Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Inclusion Criteria
2.2. Exclusion Criteria
3. Statistical Analyses
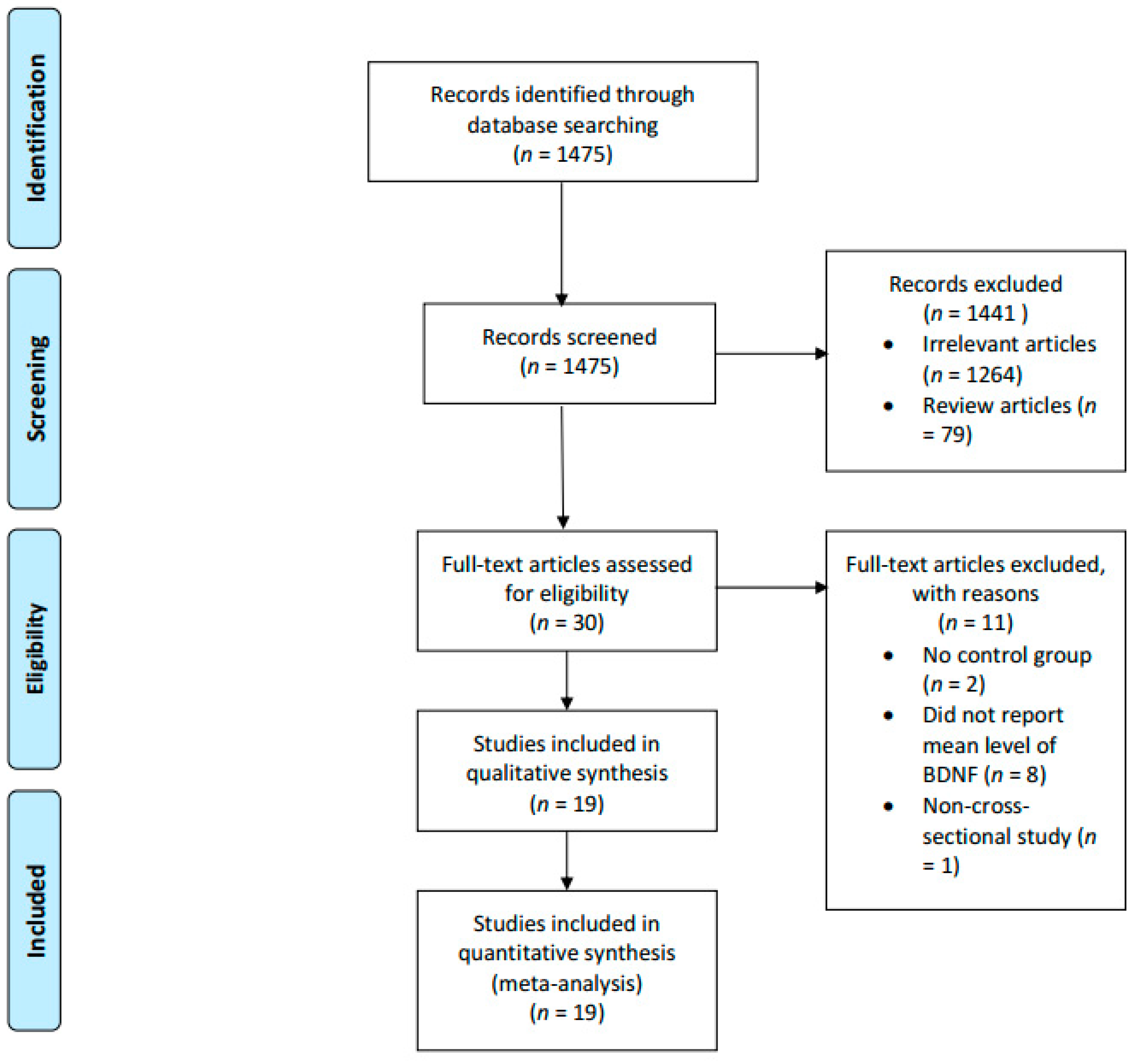
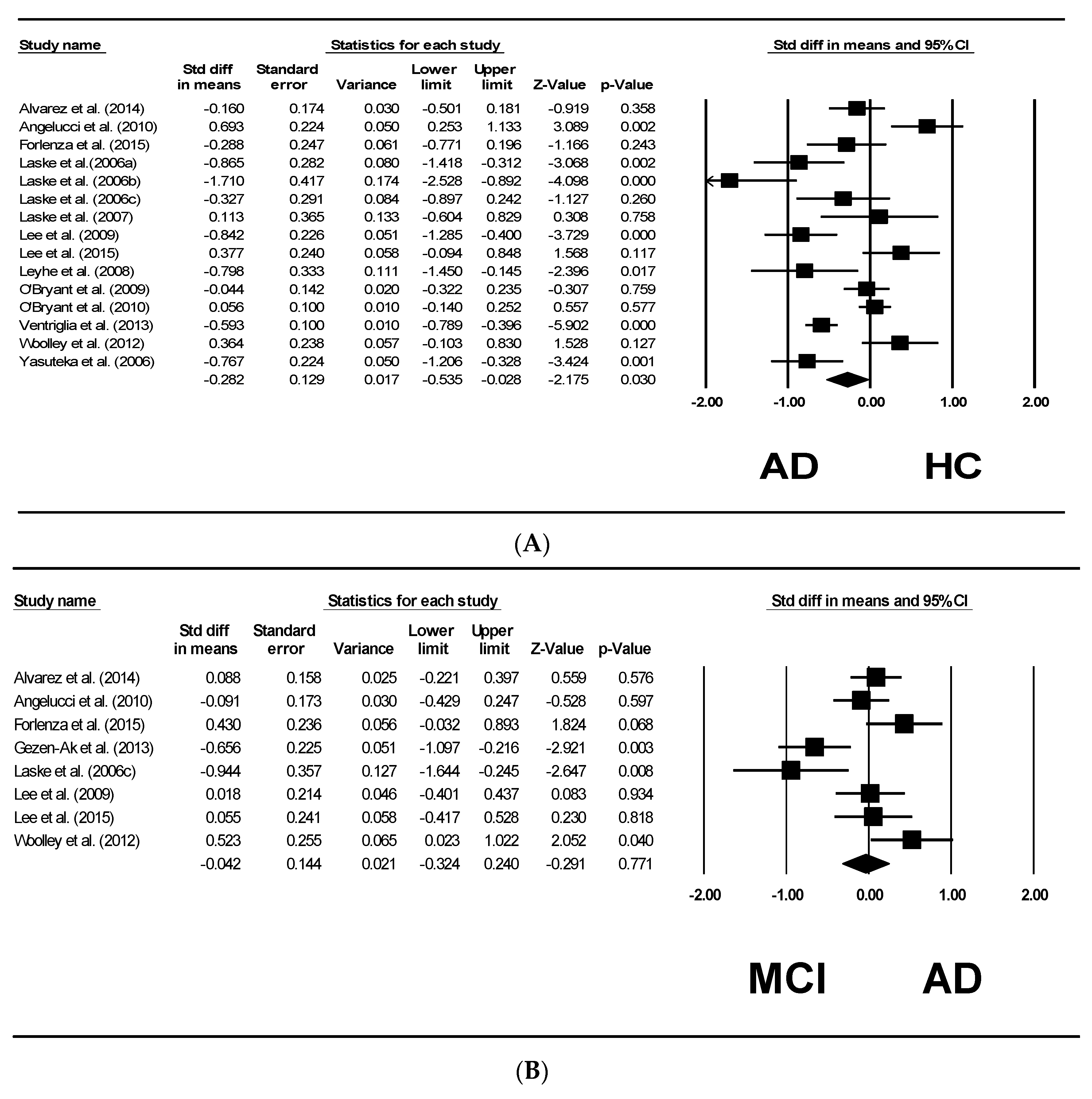
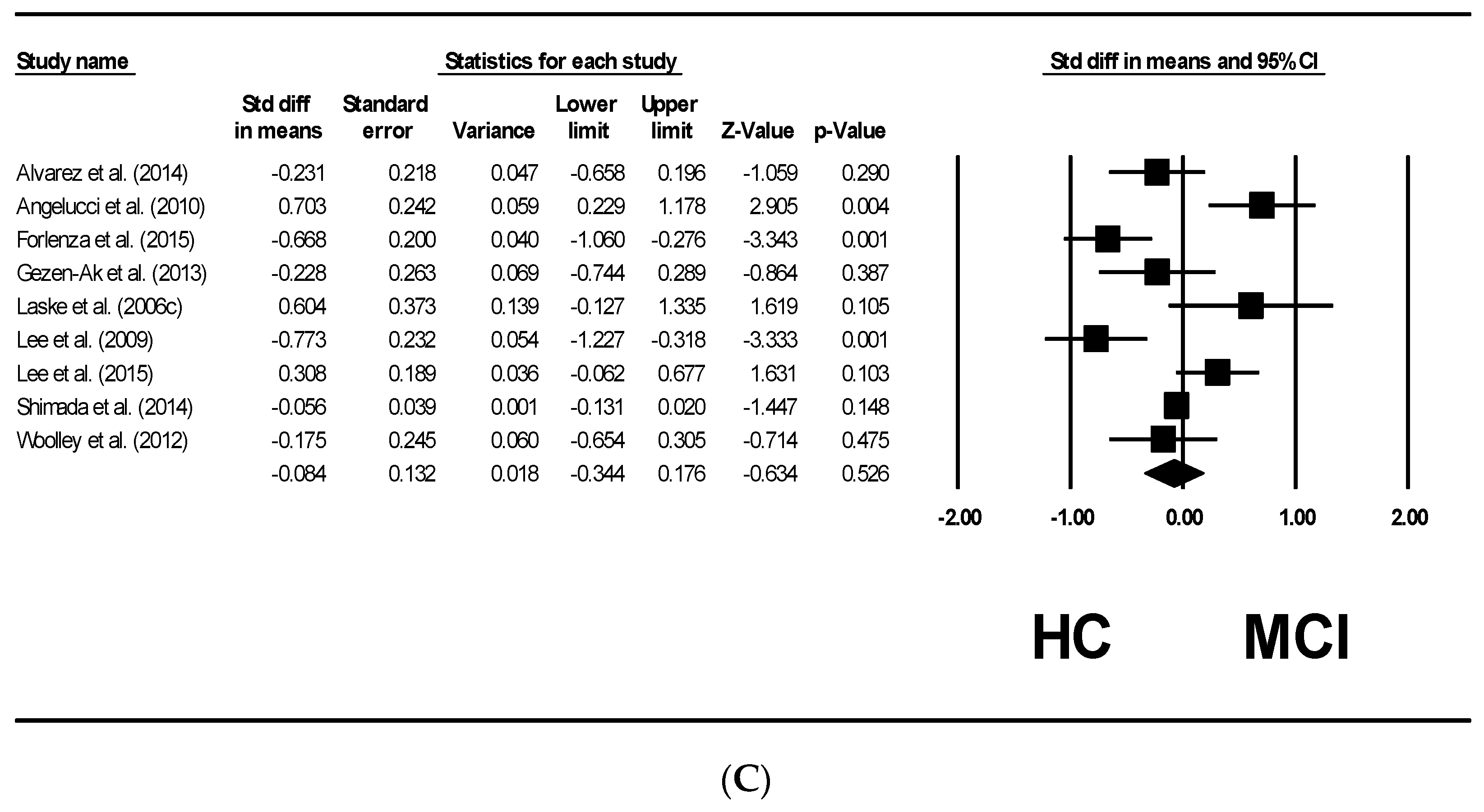
4. Results
5. Forrest Plots
6. Publication Bias
7. Discussions
8. Molecular Mechanisms of the Reduced Serum BDNF Levels in AD
9. Evidence and Protective Mechanisms of Actions of BDNF
10. Interventions to Increase BDNF Levels
11. Future Directions
12. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carrette, O.; Demalte, I.; Scherl, A.; Yalkinoglu, O.; Corthals, G.; Burkhard, P.; Hochstrasser, D.F.; Sanchez, J.C. A panel of cerebrospinal fluid potential biomarkers for the diagnosis of Alzheimer’s disease. Proteomics 2003, 3, 1486–1494. [Google Scholar] [CrossRef] [PubMed]
- Lu, B. Pro-region of neurotrophins: Role in synaptic modulation. Neuron 2003, 39, 735–738. [Google Scholar] [CrossRef]
- Angelucci, F.; Spalletta, G.; Iulio, F.D.; Ciaramella, A.; Salani, F.; Varsi, A.; Gianni, W.; Sancesario, G.; Caltagirone, C.; Bossu, P. Alzheimer’s disease (AD) and Mild Cognitive Impairment (MCI) patients are characterized by increased BDNF serum levels. Curr. Alzheimer Res. 2010, 7, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Olson, L.; Humpel, C. Growth factors and cytokines/chemokines as surrogate biomarkers in cerebrospinal fluid and blood for diagnosing Alzheimer’s disease and mild cognitive impairment. Exp. Gerontol. 2010, 45, 41–46. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Strac, D.S.; Muck-Seler, D.; Pivac, N. Neurotransmitter measures in the cerebrospinal fluid of patients with Alzheimer’s disease: A review. Psychiatr. Danub. 2015, 27, 14–24. [Google Scholar] [PubMed]
- Acheson, A.; Conover, J.C.; Fandl, J.P.; DeChiara, T.M.; Russell, M.; Thadani, A.; Squinto, S.P.; Yancopoulos, G.D.; Lindsay, R.M. A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature 1995, 374, 450–453. [Google Scholar] [CrossRef]
- Lu, B.; Nagappan, G.; Guan, X.; Nathan, P.J.; Wren, P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat. Rev. Neurosci. 2013, 14, 401–416. [Google Scholar] [CrossRef]
- Weinstein, G.; Beiser, A.S.; Choi, S.H.; Preis, S.R.; Chen, T.C.; Vorgas, D.; Au, R.; Pikula, A.; Wolf, P.A.; DeStefano, A.L.; et al. Serum brain-derived neurotrophic factor and the risk for dementia: The Framingham Heart Study. JAMA Neurol. 2014, 71, 55–61. [Google Scholar] [CrossRef]
- Blasko, I.; Lederer, W.; Oberbauer, H.; Walch, T.; Kemmler, G.; Hinterhuber, H.; Marksteiner, J.; Humpel, C. Measurement of thirteen biological markers in CSF of patients with Alzheimer’s disease and other dementias. Dement Geriatr. Cogn. Disord. 2006, 21, 9–15. [Google Scholar] [CrossRef]
- Woolley, J.D.; Strobl, E.V.; Shelly, W.B.; Karydas, A.M.; Ketelle, R.; Wolkowitz, O.M.; Miller, B.L.; Rankin, K.P. BDNF serum concentrations show no relationship with diagnostic group or medication status in neurodegenerative disease. Curr. Alzheimer Res. 2012, 9, 815–821. [Google Scholar] [CrossRef] [PubMed]
- Álvarez, A.; Aleixandre, M.; Linares, C.; Masliah, E.; Moessler, H. Apathy and APOE4 are associated with Reduced BDNF Levels in Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 42, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, O.V.; Miranda, A.S.; Guimar, I.; Talib, L.L.; Diniz, B.S.; Gattaz, W.F.; Teixeira, A.L. Decreased Neurotrophic Support is Associated with Cognitive Decline in Non-Demented Subjects. J. Alzheimer’s Dis. 2015, 46, 423–429. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Stransky, E.; Leyhe, T.; Eschweiler, G.; Wittorf, A.; Richartz, E.; Bartels, M.; Buchkremer, G.; Schott, K. Stage-dependent BDNF serum concentrations in Alzheimer’s disease. J. Neural Transm. 2006, 113, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Stransky, E.; Leyhe, T.; Koehler, N.; Schott, K. P3-340: Decrease of BDNF serum concentration from MCI to early Alzheimer’s disease. Alzheimer’s Dement. 2006, 2, S475. [Google Scholar] [CrossRef]
- Lee, J.G.; Shin, B.S.; You, Y.S.; Kim, J.E.; Yoon, S.W.; Jeon, D.W.; Baek, J.H.; Park, S.W.; Kim, Y.H. Decreased serum brain-derived neurotrophic factor levels in elderly Korean with dementia. Psychiatry Investig. 2009, 6, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.J.; Baek, J.-H.; Kim, Y.-H. Brain-derived Neurotrophic Factor Is Associated with Cognitive Impairment in Elderly Korean Individuals. Clin. Psychopharmacol. Neurosci. 2015, 13, 283–287. [Google Scholar] [CrossRef]
- Shimada, H.; Makizako, H.; Doi, T.; Yoshida, D.; Tsutsumimoto, K.; Anan, Y.; Uemura, K.; Lee, S.; Park, H.; Suzuki, T. A large, cross-sectional observational study of serum BDNF, cognitive function, and mild cognitive impairment in the elderly. Front. Aging Neurosci. 2014, 6, 69. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Green, S.; Higgins, J. Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Collaboration: London, UK, 2005. [Google Scholar]
