TNF-α Inhibitors Decrease Classical CD14hiCD16− Monocyte Subsets in Highly Active, Conventional Treatment Refractory Rheumatoid Arthritis and Ankylosing Spondylitis
Abstract
1. Introduction
2. Results
2.1. Effects of TNFi on Disease Activity
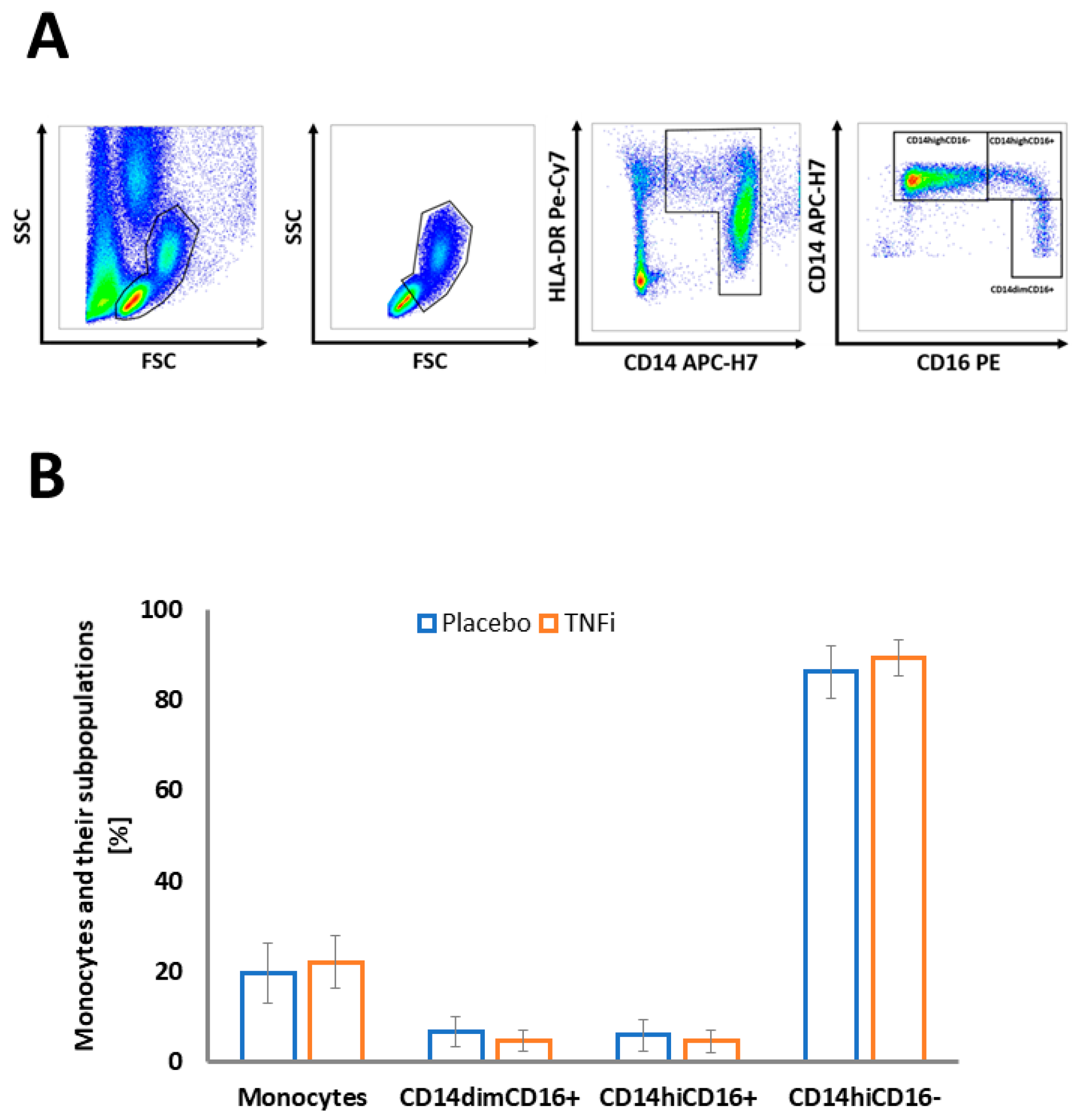
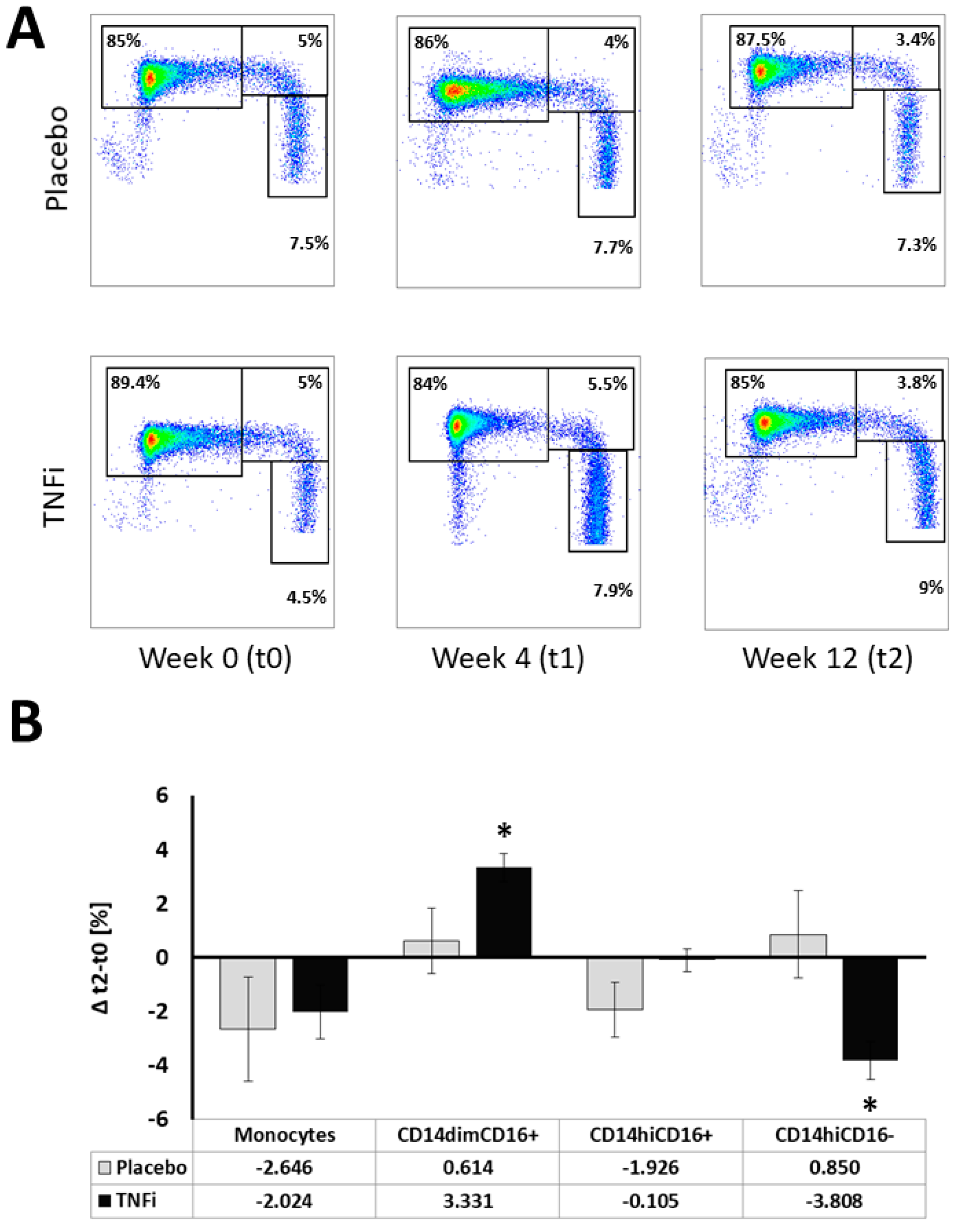
2.2. Effects of TNFi on Monocyte Subpopulations
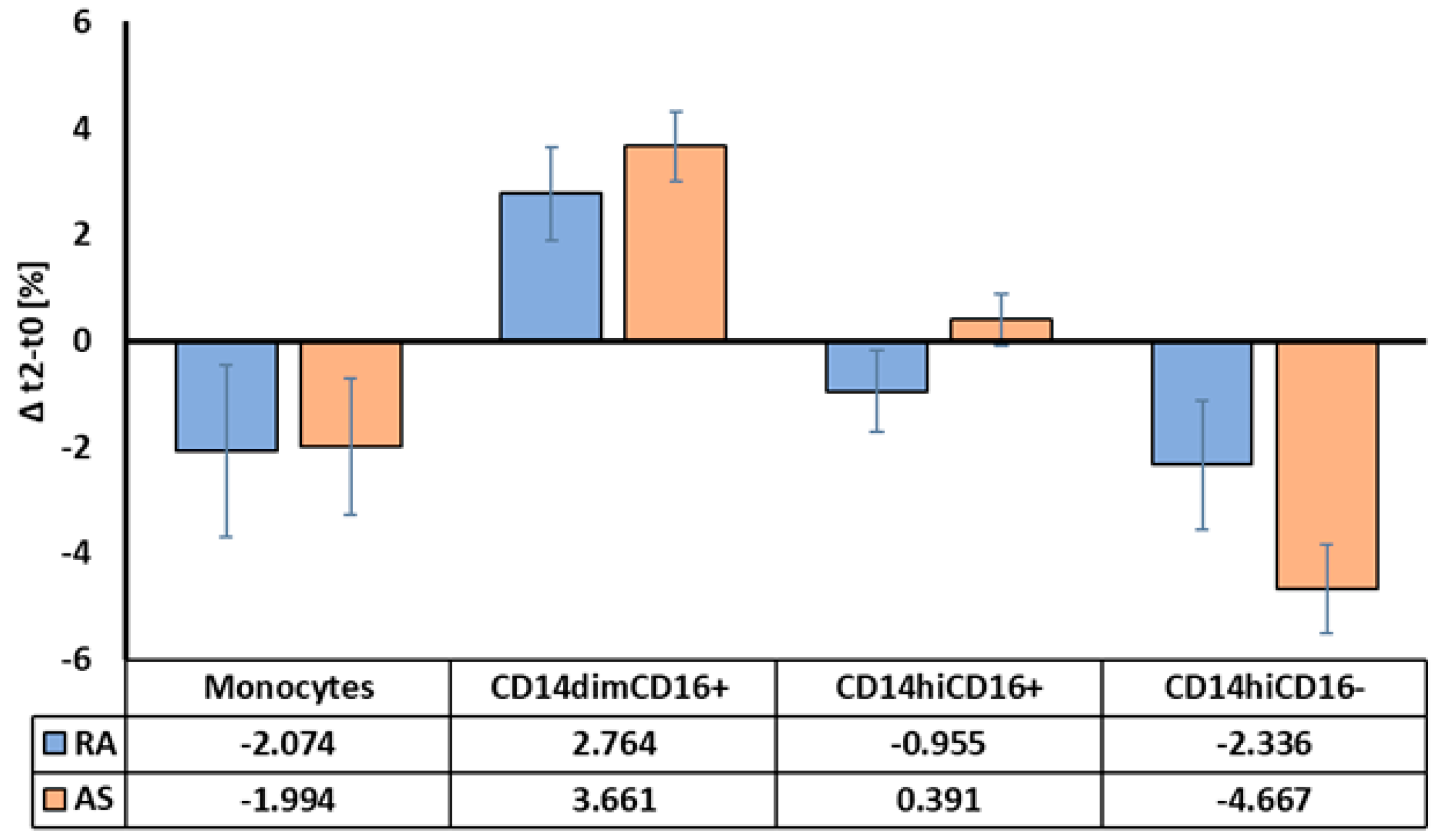
2.3. Monocyte Responses to TNFi between AS and RA Patients
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Blood Collection
4.3. Flow Cytometry
4.4. Statistics
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Szekanecz, Z.; Koch, A.E. Macrophages and their products in rheumatoid arthritis. Curr. Opin. Rheumatol. 2007, 19, 289–295. [Google Scholar] [CrossRef] [PubMed]
- Wright, C.; Edelmann, M.; DiGleria, K.; Kollnberger, S.; Kramer, H.; McGowan, S.; McHugh, K.; Taylor, S.; Kessler, B.; Bowness, P. Ankylosing spondylitis monocytes show upregulation of proteins involved in inflammation and the ubiquitin proteasome pathway. Ann. Rheum. Dis. 2009, 68, 1626–1632. [Google Scholar] [CrossRef]
- Robbins, C.S.; Swirski, F.K. The multiple roles of monocyte subsets in steady state and inflammation. Cell. Mol. Life Sci. 2010, 67, 2685–2693. [Google Scholar] [CrossRef] [PubMed]
- Ziegler-Heitbrock, L.; Ancuta, P.; Crowe, S.; Dalod, M.; Grau, V.; Hart, D.N.; Leenen, P.J.M.; Liu, Y.-J.; MacPherson, G.; Randolph, G.J.; et al. Nomenclature of monocytes and dendritic cells in blood. Blood 2010, 116, e74–e80. [Google Scholar] [CrossRef]
- Ohtsuji, M.; Lin, Q.; Okazaki, H.; Takahashi, K.; Amano, H.; Yagita, H.; Nishimura, H.; Hirose, S. Anti-CD11b antibody treatment suppresses the osteoclast generation, inflammatory cell infiltration, and autoantibody production in arthritis-prone FcγRIIB-deficient mice. Arthritis Res. Ther. 2018, 20, 25. [Google Scholar] [CrossRef] [PubMed]
- Baeten, D.; Boots, A.M.H.; Steenbakkers, P.G.A.; Elewaut, D.; Bos, E.; Verheijden, G.F.M.; Verbruggen, G.; Miltenburg, A.M.M.; Rijnders, A.W.M.; Veys, E.M.; et al. Human cartilage gp-39+,CD16+ monocytes in peripheral blood and synovium: Correlation with joint destruction in rheumatoid arthritis. Arthritis Rheum. 2000, 43, 1233–1243. [Google Scholar] [CrossRef]
