Improved In Vitro Test Procedure for Full Assessment of the Cytocompatibility of Degradable Magnesium Based on ISO 10993-5/-12
Abstract
1. Introduction
2. Results
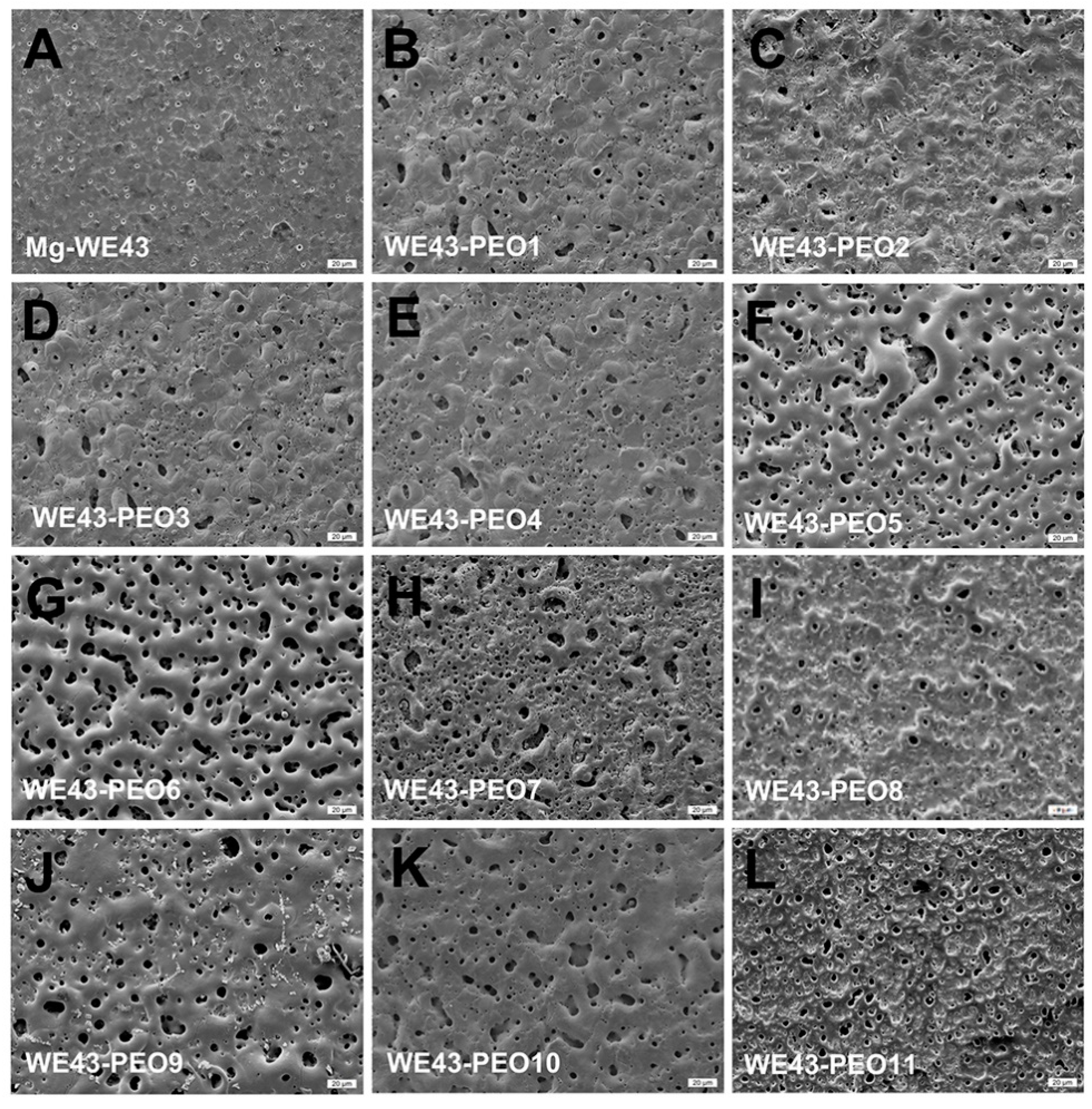
2.1. Physical Features of WE43-PEO Variants
2.2. First Material Analyses
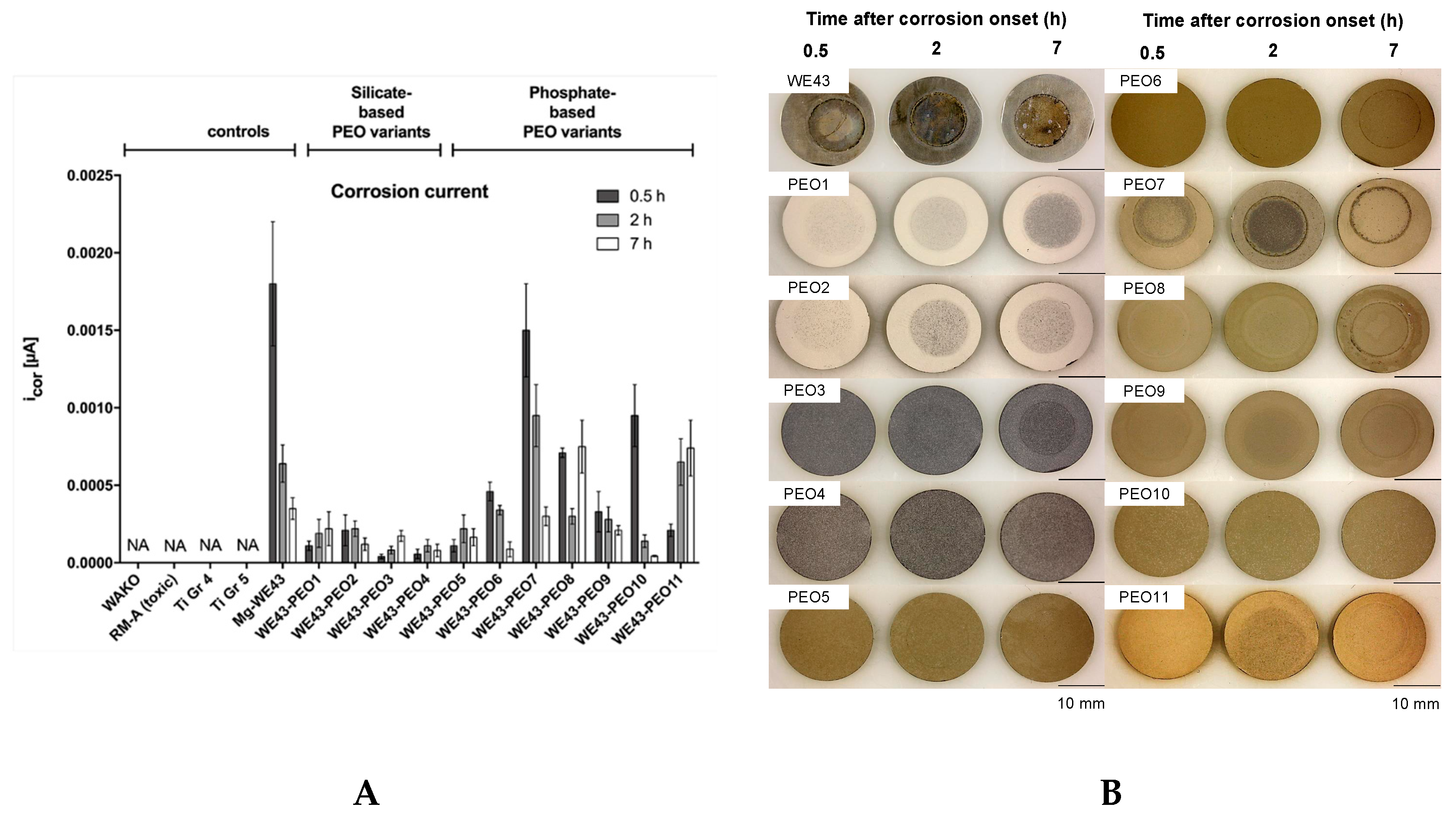
2.2.1. Corrosion Measurements
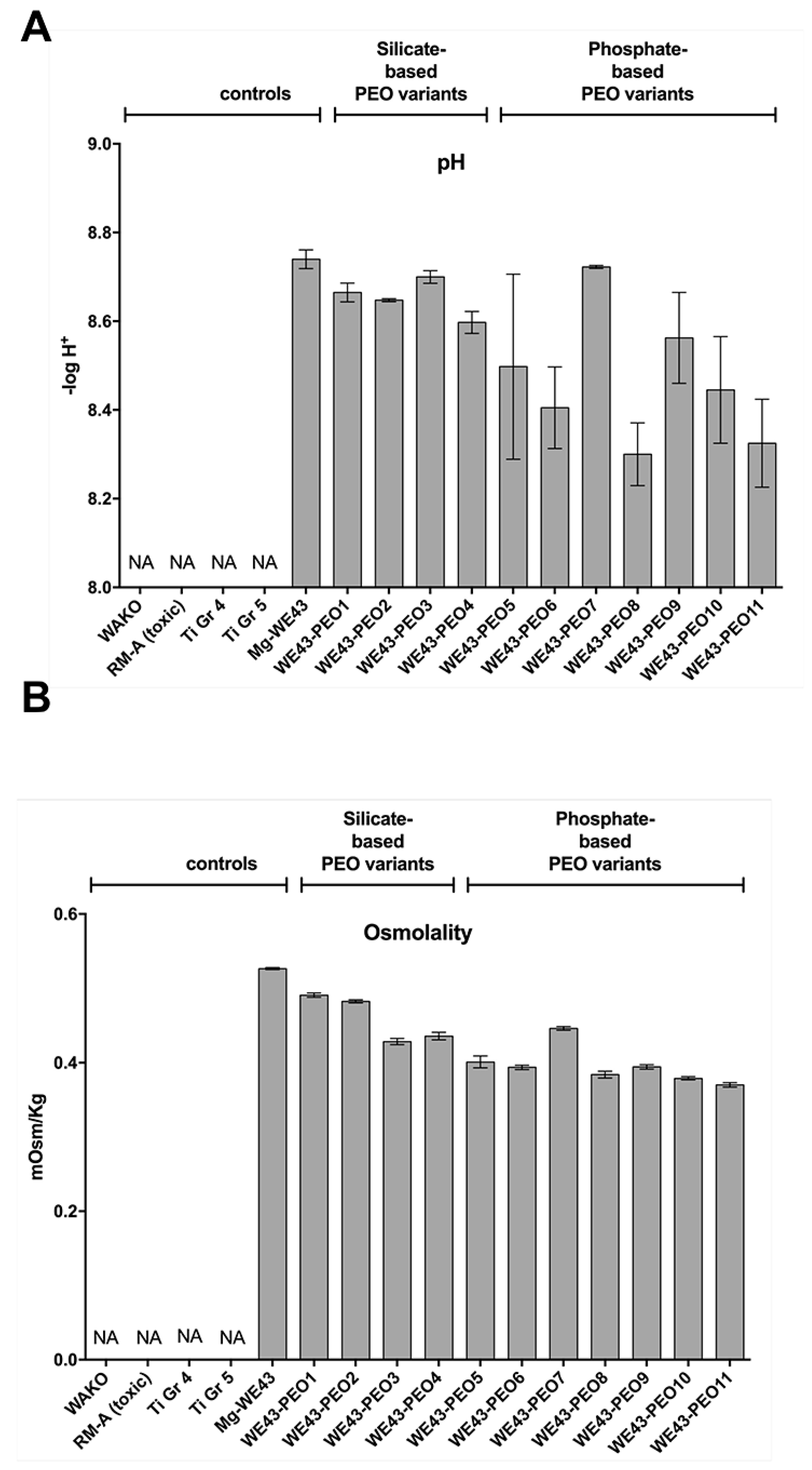
2.2.2. pH Measurements
2.2.3. Osmolality Measurements
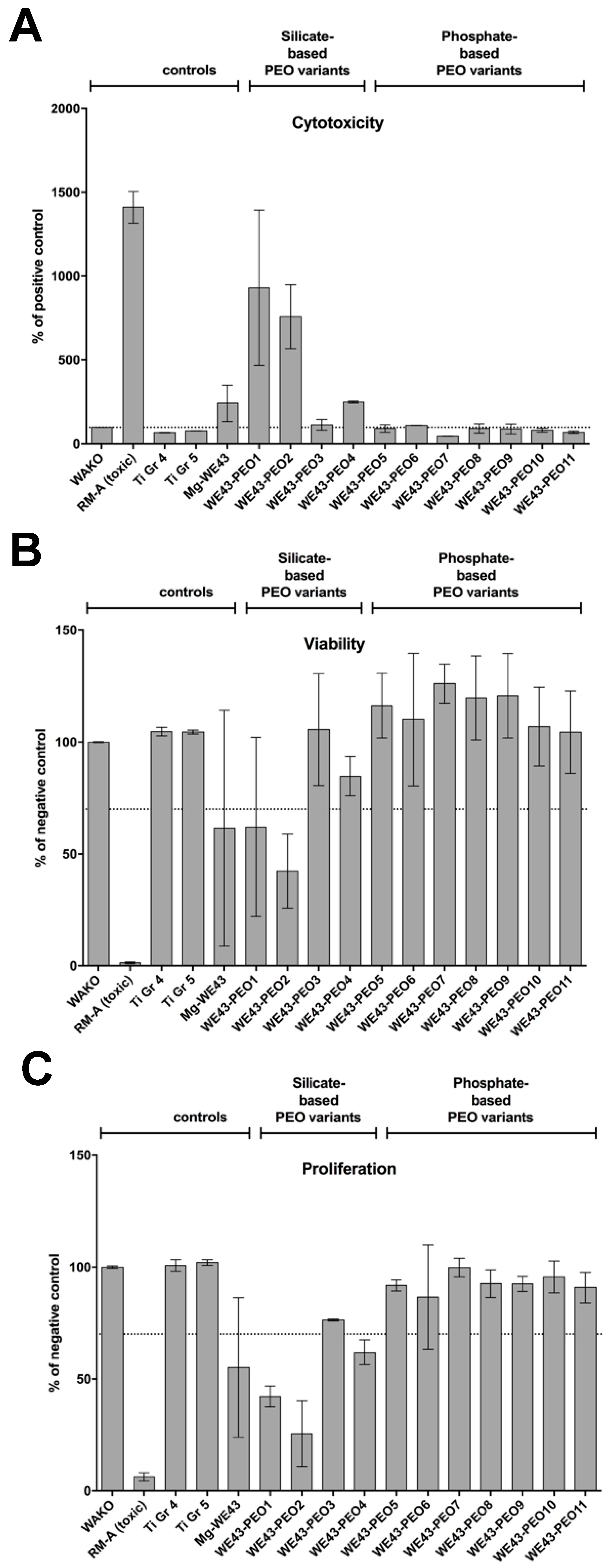
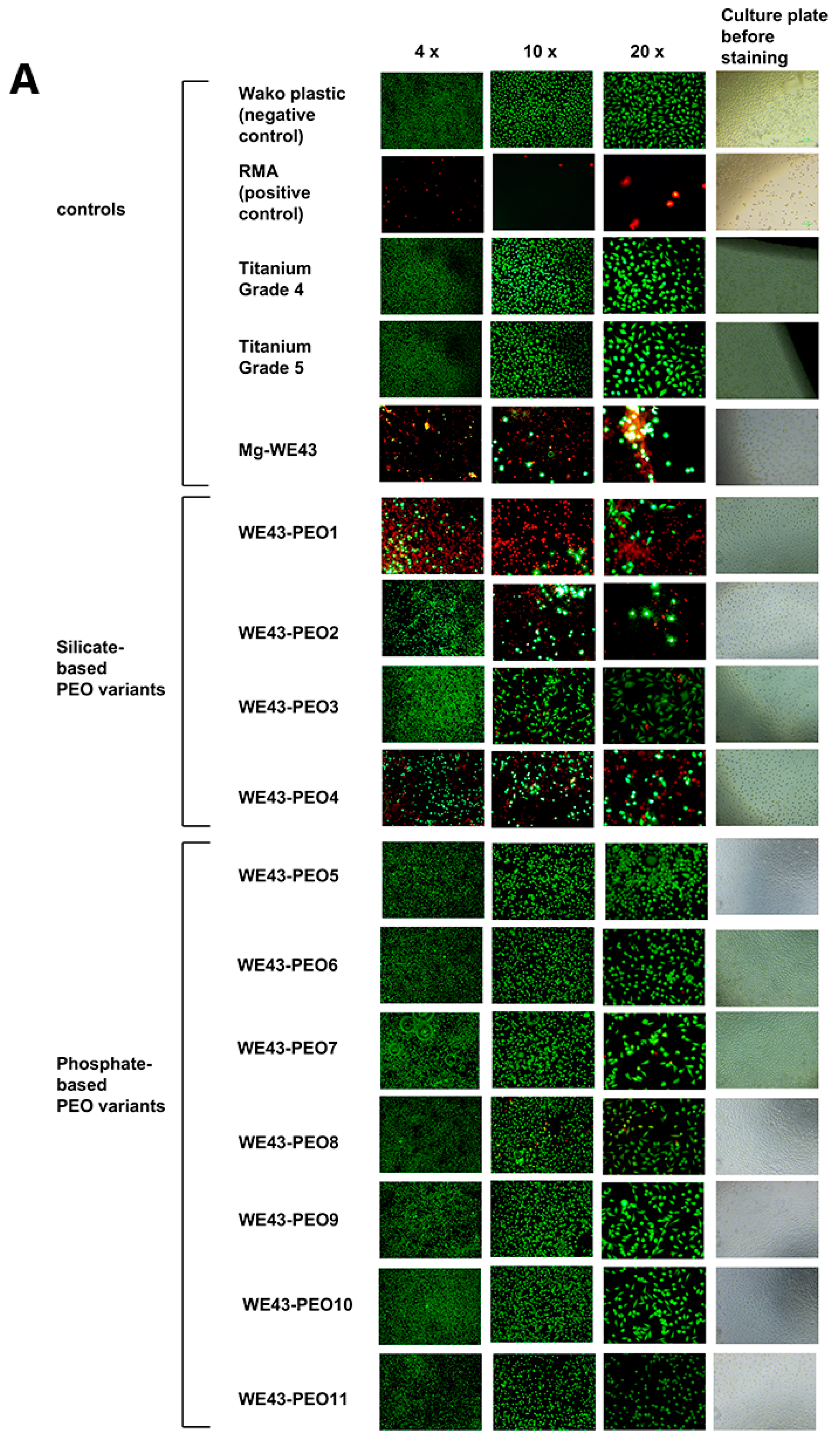
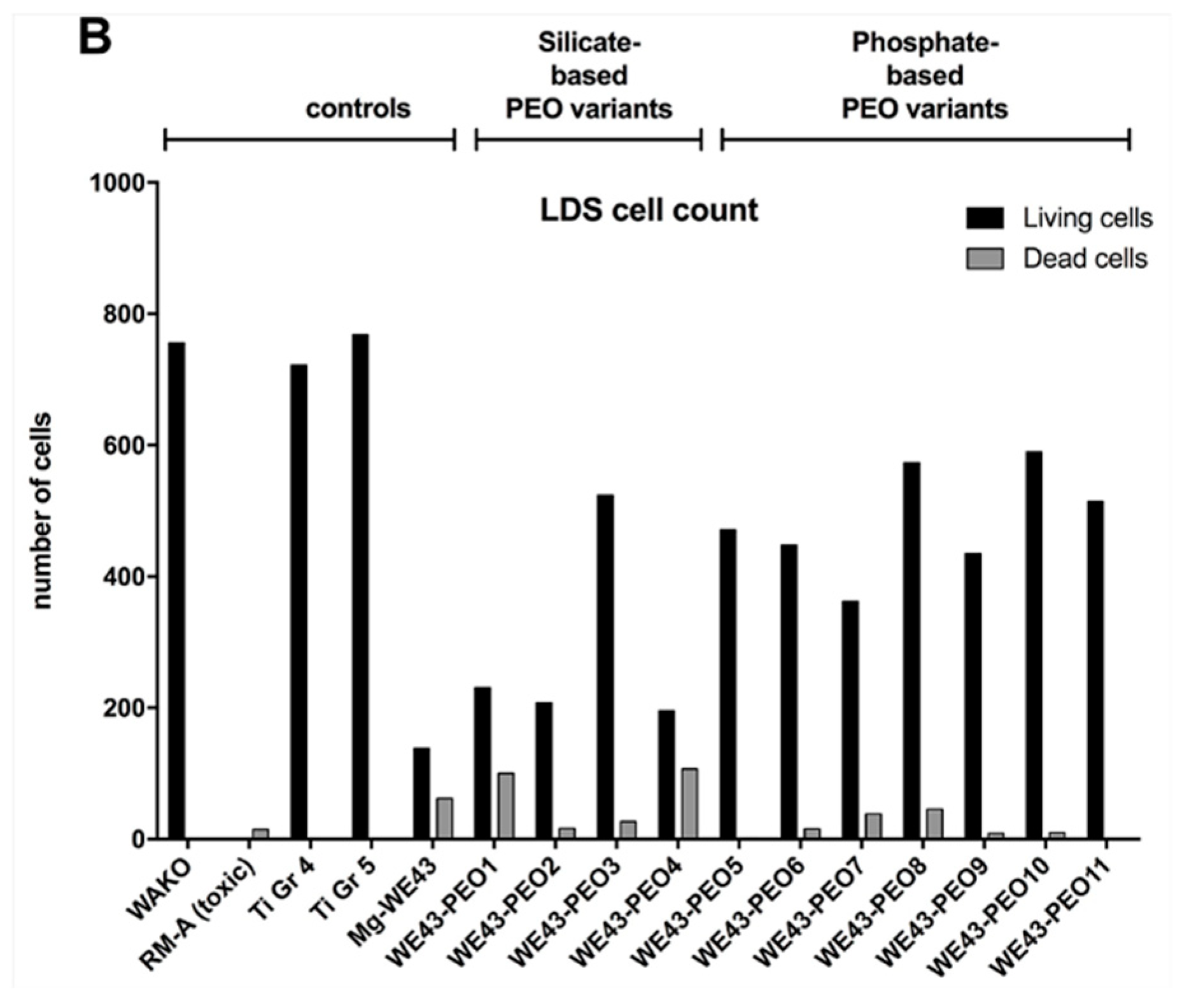
2.2.4. Cytocompatibility Analyses
2.3. Second Material Analyses
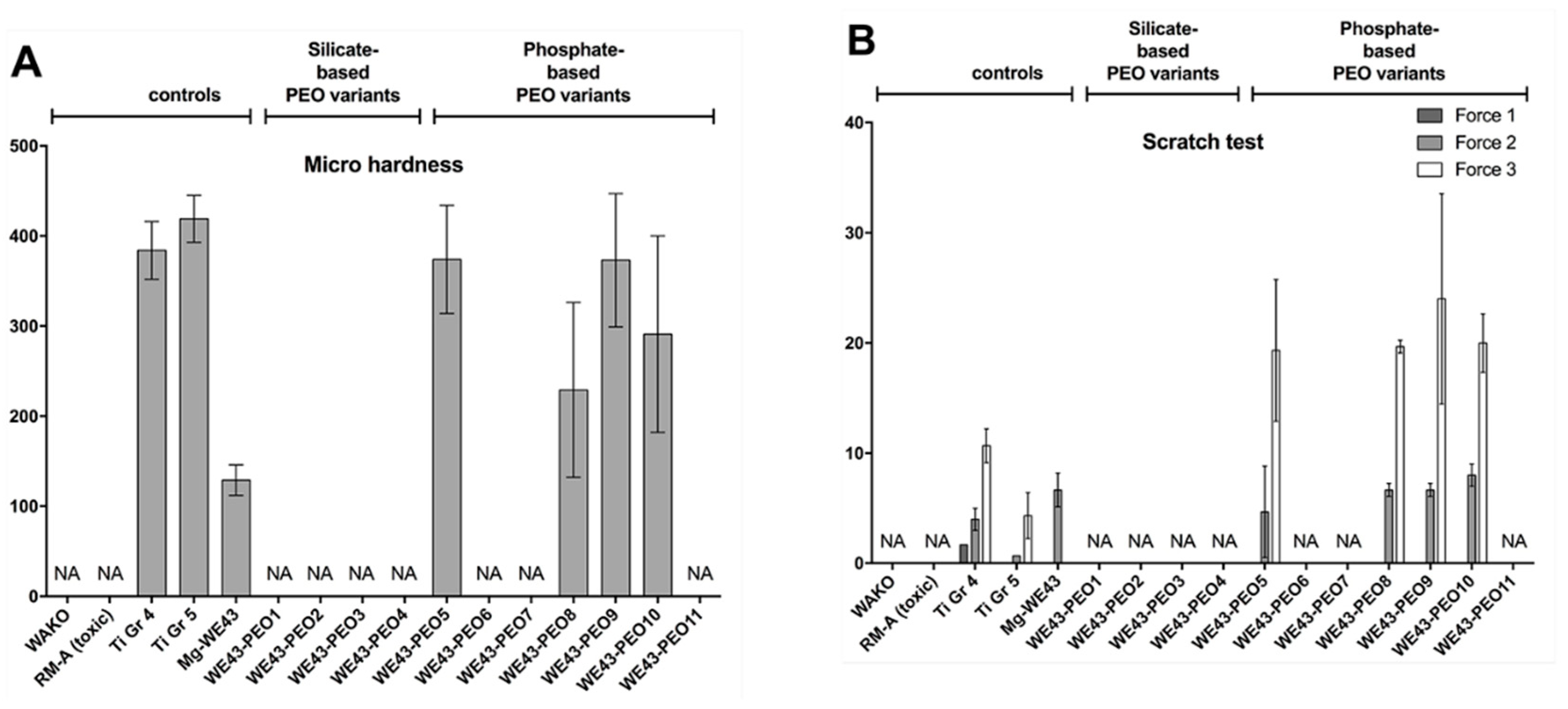
2.3.1. Mechanical Analyses
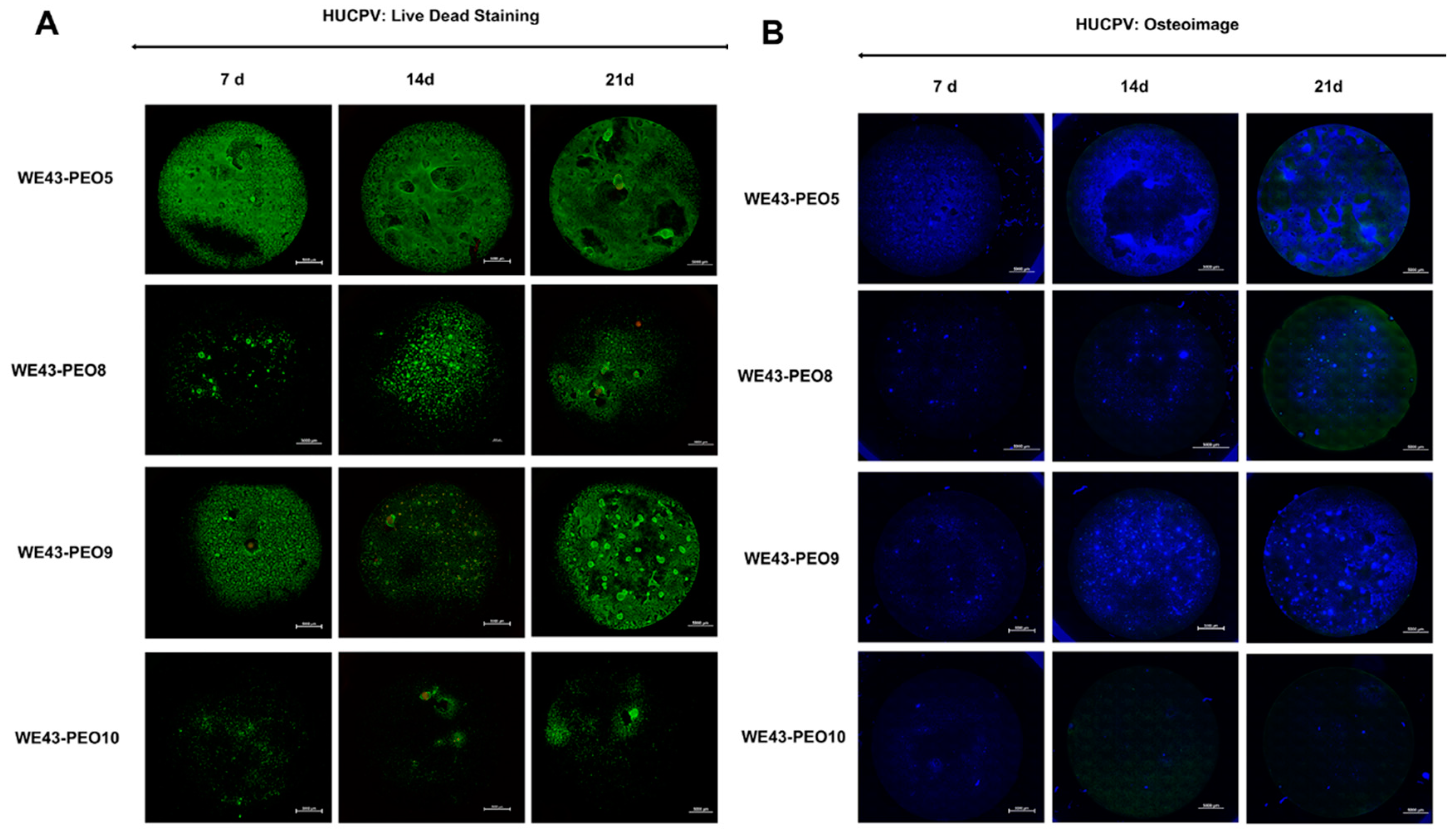
2.3.2. Analysis of the Osteogenic Differentiation of HUCPV Cells
3. Discussion
4. Materials and Methods
4.1. Biomaterial Preparation and Characterization
4.1.1. Plasma Electrolytic Oxidation (PEO) Treatment of Magnesium Specimens
4.1.2. Physicochemical Material Characterization
4.1.3. Assessment of Corrosion by Potentiodynamic Polarization Measurements (PDP)
4.1.4. Determination of pH and Osmolality of Immersion Medium
4.2. Cytocompatibility Analyses
4.2.1. Reference Materials (Positive and Negative Controls)
4.2.2. Cell Culture
4.2.3. Extract Analysis
Extraction
Assay Procedure
Bromodeoxyuridine/5-Bromo-2′-Deoxyuridine (BrdU)-Assay
Sodium 3,3′-[1(Phenylamino)Carbonyl]-3,4-Tetrazolium]-3is(4-Methoxy-6-Nitro) Benzene Sulfonic acid Hydrate (XTT)-Assay
Lactate Dehydrogenase (LDH)-Assay
4.2.4. Live-Dead Staining
4.3. Analyses of the Bone Regeneration Characteristics
4.3.1. Mechanical Characterization
4.3.2. Osteogenic Differentiation Assay