- Laske, C.; Stransky, E.; Leyhe, T.; Eschweiler, G.W.; Maetzler, W.; Wittorf, A.; Soekadar, S.; Richartz, E.; Koehler, N.; Bartels, M.; et al. BDNF serum and CSF concentrations in Alzheimer’s disease, normal pressure hydrocephalus and healthy controls. J. Psychiatr. Res. 2007, 41, 387–394. [Google Scholar] [CrossRef]
- Laske, C.; Stransky, E.; Leyhe, T.; Eschweiler, G.W.; Schott, K.; Langer, H.; Gawaz, M. Decreased brain-derived neurotrophic factor (BDNF)-and beta-thromboglobulin (beta-TG)-blood levels in Alzheimer’s disease. Thromb. Haemost. 2006, 96, 102. [Google Scholar] [PubMed]
- Leyhe, T.; Stransky, E.; Eschweiler, G.; Buchkremer, G.; Laske, C. Increase of BDNF serum concentration during donepezil treatment of patients with early Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 2008, 258, 124–128. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Hobson, V.; Hall, J.R.; Waring, S.C.; Chan, W.; Massman, P.; Lacritz, L.; Cullum, C.M.; Diaz-Arrastia, R. Brain-derived neurotrophic factor levels in Alzheimer’s disease. J. Alzheimer’s Dis. 2009, 17, 337–341. [Google Scholar] [CrossRef] [PubMed]
- O’Bryant, S.E.; Hobson, V.L.; Hall, J.R.; Barber, R.C.; Zhang, S.; Johnson, L.; Diaz-Arrastia, R. Serum brain-derived neurotrophic factor levels are specifically associated with memory performance among Alzheimer’s disease cases. Dement. Geriatr. Cogn. Disord. 2010, 31, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Pláteník, J.; Fišar, Z.; Buchal, R.; Jirák, R.; Kitzlerová, E.; Zvěřová, M.; Raboch, J. GSK3β, CREB, and BDNF in peripheral blood of patients with Alzheimer’s disease and depression. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 50, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Ventriglia, M.; Zanardini, R.; Bonomini, C.; Zanetti, O.; Volpe, D.; Pasqualetti, P.; Gennarelli, M.; Bocchio-Chiavetto, L. Serum Brain-Derived Neurotrophic Factor Levels in Different Neurological Diseases. BioMed Res. Int. 2013, 2013, 901082. [Google Scholar] [CrossRef] [PubMed]
- Yasutake, C.; Kuroda, K.; Yanagawa, T.; Okamura, T.; Yoneda, H. Serum BDNF, TNF-α and IL-1β levels in dementia patients. Eur. Arch. Psychiatry Clin. Neurosci. 2006, 256, 402–406. [Google Scholar] [CrossRef]
- Gezen-Ak, D.; Dursun, E.; Hanağası, H.; Bilgiç, B.; Lohman, E.; Araz, Ö.S.; Atasoy, İ.L.; Alaylıoğlu, M.; Önal, B.; Gürvit, H.; et al. BDNF, TNFα, HSP90, CFH, and IL-10 serum levels in patients with early or late onset Alzheimer’s disease or mild cognitive impairment. J. Alzheimer’s Dis. 2013, 37, 185–195. [Google Scholar] [CrossRef]
- Shimizu, E.; Hashimoto, K.; Okamura, N.; Koike, K.; Komatsu, N.; Kumakiri, C.; Nakazato, M.; Watanabe, H.; Shinoda, N.; Okada, S.-I.; et al. Alterations of serum levels of brain-derived neurotrophic factor (BDNF) in depressed patients with or without antidepressants. Biol. Psychiatry 2003, 54, 70–75. [Google Scholar] [CrossRef]
- Naegelin, Y.; Dingsdale, H.; Säuberli, K.; Schädelin, S.; Kappos, L.; Barde, Y.-A. Measuring and Validating the Levels of Brain-Derived Neurotrophic Factor in Human Serum. eNeuro 2018. [Google Scholar] [CrossRef]
- Ferretti, M.T.; Iulita, M.F.; Cavedo, E.; Chiesa, P.A.; Dimech, A.S.; Chadha, A.S.; Baracchi, F.; Girouard, H.; Misoch, S.; Giacobini, E.; et al. Sex differences in Alzheimer disease—The gateway to precision medicine. Nat. Rev. Neurol. 2018, 14, 457–469. [Google Scholar] [CrossRef] [PubMed]
- Polacchini, A.; Metelli, G.; Francavilla, R.; Baj, G.; Florean, M.; Mascaretti, L.G.; Tongiorgi, E. A method for reproducible measurements of serum BDNF: Comparison of the performance of six commercial assays. Sci. Rep. 2015, 5, 17989. [Google Scholar] [CrossRef] [PubMed]
- Evans, S.F.; Irmady, K.; Ostrow, K.; Kim, T.; Nykjaer, A.; Saftig, P.; Blobel, C.; Hempstead, B.L. Neuronal brain-derived neurotrophic factor is synthesized in excess, with levels regulated by sortilin-mediated trafficking and lysosomal degradation. J. Biol. Chem. 2011, 286, 29556–29567. [Google Scholar] [CrossRef] [PubMed]
- Keleshian, V.L.; Modi, H.R.; Rapoport, S.I.; Rao, J.S. Aging is associated with altered inflammatory, arachidonic acid cascade, and synaptic markers, influenced by epigenetic modifications, in the human frontal cortex. J. Neurochem. 2013, 125, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Varendi, K.; Mätlik, K.; Andressoo, J.-O. From microRNA target validation to therapy: Lessons learned from studies on BDNF. Cell. Mol. Life Sci. 2015, 72, 1779–1794. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, H.; Altar, C.A.; Chen, R.; Nakamura, T.; Nakahashi, T.; Kambayashi, J.-I.; Sun, B.; Tandon, N.N. Brain-derived neurotrophic factor is stored in human platelets and released by agonist stimulation. Thromb. Haemost. 2002, 87, 728–734. [Google Scholar] [CrossRef]
- Mowla, S.J.; Pareek, S.; Farhadi, H.F.; Petrecca, K.; Fawcett, J.P.; Seidah, N.G.; Morris, S.J.; Sossin, W.S.; Murphy, R.A. Differential sorting of nerve growth factor and brain-derived neurotrophic factor in hippocampal neurons. J. Neurosci. 1999, 19, 2069–2080. [Google Scholar] [CrossRef]
