Abstract
DNA methylation and other epigenetic factors are important in the pathogenesis of late-onset Alzheimer’s disease (LOAD). Methylenetetrahydrofolate reductase (MTHFR) gene mutations occur in most elderly patients with memory loss. MTHFR is critical for production of S-adenosyl-l-methionine (SAM), the principal methyl donor. A common mutation (1364T/T) of the cystathionine-γ-lyase (CTH) gene affects the enzyme that converts cystathionine to cysteine in the transsulfuration pathway causing plasma elevation of total homocysteine (tHcy) or hyperhomocysteinemia—a strong and independent risk factor for cognitive loss and AD. Other causes of hyperhomocysteinemia include aging, nutritional factors, and deficiencies of B vitamins. We emphasize the importance of supplementing vitamin B12 (methylcobalamin), vitamin B9 (folic acid), vitamin B6 (pyridoxine), and SAM to patients in early stages of LOAD.
1. Introduction
Most genetic research on late-onset Alzheimer’s disease (LOAD) has focused on genome-wide association studies (GWAS) that have provided low effect size results in general, with the exception of apolipoprotein E (ApoE) [1,2]. Studies of monozygotic twins with Alzheimer’s disease (AD) showed discordance in onset and progression indicating a role for nongenetic factors in disease pathogenesis [3]. For these reasons, genetic research turned to epigenetic modifications using epigenome-wide association studies (EWAS) in the last few years [4,5]. Bonasio et al. [6] defined epigenetics as ‘‘the study of molecular signatures that provide a memory of previously experienced stimuli, without irreversible changes in the genetic information’’. Therefore, epigenetic refers to potentially heritable and nonheritable modifications in gene expression induced by environmental factors without changes in DNA base sequences [1]. These epigenetic processes include DNA methylation, histone modification and expression of long noncoding RNAs and noncoding microRNAs (miRNAs) that primarily repress target messenger RNAs (mRNAs) [7,8,9,10].
This review focuses on DNA methylation dynamics and other epigenetic changes, including the role of methylenetetrahydrofolate reductase (MTHFR) gene polymorphisms and its metabolic pathways particularly in aging and LOAD pathology [11]. We also review polymorphisms of the cystathionine-gamma(γ)-lyase (CTH) gene [12], the enzyme that converts cystathionine to cysteine in the transsulfuration pathway and is responsible for plasma elevation of total homocysteine (tHcy). The role of relevant nutritional factors including the B-vitamins folate, vitamin B12, and vitamin B6 status is summarized. Elevation of Hcy is important in oxidative stress contributing to the decrease of S-adenosyl-l-methionine (SAM) levels, which induce demethylation of DNA resulting in overexpression of genes involved in AD pathology such as presenilin (PSEN1) and beta-secretase (BACE1), the β-site amyloid precursor protein (APP)-cleaving enzyme that increases hypomethylation and Aβ1-42 deposition [9]. Moreover, epigenetic markers have also been demonstrated to be critical regulatory factors of brain function [9], not only in AD but also in other neurodegenerative diseases [1,2] as well as in aging [9]. Experimental antiaging epigenetic interventions attempt to reverse age-related changes in DNA methylation [10].
2. DNA Methylation Studies
2.1. 5-Cytosine Methylation and DNA Methyltransferases
Methylation at the 5-position of the cytosine base (5mC) is considered a critical phase of epigenetic regulation in pathways related with neuronal development. Methylation and demethylation of cytosine-phosphate-guanine (CpG) islands is associated with alterations in local chromatin producing a long-term regulation of transcription tagging genome into active and inactive territories introducing a “masking” function [13]. Decreased levels of 5mC [1] and targeted mutations of DNA methyltransferases introduced into the germline produce severe developmental restriction [13] and finally a lethal phenotype in mice [14]. Cytosine base methylation occurs mainly at CpG dinucleotides [1]. Gene regulation is achieved by 5mC silencing gene expression via high-density CpG areas, known as CpG islands, which remain largely unmethylated [13]. In humans, genomic DNA methylation of cytosine results from the addition of a methyl group from SAM to the cytosine, catalyzed by DNA methyltransferases (DNMT1, DNMT3A, and DNMT3B) [9]. In addition to 5mC, hydroxymethylation at the 5-position of the cytosine base (5hmC) derived from the oxidation of methylated cytosines by ten-eleven translocation (TET) enzymes is another epigenetic regulatory mechanism, which is particularly abundant in the brain. The TET family of enzymes catalyzes Fe (II)- and alpha-ketoglutarate (α-KG)–dependent oxidation reactions, [9] and produces the initial step of oxidation of 5mC to 5hmC. TET enzymes also participate in the conversion of 5-formylcytosine (5fC) to 5-carboxylcytosine (5caC); this cycle ends when 5caC is excised by a thymine-DNA glycosylase (TDG) [9].
In humans, DNA methyltransferases are involved in tumor transformation and progression resulting in genome-wide hypomethylation of tumor cells and silencing of tumor-suppressor genes [15]; also, DNMT3A mutations have been associated with poor prognosis in acute myeloid leukemia [15]. DNMT1 mutations occur in hereditary sensory and autonomic neuropathy type 1 (HSAN1) [15]. In mice, DNMT1 mutations induce global hypomethylation along with cortical and hippocampal neuronal dysfunction causing neurodegeneration with severe deficits in learning, memory and behavior [16]. Hypomethylated excitatory neurons have postnatal maturation defects including abnormal dendritic arborization and impaired neuronal excitability [16]. Grossi et al. [17] used artificial neural network analysis to illustrate how low cobalamin; low folate and high Hcy are linked to AD. Low PSEN1 methylation was linked to low folate levels and low promoter methylation of BACE1 and DNMT genes. High levels of folate-vitamin B12 and low Hcy promoted methylation of genes required for DNA methylation reactions (DNMT1, DNMT3A, DNMT3B, and MTHFR) [18].
2.2. DNA Methylation in Alzheimer’s Disease
Early studies of DNA methylation in LOAD from peripheral blood lymphocytes [19,20], brain biopsies and autopsy material [21,22,23,24,25,26,27,28,29] demonstrated variable results of cytosine methylation at CpG dinucleotides. Wang and coworkers [30] studied postmortem prefrontal cortex tissue and peripheral lymphocytes of AD patients and showed that specific loci in MTHFR gene promoter regions were hypermethylated compared to healthy controls. Ellison and collaborators [31] using gas chromatography/mass spectrometry found abnormal levels of 5mC and 5hmC in the superior and middle temporal gyri, hippocampus and parahippocampal gyrus in early stages of AD, as well as in frontotemporal lobe degeneration and Lewy body dementia; these global values returned to control levels as the disease progressed suggesting that methylation changes occur in early stages of neurodegenerative dementias. Chouliaras et al. [32] confirmed the presence of significant decreases in levels of 5mC and 5hmC in the hippocampus of AD patients compared with negative controls. Levels of 5mC were inversely proportional to the deposition of neurofibrillary tangles in the same hippocampal cells. Hernández et al. [33] studied DNA methylation patterns of cortical pyramidal layers in 32 brains of patients with LOAD demonstrating hypermethylation of synaptic genes and genes related to oxidative stress including HOXA3, GSTP1, CXXC1-3 and BIN1.
One of the major problems of initial methylation studies was the small sample size. This was solved by De Jager and collaborators [4] utilizing one of the largest clinicopathological studies to date, the Religious Orders Study, with 708 brains to assess the methylation state of the brain’s DNA correlated with AD pathology. Almost a half-million CpGs were interrogated including CpGs in the ABCA7 and BIN1 regions. The authors also identified genes whose RNA expression was altered in AD including ANK1, CDH23, DIP2A, RHBDF2, RPL13, SERPINF1 and SERPINF2. ANKYRIN 1 (ANK1) and RHOMBOID5 (RHBDF2) genes are involved in the protein tyrosine kinase 2-beta (PTK2B) gene network, a LOAD gene that is a key element of the calcium-induced signaling cascade involved in modulating the activation of microglia and macrophages, as well as in the transport of TNFα converting enzyme (ADAM17) from the cell surface.