4.4. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wolf, F.I.; Cittadini, A. Chemistry and biochemistry of magnesium. Mol. Aspects Med. 2003, 24, 3–9. [Google Scholar] [CrossRef]
- Jahnen-Dechent, W.; Ketteler, M. Magnesium basics. Clin. Kidney J. 2012, 5, i3–i14. [Google Scholar] [CrossRef] [PubMed]
- Saris, N.E.; Mervaala, E.; Karppanen, H.; Khawaja, J.A.; Lewenstam, A. Magnesium: An update on physiological, clinical and analytical aspects. Clin. Chim. Acta 2000, 294, 1–26. [Google Scholar] [CrossRef]
- Farraro, K.F.; Kim, K.E.; Woo, S.L.; Flowers, J.R.; McCullough, M.B. Revolutionizing orthopaedic biomaterials: The potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering. J. Biomech. 2014, 47, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.M.; Touny, A.H.; Al-Omair, M.A. Biodegradable/biocompatible coated metal implants for orthopedic applications. Biomed. Mater. Eng. 2016, 27, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Witte, F.; Lu, F.; Wang, J.; Li, J.; Qin, L. Current status on clinical applications of magnesium-based orthopaedic implants: A review from clinical translational perspective. Biomaterials 2017, 112, 287–302. [Google Scholar] [CrossRef]
- Staiger, M.P.; Pietak, A.M.; Huadmai, J.; Dias, G. Magnesium and its alloys as orthopedic biomaterials: A review. Biomaterials 2006, 27, 1728–1734. [Google Scholar] [CrossRef]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The effect of hydrogen gas evolution of magnesium implant on the postimplantation mortality of rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef]
- Schneider, L.A.; Korber, A.; Grabbe, S.; Dissemond, J. Influence of pH on wound-healing: A new perspective for wound-therapy? Arch. Dermatol. Res. 2007, 298, 413–420. [Google Scholar] [CrossRef]