- Seeling, M.; Hillenhoff, U.; David, J.P.; Schett, G.; Tuckermann, J.; Lux, A.; Nimmerjahn, F. Inflammatory monocytes and Fcγ receptor IV on osteoclasts are critical for bone destruction during inflammatory arthritis in mice. Proc. Natl. Acad. Sci. USA 2013, 110, 10729. [Google Scholar] [CrossRef] [PubMed]
- Puchner, A.; Saferding, V.; Bonelli, M.; Mikami, Y.; Hofmann, M.; Brunner, J.S.; Caldera, M.; Goncalves-Alves, E.; Binder, N.B.; Fischer, A.; et al. Non-classical monocytes as mediators of tissue destruction in arthritis. Ann. Rheum. Dis. 2018, 77, 1490–1497. [Google Scholar] [CrossRef] [PubMed]
- Guzik, T.J.; Skiba, D.S.; Touyz, R.M.; Harrison, D.G. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc. Res. 2017, 113, 1009–1023. [Google Scholar] [CrossRef]
- Cros, J.; Cagnard, N.; Woollard, K.; Patey, N.; Zhang, S.-Y.; Senechal, B.; Puel, A.; Biswas, S.K.; Moshous, D.; Picard, C.; et al. Human CD14dim Monocytes Patrol and Sense Nucleic Acids and Viruses via TLR7 and TLR8 Receptors. Immunity 2010, 33, 375–386. [Google Scholar] [CrossRef]
- Moots, R.J.; Curiale, C.; Petersel, D.; Rolland, C.; Jones, H.; Mysler, E. Efficacy and Safety Outcomes for Originator TNF Inhibitors and Biosimilars in Rheumatoid Arthritis and Psoriasis Trials: A Systematic Literature Review. BioDrugs 2018, 32, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Panahi, M.; Papanikolaou, A.; Torabi, A.; Zhang, J.-G.; Khan, H.; Vazir, A.; Hasham, M.G.; Cleland, J.G.F.; Rosenthal, N.A.; Harding, S.E.; et al. Immunomodulatory interventions in myocardial infarction and heart failure: A systematic review of clinical trials and meta-analysis of IL-1 inhibition. Cardiovasc. Res. 2018, 114, 1445–1461. [Google Scholar] [CrossRef]
- Koch, S.; Kucharzik, T.; Heidemann, J.; Nusrat, A.; Luegering, A. Investigating the role of proinflammatory CD16+ monocytes in the pathogenesis of inflammatory bowel disease. Clin. Exp. Immunol. 2010, 161, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Rosetti, F.; Mayadas, T.N. The many faces of Mac-1 in autoimmune disease. Immunol. Rev. 2016, 269, 175–193. [Google Scholar] [CrossRef] [PubMed]