Absence of RHBDF2 in mice impacts the normal release of TNFα [4] activated astrocytes in the vicinity of neuritic plaques that overexpress CADHERIN23 (CDH23) gene. DIP2A functions as a cell surface protein and connects directly to the known SORTILIN RELATED RECEPTOR 1 (SORL1) susceptibility gene that is involved in the APP susceptibility network and amyloid processing [4]. Both SERPIN PEPTIDASE INHIBITORS (SERPINF1 and SERPINF2) interact with elements of amyloid processing. SERPINF1 mRNA expression is reduced in LOAD and when knocked-out in vitro leads to reduced neurite outgrowth [4].
A Religious Orders companion study by Lunnon and coworkers [5] found robust association between differences in methylation, mRNA levels, and Braak & Braak staging. The severity of Alzheimer’s disease is defined in neuropathology by the presence of tau-based neurofibrillary tangles ranging from early stages (I and II) to extensive neocortical involvement in Braak & Braak stages V and VI in advanced disease. Dysregulation of DNA methylation occurred earlier in brain areas affected at onset by AD and appeared to have stronger effects (28.7%) than the combination of ApoE and other risk genes (13.9%) identified by GWAS [1,2], indicating the importance of epigenetic changes in AD. Additional studies by Yu et al. [34] confirmed the association of DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 genes with pathological diagnosis of AD including both Aβ load and tau tangle density. RNA expression of transcripts of SORL1 and ABCA7 was associated with tau tangle density, and the expression of BIN1 was associated with Aβ load [34]. Moreover, Lunnon et al. [5] found hypermethylation of the ANK1 gene in the entorhinal cortex, superior temporal gyrus and prefrontal cortex in LOAD. These findings confirm that AD involves significant disruption of DNA methylation. Epigenetic age-associated alterations of DNA methylation have also been reported in animal models of AD, in particular global DNA hypomethylation in the J20 model and DNA hypermethylation in the triple transgenic 3xTg-AD model [35].
3. miRNAs Epigenetic Effects
Long noncoding RNAs and noncoding microRNAs (miRNAs) that primarily repress target messenger RNAs (mRNAs) play a pivotal role in oncology, cardiovascular diseases and dementia [7,8]. In AD, the miRNA-125b is overexpressed enhancing neuronal apoptosis and tau phosphorylation by activation of cyclin-dependent kinase 5 (CDK5) and p35/25. FORKHEAD BOX Q1 (FOXQ1) is the direct target gene of miR-125b [7]. Patrick and coworkers [8] studied the role of miRNA-132, miRNA-129 and miRNA-99 in the dorsolateral prefrontal cortex of more than 500 brain samples demonstrating a small number of specific alterations on target genes such as EP300 that encodes p300, a histone acetyltransferase that regulates transcription in the cortex of subjects with AD.
4. Transsulfuration Metabolic Pathways and Remethylation Defects
The metabolism of sulfur-containing amino acids in the transsulfuration pathway involves the transfer of the sulfur atom of methionine to serine to produce cysteine (Figure 1). Methionine first reacts with ATP to form S-adenosyl-l-methionine (SAM), then S-adenosyl-homocysteine (SAH) and finally, homocysteine. Plasma elevation of total homocysteine (tHcy) or hyperhomocysteinemia may result from congenital deficiency of cystathionine β-synthase (CBS) leading to homocystinuria, or more frequently from polymorphisms of the cystathionine γ lyase (CTH) gene (OMIM *607657; EC 4.4.1.1.) in chromosome 1 (1p31.1) [36]. CTH is the enzyme that converts cystathionine to cysteine, the last step in the transsulfuration pathway. Wang et al [12] demonstrated that a single nucleotide polymorphism (SNP), namely c.1364G > T in exon 12 of the CTH gene causes elevation of tHcy and cystathioninuria. Caucasian subjects homozygous for the CTH 1364T/T SNP showed elevation of tHcy that reached effects sizes similar to those caused by the 677C > T MTHFR gene polymorphism [12].
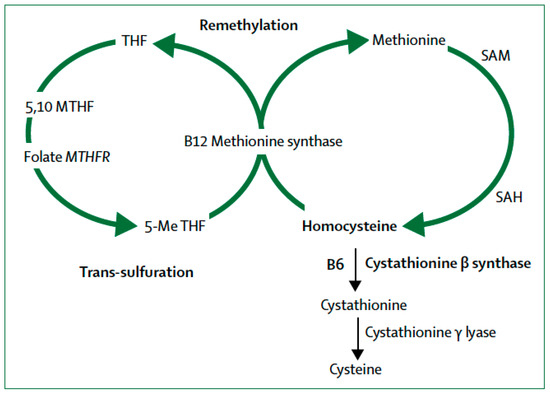
Figure 1.
Homocysteine metabolism: B12 = cobalamin (vitamin B12). B6 = pyridoxine (vitamin B6). MTH = methylenetetrahydrofolate. MTHFR = methylenetetrahydrofolate reductase. SAM = S-adenosyl- methionine. SAH = S-adenosylhomocysteine. 5-Me THF = 5-methyl tetrahydrofolate. (From [53]).
Closely related to the transsulfuration pathway are the remethylation defects resulting from the failure to convert homocysteine to the amino acid methionine (Figure 1). This pathway requires the integrity of the gene encoding methylenetetrahydrofolate reductase (MTHFR) required for the interaction of folate and cobalamin (vitamin B12). Folate provides the methyl group required for the remethylation pathway (Figure 1) to finally produce SAM, the main methyl donor for epigenetic processes. The human MTHFR gene (OMIM *607093; EC 1.5.1.20) is localized in chromosome 1 (1p36.3) and it encodes for 5,10-methylenetetrahydrofolate reductase (MTHFR) [37]. This enzyme catalyzes the conversion of 5,10-methylenetetrahydrofolate to 5-methyltetrahydrofolate, a co-substrate with vitamin B12 for the remethylation of homocysteine to methionine [11]. Mutations of this gene occur in 10–15% of the population and the resulting MTHFR deficiency affects the production of methionine and SAM. Patients with AD have low levels of SAM in the CSF [38].
MTHFR gene polymorphisms cause enzyme thermolability and involve C-to-T substitution at nucleotide 667 and A-to-C at nucleotide 1298; these MTHFR mutations have been associated with homocystinuria, neural tube defects, preeclampsia, cleft lip and cleft palate, cerebrovascular disease, and psychiatric disorders including susceptibility to depression and schizophrenia [39,40]. Population-based international studies showed no increased risk of dementia in subjects with MTHFR polymorphisms [41,42]. In Japan, Nishiyama et al. [43] found a slight association of the MTHFR-C667T polymorphism with senile cognitive decline in men but not with AD. In Australia, a causal link between high tHcy and incident dementia was demonstrated [44] but the study lacked power to determine an effect of the MTHFR-C667T genotype. In contrast, de Lau and collaborators [45] in the normal elderly population of the Rotterdam Study observed that the MTHFR-C665T genotype was associated with elevated tHcy but not with cognitive loss or white matter lesions. In a small patient population in Tunisia [46], the MTHFR-A1298C mutation was associated with susceptibility to AD. As mentioned earlier, Román [11] found a very high frequency (above 90%) of MTHFR gene mutations in an elderly population attending a memory clinic in the USA, with diagnoses ranging from mild cognitive impairment (MCI) to LOAD; about 65% had single mutations; the MTHFR-C667T mutation was found in 58.5% of the patients and 41.5% had the MTHFR-A1298C mutation whereas 20% were compound heterozygous for both mutations [11].
