus aureus; Escherichia coli; Candida albicans | Not tested | [59] |
| Cu2+ Mg2+ | Mg-Cu alloy | Staphylococcus aureus | Biocompatible (studies on MC3T3-E1 and HUVEC lines) | [39] |
| Cu2+ | Chitosan biomaterial with Cu incorporated | Escherichia coli; Staphylococcus carnosus | Non-toxic at low Cu concentrations, toxic at high Cu concentrations (studies on mouse embryonic fibroblast cell line—MEF) | [60] |
| Cu2+ | Mesoporous BG containing Cu | Staphylococcus epidermidis; Staphylococcus aureus; Escherichia coli | Not tested | [61] |
| Ce3+ | Ce-doped nanoHA | Escherichia coli; Staphylococcus aureus | Not tested | [62] |
| Li+ | Li-doped 58S BG | Staphylococcus aureus | Biocompatible (studies on MC3T3-E1 cell line) | [63] |
| Type of Biomaterial | Loading Regime | In Vitro Cellular Model | Effect on Eukaryotic Cells | Ref. |
|---|---|---|---|---|
| Porous PVDF and PVDF-TrFE membranes | Static | MC3T3-E1 cell line mouse myoblast cell line (C2C12) | Enhanced cell proliferation | [110] |
| PVDF film on Ti substrate | Static | BMDSCs | Enhanced cell proliferation and osteogenic differentiation | [111] |
| PVDF film with Ti layer | Dynamic (mechanical stimulation in a bioreactor) | MC3T3-E1 cell line | Enhanced cell proliferation | [112] |
| PVDF film | Dynamic (mechanical stimulation in a bioreactor) | Human ADSCs | Enhanced osteogenic differentiation | [113] |
| Porous HA scaffold | Static | MC3T3-E1 cell line | Enhanced proliferation and matrix mineralization | [114] |
| HA disc | Static | MC3T3-E1 cell line | Increased cell adhesion, proliferation, and metabolic activity | [115] |
| KNN ceramics | Static | MC3T3-E1 cell line | Enhanced cell proliferation | [105] |
| HA/BaTiO3 composite | Dynamic (mechanical stimulation with loading device: 3 Hz and 60 N) | Osteoblasts | Enhanced osteoblast growth and bone-inducing activity | [107] |
| HA/BaTiO3 composite | Dynamic (electrical stimulation) | Primary culture of human osteoblasts | Enhanced cell proliferation | [116] |
| HA/BaTiO3 composite | Static | MG-63 cell line (osteosarcoma) | Improved cell adhesion, proliferation, and osteogenic differentiation | [117] |
| HA biomaterial with KNN layers | Static | Saos-2 cell line (osteosarcoma) | Enhanced cell proliferation | [118] |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Przekora, A. Current Trends in Fabrication of Biomaterials for Bone and Cartilage Regeneration: Materials Modifications and Biophysical Stimulations. Int. J. Mol. Sci. 2019, 20, 435. https://doi.org/10.3390/ijms20020435
Przekora A. Current Trends in Fabrication of Biomaterials for Bone and Cartilage Regeneration: Materials Modifications and Biophysical Stimulations. International Journal of Molecular Sciences. 2019; 20(2):435. https://doi.org/10.3390/ijms20020435
Chicago/Turabian StylePrzekora, Agata. 2019. "Current Trends in Fabrication of Biomaterials for Bone and Cartilage Regeneration: Materials Modifications and Biophysical Stimulations" International Journal of Molecular Sciences 20, no. 2: 435. https://doi.org/10.3390/ijms20020435
APA StylePrzekora, A. (2019). Current Trends in Fabrication of Biomaterials for Bone and Cartilage Regeneration: Materials Modifications and Biophysical Stimulations. International Journal of Molecular Sciences, 20(2), 435. https://doi.org/10.3390/ijms20020435