- Aeberli, D.; Kamgang, R.; Balani, D.; Hofstetter, W.; Villiger, P.M.; Seitz, M. Regulation of peripheral classical and non-classical monocytes on infliximab treatment in patients with rheumatoid arthritis and ankylosing spondylitis. RMD Open 2016, 2, e000079. [Google Scholar] [CrossRef] [PubMed]
- Lügering, A.; Schmidt, M.; Lügering, N.; Pauels, H.G.; Domschke, W.; Kucharzik, T. Infliximab induces apoptosis in monocytes from patients with chronic active Crohn’s disease by using a caspase-dependent pathway. Gastroenterology 2001, 121, 1145–1157. [Google Scholar] [CrossRef]
- Shi, C.; Pamer, E.G. Monocyte recruitment during infection and inflammation. Nat. Rev. Immunol. 2011, 11, 762–774. [Google Scholar] [CrossRef]
- Kawanaka, N.; Yamamura, M.; Aita, T.; Morita, Y.; Okamoto, A.; Kawashima, M.; Iwahashi, M.; Ueno, A.; Ohmoto, Y.; Makino, H. CD14+,CD16+ blood monocytes and joint inflammation in rheumatoid arthritis. Arthritis Rheum. 2002, 46, 2578–2586. [Google Scholar] [CrossRef]
- Klimek, E.; Mikołajczyk, T.; Sulicka, J.; Kwaśny-Krochin, B.; Korkosz, M.; Osmenda, G.; Wizner, B.; Surdacki, A.; Guzik, T.; Grodzicki, T.K.; et al. Blood monocyte subsets and selected cardiovascular risk markers in rheumatoid arthritis of short duration in relation to disease activity. Biomed Res. Int. 2014, 2014, 736853. [Google Scholar] [CrossRef]
- Telesinska-Jasiówka, G.; Klimek, E.; Kierzkowska, I.; Guzik, T.; Andrzej Surdacki, T.K.; Sulicka, J.; Korkosz, M.; Mikolajczyk, T.; Surdacki, A.; Sulicka, J.; et al. Blood Monocyte Heterogeneity and Markers of Endothelial Activation in Ankylosing Spondylitis. J. Rheumatol. 2014, 41, 481–489. [Google Scholar]
- Zhao, J.; Yuan, W.; Tao, C.; Sun, P.; Yang, Z.; Xu, W. M2 polarization of monocytes in ankylosing spondylitis and relationship with inflammation and structural damage. Apmis 2017, 125, 1070–1075. [Google Scholar] [CrossRef]
- Chimen, M.; Yates, C.M.; McGettrick, H.M.; Ward, L.S.C.; Harrison, M.J.; Apta, B.; Dib, L.H.; Imhof, B.A.; Harrison, P.; Nash, G.B.; et al. Monocyte Subsets Coregulate Inflammatory Responses by Integrated Signaling through TNF and IL-6 at the Endothelial Cell Interface. J. Immunol. 2017, 198, 2834–2843. [Google Scholar] [CrossRef]