- Gonzalez, J.; Hou, R.Q.; Nidadavolu, E.P.S.; Willumeit-Romer, R.; Feyerabend, F. Magnesium degradation under physiological conditions—Best practice. Bioact. Mater. 2018, 3, 174–185. [Google Scholar] [CrossRef] [PubMed]
- Jung, O.; Smeets, R.; Porchetta, D.; Kopp, A.; Ptock, C.; Muller, U.; Heiland, M.; Schwade, M.; Behr, B.; Kroger, N.; et al. Optimized in vitro procedure for assessing the cytocompatibility of magnesium-based biomaterials. Acta Biomater. 2015, 23, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Martinez Sanchez, A.H.; Luthringer, B.J.; Feyerabend, F.; Willumeit, R. Mg and Mg alloys: How comparable are in vitro and in vivo corrosion rates? A review. Acta Biomater. 2015, 13, 16–31. [Google Scholar] [CrossRef] [PubMed]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Stormer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.E.; Luan, B. Protective coatings on magnesium and its alloys—A critical review. J. Alloy. Compd. 2002, 336, 88–113. [Google Scholar] [CrossRef]
- Thomann, M.; Krause, C.; Angrisani, N.; Bormann, D.; Hassel, T.; Windhagen, H.; Meyer-Lindenberg, A. Influence of a magnesium-fluoride coating of magnesium-based implants (MgCa0.8) on degradation in a rabbit model. J. Biomed. Mater. Res. A 2010, 93, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Cui, W.; Beniash, E.; Gawalt, E.; Xu, Z.; Sfeir, C. Biomimetic coating of magnesium alloy for enhanced corrosion resistance and calcium phosphate deposition. Acta Biomater. 2013, 9, 8650–8659. [Google Scholar] [CrossRef] [PubMed]
- Imwinkelried, T.; Beck, S.; Iizuka, T.; Schaller, B. Effect of a plasmaelectrolytic coating on the strength retention of in vivo and in vitro degraded magnesium implants. Acta Biomat. 2013, 9, 8643–8649. [Google Scholar] [CrossRef]