- Landfield, P.W.; Blalock, E.M.; Chen, K.-C.; Porter, N.M. A new glucocorticoid hypothesis of brain aging: Implications for Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 205–212. [Google Scholar] [CrossRef]
- Schaaf, M.; De Kloet, E.; Vreugdenhil, E. Corticosterone effects on BDNF expression in the hippocampus implications for memory formation. Stress 2000, 3, 201–208. [Google Scholar] [CrossRef]
- Connor, B.; Young, D.; Yan, Q.; Faull, R.; Synek, B.; Dragunow, M. Brain-derived neurotrophic factor is reduced in Alzheimer’s disease. Mol. Brain Res. 1997, 49, 71–81. [Google Scholar] [CrossRef]
- Peskind, E.; Wilkinson, C.; Petrie, E.; Schellenberg, G.; Raskind, M. Increased CSF cortisol in AD is a function of APOE genotype. Neurology 2001, 56, 1094–1098. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ku, B.; Tie, L.; Yao, H.; Jiang, W.; Ma, X.; Li, X. Curcumin reverses the effects of chronic stress on behavior, the HPA axis, BDNF expression and phosphorylation of CREB. Brain Res. 2006, 1122, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Karege, F.; Bondolfi, G.; Gervasoni, N.; Schwald, M.; Aubry, J.-M.; Bertschy, G. Low brain-derived neurotrophic factor (BDNF) levels in serum of depressed patients probably results from lowered platelet BDNF release unrelated to platelet reactivity. Biol. Psychiatry 2005, 57, 1068–1072. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kim, J.H.; Huh, J.Y.; Yoon, H.; Park, D.K.; Lim, J.Y.; Kim, J.M.; Jeon, D.; et al. miR-206 regulates brain-derived neurotrophic factor in Alzheimer disease model. Ann. Neurol. 2012, 72, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Cattaneo, A.; Cattane, N.; Begni, V.; Pariante, C.; Riva, M. The human BDNF gene: Peripheral gene expression and protein levels as biomarkers for psychiatric disorders. Transl. Psychiatry 2016, 6, e958. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.L.; Everitt, B.J.; Thomas, K.L. Independent cellular processes for hippocampal memory consolidation and reconsolidation. Science 2004, 304, 839–843. [Google Scholar] [CrossRef] [PubMed]
- Duan, W.; Lee, J.; Guo, Z.; Mattson, M.P. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J. Mol. Neurosci. 2001, 16, 1–12. [Google Scholar] [CrossRef]
- Marvanová, M.; Lakso, M.; Pirhonen, J.; Nawa, H.; Wong, G.; Castrén, E. The neuroprotective agent memantine induces brain-derived neurotrophic factor and trkB receptor expression in rat brain. Mol. Cell. Neurosci. 2001, 18, 247–258. [Google Scholar] [CrossRef]
- Meisner, F.; Scheller, C.; Kneitz, S.; Sopper, S.; Neuen-Jacob, E.; Riederer, P.; ter Meulen, V.; Koutsilieri, E. Memantine upregulates BDNF and prevents dopamine deficits in SIV-infected macaques: A novel pharmacological action of memantine. Neuropsychopharmacology 2008, 33, 2228–2236. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Long, J.M.; Ingram, D.K.; Mattson, M.P. Dietary restriction increases the number of newly generated neural cells, and induces BDNF expression, in the dentate gyrus of rats. J. Mol. Neurosci. 2000, 15, 99–108. [Google Scholar] [CrossRef]
- Pillai, A.; Bruno, D.; Sarreal, A.S.; Hernando, R.T.; Saint-Louis, L.A.; Nierenberg, J.; Ginsberg, S.D.; Pomara, N.; Mehta, P.D.; Zetterberg, H.; et al. Plasma BDNF levels vary in relation to body weight in females. PLoS ONE 2012, 7, e39358. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, M.R.; Kim, Y.; Karpova, S.A.; McCarley, R.W.; Strecker, R.E.; Gerashchenko, D. Chronic sleep restriction elevates brain interleukin-1 beta and tumor necrosis factor-alpha and attenuates brain-derived neurotrophic factor expression. Neurosci. Lett. 2014, 580, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Giese, M.; Unternaehrer, E.; Brand, S.; Calabrese, P.; Holsboer-Trachsler, E.; Eckert, A. The interplay of stress and sleep impacts BDNF level. PLoS ONE 2013, 8, e76050. [Google Scholar] [CrossRef] [PubMed]
- Ferris, L.T.; Williams, J.S.; Shen, C.-L. The effect of acute exercise on serum brain-derived neurotrophic factor levels and cognitive function. Med. Sci. Sports Exerc. 2007, 39, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.I.; Voss, M.W.; Prakash, R.S.; Basak, C.; Szabo, A.; Chaddock, L.; Kim, J.S.; Heo, S.; Alves, H.; White, S.M. Exercise training increases size of hippocampus and improves memory. Proc. Natl. Acad. Sci. USA 2011, 108, 3017–3022. [Google Scholar] [CrossRef] [PubMed]
- Laske, C.; Banschbach, S.; Stransky, E.; Bosch, S.; Straten, G.; Machann, J.; Fritsche, A.; Hipp, A.; Niess, A.; Eschweiler, G.W. Exercise-induced normalization of decreased BDNF serum concentration in elderly women with remitted major depression. Int. J. Neuropsychopharmacol. 2010, 13, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Coelho, F.; Pereira, D.; Lustosa, L.; Silva, J.; Dias, J.; Dias, R.; Queiroz, B.; Teixeira, A.; Teixeira, M.; Pereira, L. Physical therapy intervention (PTI) increases plasma brain-derived neurotrophic factor (BDNF) levels in non-frail and pre-frail elderly women. Arch. Gerontol. Geriatr. 2012, 54, 415–420. [Google Scholar] [CrossRef]