- Abbadi, D.; Laroumanie, F.; Bizou, M.; Pozzo, J.; Daviaud, D.; Delage, C.; Calise, D.; Gaits-Iacovoni, F.; Dutaur, M.; Tortosa, F.; et al. Local production of tenascin-C acts as a trigger for monocyte/macrophage recruitment that provokes cardiac dysfunction. Cardiovasc. Res. 2018, 114, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Lundahl, J.; Hed, J. Differences in altered expression of L-selectin and Mac-1 in monocytes and neutrophils. Inflammation 1994, 18, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yago, T.; Nanke, Y.; Ichikawa, N.; Kobashigawa, T.; Mogi, M.; Kamatani, N.; Kotake, S. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-α antibody: A novel mechanism of osteoclastogenesis by IL-17. J. Cell. Biochem. 2009, 108, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Mei, S.; Liu, Y.; Wu, X.; He, Q.; Min, S.; Li, L.; Zhang, Y.; Yang, R. TNF-α-mediated microRNA-136 induces differentiation of myeloid cells by targeting NFIA. J. Leukoc. Biol. 2016, 99, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Torsteinsdóttir, I.; Arvidson, N.G.; Hällgren, R.; Håkansson, L. Monocyte activation in rheumatoid arthritis (RA): Increased integrin, Fc gamma and complement receptor expression and the effect of glucocorticoids. Clin. Exp. Immunol. 1999, 115, 554–560. [Google Scholar] [CrossRef]
- Stuhlmüller, B.; Häupl, T.; Hernandez, M.M.; Grützkau, A.; Kuban, R.-J.; Tandon, N.; Voss, J.W.; Salfeld, J.; Kinne, R.W.; Burmester, G.R. CD11c as a Transcriptional Biomarker to Predict Response to Anti-TNF Monotherapy With Adalimumab in Patients With Rheumatoid Arthritis. Clin. Pharmacol. Ther. 2010, 87, 311–321. [Google Scholar] [CrossRef]
- Conrad, K.; Wu, P.; Sieper, J.; Syrbe, U. In vivo pre-activation of monocytes in patients with axial spondyloarthritis. Arthritis Res. Ther. 2015, 17, 179. [Google Scholar] [CrossRef]
- Liu, B.; Dhanda, A.; Hirani, S.; Williams, E.L.; Sen, H.N.; Martinez Estrada, F.; Ling, D.; Thompson, I.; Casady, M.; Li, Z.; et al. CD14++CD16+ Monocytes Are Enriched by Glucocorticoid Treatment and Are Functionally Attenuated in Driving Effector T Cell Responses. J. Immunol. 2015, 194, 5150–5160. [Google Scholar] [CrossRef]