- Ma, W.H.; Liu, Y.J.; Wang, W.; Zhang, Y.Z. Improved biological performance of magnesium by micro-arc oxidation. Braz. J. Med. Biol. Res. 2015, 48, 214–225. [Google Scholar] [CrossRef]
- Jung, O.; Smeets, R.; Kopp, A.; Porchetta, D.; Hiester, P.; Heiland, M.; Friedrich, R.E.; Precht, C.; Hanken, H.; Grobe, A.; et al. PEO-generated Surfaces Support Attachment and Growth of Cells In Vitro with No Additional Benefit for Micro-roughness in Sa (0.2–4 mum). In Vivo 2016, 30, 27–33. [Google Scholar]
- Barati Darband, G.; Aliofkharaei, M.; Hamghalam, P.; Valizade, N. Plasma electrolytic oxidation of magnesium and its alloys: Mechanism, properties and applications. J. Magnes. Alloy. 2017, 5, 74–132. [Google Scholar] [CrossRef]
- Hartjen, P.; Hoffmann, A.; Henningsen, A.; Barbeck, M.; Kopp, A.; Kluwe, L.; Precht, C.; Quatela, O.; Gaudin, R.; Heiland, M.; et al. Plasma Electrolytic Oxidation of Titanium Implant Surfaces: Microgroove-Structures Improve Cellular Adhesion and Viability. In Vivo 2018, 32, 241–247. [Google Scholar] [PubMed]
- DIN EN ISO 10993-5: Biologische Beurteilung von Medizinprodukten—Teil 5: Prüfungen auf In-vitro-Zytotoxizität (ISO 10993-5:2009); German Version of EN ISO 10993-5:2009; International Organisation for Standardization: Geneva, Switzerland, 2009.
- Walker, J.; Shadanbaz, S.; Kirkland, N.T.; Stace, E.; Woodfield, T.; Staiger, M.P.; Dias, G.J. Magnesium alloys: Predicting in vivo corrosion with in vitro immersion testing. J. Biomed. Mater. Res. B Appl. Biomater. 2012, 100, 1134–1141. [Google Scholar] [CrossRef] [PubMed]