- Xiong, G.L.; Doraiswamy, P.M. Does meditation enhance cognition and brain plasticity? Ann. N. Y. Acad. Sci. 2009, 1172, 63–69. [Google Scholar] [CrossRef]
- Walton, K.G.; Fields, J.Z.; Levitsky, D.K.; Harris, D.A.; Pugh, N.D.; Schneider, R.H. Lowering cortisol and CVD risk in postmenopausal women: A pilot study using the Transcendental Meditation program. Ann. N. Y. Acad. Sci. 2004, 1032, 211–215. [Google Scholar] [CrossRef]
- Naveen, G.; Thirthalli, J.; Rao, M.; Varambally, S.; Christopher, R.; Gangadhar, B. Positive therapeutic and neurotropic effects of yoga in depression: A comparative study. Indian J. Psychiatry 2013, 55, S400–S404. [Google Scholar]
- Cullum, S.; Huppert, F.A.; McGee, M.; Dening, T.; Ahmed, A.; Paykel, E.S.; Brayne, C. Decline across different domains of cognitive function in normal ageing: Results of a longitudinal population-based study using CAMCOG. Int. J. Geriatr. Psychiatry 2000, 15, 853–862. [Google Scholar] [CrossRef]
- Wang, R.; Li, Y.-B.; Li, Y.-H.; Xu, Y.; Wu, H.-L.; Li, X.-J. Curcumin protects against glutamate excitotoxicity in rat cerebral cortical neurons by increasing brain-derived neurotrophic factor level and activating TrkB. Brain Res. 2008, 1210, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Cole, G.M.; Frautschy, S.A. DHA may prevent age-related dementia. J. Nutr. 2010, 140, 869–874. [Google Scholar] [CrossRef] [PubMed]
- Gundimeda, U.; McNeill, T.H.; Fan, T.K.; Deng, R.; Rayudu, D.; Chen, Z.; Cadenas, E.; Gopalakrishna, R. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: Role of 67-kDa laminin receptor and hydrogen peroxide. Biochem. Biophys. Res. Commun. 2014, 445, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Wu, A.; Ying, Z.; Gomez-Pinilla, F. Docosahexaenoic acid dietary supplementation enhances the effects of exercise on synaptic plasticity and cognition. Neuroscience 2008, 155, 751–759. [Google Scholar] [CrossRef] [PubMed]
| Study Authors and Years of Publications | Locations of the Studies | Patients with ad Sites of Recruitment | Case Definitions | AD Serum BDNF Mean (SD) | Numbers of Patients with AD; Ratio Male/Female | Average Age (SD) | Healthy Controls Sites of Recruitment | Methods of Cognitive Assessments | Control Serum BDNF Mean (SD) | Number of Healthy Controls; Ratio Male/Female | Average Age | Total Numbers of Subjects; Ratio Male/Female |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alvarez et al. (2014) [12] | A Coruña, Granada and Málaga, Spain | Three institutions specialized in cognitive disorders | DSM-IV and NINCDS-ADRDA criteria | 15.16 (9.48) | 252; M = 56, F = 196 | 74.99 (7.35) | Three institutions specialized in cognitive disorders | MMSE and ADAS-cog+ | 16.73 (11.83) | 38; M = 8, F = 30 | 72.74 (5.69) | 290; M = 64, F = 226 |
| Angelucci et al. (2010) [3] | Rome, Italy | NA | NINCDS-ADRDA criteria and MMSE | 6.07 (1.27) | 89 Mild AD: 54; M = 19, F = 35 Moderate-severe AD: 35; M = 8, F = 22 | Mild AD (n = 54) 74.31 (6.97) Moderate-severe AD (n = 35) 77.42 (8.27) | NA | MMSE score >24 and not satisfying the NINCDS-ADRDA criteria for the diagnosis of AD or the MCI Petersen criteria, confirmed by the memory tests of the MDB | 5.17 (1.39) | 27; M = 10, F = 17 | 68.48 (6.12) | 116; M = 64, F = 54 |
| Forlenza et al. (2015) [13] | São Paulo, Brazil | Community-dwelling elders recruited from an ongoing cohort study | NINCDS-ADRDA criteria | 0.66 (0.49) | 26; M = 8, F = 18 | 76.8 (6.6) | Community-dwelling elders recruited from an ongoing cohort study | Cambridge Cognitive Test, MMSE and neuropsychological tests | 0.84 (0.69) | 46; M = 9, F = 37 | 68.8 (6.8) | 72; M = 17, F = 55 |
| Laske et al. (2007) [21] | Tübingen, Germany | Outpatients from memory clinic | DSM-V, ICD-10, NINCDS-ADRDA criteria and MMSE | 18.60 (2.67) | 27; M = 10, F = 17 | 70.2 (8.6) | Healthy elderly volunteers | Without any organic brain disorders and had to reach MMSE score >27 | 21.63 (4.70) | 28; M = 9, F = 19 | 70.6 (7.1) | 55; M = 19, F = 36 |
| Laske et al. (2006A) [22] | Tübingen, Germany | Outpatients from memory clinic | DSM-V, ICD-10, NINCDS-ADRDA criteria and MMSE | 18.30 (2.00) | 28; M = 11, F = 17 | 70.4 (NA) | Healthy elderly volunteers | Normal clinical and cognitive status according to clinical examination and MMSE score | 21.60 (3.96) | 10; M = 6, F = 4 | 69.1 (NA) | 38 |
| Laske et al. (2006B) [14] | Tübingen, Germany | Outpatients from memory clinic | DSM-V, ICD-10, NINCDS-ADRDA criteria and MMSE | 21.20 (4.18) | 30; M = 9, F = 21 | 71.7 (7.0) | Patients who underwent lumbar puncture for orthopedic or neurologic diagnostic purposes | Have normal CSF cell counts and total protein levels, absence of signs of blood–brain barrier dysfunction or cerebral immunoglobulin G (IgG) synthesis, no cerebral disorders | 20.70 (5.20) | 10, M = 2, F = 8 | 70.3 (5.4) | 40; M = 11, F = 29 |
| Laske et al. (2006C) [15] | Tübingen, Germany | NA | MMSE <26 and ≥21 | 19.60 (4.50) | 30; NA | NA | NA | NA | 21.10 (4.70) | 20; NA | NA | 50; NA |