- Chara, L.; Sánchez-Atrio, A.; Pérez, A.; Cuende, E.; Albarrán, F.; Turrión, A.; Chevarria, J.; Sánchez, M.A.; Monserrat, J.; de la Hera, A.; et al. Monocyte populations as markers of response to adalimumab plus MTX in rheumatoid arthritis. Arthritis Res. Ther. 2012, 14, R175. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, M.; Seta, N.; Yoshimoto, K.; Suzuki, K.; Yamaoka, K.; Takeuchi, T. CD14brightCD16+ intermediate monocytes are induced by interleukin-10 and positively correlate with disease activity in rheumatoid arthritis. Arthritis Res. Ther. 2017, 19, 28. [Google Scholar] [CrossRef] [PubMed]
- van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O.; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Heimbeck, I.; Hofer, T.P.J.; Eder, C.; Wright, A.K.; Frankenberger, M.; Marei, A.; Boghdadi, G.; Scherberich, J.; Ziegler-Heitbrock, L. Standardized single-platform assay for human monocyte subpopulations: Lower CD14+CD16++ monocytes in females. Cytom. Part A 2010, 77A, 823–830. [Google Scholar] [CrossRef] [PubMed]
| Clinical Characteristic | Anti-TNF-α Treatment (n = 38) Mean (SD) | Placebo Comparator (n = 12) Mean (SD) | p Value |
|---|---|---|---|
| Demographic Factors | |||
| Age (years) | 40.37 (11.74) | 42.75 (7.40) | 0.41 |
| Gender (%) | |||
| Male (n) | 57.89% (22) | 58.33% (7) | 0.98 |
| Female (n) | 42.11% (16) | 41.67% (5) | 0.98 |
| Smoking history | |||
| Never smoker (n) | 42.11% (16) | 33.33% (4) | 0.85 |
| Ever smoker (n) | 34.21% (13) | 41.67% (5) | 0.85 |
| Active smoker (n) | 23.68% (9) | 25.00% (3) | 0.85 |
| BMI (kg/m2) | 27.04 (4.05) | 26.89 (4.43) | 0.92 |
| Disease Related Factors | |||
| Duration of the disease (years) | 8.37 (7.56) | 13.46 (10.50) | 0.14 |
| CRP (mg/l) | 21.16 (38.67) | 12.46 (9.55) | 0.21 |
| BASDAI (AS), n = 32 | 7.14 (1.05) | 6.34 (1.75) | 0.25 |
| HLA-B27 (AS) | 87.5% (n = 21) | 100% (n = 8) | 0.29 |
| DAS 28 (RA), n =18 | 5.50 (0.71) | 5.21 (0.22) | 0.13 |
| RF (RA) | 71.43% (n = 10) | 100% (n = 4) | 0.23 |
| aCCP (RA) | 78.57% (n = 11) | 75% (n = 3) | 0.31 |
| Drugs | |||
| csDMARDS | |||
| Methotrexate (n) | 39.47% (15) | 25% (3) | 0.36 |
| Sulfasalazine (n) | 18.42% (7) | 25% (3) | 0.62 |
| Leflunomide (n) | 2.6% (1) | 0% (0) | 0.57 |