MTHFR and Epigenetic Drift
In 2005, a multinational study of identical twins by Fraga and collaborators [47] first demonstrated that whereas DNA methylation and histone acetylation in young identical twins are indistinguishable, older identical twins showed substantial differences; epigenetic changes were up to four times greater than those of young twin pairs. The authors concluded that this “epigenetic drift” was associated with aging [47]. Epigenetic drift of identical twins with aging also occurs among a large number of animal species [48] following a non-Mendelian pattern. In identical twins with AD, the prognosis and onset of AD can differ by more than ten years [3,49,50]; young identical twin pairs are essentially indistinguishable in their epigenetic markings while older identical twin pairs show substantial variations. Breitner et al. [50] suggested that twins with a history of systemic infection developed AD at an earlier onset than their identical twin. Epigenetic drift can be caused by lifestyle, diet, infections, folate status, homocysteine status or toxic exposure [51]. Wang et al. [30] demonstrated that the MTHFR gene promoter in the brain displayed high interindividual variance in DNA methylation among twins. The methylation level of MTHFR and APOE in individuals 30 years of age apart decreased by 10.6%, whereas in patients with AD the methylation level increased by 6.8%. The epigenetic drift increases with age particularly in genes that play pivotal roles in removing β-amyloid such as APOE and among methylation genes such as MTHFR and DNMT1 [9,52].
5. Homocysteine (Hcy): A Risk Factor for Cognitive Loss and Dementia
Hcy is a sulfur-containing amino acid produced in the transsulfuration pathway (Figure 1) from the reaction of methionine with ATP to form SAM, then SAH and finally homocysteine. Homocystinuria due to congenital deficiency of the CBS gene causes hyperhomocysteinemia. Polymorphisms of the CTH and MTHFR genes are common genetic causes of hyperhomocysteinemia [38,39]. The remethylation pathway (Figure 1) involves reactions enzymatically mediated by MTHFR requiring as co-substrates the B-group vitamins folic acid (vitamin B9) and cobalamin (vitamin B12) for the remethylation of homocysteine to methionine. Pyridoxine (vitamin B6) is required by CBS for the conversion of homocysteine to cysteine (Figure 1).
5.1. Hyperhomocysteinemia is an Independent Vascular Risk Factor
Elevation of plasma or serum tHcy (hyperhomocysteinemia) is an independent vascular risk factor linked to coronary disease, peripheral vascular disease, stroke and small-vessel cerebrovascular disease [53]. More importantly, elevated tHcy is considered a risk factor for dementia and cognitive decline in the elderly, particularly in association with low levels of folate and cobalamin [54,55]. A number of studies in cognitively normal elderly subjects, demonstrated that baseline tHcy is a strong and independent predictor of cognitive decline after observation periods ranging from 3 years (USA, n = 321 men [55] and Sydney, Australia, n = 889 [56]), 4 years (France, n = 1241) [57], 5 years (Wales, UK, n = 32) [58], 6 years (Norway, n = 2189) [59], 7 years (Finland n = 274) [60] and up to 10 years (UK, n = 691) [61]. In the Finland cohort [60], the magnetic resonance imaging (MRI) study demonstrated the association of higher baseline vitamin B12 and holotranscobalamin levels with a decreased rate of total brain volume loss during 8 years of the study period [62]. Increased tHcy levels were associated with faster rates of total brain volume loss and with progression of white matter hyperintensities among participants with hypertension (systolic blood pressure > 140 mm Hg) [62].
Regarding the risk of AD associated to elevated tHcy, in the Framingham Study, Seshadri and colleagues [63] demonstrated in elderly subjects (mean age, 76 years) that raised tHcy above 14 μmol/L nearly doubled the risk of LOAD over a period of 8 years. Similar findings were corroborated in two large Finnish [60,64] and Australian [65] cohorts. In 2008, Smith [66] performed a comprehensive review of cross-sectional and prospective studies involving >46,000 subjects and confirmed the association between elevated tHcy and cognitive deficit or dementia.
According to a recent international consensus statement [67], moderately raised homocysteine (>11 μmol/L) increases the relative risk of dementia in the elderly 1.15 to 2.5 fold, and the Population Attributable risk from 4.3 to 31% [67]. From the Public Health viewpoint, homocysteine-lowering treatment with B vitamins that markedly slows down the rate of brain atrophy and cognitive decline in the elderly offers the possibility that, in addition to folic acid fortification, mandatory methylcobalamin supplementation should also be considered for the prevention of LOAD [67].
5.2. Genetic and Nongenetic Causes of Hyperhomocysteinemia
Elevation of tHcy is caused by numerous factors including advancing age, diet, supplementation of B-vitamins, obstructive sleep apnea, smoking, Helicobacter pylori infection, and renal failure, among others [53,54]. As indicated earlier, both CBS gene polymorphisms and the C667T and the A1298C SNPs in the MTHFR gene decrease the activity of the MTHFR enzyme leading to hyperhomocysteinemia. Minagawa et al. [68] found that elevated Hcy inhibits the dimerization of ApoE3 and reduces ApoE3-mediated high-density lipoprotein (HDL) concentrations involved in degradation of soluble Aβ within microglia. ApoE4 was not affected; in patients with hyperhomocysteinemia the CSF levels of ApoE3 dimers were significantly lower than in controls. Minagawa and colleagues [68] suggested that the effects of elevated Hcy on ApoE3 contribute to the pathogenesis of AD. Smith and Refsum [54] reviewed the proposed mechanisms responsible for the harmful cognitive effects of hyperhomocysteinemia (Table 1). These include impaired endothelial function with reduced inducible nitric oxide synthase; augmented oxidative stress and decreased activity of key antioxidant enzymes; raised generation of the superoxide anion; alterations of lipid metabolism with increased cholesterol synthesis and reduced synthesis of apolipoprotein 1; and, carotid stenosis and induction of thrombosis [69,70].

Table 1.
Harmful effects of homocysteine on vascular function and cognition (modified from Smith & Refsum [54]).
Hyperhomocysteinemia induces a decrease in the SAM-dependent synthesis of catecholamines including dopamine, norepinephrine, and epinephrine, as well as non-catecholamine neurotransmitters such as melatonin and serotonin (5-HT) that contribute to development of depression [69]. Moreover, elevated tHcy produces two neurotoxic products, homocysteic acid (HCA) and cysteine sulfinic acid (CSA), which are agonists of the N-methyl-D-aspartate (NMDA) glutamate receptor, with neurotoxic effects on dopaminergic neurons derived from excessive Ca++ influx and reactive oxygen generation [70]. The beneficial effects of B-group vitamins on elevated tHcy will be reviewed next.
6. Folate Metabolism
Vitamin B9 or folic acid (from the Latin folium, leaf) is abundantly found in green leafy vegetables. Folate is vital for cell development and growth given its role in numerous biochemical one-carbon (methyl-group, –CH3) reactions, many of them critical for cognition. The Nun Study [71] first provided epidemiological and neuropathological data demonstrating that limited lifetime consumption of salads with low blood folate levels increased the risk of cognitive decline and dementia. Also, the severity of the atrophy in the neocortex and of the Alzheimer disease lesions were strongly correlated with low serum folate levels; none of 18 other nutrients, lipoproteins, or nutritional markers measured in the study correlated with the atrophy [71]. Further studies confirmed that normal cognitive scores were highly associated with elevated blood folate despite the neuropathological evidence of LOAD brain lesions [72].