| Lee et al. (2009) [16] | Busan, South Korea | Patients of the Busan Paik Hospital | MMSE-KC, CERAD-K, CDRS, DSM-IV, NINCDS-ADRDA criteria | 22.90 (5.00) | 47; M = 14, F = 33 | 75.1 (6.4) | Patients of the Busan Paik Hospital | MMSE-KC scores >25 | 27.90 (6.90) | 39; M = 16, F = 23 | 72.8 (5.0) | 86; M = 30, F = 56 |
| Lee et al. (2015) [17] | Busan, South Korea | Elderly individuals over 60 years of age | MMSE-KC, CERAD-K, CDRS, DSM-IV | 29.10 (7.70) | 25; NA | 73.3 (6.8) | Elderly individuals over 60 years of age | Score >1.5 standard deviations (SD) above the mean of normalized MMSE-KC score | 26.80 (5.30) | 59; NA | 72.0 (5.8) | 84; NA |
| Leyhe et al. (2008) [23] | Tübingen, Germany | NA | DSM-IV, ICD-10, NINCDS-ADRDA criteria, MMSE EEG, CT, or MRI were also performed to validate the diagnosis of AD | 19.20 (3.70) | 19; M = 4, F = 15 | 70.9 (8.7) | NA | Without any organic brain disorders and MMSE score ≥27 | 23.20 (6.00) | 20; M = 13, F = 7 | 69.6 (11.6) | 39; M = 17, F = 22 |
| O’Bryant et al. (2009) [24] | Texas, USA | Participants from TARC | NINCDS-ADRDA criteria | 23.50 (7.40) | 99; M = 43, F = 56 | 77.8 (8.17) | Participants from TARC | Normal limits on psychometric assessment and were assigned a CDR global score of 0 | 23.80 (6.30) | 99; M = 39, F = 60 | 72.01 (8.56) | 198; M = 82, F = 116 |
| O’Bryant et al. (2010) [25] | Texas, USA | Participants from TARC | NINCDS-ADRDA criteria | 31.46 (9.10) | 198; M = 68, F = 130 | 76.63 (8.33) | Participants from TARC | Judged to be within normal limits on consensus review | 30.96 (8.82) | 201; M = 64, F = 137 | 70.4 (8.86) | 399; M = 132, F = 267 |
| Platenik et al. (2014) [26] | Prague, Czech Republic | Department of Psychiatry of the First Faculty of Medicine and General University Hospital | age >50 years; NINCDS-ADRDA Alzheimer’s criteria; brain imaging (magnetic resonance imaging) measuring cortico-subcortical atrophy (atrophy in the hippocampus and temporal corners of the side chambers); no any other organic brain lesions (vascular changes, tumors, intracranial hemorrhage, etc.); MMSE score <26; and no serious unstable somatic disease. | 1.72 (NA) | 85; M = 34, F = 51 | 75.6 (7.7) | Department of Psychiatry of the First Faculty of Medicine and General University Hospital | Underwent a psychiatric examination equivalent to that of AD patients, and it was confirmed that they were non-demented, nondepressed, and without any organic brain disorder. | 1.68 (NA) | 96; M = 30, F = 66 | 47.8 (16.1) | 181; M = 64, F = 117 |
| Ventriglia et al. (2013) [27] | Brescia, Italy | Alzheimer’s Unit of a private hospital | NINCDS-ADRDA & DSM-V MMSE for examination of severity | 33.16 (12.40) | 266; M = 88, F = 178 | 80.1 (7.1) | Enrolled at the Alzheimer Unit of a private hospital | MMSE scores ≥27/30 | 39.89 (9.48) | 169; M = 83, F = 86 | 48.0 (15.7) | 435; M = 171, F = 264 |
| Woolley et al. (2012) [11] | California, USA | Memory and Aging Center | NINCDS-ADRDA criteria | 23.00 (11.00) | 34; M = 18, F = 16 | 66.1 (11.3) | Recruited through advertisements in local newspapers & recruitment talks at local senior community centers. | A normal result from neurological examination, a CDR score of 0 & MMSE score ≥28/30. | 19.00 (11.00) | 38; M = 15, F = 23 | 69.2 (9.8) | 72; M = 33, F = 39 |
| Yasutake et al. (2006) [28] | Takatsuki, Japan | NA | NINCDS-ADRDA criteria and CT scan. AD severity was rated according to FAST | 14.73 (5.88) | 60; M = 20, F = 40 | 77.93 (7.04) | NA | NA | 19.72 (7.53) | 33; M = 8, F = 25 | 71.06 (5.77Z) | 93; M = 28, F = 65 |
| Study Authors and Years of Publications | Locations of the Studies | Patients with AD Sites of Recruitment | Case Definitions | AD Serum BDNF Mean (SD) | Numbers of Patients with AD; Ratio Male/Female | Average Age | Individuals with MCI Sites of Recruitment | Case Definitions | MCI Serum BDNF Mean (SD) | Numbers of Individuals with MCI; Ratio Male/Female | Average Age | Total Numbers of Subjects; Ratio Male/Female |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alvarez et al. (2014) [12] | A Coruña, Granada and Málaga, Spain | Three institutions specialized in cognitive disorders | DSM-IV and NINCDS-ADRDA criteria | 15.16 (9.48) | 252; M = 56, F = 196 | 74.99 (7.35) | Subjects evaluated at three institutions specialized in cognitive disorders | Petersen criteria revised | 14.33 (9.12) | 48; M = 13 F = 35 | 73.46 (7.57) | 300; M = 69, F = 231 |
| Angelucci et al. (2010) [3] | Rome, Italy | NA | NINCDS-ADRDA and MMSE | 6.07 (1.38) | 89 Mild AD: 54; M = 19, F = 35 Moderate-severe AD: 35; M = 8, F = 22 | Mild AD (n = 54) 74.31 (6.97) Moderate-severe AD (n = 35) 77.42 (8.27) | NA | Peterson’s guidelines and MMSE score ≥23 | 6.20 (1.50) | 54; M = 32, F = 27 | 69.61 (6.65) | 143; M = 59, F = 84 |
| Forlenza et al. (2015) [13] | São Paulo, Brazil | Community-dwelling elders recruited from an ongoing cohort study | NINCDS-ADRDA criteria | 0.66 (0.49) | 26; M = 8, F = 18 | 76.8 (6.6) | Community-dwelling elders recruited from an ongoing cohort study | Mayo Clinic criteria | 0.51 (0.27) | 62; M = 17, F = 45 | 72.2 (6.2) | 88; M = 25, F = 63 |