| Glucocorticosteroids | 39.47% (15) | 41.67% (5) | 0.89 |
| NSAIDs | 71.05% (27) | 83.33% (10) | 0.40 |
| Anti-TNF-α | |||
| Etanercept (n) | 50.00% (19) | - | |
| Adalimumab (n) | 21.05% (8) | - | |
| Infliximab (n) | 21.05% (8) | - | |
| Certolizumab (n) | 7.89% (3) | - |
| Subpopulation | Placebo | Anti-TNF-α Treatment | p Value | ||||
|---|---|---|---|---|---|---|---|
| Timepoint | t0 | t1 | t2 | t0 | t1 | t2 | |
| Monocytes (%) | 19.44 (6.69) | 18.97 (5.70) | 16.80 (5.69) | 21.98 (5.81) | 18.15 (6.23) | 19.96 (5.96) | p = 0.124 |
| CD45RA MFI | 3188.08 (1223.47) | 3040.25 (914.15) | 3033.00 (822.31) | 2641.50 (1580.84) | 3484.53 (1806.13) | 3654.32 (2189.89) | p = 0.013 |
| CD11c MFI | 778.92 (140.42) | 901.08 (234.06) | 877.83 (229.42) | 937.24 (407.19) | 972.34 (334.35) | 980.74 (399.46) | p = 0.832 |
| CD11b MFI | 900.58 (212.85) | 873.17 (166.56) | 834.33 (227.42) | 3310.42 (2993.18) | 4138.79 (4056.15) | 2100.51 (2861.03) | p = 0.121 |
| HLA-DR MFI | 10984.50 (3181.69) | 12183.08 (4130.38) | 11438.00 (4321.06) | 9481.24 (4661.29) | 12162.82 (6078.35) | 13064.74 (5250.11) | p = 0.274 |
| CD14dimCD16+ (%) | 6.52 (3.41) | 7.28 (4.29) | 7.14 (4.13) | 4.65 (2.34) | 7.43 (4.53) | 7.98 (3.57) | p = 0.073 |
| CD45RA MFI | 11065.17 (5181.33) | 10355.00 (5370.98) | 10033.42 (4897.20) | 9254.92 (6000.12) | 10994.37 (5624.30) | 11494.16 (6599.58) | p = 0.049 |
| CD11c MFI | 2173.75 (318.64) | 2665.25 (573.03) | 2813.00 (868.16) | 2998.82 (1273.40) | 2756.58 (1124.21) | 2690.21 (1206.80) | p = 0.046 |
| CD11b MFI | 531.92 (106.24) | 556.17 (115.43) | 535.50 (123.92) | 1943.86 (1665.57) | 1931.00 (1835.86) | 1012.83 (1084.80) | p = 0.028 |
| HLA-DR MFI | 25946.50 (4698.43) | 29249.33 (7221.83) | 27596.17 (6016.55) | 24027.34 (10224.44) | 24758.55 (16111.57) | 26336.08 (17167.62) | p = 0.771 |
| CD14hiCD16+ | 5.71 (3.54) | 5.03 (2.86) | 3.79 (2.12) | 4.41 (2.40) | 4.97 (3.51) | 4.31 (1.97) | p = 0.170 |
| CD45RA MFI | 2871.33 (1139.52) | 3070.92 (1566.17) | 2804 (1197.48) | 3046.66 (2779.49) | 3371 (2334.81) | 3456.95 (3063.03) | p = 0.593 |
| CD11c MFI | 1838.08 (394.12) | 2232.67 (586.69) | 2193.83 (478.66) | 2458.05 (999.10) | 2417 (796.66) | 2435.16 (748.38) | p = 0.315 |