The primary methyl-group donor for DNA methylation reactions is 5-methyl-tetrahydrofolate (CH3-THF) required for the transformation of homocysteine into methionine mediated by methionine synthase with cobalamin (vitamin B12) as a cosubstrate (Figure 1), leading to the synthesis of SAM. Also, CH3-THF is critical in the de novo purine synthesis to convert dUMP (deoxyuridylate) into dTMP (thymidylate) for DNA and RNA synthesis, DNA repair or replication. Several forms of cancer are associated with epigenetic differential methylation causing disturbances in nucleotide synthesis; for instance, hypermethylation may inhibit tumor suppressors. Folate, therefore, is a B-vitamin that plays an important role as a precursor in the epigenetic regulation of gene expression, DNA stability, DNA integrity and mutagenesis. Abnormal folate status has been associated with neural tube defects, cardiovascular and cerebrovascular diseases, cleft lip and palate, neurodegenerative diseases, schizophrenia and depression [40,73,74].
Telomeres and Folate Levels
Telomeres protect chromosomes from abnormal combination and degradation. The shortening of telomeres’ cap serve as a signature of cell division history, acting as biomarker of aging. In peripheral leukocytes, short telomere length is associated with increased risk of cognitive decline and LOAD [75,76]. Low folate levels are associated with short telomeres due to DNA damage in the telomeric region. Telomere length is epigenetically regulated by DNA methylation and directly influenced by folate status, a process independent of DNA damage due to uracil incorporation. Shorter telomeres occur with age, infection, stress, and chronic diseases including LOAD [75].
Paul and collaborators [76] observed that decreased plasma folate concentration to <11.6 μmol/L was correlated with a decrease in mean telomere length. In this population, homozygous carriers of the MTHFR-C677T gene mutation showed decreased levels of plasma folate [77]. Decreased serum folate induces anomalous integration of uracil in place of thymidine in DNA [78], a mechanism corrected by folic acid supplementation. Troesch, Weber and Mohajeri [79] summarized the importance for the development of LOAD of reduced SAM-dependent methylation reactions due to genetic factors along with reduction of folate, vitamin B6 and vitamin B12 levels. The resulting elevation of Hcy levels and the reduced capacity to synthetize, methylate and repair DNA, along with the impaired modulation of neurotransmission, appears to favor the development of AD particularly when combined with increased oxidative stress, particularly in ApoE ε4 carriers [80].
7. Vitamin B12 Deficiency and β-amyloid Deposition
Smith, Warren and Refsum [81] have recently provided a comprehensive review of vitamin B12. Only bacteria can biosynthesize vitamin B12; in humans B12 from the diet is a cofactor for the enzymes methionine synthase and l-methyl-malonyl-CoA mutase. B12 deficiency results in build-up of homocysteine and lack of interaction with folate that is trapped as CH3-THF leading to depletion of tetrahydrofolates used in thymidylate and purine synthesis blocking DNA for the production of red cells in the bone marrow. B12 deficiency impedes cellular proliferation and protein synthesis and thereby causes development of megaloblastic anemia [81].
7.1. Clinical Manifestations of Vitamin B12 Deficiency
In 1920, pernicious anemia—a fatal form of a megaloblastic anemia—was successfully treated by adding liver to the diet. In 1955, Dorothy Hodgkin used crystallography to first identify the molecular structure of cyanocobalamin or vitamin B12 from the deep-red cyanide-containing pigment isolated from liver tissue. Pernicious anemia was the first disease to be identified as caused by vitamin B12 deficiency [81].
Stabler [82] reviewed the clinical manifestations of vitamin B12 deficiency. In addition to megaloblastic anemia, acidemia from elevation of serum methylmalonic acid (MMA), and methylmalonic aciduria, the neurological manifestations of pernicious anemia include memory loss and cognitive decline, visual disturbances from optic nerve neuropathy, burning and painful sensations in hands and feet from peripheral neuropathy, and spinal cord involvement with subacute combined degeneration resulting in loss of proprioception from dorsal column involvement and pyramidal tract symptoms such as paralysis and incontinence.
7.2. Measuring Total Serum B12 Levels
Dietary sources of B12 include liver, meat, fish, shellfish and dairy products; vegans are prone to B12 deficiency [81,82]. Vitamin B12 deficiency occurs from inborn metabolic errors, alterations of B12-binding proteins including haptocorrin (HC) found in saliva, intrinsic factor (IF) produced by parietal cells in the stomach (pernicious anemia is associated with anti-parietal-cell and anti-IF auto-antibodies), and transcobalamin (TC), which binds B12 to facilitate uptake by the cells [81]. According to Stabler [82], measurement of total serum B12 levels is unsatisfactory because it reflects B12 that is bound to either HC or TC, and up to 60% of bound materials are cobalamin analogues (corrinoids). Therefore, “normal” total serum B12 levels can mask deficiency if serum contains relatively large amounts of cobalamin analogues [83]. Levels below 200 pg/mL usually indicate biochemical B12 insufficiency. Serum B12 < 350 pg/mL along with tHcy > 14 µmol/L indicate metabolic B12 deficiency [81,82]. For this reason, holotranscobalamin, MMA and tHcy levels should be included in the evaluation of a patient suspected of having B12 deficiency [83].
7.3. Causes of Vitamin B12 Deficiency
Other than pernicious anemia resulting from presence of anti-parietal-cell and anti-IF autoantibodies, other causes of B12 deficiency include atrophic body gastritis, Helicobacter pylori infection, malabsorption of vitamin B12, gastrectomy, gastric bypass or other bariatric surgery, inflammatory bowel disease, tropical sprue, use of metformin, anticonvulsants, proton-pump inhibitors and other drugs to block stomach acid, and vegetarian diets low in meat and dairy products. Hemodialysis patients, nitrous oxide inhalation, and cholinesterase inhibitors in LOAD patients [84] also increase the risk of vitamin B12 deficiency.
Epidemiological studies have shown that prevalence of vitamin B12 deficiency increases with age [85,86], due to decreased saliva (e.g., dry eyes-dry mouth of Sjögren syndrome) [87] and gastric atrophy with deficits respectively of haptocorrin and intrinsic factor. Andrès and colleagues [88] have emphasized that as many as 20% of elderly people may have unrecognized B12 deficiency due to food-cobalamin malabsorption plus insufficient dietary intake. According to Spence [89], metabolic B12 deficiency occurs in 30% of vascular patients older than 71 years, increasing to as many as 40% in patients above age 80 years; these patients usually have plasma levels of tHcy >14 µmol/L resulting from B12 deficiency. Inadequate supply of B12 and folic acid is not only a strong and independent vascular risk factor particularly for subcortical ischemic small-vessel disease [90], a common and important contributor to cognitive impairment and memory complaints in the elderly, but also enhancing the development of LOAD [91]. Animal experimental data confirms the importance of B-vitamin deprivation in the expression of AD [92].
7.4. Effects of B-Group Vitamins on Cognition: Negative Clinical Trials
An international consensus [67] provided a comprehensive explanation of the negative results of meta-analyses [93] based on reviews of the results from a number of inadequately controlled clinical trials; most participants in those trials were enrolled in post-hoc studies which were not designed primarily to assess cognition. Usually, these were short-duration trials without baseline cognitive assessment and results were based on post-hoc brief cognitive assessments; only a few of these studies assessed the incidence of dementia or mild cognitive impairment.
In contrast, solid positive results were obtained in the Oxford Project to Investigate Memory and Ageing (OPTIMA) trial [94,95,96] that used comprehensive neuropsychological evaluations plus brain imaging end-points. The results of this trial indicate that supplementation of B12, pyridoxine, and folic acid in subjects with MCI and hyperhomocysteinemia decreases tHcy resulting in improved episodic memory and global cognition [95], and most importantly, brain imaging demonstration of slowing of the progression of the brain atrophy in areas affected by AD [96]. Current recommendation is to provide oral supplementation of methylcobalamin 1000 µg/d, folic acid 800 µg/d and pyridoxine 100 mg/d.