| Gezen-ak et al. (2013) [29] | Istanbul, Turkey | Behavioral Neurology and Movement Disorder Clinic | DSM-VI and MMSE | 0.93 (0.31) | 76; NA; EOAD = 22, LOAD = 54 | EOAD = 61.1 (4.8); LOAD = 74.22 (3.73) | Behavioral Neurology and Movement Disorder Clinic | NA | 1.15 (0.40) | 30; NA | 74.4 (2.9) | 101; NA |
| Laske et al. (2006C) [15] | Tübingen, Germany | NA | MMSE <26 + > or =21 | 19.60 (4.50) | 30; NA | NA | NA | MMSE ≥26 | 24.10 (5.40) | 12; NA | NA | 42; NA |
| Lee et al. (2009) [16] | Busan, South Korea | Patients of the Busan Paik Hospital | MMSE-KC, CERAD-K, CDRS, DSM-IV, NINCDS-ADRDA criteria | 22.90 (5.00) | 47; NA | NA | Patients of the Busan Paik Hospital | CDRS and Peterson’s Criteria | 22.80 (6.30) | 41; NA | NA | 88; NA |
| Lee et al. (2015) [17] | Busan, South Korea | Elderly individuals over 60 years of age | MMSE-KC, CERAD-K, CDRS, DSM-IV | 29.10 (7.70) | 25; NA | 73.3 (6.8) | Elderly individuals over 60 years of age | MMSE-KC, CERAD-K, CDRS, DSM-IV | 28.70 (7.00) | 55; NA | 71.5 (4.7) | 80; NA |
| Woolley et al. (2012) [11] | California, USA | Memory and Aging Center | NINCDS-ADRDA criteria | 23.00 (11.00) | 34; M = 18, F = 16 | 66.1 (11.3) | Memory and Aging Center | Peterson’s Criteria | 17.00 (12.00) | 30; M = 17, F = 13 | 71.3 (11.5) | 64; M = 35, F = 29 |
| Study Authors and Years of Publications | Locations of the Studies | Individuals with Mci Sites of Recruitment | Case Definitions | MCI Serum BDNF Mean (SD) | Numbers of Individuals with MCI; Ratio Male/Female | Average Age | Healthy Controls Sites of Recruitment | Methods of Cognitive Assessments | Control Serum BDNF Mean (SD) | Numbers of Healthy Controls; Ratio Male/Female | Average Age | Total Numbers of Subjects; Ratio Male/Female |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alvarez et al. (2014) [12] | A Coruña, Granada and Málaga, Spain | Three institutions specialized in cognitive disorders | Petersen’s criteria revised | 14.33 (9.12) | 48; M = 13, F = 35 | 73.46 (7.57) | Subjects evaluated at three institutions specialized in cognitive disorders | MMSE and ADAS-cog+ | 16.73 (11.83) | 38; M = 8, F = 30 | 72.74 (5.69) | 86; M = 21, F = 65 |
| Angelucci et al. (2010) [3] | Rome, Italy | NA | Peterson’s guidelines and MMSE score = or > 23 | 6.20 (1.50) | 54; M = 32, F = 22 | 69.61 (6.65) | NA | MMSE score >24 and not satisfying the NINCDS-ADRDA criteria nor the Petersen’s criteria, confirmed by the memory tests of the MBD | 5.17 (1.39) | 27; M = 10, F = 17 | 68.48 (6.12) | 81; M = 42, F = 39 |
| Forlenza et al. (2015) [13] | São Paulo, Brazil | Community-dwelling elders recruited from an ongoing cohort study | Mayo Clinic criteria | 0.51 (0.27) | 62; M = 17, F = 45 | 72.2 (6.2) | Community-dwelling elders recruited from an ongoing cohort study | Cambridge Cognitive Test, MMSE, and neuropsychological tests | 0.84 (0.69) | 46; M = 9, F = 37 | 68.8(6.8) | 108; M = 26, F = 82 |
| Gezen-ak et al. (2013) [29] | Istanbul, Turkey | Behavioral Neurology and Movement Disorder Clinic | DSM-VI and MMSE | 1.15 (0.40) | 29; NA | 74.4 (2.9) | Behavioral Neurology and Movement Disorder Clinic | NA | 1.25 (0.48) | 29; NA | 72.1 (3.4) | 58; NA |
| Laske et al. (2006C) [15] | Tübingen, Germany | NA | MMSE > or = 26 | 24.10 (5.40) | 12; NA | NA | NA | NA | 21.10 (4.70) | 20; NA | NA | 32; NA |
| Lee et al. (2009) [16] | Busan, South Korea | Patients of the Busan Paik Hospital | CDRS and Peterson’s Criteria | 22.80 (6.30) | 41; M = 17, F = 24 | 74.1 (5.7) | Patients of the Busan Paik Hospital | MMSE-KC scores >25 | 27.90 (6.90) | 39; M = 16, F = 23 | 72.8 (5.0) | 80; M = 33, F = 47 |
| Lee et al. (2015) [17] | Busan, South Korea | Elderly individuals over 60 years of age | MMSE-KC, CERAD-K, CDRS, DSM-IV | 28.70 (7.00) | 55; NA | 71.5 (4.7) | Elderly individuals over 60 years of age | Score >1.5 standard deviations (SD) above the mean MMSE-KC score | 26.80 (5.30) | 59; NA | 72.0 (5.8) | 114; NA |
| Shimada et al. (2014) [18] | Obu, Japan | Subjects enrolled in the OSHPE | ≤23 points on the MMSE and NCGG-FAT (for screening), Petersen’s Criteria for diagnosis | 20.90 (5.30) | 827; NA | NA | Enrolled in the OSHPE | NA | 21.20 (5.40) | 3636; NA | NA | 4463; NA |
| Woolley et al. (2012) [11] | California, USA | Memory and Aging Center | Peterson’s Criteria | 17.00 (12.00) | 30; M = 17, F = 13 | 71.3 (11.5) | Recruited through advertisements in local newspapers and recruitment talks at local senior community centers. | A normal result from neurological examination, a CDR score of 0, and MMSE score ≥28/30. | 19.00 (11.00) | 38; M = 15, F = 23 | 69.2 (9.8) | 68; M = 32, F = 36 |
| Study Authors and Years of Publications | Study Design | Power Calculation and Sample Size Calculation (YES/NO) | Bdnf Lab Technician Performing Elisa Procedure Masked (Yes/No) | Type of BDNF Elisa Assay Kit Used and Manufacturer | Intra-Assay and Inter-Assay Cv (%) | Disease Duration | Psychotropic and Other Medications Usage | Exclusion Criteria; Other Potential Moderators/Confounders Reported |