- Mueller, W.D.; de Mele, M.F.; Nascimento, M.L.; Zeddies, M. Degradation of magnesium and its alloys: Dependence on the composition of the synthetic biological media. J. Biomed. Mater. Res. A 2009, 90, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Hu, T.; Chu, P.K. In vitro studies of biomedical magnesium alloys in a simulated physiological environment: A review. Acta Biomater. 2011, 7, 1452–1459. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.F.; Chiu, K.Y.; Cheng, F.T. Effect of pH on the in vitro corrosion rate of magnesium degradable implant material. Mater. Sci. Eng. C 2010, 30, 898–903. [Google Scholar] [CrossRef]
- Marco, I.; Feyerabend, F.; Willumeit-Romer, R.; Van der Biest, O. Degradation testing of Mg alloys in Dulbecco’s modified eagle medium: Influence of medium sterilization. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 62, 68–78. [Google Scholar] [CrossRef]
- Nidadavolu, E.P.S.; Feyerabend, F.; Ebel, T.; Willumeit-Romer, R.; Dahms, M. On the Determination of Magnesium Degradation Rates under Physiological Conditions. Materials 2016, 9, 627. [Google Scholar] [CrossRef]
- Cecchinato, F.; Agha, N.A.; Martinez-Sanchez, A.H.; Luthringer, B.J.; Feyerabend, F.; Jimbo, R.; Willumeit-Romer, R.; Wennerberg, A. Influence of Magnesium Alloy Degradation on Undifferentiated Human Cells. PLoS ONE 2015, 10, e0142117. [Google Scholar] [CrossRef]
- Fischer, J.; Prosenc, M.H.; Wolff, M.; Hort, N.; Willumeit, R.; Feyerabend, F. Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays. Acta Biomater. 2010, 6, 1813–1823. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, J.; Zhao, C.; Zhang, S.; Yu, S.; Wang, Z.; Wang, X.; Zhang, X.; Zheng, Q. In vitro and in vivo assessment of the biocompatibility of an Mg-6Zn alloy in the bile. J. Mater. Sci. Mater. Med. 2014, 25, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Cipriano, A.F.; Sallee, A.; Guan, R.G.; Zhao, Z.Y.; Tayoba, M.; Sanchez, J.; Liu, H. Investigation of magnesium-zinc-calcium alloys and bone marrow derived mesenchymal stem cell response in direct culture. Acta Biomater. 