8. SAM in Depression and Cognitive Loss
As described above (Figure 1) SAM is the main methyl-group donor for the methylation reactions reviewed here; as well as for synthesis of neurotransmitters, proteins, nucleic acids, phospholipids, and myelin. SAM has been used as an adjuvant for the treatment of depression [97]. Linnebank et al. [38] demonstrated a decrease of SAM in the cerebrospinal fluid (CSF) of patients with LOAD, affecting mainly ApoE ε4 carriers. According to Dayon et al. [98], plasma levels of one-carbon metabolites predicted cognitive decline. Despite the enhancing effects of SAM on antidepressants, no conclusive clinical trials of SAM have been reported [99].
9. Conclusions
It is established that the damaging effects of deficiencies of folate and cobalamin and the resulting elevation of tHcy contribute to the development of LOAD [67]. The numerous detrimental effects of elevated tHcy include, among others, endothelial and cerebrovascular damage of large-vessels as well as small-vessel disease [90]; activation of tau kinases; inhibition of methylation reactions; epigenetic effects on the β-amyloid pathway; reduced protein phosphatase-2A; and, impaired formation of phosphatidylcholine. Adequate supply of B-vitamins in the elderly, particularly in subjects with MTHFR and CTH gene mutations, appears to be critical to prevent the development of cognitive decline and to halt the progression of LOAD.
Author Contributions
Conceptualization, G.C.R. and O.M.-P.; methodology, G.C.R.; bibliographic investigation, G.C.R., O.M.-P. and C.B.; writing—original draft preparation, G.C.R., O.M.-P. and C.B.; writing—review and editing, G.C.R. and O.M.-P.; funding acquisition, G.C.R.
Funding
This research was funded by the Jack Blanton Presidential Distinguished Chair, the Fondren Fund and the Wareing Family Fund at Houston Methodist Hospital to G.C.R.
Acknowledgments
The authors would like to thank Houston Methodist Hospital for constant support to clinical research. Mancera-Páez was supported by the David Cabello International Alzheimer Disease Scholarship Fund. J David Spence, London, ON Canada provided valuable comments and bibliography.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Roubroeks, J.A.Y.; Smith, R.G.; van den Hove, D.L.A.; Lunnon, K. Epigenetics and DNA methylomic profiling in Alzheimer’s disease and other neurodegenerative diseases. J. Neurochem. 2017, 143, 158–170. [Google Scholar] [CrossRef]
- Millan, M.J. An epigenetic framework for neurodevelopmental disorders: From pathogenesis to potential therapy. Neuropharmacology 2013, 68, 2–82. [Google Scholar] [CrossRef] [PubMed]
- Gatz, M.; Pedersen, N.L.; Berg, S.; Johansson, B.; Johansson, K.; Mortimer, J.A.; Posner, S.F.; Viitanen, M.; Winblad, B.; Ahlbom, A. Heritability for Alzheimer’s disease: The study of dementia in Swedish twins. J. Gerontol. A Biol. Sci. Med. Sci. 1997, 52, M117–M125. [Google Scholar] [CrossRef] [PubMed]
- De Jager, P.L.; Srivastava, G.; Lunnon, K.; Burgess, J.; Schalkwyk, L.C.; Yu, L.; Eaton, M.L.; Keenan, B.T.; Ernst, J.; McCabe, C.; et al. Alzheimer’s disease: Early alterations in brain DNA methylation at ANK1, BIN1, RHBDF2 and other loci. Nat. Neurosci. 2014, 17, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Lunnon, K.; Smith, R.; Hannon, E.; De Jager, P.L.; Srivastava, G.; Volta, M.; Troakes, C.; Al-Sarraj, S.; Burrage, J.; Macdonald, R.; et al. Methylomic profiling implicates cortical deregulation of ANK1 in Alzheimer’s disease. Nat. Neurosci. 2014, 17, 1164–1170. [Google Scholar] [CrossRef]
- Bonasio, R.; Tu, S.; Reinberg, D. Molecular signals of epigenetic states. Science 2010, 330, 612–616. [Google Scholar] [CrossRef]
- Ma, X.; Liu, L.; Meng, J. MicroRNA-125b promotes neurons cell apoptosis and tau phosphorylation in Alzheimer’s disease. Neurosci. Lett. 2017. [Google Scholar] [CrossRef] [PubMed]
- Patrick, E.; Rajagopal, S.; Wong, H.A.; McCabe, C.; Xu, J.; Tang, A.; Imboywa, S.H.; Schneider, J.A.; Pochet, N.; Krichevsky, A.M.; et al. Dissecting the role of non-coding RNAs in the accumulation of amyloid and tau neuropathologies in Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 51. [Google Scholar] [CrossRef] [PubMed]
- Irier, H.A.; Jin, P. Dynamics of DNA methylation in aging and Alzheimer’s disease. DNA Cell Biol. 2012, 31 (Suppl. 1), S42–S48. [Google Scholar] [CrossRef] [PubMed]
- Unnikrishnan, A.; Freeman, W.M.; Jackson, J.; Wren, J.D.; Porter, H.; Richardson, A. The role of DNA methylation in epigenetics of aging. Pharmacol. Ther. 2018. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C. MTHFR gene mutations: A potential marker of late-onset Alzheimer’s disease? J. Alzheimer’s Dis. 2015, 47, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Huff, A.M.; Spence, J.D.; Hegele, R.A. Single nucleotide polymorphism in CTH associated with variation in plasma homocysteine concentration. Clin. Genet. 2004, 65, 483–486. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.P.; Gavin, D.P.; Grayson, D.R. CpG methylation in neurons: Message, memory, or mask? Neuropsychopharmacology 2010, 35, 2009–2020. [Google Scholar] [CrossRef] [PubMed]
- Li, E.; Bestor, T.H.; Jaenisch, R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell 1992, 69, 915–926. [Google Scholar] [CrossRef]
- Zhang, W.; Xu, J. DNA methyltransferases and their roles in tumorigenesis. Biomark. Res. 2017, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Hutnick, L.K.; Golshani, P.; Namihira, M.; Xue, Z.; Matynia, A.; Yang, X.W.; Silva, A.J.; Schweizer, F.E.; Fan, G. DNA hypomethylation restricted to the murine forebrain induces cortical degeneration and impairs postnatal neuronal maturation. Hum. Mol. Genet. 2009, 18, 2875–2888. [Google Scholar] [CrossRef]
- Grossi, E.; Stoccoro, A.; Tannorella, P.; Migliore, L.; Coppedè, F. Artificial neural networks link one-carbon metabolism to gene-promoter methylation in Alzheimer’s disease. J. Alzheimers Dis. 2016, 53, 1517–1522. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.A.; Liang, G. Rethinking how DNA methylation patterns are maintained. Nat. Rev. Genet. 2009, 10, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.Z.; Guan, W.P.; Maeda, T.; Makino, N. Analysis of telomere length and subtelomeric methylation of circulating leukocytes in women with Alzheimer’s disease. Aging Clin. Exp. Res. 2013, 25, 17–23. [Google Scholar] [CrossRef]
- Piaceri, I.; Raspanti, B.; Tedde, A.; Bagnoli, S.; Sorbi, S.; Nacmias, B. Epigenetic modifications in Alzheimer’s disease: Cause or effect? J. Alzheimers Dis. 2015, 43, 1169–1173. [Google Scholar] [CrossRef] [PubMed]