|---|---|---|---|---|---|---|---|---|
| Alvarez et al. (2014) [12] | Case-control study | - | - | ELISA kit specific for the quantitative determination of both natural and recombinant human BDNF in cell culture supernatant, serum and plasma (R&D Systems, Inc., Minneapolis, MN, USA) provided by Vitro SA (Spain) | Both <10% | - | SSRIs treatment (yes, no) | Subjects having any other significant neurological or psychiatric disease, active allergies, unstable medical conditions, or clinically significant laboratory abnormalities. Not taking systemic corticosteroids, antiparkinsonian agents, narcotics, or cholinesterase inhibitors for at least one month prior to blood sampling. No clinically significant depression in the medical evaluation and/or scores higher than 15 in the 17-item subscale of the Hamilton Depression Scale. Subjects were not on specific exercise programs. APOE4, apathy (present, absent), dysphoria (present, absent), disease severity (CIBIS+ score), dysphoria, total NPI score and CIBIC+ score |
| Angelucci et al. (2010) [3] | Case-control study | - | - | Sandwich ELISAs (R and D Systems, Minneapolis, MN, USA). This ELISA kit is set in order to measure human mature BDNF. | 8,14 | - | AChEI or antidepressant drugs; 15.7% of total AD patients were free of treatments at the time of blood collection. 76.5% of AD patients were treated with AChEI and 38% with antidepressants. 14.6% of AD patients, 12.9% of MCI, 11.1% of healthy subjects were prescribed statins at the time of the study. | Exclusion criteria: diabetes, obstructive pulmonary disease or asthma, hematological/oncological disorders, B12 or folate deficiency, pernicious anemia, active gastrointestinal, renal, hepatic, endocrine or cardiovascular system disease, newly treated hypothyroidism, liver function tests greater than three times the upper normal limit, creatinine concentrations greater than 150 mol/L; comorbidity of primary psychiatric (i.e., schizophrenia, major depression onset before the AD onset) or neurological disorders (i.e., stroke, Parkinson disease, seizure disorder, or head injury with loss of consciousness within the past year); known suspected history of alcoholism or drug abuse; computed tomography or magnetic resonance imaging evidence of focal parenchymal abnormalities; Structured Clinical Interview for the DSM-IV (SCID-P). All of the patients were accurately screened for the onset of depression after the onset of the cognitive symptoms of dementia. Subjects whose depression onset preceded the onset of dementia were excluded. |
| Forlenza et al. (2015) [13] | Case-control study from on-going cohort | - | - | ELISA (DuoSet, 136 R&D Systems, Minneapolis, MN, USA) | - | - | AD patients were under treatment with cholinesterase inhibitors for at least three months at the time of enrolment. | The subjects should not have any evidence of depressive disorder, based on the Hamilton Rating Scale for Depression-21; HDRS-21, Hamilton Rating Scale for Depression-21, APOE genotype. |
| Gezen-ak et al. (2013) [29] | Age-matched case-control study | - | - | ChemiKineTMSandwich ELISA Kit (CYT306, Millipore Corporation, Bil150 lerica, MA, USA) | -, <10% | EOAD: <65 (age at AD onset 50 to 63), LOAD: >65 (age at AD onset 65 to 80), MCI = age at MCI onset 60 to 78 | - | Patients with chronic heart disease, inflammatory diseases, autoimmune disease, infectious or psychiatric disease, non-Alzheimer’s dementia, patients taking antibiotics or non-steroidal anti-inflammatory drugs, had significant laboratory abnormalities, did not have erythrocyte sedimentation rates within reference values; - |
| Laske et al. (2007) [21] | Age-matched case-control study | - | - | ELISA kit (R&D Systems GmbH Wiesbaden-Nordenstadt, Germany) | Both <10% | - | - | Excluded those with depressive or psychotic episodes; - |
| Laske et al. (2006A) [22] | Case-control study | - | - | ELISA kit (R&D Systems GmbH Wiesbaden-Nordenstadt, Germany) | Both <10% | - | - | Excluded patients or control subjects with current or a history of depression or psychosis, with neurologic disorders, major physical illness, alcohol or substance abuse, or use of psychoactive medications; NA |
| Laske et al. (2006B) [14] | Age-matched case-control study | - | - | ELISA (R&D Systems GmbH Wiesbaden-Nordenstadt, Germany) | Both <10% | - | - | Excluded those with depressive or psychotic episodes; - |
| Laske et al. (2006C) [15] | Age-matched case-control study | - | - | - | - | - | - | - |
| Lee et al. (2009) [16] | Case-control study | - | - | ELISA kits (Promega, Madison, WI, USA) | - | - | - | With psychotic features or history of depressive episodes according to DSMIV criteria, had clinically significant physical abnormalities based on both physical and laboratory examination, had a history of organic brain abnormality or psychotropic drug misuse; Korean version of the Geriatric Depression Scale (GDS-K). Subjects with total GDS-K scores higher than 20 were considered to have depression. |