2015, 12, 298–321. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.; Saha, P.; Chou, D.T.; Lee, B.; Collins, B.E.; Tan, Z.; Dong, Z.; Kumta, P.N. In vitro degradation and cytotoxicity response of Mg-4% Zn-0.5% Zr (ZK40) alloy as a potential biodegradable material. Acta Biomater. 2013, 9, 8534–8547. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.B.; Xie, X.H.; Li, H.F.; Wang, X.L.; Zhao, M.Z.; Zhang, E.W.; Bai, Y.J.; Zheng, Y.F.; Qin, L. Biodegradable CaMgZn bulk metallic glass for potential skeletal application. Acta Biomater. 2011, 7, 3196–3208. [Google Scholar] [CrossRef] [PubMed]
- Diez, M.; Kang, M.H.; Kim, S.M.; Kim, H.E.; Song, J. Hydroxyapatite (HA)/poly-L-lactic acid (PLLA) dual coating on magnesium alloy under deformation for biomedical applications. J. Mater. Sci. Mater. Med. 2016, 27, 34. [Google Scholar] [CrossRef]
- Tan, L.L.; Yu, X.M.; Wan, P.; Yang, K. Biodegradable Materials for Bone Repairs: A Review. J. Mater. Sci. Technol. 2013, 29, 503–513. [Google Scholar] [CrossRef]
- Song, G.L.; Atrens, A. Understanding magnesium corrosion—A framework for improved alloy performance. Adv. Eng. Mater. 2003, 5, 837–858. [Google Scholar] [CrossRef]
- Xin, Y.C.; Hu, T.; Chu, P.K. Degradation behaviour of pure magnesium in simulated body fluids with different concentrations of HCO3−. Corros. Sci. 2011, 53, 1522–1528. [Google Scholar] [CrossRef]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. Correlation between electrochemical impedance measurements and corrosion rate of magnesium investigated by real-time hydrogen measurement and optical imaging. Electrochim. Acta 2015, 166, 372–384. [Google Scholar]