- West, R.L.; Lee, J.M.; Maroun, L.E. Hypomethylation of the amyloid precursor protein gene in the brain of an Alzheimer’s disease patient. J. Mol. Neurosci. 1995, 6, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Tohgi, H.; Utsugisawa, K.; Nagane, Y.; Yoshimura, M.; Genda, Y.; Ukitsu, M. Reduction with age in methylcytosine in the promoter region -224 approximately -101 of the amyloid precursor protein gene in autopsy human cortex. Brain Res. Mol. Brain Res. 1999, 70, 288–292. [Google Scholar] [CrossRef]
- Barrachina, M.; Ferrer, I. DNA methylation of Alzheimer disease and tauopathy-related genes in postmortem brain. J. Neuropathol. Exp. Neurol. 2009, 68, 880–891. [Google Scholar] [CrossRef] [PubMed]
- Chouliaras, L.; Rutten, B.P.; Kenis, G.; Peerbooms, O.; Visser, P.J.; Verhey, F.; van Os, J.; Steinbusch, H.W.; van den Hove, D.L. Epigenetic regulation in the pathophysiology of Alzheimer’s disease. Prog. Neurobiol. 2010, 90, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Bakulski, K.M.; Dolinoy, D.C.; Sartor, M.A.; Paulson, H.L.; Konen, J.R.; Lieberman, A.P.; Albin, R.L.; Hu, H.; Rozek, L.S. Genome-wide DNA methylation differences between late-onset Alzheimer’s disease and cognitively normal controls in human frontal cortex. J. Alzheimers Dis. 2012, 29, 571–588. [Google Scholar] [CrossRef] [PubMed]
- Bradley-Whitman, M.; Lovell, M.A. Epigenetic changes in the progression of Alzheimer’s disease. Mech. Ageing Dev. 2013, 134, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, N.; Dieriks, B.V.; Lill, C.; Faull, R.L.; Curtis, M.A.; Dragunow, M. Global changes in DNA methylation and hydroxymethylation in Alzheimer’s disease human brain. Neurobiol. Aging 2014, 35, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Iwata, A.; Nagata, K.; Hatsuta, H.; Takuma, H.; Bundo, M.; Iwamoto, K.; Tamaoka, A.; Murayama, S.; Saido, T.; Tsuji, S. Altered CpG methylation in sporadic Alzheimer’s disease is associated with APP and MAPT dysregulation. Hum. Mol. Genet. 2014, 23, 648–656. [Google Scholar] [CrossRef]
- Humphries, C.E.; Kohli, M.A.; Nathanson, L.; Whitehead, P.; Beecham, G.; Martin, E.; Mash, D.C.; Pericak-Vance, M.A.; Gilbert, J. Integrated whole transcriptome and DNA methylation analysis identifies gene networks specific to late-onset Alzheimer’s disease. J. Alzheimers Dis. 2015, 44, 977–987. [Google Scholar] [CrossRef]
- Wang, S.C.; Oelze, B.; Schumacher, A. Age-specific epigenetic drift in late-onset Alzheimer’s disease. PLoS ONE 2008, 3, e2698. [Google Scholar] [CrossRef]
- Ellison, E.M.; Abner, E.L.; Lovell, M.A. Multiregional analysis of global 5-methylcytosine and 5-hydroxymethylcytosine throughout the progression of Alzheimer’s disease. J. Neurochem. 2017, 140, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Chouliaras, L.; Mastroeni, D.; Delvaux, E.; Grover, A.; Kenis, G.; Hof, P.R.; Steinbusch, H.W.; Coleman, P.D.; Rutten, B.P.; van den Hove, D.L. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of Alzheimer’s disease patients. Neurobiol. Aging. 2013, 34, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Hernández, H.G.; Sandoval-Hernández, A.G.; Garrido-Gil, P.; Labandeira-Garcia, J.L.; Zelaya, M.V.; Bayon, G.F.; Fernández, A.F.; Fraga, M.F.; Arboleda, G.; Arboleda, H. Alzheimer’s disease DNA methylome of pyramidal layers in frontal cortex: Laser-assisted microdissection study. Epigenomics 2018. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chibnik, L.B.; Srivastava, G.P.; Pochet, N.; Yang, J.; Xu, J.; Kozubek, J.; Obholzer, N.; Leurgans, S.E.; Schneider, J.A.; et al. Association of brain DNA methylation in SORL1, ABCA7, HLA-DRB5, SLC24A4, and BIN1 with pathological diagnosis of Alzheimer disease. JAMA Neurol. 2015, 72, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Lardenoije, R.; van den Hove, D.L.A.; Havermans, M.; van Casteren, A.; Le, K.X.; Palmour, R.; Lemere, C.A.; Rutten, B.P.F. Age-related epigenetic changes in hippocampal subregions of four animal models of Alzheimer’s disease. Mol. Cell. Neurosci. 2018, 86, 1–15. [Google Scholar] [CrossRef] [PubMed]
- OMIM®. Online Mendelian Inheritance in Man® Cystathionine Gamma-Lyase; CTH. Available online: http://www.omim.org/entry/607657 (accessed on 12 December 2018).
- OMIM®. Online Mendelian Inheritance in Man® 5,10-Methylenetetrahydrofolate Reductase; MTHFR. Available online: http://www.omim.org/entry/607093 (accessed on 12 December 2018).
- Linnebank, M.; Popp, J.; Smulders, Y.; Smith, D.; Semmler, A.; Farkas, M.; Kulic, L.; Cvetanovska, G.; Blom, H.; Stoffel-Wagner, B.; et al. S-adenosylmethionine is decreased in the cerebrospinal fluid of patients with Alzheimer’s disease. Neurodegener. Dis. 2010, 7, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Kirsch, S.H.; Herrmann, W.; Obeid, R. Genetic defects in folate and cobalamin pathways affecting the brain. Clin. Chem. Lab. Med. 2013, 51, 139–155. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, E.S.; Conus, N.; Kaput, J. B vitamin polymorphisms and behavior: Evidence of associations with neurodevelopment, depression, schizophrenia, bipolar disorder and cognitive decline. Neurosci. Biobehav. Rev. 2014, 47, 307–320. [Google Scholar] [CrossRef]
- Seripa, D.; Forno, G.D.; Matera, M.G.; Gravina, C.; Margaglione, M.; Palermo, M.T.; Wekstein, D.R.; Antuono, P.; Davis, D.G.; Daniele, A.; et al. Methylenetetrahydrofolate reductase, angiotensin converting enzyme gene polymorphisms in two genetically, and diagnostically distinct cohort of Alzheimer patients. Neurobiol. Aging 2003, 24, 933–939. [Google Scholar] [CrossRef]
- Da Silva, V.C.; da Costa Ramos, F.J.; Malaquias Freitas, E.; de Brito-Marques, P.R.; de Holanda Cavalcanti, M.N.; D’Almeida, V.; Cabral-Filho, J.E.; Cartaxo Muniz, M.T. Alzheimer’s disease in Brazilian elderly has a relation with homocysteine but not with MTHFR polymorphisms. Arq. Neuro-Psiquiatr. 2006, 64, 941–945. [Google Scholar] [CrossRef]
- Nishiyama, M.; Kato, Y.; Hashimoto, M.; Yukawa, S.; Omori, K. Apolipoprotein E, methylenetetrahydrofolate reductase (MTHFR) mutation and the risk of senile dementia—An epidemiological study using the polymerase chain reaction (PCR) method. Epidemiology 2000, 10, 163–172. [Google Scholar] [CrossRef]
- Ford, A.H.; Flicker, L.; Alfonso, H.; Hankey, G.J.; Norman, P.E.; van Bockxmeer, F.M.; Almeida, O.P. Plasma homocysteine and MTHFRC667T polymorphism as risk factors for incident dementia. J. Neurol. Neurosurg. Psychiatry 2012, 83, 70–75. [Google Scholar] [CrossRef] [PubMed]
