Transcriptome Analysis Reveals the Mechanism of Fluoride Treatment Affecting Biochemical Components in Camellia sinensis
Abstract
1. Introduction
2. Results
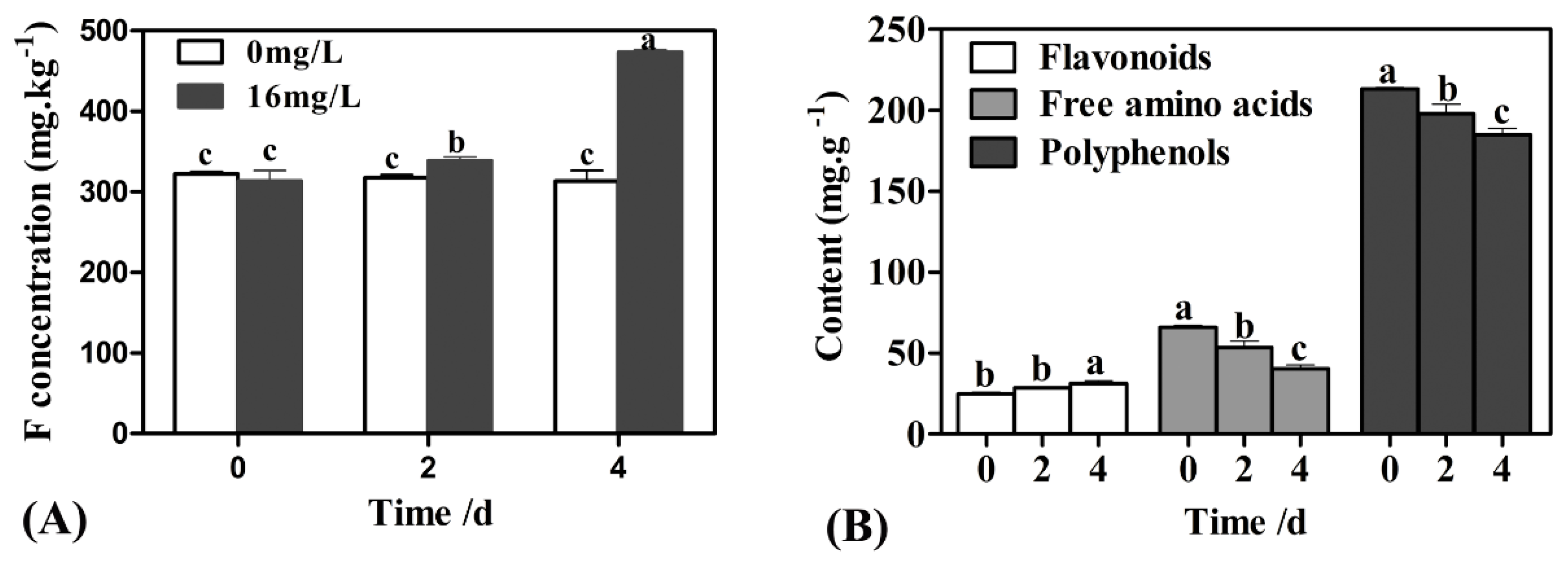
2.1. The Changes in F, Polyphenol, Flavonoid, and Free Amino Acid Contents under F Treatment
2.2. The Effect of F Treatment on Catechins and Caffeine Content