| CD11b MFI | 1084.00 (266.68) | 1068.25 (209.36) | 1045.50 (265.60) | 4071.86 (3416.93) | 5051.47 (4754.07) | 2548.31 (3371.88) | p = 0.102 |
| HLA-DR MFI | 49088.17 (11819.53) | 54980.00 (11033.93) | 54020.42 (15785.05) | 45712.00 (19544.37) | 49330.89 (19217.99) | 55932.68 (21573.64) | p = 0.466 |
| CD14hiCD16− (%) | 86.28 (5.77) | 86.17 (6.87) | 87.13 (5.93) | 89.35 (4.04) | 85.19 (7.47) | 85.54 (4.50) | p = 0.026 |
| CD45RA MFI | 2002.58 (671.09) | 1935.42 (510.77) | 1853.42 (515.27) | 1862.82 (1281.84) | 1831.71 (968.78) | 1997.26 (1569.28) | p = 0.297 |
| CD11c MFI | 588.833 (122.13) | 663.25 (144.66) | 644.667 (126.93) | 738.789 (340.83) | 719.447 (254.83) | 723.763 (307.49) | p = 0.725 |
| CD11b MFI | 909.75 (220.24) | 883.25 (173.51) | 841.42 (231.86) | 3355.67 (3058.44) | 4317.13 (4268.82) | 2210.06 (3167.63) | p = 0.139 |
| HLA-DR MFI | 7218.33 (2526.62) | 8054.08 (2533.40) | 7921.17 (2929.59) | 6706.42 (3353.68) | 8473.97 (3954.01) | 9368.45 (3641.07) | p = 0.340 |
| Placebo | TNFi | p Value | |
|---|---|---|---|
| Monocytes | |||
| CD11b (%) | −1.08 (2.10) | −3.51 (3.52) | 0.008 |
| CD11b, MFI | −66.25 (379.01) | −1540.21 (2789.60) | 0.006 |
| CD11c (%) | 1.69 (8.58) | −0.84 (9.88) | 0.400 |
| CD11c, MFI | 98.92 (289.18) | 43.50 (482.08) | 0.631 |
| CD45RA (%) | −3.48 (7.86) | 3.58 (10.44) | 0.020 |
| CD45RA, MFI | −155.08 (689.07) | 1012.82 (1203.48) | <0.001 |
| HLA-DR (%) | −0.05 (0.26) | 0.55 (1.73) | 0.045 |
| HLA-DR, MFI | 453.50 (3829.02) | 3583.50 (6990.94) | 0.056 |
| CD14dimCD16+ | |||
| CD11b (%) | −5.83 (15.24) | −21.97 (27.99) | 0.019 |
| CD11b, MFI | 3.58 (120.93) | −1071.09 (1252.97) | <0.001 |
| CD11c (%) | 0.08 (0.20) | 0.08 (0.26) | 0.923 |
| CD11c, MFI | 639.25 (868.90) | −308.61 (1395.26) | 0.009 |
| CD45RA (%) | −0.10 (0.47) | 1.58 (4.49) | 0.030 |
| CD45RA, MFI | −1031.75 (3329.37) | 2239.24 (4509.90) | 0.012 |
| HLA-DR (%) | −0.08 (0.22) | −0.06 (1.35) | 0.950 |
| HLA-DR, MFI | 1649.67 (4163.04) | 2308.74 (18560.71) | 0.840 |
| CD14hiCD16+ | |||
| CD11b (%) | −0.44 (0.72) | −0.63 (1.12) | 0.513 |
| CD11b, MFI | −38.50 (424.72) | −1841.12 (3118.36) | 0.003 |
| CD11c (%) | −0.29 (1.03) | 0.05 (1.46) | 0.378 |