- De Lau, L.M.L.; van Meurs, J.B.; Uitterlinden, A.G.; Smith, A.D.; Refsum, H.; Johnston, C.; Breteler, M.M. Genetic variation in homocysteine metabolism, cognition, and white matter lesions. Neurobiol. Aging 2010, 31, 2020–2022. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, L.; Fekih-Mrissa, N.; Klai, S.; Mansour, M.; Gritli, N.; Mrissa, R. Association of methylenetetrahydrofolate reductase polymorphisms with susceptibility to Alzheimer’s disease. Clin. Neurol. Neurosurg. 2013, 115, 1693–1696. [Google Scholar] [CrossRef] [PubMed]
- Fraga, M.F.; Ballestar, E.; Paz, M.F.; Ropero, S.; Setien, F.; Ballestar, M.L.; Heine-Suñer, D.; Cigudosa, J.C.; Urioste, M.; Benitez, J.; et al. Epigenetic differences arise during the lifetime of monozygotic twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10604–10609. [Google Scholar] [CrossRef]
- Martin, G.M. Epigenetic drift in aging identical twins. Proc. Natl. Acad. Sci. USA 2005, 102, 10413–10414. [Google Scholar] [CrossRef]
- Cook, R.H.; Schneck, S.A.; Clark, D.B. Twins with Alzheimer’s disease. Arch. Neurol. 1981, 38, 300–301. [Google Scholar] [CrossRef]
- Breitner, J.C.; Gatz, M.; Bergem, A.L.; Christian, J.C.; Mortimer, J.A.; McClearn, G.E.; Heston, L.L.; Welsh, K.A.; Anthony, J.C.; Folstein, M.F. Use of twin cohorts for research in Alzheimer’s disease. Neurology 1993, 43, 261–267. [Google Scholar] [CrossRef]
- Nee, L.E.; Lippa, C.F. Alzheimer’s disease in 22 twin pairs—13-year follow-up: Hormonal, infectious and traumatic factors. Dement. Geriatr. Cogn. Disord. 1999, 10, 148–151. [Google Scholar] [CrossRef]
- Coppedè, F. One-carbon metabolism and Alzheimer’s disease: Focus on epigenetics. Curr. Genom. 2010, 11, 246–260. [Google Scholar] [CrossRef]
- Spence, J.D.; Yi, Q.; Hankey, G.J. B vitamins in stroke prevention: Time to reconsider. Lancet Neurol. 2017, 16, 750–760. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine, B vitamins, and cognitive impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef] [PubMed]
- Tucker, K.L.; Qiao, N.; Scott, T.; Rosenberg, I.; Spiro, A. High homocysteine and low B vitamins predict cognitive decline in aging men: The Veterans Affairs Normative Aging Study. Am. J. Clin. Nutr. 2005, 82, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Lipnicki, D.M.; Sachdev, P.S.; Crawford, J.; Reppermund, S.; Kochan, N.A.; Trollor, J.N.; Draper, B.; Slavin, M.J.; Kang, K.; Lux, O.; et al. Risk factors for late-life cognitive decline and variation with age and sex in the Sydney Memory and Ageing Study. PLoS ONE 2013, 8, e65841. [Google Scholar] [CrossRef] [PubMed]
- Dufouil, C.; Alperovitch, A.; Ducros, V.; Tzourio, C. Homocysteine, white matter hyperintensities, and cognition in healthy elderly people. Ann. Neurol. 2003, 53, 214–221. [Google Scholar] [CrossRef] [PubMed]
- McCaddon, A.; Hudson, P.; Davies, G.; Hughes, A.; Williams, J.H.; Wilkinson, C. Homocysteine and cognitive decline in healthy elderly. Dement. Geriatr. Cogn. Disord. 2001, 12, 309–313. [Google Scholar] [CrossRef] [PubMed]
- Nurk, E.; Refsum, H.; Tell, G.S.; Engedal, K.; Vollset, S.E.; Ueland, P.M.; Nygaard, H.A.; Smith, A.D. Plasma total homocysteine and memory in the elderly: The Hordaland Homocysteine study. Ann. Neurol. 2005, 58, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Solomon, A.; Kåreholt, I.; Rusanen, M.; Hänninen, T.; Leiviskä, J.; Winblad, B.; Laatikainen, T.; Soininen, H.; Kivipelto, M. Associations between serum homocysteine, holotranscobalamin, folate and cognition in the elderly: A longitudinal study. J. Intern. Med. 2012, 271, 204–221. [Google Scholar] [CrossRef] [PubMed]
- Clarke, R.; Birks, J.; Nexo, E.; Ueland, P.M.; Schneede, J.; Scott, J.; Molloy, A.; Evans, J.G. Low vitamin B-12 status and risk of cognitive decline in older adults. Am. J. Clin. Nutr. 2007, 86, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Mangialasche, F.; Kalpouzos, G.; Solomon, A.; Kåreholt, I.; Smith, A.D.; Refsum, H.; Wang, R.; Mühlmann, M.; Ertl-Wagner, B.; et al. Association of vitamin B12, folate, and sulfur amino acids with brain magnetic resonance imaging measures in older adults: A longitudinal population-based study. JAMA Psychiatry 2016, 73, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and Alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Hooshmand, B.; Solomon, A.; Kåreholt, I.; Leiviskä, J.; Rusanen, M.; Ahtiluoto, S.; Winblad, B.; Laatikainen, T.; Soininen, H.; Kivipelto, M. Homocysteine and holotranscobalamin and the risk of Alzheimer disease: A longitudinal study. Neurology 2010, 75, 1408–1414. [Google Scholar] [CrossRef]
- Faux, N.G.; Ellis, K.A.; Porter, L.; Fowler, C.J.; Laws, S.M.; Martins, R.N.; Pertile, K.K.; Rembach, A.; Rowe, C.C.; Rumble, R.L.; et al. Homocysteine, vitamin B12, and folic acid levels in Alzheimer’s disease, mild cognitive impairment, and healthy elderly: Baseline characteristics in subjects of the Australian Imaging Biomarker Lifestyle study. J. Alzheimers Dis. 2011, 27, 909–922. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D. The worldwide challenge of the dementias: A role for B vitamins and homocysteine? Food Nutr. Bull. 2008, 29, S143–S172. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and dementia: An international consensus statement. J. Alzheimers Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Minagawa, H.; Watanabe, A.; Akatsu, H.; Adachi, K.; Ohtsuka, C.; Terayama, Y.; Hosono, T.; Takahashi, S.; Wakita, H.; Jung, C.G.; et al. Homocysteine, another risk factor for Alzheimer disease, impairs apolipoprotein E3 function. J. Biol. Chem. 2010, 285, 38382–38388. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, P.; Singh, N. Homocysteine excess: Delineating the possible mechanism of neurotoxicity and depression. Fundam. Clin. Pharmacol. 2015, 29, 522–528. [Google Scholar] [CrossRef]
- Lipton, S.A.; Kim, W.K.; Choi, Y.B.; Kumar, S.; D’Emilia, D.M.; Rayudu, P.V.; Arnelle, D.R.; Stamler, J.S. Neurotoxicity associated with dual actions of homocysteine at the N-methyl-D-aspartate receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5923–5928. [Google Scholar] [CrossRef]
- Snowdon, D.A.; Tully, C.L.; Smith, C.D.; Riley, K.P.; Markesbery, W.R. Serum folate and the severity of atrophy of the neocortex in Alzheimer disease: Findings from the Nun study. Am. J. Clin. Nutr. 2000, 71, 993–998. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Odegaard, A.; Thyagarajan, B.; Hayes, J.; Cruz, K.S.; Derosiers, M.F.; Tyas, S.L.; Gross, M.D. Blood folate is associated with asymptomatic or partially symptomatic Alzheimer’s disease in the Nun study. J. Alzheimers Dis. 2012, 28, 637–645. [Google Scholar] [CrossRef]