- Ascencio, M.; Pekguleryuz, M.; Omanovic, S. An investigation of the corrosion mechanisms of WE43 Mg alloy in a modified simulated body fluid solution: The influence of immersion time. Corros. Sci. 2014, 87, 489–503. [Google Scholar] [CrossRef]
| Cor | pH | Osm | Cytotox | Viability | Prolifer | LDS Ql. | LDS Qn. | 1st Step | Micro | Scratch | Osteo | 2nd Step | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg-WE43 | 0 | 0 | |||||||||||
| WE43-PEO1 | X | 1 | |||||||||||
| WE43-PEO2 | X | 1 | |||||||||||
| WE43-PEO3 | X | X | X | X | 3 | ||||||||
| WE43-PEO4 | X | X | 1 | ||||||||||
| WE43-PEO5 | X | X | X | X | X | X | X | X | 8 | X | X | 2 | |
| WE43-PEO6 | X | X | X | X | X | X | X | 7 | |||||
| WE43-PEO7 | X | X | X | X | X | 5 | |||||||
| WE43-PEO8 | X | X | X | X | X | X | X | 7 | X | 1 | |||
| WE43-PEO9 | X | X | X | X | X | X | X | X | 8 | X | X | 2 | |
| WE43-PEO10 | X | X | X | X | X | X | X | X | 8 | X | 1 | ||
| WE43-PEO11 | X | X | X | X | X | X | 6 |
| Variant | Electrolytes |
|---|---|
| Untreated | Magnesium WE43 |
| Silicate-based | |
| PEO1 | Silicate + Potassium Hydroxide |
| PEO2 | Silicate + Potassium Hydroxide + Borate |
| PEO3 | Silicate + Potassium Hydroxide + Titanate |
| PEO4 | Silicate + Potassium Hydroxide + Borate + Titanate |
| Phosphate-based | |
| PEO5 | Phosphate + Potassium Hydroxide |
| PEO6 | Phosphate + Ammonium Hydroxide |
| PEO7 | Phosphate + Potassium Hydroxide + Aluminate |
| PEO8 | Phosphate + Ammonium Hydroxide + Urea |
| PEO9 | Phosphate + Ammonium Hydroxide + EDTA |