| Lee et al. (2015) [17] | Case-control study | - | - | ELISA kits (Promega, Maidison, WI, USA) | - | - | - | Subjects with a history of organic brain abnormalities (e.g., vascular dementia, Parkinson’s disease, etc.); - |
| Leyhe et al. (2008) [23] | Age-matched case-control study | - | - | ELISA kit (R&D Systems GmbH Wiesbaden-Norderstadt, Germany) | Both <10% | - | No known concomitant medication that could interfere with BDNF, specifically no patient received antidepressants, non-steroidal antiphlogistics, or statins. | Excluded those with depressive or psychotic episodes;- |
| Platenik et al. (2014) [26] | Age-matched case-control study | - | - | Human BDNF DuoSet ELISA development kit (cat. no. DY248), Human BDNF Quantikine Immunoassay (cat. no. DBD00) | - | - | AD patients were treated with reversible acetylcholinesterase inhibitors and/or NMDA receptor antagonists, as well as other drugs according to their somatic illnesses. Those with co-morbid depression were treated with antidepressants as well. | Other causes of dementia were excluded, including pseudodementia. Serious somatic disease or chronic somatic pharmacotherapy was not present, and patients were without organic brain disease, without cognitive impairment, and without abuse of psychoactive substances; BMI, platelet concentration, and clinical variables (GDS and MMSE for AD patients). |
| O’Bryant et al. (2009) [24] | Case-control study from a longitudinal research cohort | - | - | Multiplexed immunoassay via human MAP | NA, ≤7% | - | - | NA; NA |
| O’Bryant et al. (2010) [25] | Case-control study from a longitudinal research cohort | - | - | Multiplexed immunoassay via human MAP | NA, ≤7% | - | - | -; GDS and APOE4 status (present versus absent) |
| Shimada et al. (2014) [18] | Case-control study from an observational study | - | - | DuoSet ELISA Development Kit from R&D Systems (Minneapolis, MN, USA). Assays were performed using a specific human BDNF antibody (Minneapolis, MN, USA); no significant cross-reactivity or interference reported in this assay. | 3.8, 7.6 | - | - | Excluded participants who had missing BDNF data and characteristics, diagnosed neurological disorders, included stroke, Parkinson’s disease, AD, and depression, certified long-term care insurance, or functional decline of activities of daily living (ADL); Walking speed: start and end of 2.4 m walkway, histories of heart diseases and diabetes, smoking status, exercise, frequencies of going outdoors. |
| Ventriglia et al. (2013) [27] | Case-control study | - | - | Human BDNF Quantikine kit (R&D system, Minneapolis, MN, USA) | NA, approximately 8% | - | Most subjects took more than one medication concurrently. Psychotropic medications (neuroleptics, benzodiazepines, antidepressants, mood stabilizers/antiepileptics, L-DOPA, and cholinesterase inhibitors) were recorded and taken into account in the analyses. | - |
| Woolley et al. (2012) [11] | Case-control study; inclusion of multiple neurodegenerative diseases in a single study. | YES; >0.8 power to detect differences in BDNF concentrations in each neurodegenerative disease group when comparing against healthy subjects, assuming the predetermined effect sizes and SDs inthe AD literature. | - | BDNF ELISA kit (R&D Systems, Minneapolis, MN, USA) | <10; 8–14 | - | Use of AChEIs or selective serotonin reuptake inhibitors (SSRIs) and/or serotonin and norepinephrine reuptake inhibitors (SNRIs) was analyzed in relation to BDNF concentrations. | - |
| Yasutake et al. (2006) [28] | Age and gender-matched case-control study | - | - | ELISA kit (Quantikine R&D System, Minneapolis, MN, USA) | 5, 11.3 | - | - | Subjected to a structural interview and physical examination, and those with malignant diseases or severe infections were excluded from all of the study groups. |
| Moderators | Number of Studies | β | z | p |
|---|---|---|---|---|
| Age | 15 | 0.0548 | 3.32 | <0.001 |
| MMSE Scores | 15 | 0.0518 | 3.52 | <0.001 |
| Sex (% of female) | 13 | 0.0104 | 1.35 | 0.1764 |
| Years of Education | 7 | 0.0181 | 1.06 | 0.2909 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ng, T.K.S.; Ho, C.S.H.; Tam, W.W.S.; Kua, E.H.; Ho, R.C.-M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2019, 20, 257. https://doi.org/10.3390/ijms20020257
Ng TKS, Ho CSH, Tam WWS, Kua EH, Ho RC-M. Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences. 2019; 20(2):257. https://doi.org/10.3390/ijms20020257
Chicago/Turabian StyleNg, Ted Kheng Siang, Cyrus Su Hui Ho, Wilson Wai San Tam, Ee Heok Kua, and Roger Chun-Man Ho. 2019. "Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis" International Journal of Molecular Sciences 20, no. 2: 257. https://doi.org/10.3390/ijms20020257
APA StyleNg, T. K. S., Ho, C. S. H., Tam, W. W. S., Kua, E. H., & Ho, R. C.-M. (2019). Decreased Serum Brain-Derived Neurotrophic Factor (BDNF) Levels in Patients with Alzheimer’s Disease (AD): A Systematic Review and Meta-Analysis. International Journal of Molecular Sciences, 20(2), 257. https://doi.org/10.3390/ijms20020257