- Blom, H.J.; Smulders, Y. Overview of homocysteine and folate metabolism. With special references to cardiovascular disease and neural tube defects. J. Inherit. Metab. Dis. 2011, 34, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Nazki, F.H.; Sameer, A.S.; Ganaie, B.A. Folate: Metabolism, genes, polymorphisms and the associated diseases. Gene 2014, 533, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Panossian, L.A.; Porter, V.R.; Valenzuela, H.F.; Zhu, X.; Reback, E.; Masterman, D.; Cummings, J.L.; Effros, R.B. Telomere shortening in T cells correlates with Alzheimer’s disease status. Neurobiol. Aging 2003, 24, 77–84. [Google Scholar] [CrossRef]
- Paul, L.; Cattaneo, M.; D’Angelo, A.; Sampietro, F.; Fermo, I.; Razzari, C.; Fontana, G.; Eugene, N.; Jacques, P.F.; Selhub, J. Telomere length in peripheral blood mononuclear cells is associated with folate status in men. J. Nutr. 2009, 139, 1273–1278. [Google Scholar] [CrossRef] [PubMed]
- Friso, S.; Choi, S.-W.; Girelli, D.; Mason, J.B.; Dolnikowski, G.G.; Bagley, P.J.; Olivieri, O.; Jacques, P.F.; Rosenberg, I.H.; Corrocher, R.; et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 2002, 99, 5606–5811. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Mack, M.M.; Wehr, C.M.; MacGregor, J.T.; Hiatt, R.A.; Wang, G.; Wickramasinghe, S.N.; Everson, R.B.; Ames, B.N. Folate deficiency causes uracil misincorporation into human DNA and chromosome breakage: Implications for cancer and neuronal damage. Proc. Natl. Acad. Sci. USA 1997, 94, 3290–3295. [Google Scholar] [CrossRef] [PubMed]
- Troesch, B.; Weber, P.; Mohajeri, M. Potential links between impaired one-carbon metabolism due to polymorphisms, inadequate B-vitamin status, and the development of Alzheimer’s disease. Nutrients 2016, 8, 803. [Google Scholar] [CrossRef]
- Religa, D.; Styczynska, M.; Peplonska, B.; Gabryelewicz, T.; Pfeffer, A.; Chodakowska, M.; Luczywek, E.; Wasiak, B.; Stepien, K.; Golebiowski, M.; et al. Homocysteine, apolipoprotein E and methylenetetrahydrofolate reductase in Alzheimer’s disease and mild cognitive impairment. Dement. Geriatr. Cogn. Disord. 2003, 16, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.A.D.; Warren, M.J.; Refsum, H. Chapter Six—Vitamin B12. Adv. Food Nutr. Res. 2018, 83, 215–279. [Google Scholar] [CrossRef] [PubMed]
- Stabler, S.P. Vitamin B12 deficiency. N. Engl. J. Med. 2013, 368, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Valente, E.; Scott, J.M.; Ueland, P.-M.; Cunningham, C.; Casey, M.; Molloy, A.M. Diagnostic accuracy of holotranscobalamin, methylmalonic acid, serum cobalamin, and other indicators of tissue vitamin B12 status in the elderly. Clin. Chem. 2011, 57, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.S.; Huang, L.K.; Lee, Y.T.; Chan, L.; Hong, C.T. Suboptimal baseline serum vitamin B12 is associated with cognitive decline in people with Alzheimer’s disease undergoing cholinesterase inhibitor treatment. Front. Neurol. 2018, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.; Haron, Y.; Evans, L.; Smith, M.; Freedman, M.; Román, G. Metabolic markers of cobalamin deficiency and cognitive function in normal older adults. J. Am. Geriatr. Soc. 2004, 52, 66–71. [Google Scholar] [CrossRef]
- Garcia, A.; Zanibbi, K. Homocysteine and cognitive function in elderly people. CMAJ 2004, 171, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Román, G.C.; Ruiz, P.J. Neurologic complications of Sjögren syndrome. MedLink Neurol. 2010, 11, 1034. [Google Scholar]
- Andrès, E.; Loukili, N.H.; Noel, E.; Kaltenbach, G.; Abdelgheni, M.B.; Perrin, A.E.; Noblet-Dick, M.; Maloisel, F.; Schlienger, J.L.; Blicklé, J.F. Vitamin B12 (cobalamin) deficiency in elderly patients. CMAJ 2004, 171, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Spence, D. Mechanisms of thrombogenesis in atrial fibrillation. Lancet 2009, 373, 1006. [Google Scholar] [CrossRef]
- Wallin, A.; Román, G.C.; Esiri, M.; Kettunen, P.; Svensson, J.; Paraskevas, G.P.; Kapaki, E. Update on vascular cognitive impairment associated with subcortical small-vessel disease. J. Alzheimers Dis. 2018, 62, 1417–1441. [Google Scholar] [CrossRef] [PubMed]
- Mohajeri, M.H.; Troesch, B.; Weber, P. Inadequate supply of vitamins and DHA in the elderly: Implications for brain aging and Alzheimer-type dementia. Nutrition 2015, 31, 261–275. [Google Scholar] [CrossRef]
- Fuso, A.; Nicolia, V.; Cavallaro, R.A.; Ricceri, L.; D’Anselmi, F.; Coluccia, P.; Calamandrei, G.; Scarpa, S. B-vitamin deprivation induces hyperhomocysteinemia and brain S-adenosylhomocysteine, depletes brain S-adenosylmethionine, and enhances PS1 and BACE expression and amyloid-β deposition in mice. Mol. Cell. Neurosci. 2008, 37, 731–746. [Google Scholar] [CrossRef] [PubMed]
- McCleery, J.; Abraham, R.P.; Denton, D.A.; Rutjes, A.W.; Chong, L.Y.; Al-Assaf, A.S.; Griffith, D.J.; Rafeeq, S.; Yaman, H.; Malik, M.A.; et al. Vitamin and mineral supplementation for preventing dementia or delaying cognitive decline in people with mild cognitive impairment. Cochrane Database Syst. Rev. 2018, 11, CD011905. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, S.M.; de Jager, C.A.; Whitbread, P.; Johnston, C.; Agacinski, G.; Oulhaj, A.; Bradley, K.M.; Jacoby, R.; Refsum, H. Homocysteine-lowering by B vitamins slows the rate of accelerated brain atrophy in mild cognitive impairment. A randomized controlled trial. PLoS ONE 2010, 5, e12244. [Google Scholar] [CrossRef] [PubMed]
- Jager, C.A.; Oulhaj, A.; Jacoby, R.; Refsum, H.; Smith, A.D. Cognitive and clinical outcomes of homocysteine-lowering B-vitamin treatment in mild cognitive impairment: A randomized controlled trial. Int. J. Geriatr. Psychiatry 2012, 27, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Douauda, G.; Refsum, H.; de Jager, C.A.; Jacoby, R.; Nichols, T.E.; Smith, S.M.; Smith, A.D. Preventing Alzheimer’s disease-related gray matter atrophy by B-vitamin treatment. PNAS Proc. Natl. Acad. Sci. USA 2013, 110, 9523–9528. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Gerbarg, P.; Bottiglieri, T.; Massoumi, L.; Carpenter, L.L.; Lavretsky, H.; Muskin, P.R.; Brown, R.P.; Mischoulon, D. As Work Group of the American Psychiatric Association Council on Research S-Adenosylmethionine (SAMe) for Neuropsychiatric Disorders: A Clinician-Oriented Review of Research. J. Clin. Psychiatry 2017, 78, e656–e667. [Google Scholar] [CrossRef] [PubMed]
- Dayon, L.; Guiraud, S.P.; Corthésy, J.; Da Silva, L.; Migliavacca, E.; Tautvydaitė, D.; Oikonomidi, A.; Moullet, B.; Henry, H.; Métairon, S.; et al. One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: Homocysteine and beyond. Alzheimers. Res. Ther. 2017, 9, 43. [Google Scholar] [CrossRef]
- Galizia, I.; Oldani, L.; Macritchie, K.; Amari, E.; Dougall, D.; Jones, T.N.; Lam, R.W.; Massei, G.J.; Yatham, L.N.; Young, A.H. S-adenosyl methionine (SAMe) for depression in adults. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).