| PEO10 | Phosphate + Ammonium Hydroxide + Flouride + Urotropin |
| PEO11 | Phosphate + Ammonium Hydroxide + Fluoride + Borate + Urotropin |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jung, O.; Smeets, R.; Hartjen, P.; Schnettler, R.; Feyerabend, F.; Klein, M.; Wegner, N.; Walther, F.; Stangier, D.; Henningsen, A.; et al. Improved In Vitro Test Procedure for Full Assessment of the Cytocompatibility of Degradable Magnesium Based on ISO 10993-5/-12. Int. J. Mol. Sci. 2019, 20, 255. https://doi.org/10.3390/ijms20020255
Jung O, Smeets R, Hartjen P, Schnettler R, Feyerabend F, Klein M, Wegner N, Walther F, Stangier D, Henningsen A, et al. Improved In Vitro Test Procedure for Full Assessment of the Cytocompatibility of Degradable Magnesium Based on ISO 10993-5/-12. International Journal of Molecular Sciences. 2019; 20(2):255. https://doi.org/10.3390/ijms20020255
Chicago/Turabian StyleJung, Ole, Ralf Smeets, Philip Hartjen, Reinhard Schnettler, Frank Feyerabend, Martin Klein, Nils Wegner, Frank Walther, Dominic Stangier, Anders Henningsen, and et al. 2019. "Improved In Vitro Test Procedure for Full Assessment of the Cytocompatibility of Degradable Magnesium Based on ISO 10993-5/-12" International Journal of Molecular Sciences 20, no. 2: 255. https://doi.org/10.3390/ijms20020255
APA StyleJung, O., Smeets, R., Hartjen, P., Schnettler, R., Feyerabend, F., Klein, M., Wegner, N., Walther, F., Stangier, D., Henningsen, A., Rendenbach, C., Heiland, M., Barbeck, M., & Kopp, A. (2019). Improved In Vitro Test Procedure for Full Assessment of the Cytocompatibility of Degradable Magnesium Based on ISO 10993-5/-12. International Journal of Molecular Sciences, 20(2), 255. https://doi.org/10.3390/ijms20020255