| CD11c, MFI | 355.75 (582.27) | −22.90 (1065.56) | 0.125 |
| CD45RA (%) | −2.72 (4.21) | 3.50 (8.57) | 0.002 |
| CD45RA, MFI | −67.33 (884.13) | 410.29 (1210.45) | 0.150 |
| HLA-DR (%) | −0.80 (1.20) | −2.00 (6.96) | 0.314 |
| HLA-DR, MFI | 4932.25 (9930.15) | 10220.68 (25135.67) | 0.294 |
| CD14hiCD16− | |||
| CD11b (%) | −0.17 (0.22) | −0.52 (0.57) | 0.004 |
| CD11b, MFI | −68.33 (392.09) | −1504.85 (3016.97) | 0.011 |
| CD11c (%) | 2.19 (9.88) | −1.14 (11.63) | 0.341 |
| CD11c, MFI | 55.83 (197.20) | −15.03 (409.84) | 0.423 |
| CD45RA (%) | −4.20 (9.28) | 2.37 (11.31) | 0.055 |
| CD45RA, MFI | −149.17 (234.61) | 134.45 (627.74) | 0.025 |
| HLA-DR (%) | 0.00 (0.27) | 0.67 (1.74) | 0.026 |
| HLA-DR, MFI | 702.83 (2131.76) | 2662.03 (4829.73) | 0.056 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Batko, B.; Schramm-Luc, A.; Skiba, D.S.; Mikolajczyk, T.P.; Siedlinski, M. TNF-α Inhibitors Decrease Classical CD14hiCD16− Monocyte Subsets in Highly Active, Conventional Treatment Refractory Rheumatoid Arthritis and Ankylosing Spondylitis. Int. J. Mol. Sci. 2019, 20, 291. https://doi.org/10.3390/ijms20020291
Batko B, Schramm-Luc A, Skiba DS, Mikolajczyk TP, Siedlinski M. TNF-α Inhibitors Decrease Classical CD14hiCD16− Monocyte Subsets in Highly Active, Conventional Treatment Refractory Rheumatoid Arthritis and Ankylosing Spondylitis. International Journal of Molecular Sciences. 2019; 20(2):291. https://doi.org/10.3390/ijms20020291
Chicago/Turabian StyleBatko, Bogdan, Agata Schramm-Luc, Dominik S. Skiba, Tomasz P. Mikolajczyk, and Mateusz Siedlinski. 2019. "TNF-α Inhibitors Decrease Classical CD14hiCD16− Monocyte Subsets in Highly Active, Conventional Treatment Refractory Rheumatoid Arthritis and Ankylosing Spondylitis" International Journal of Molecular Sciences 20, no. 2: 291. https://doi.org/10.3390/ijms20020291
APA StyleBatko, B., Schramm-Luc, A., Skiba, D. S., Mikolajczyk, T. P., & Siedlinski, M. (2019). TNF-α Inhibitors Decrease Classical CD14hiCD16− Monocyte Subsets in Highly Active, Conventional Treatment Refractory Rheumatoid Arthritis and Ankylosing Spondylitis. International Journal of Molecular Sciences, 20(2), 291. https://doi.org/10.3390/ijms20020291