2.3. The Effect of the F Treatment on the Content of Theanine and Hydrolyzed Amino Acids
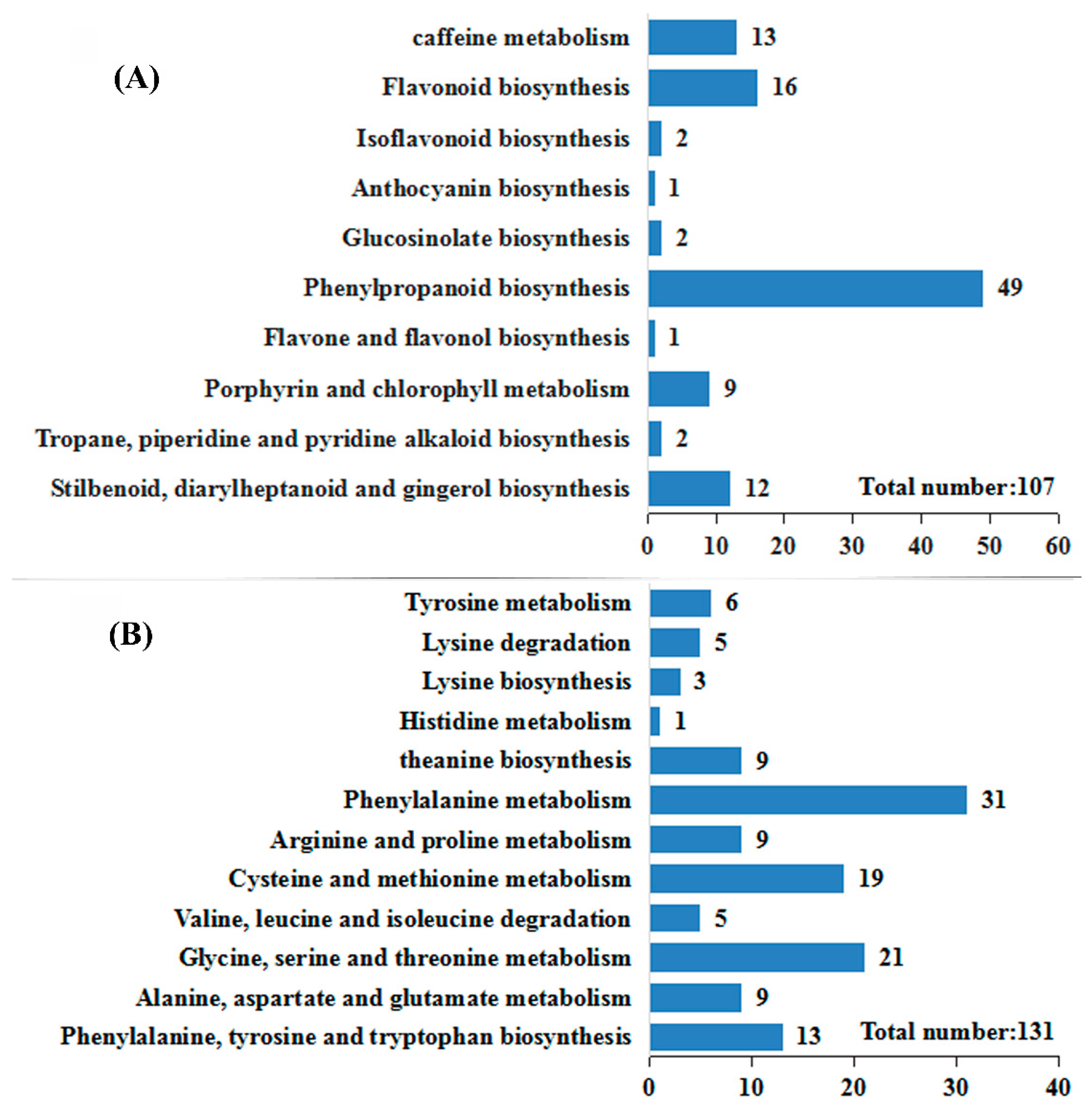
2.4. Identification and Analysis of DEGs Associated with Amino Acid Metabolism and Secondary Metabolism
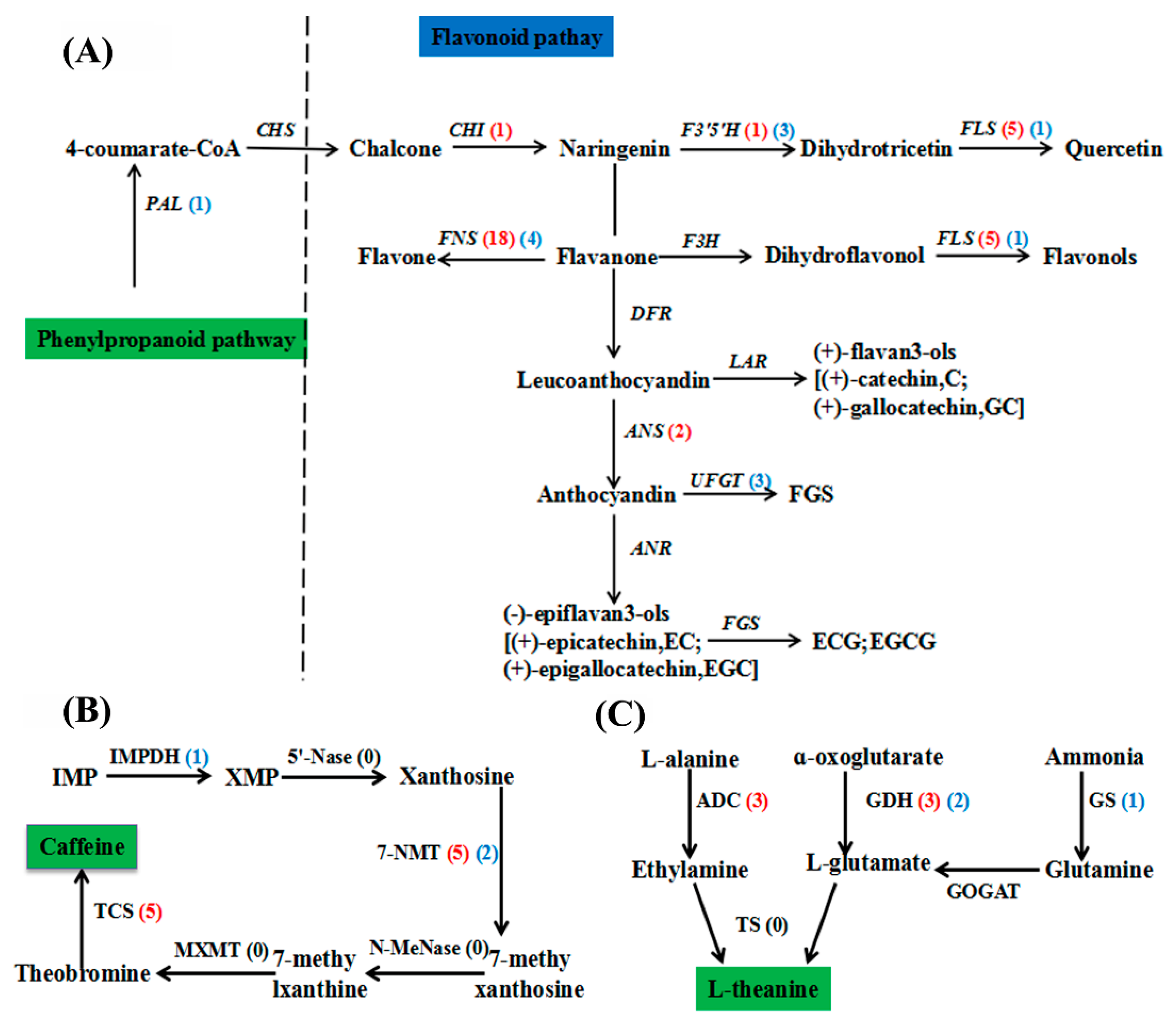
2.5. Changes in the Flavonoid, Caffeine, and Theanine Biosynthesis Pathways in C. sinensis in Response to F Treatment
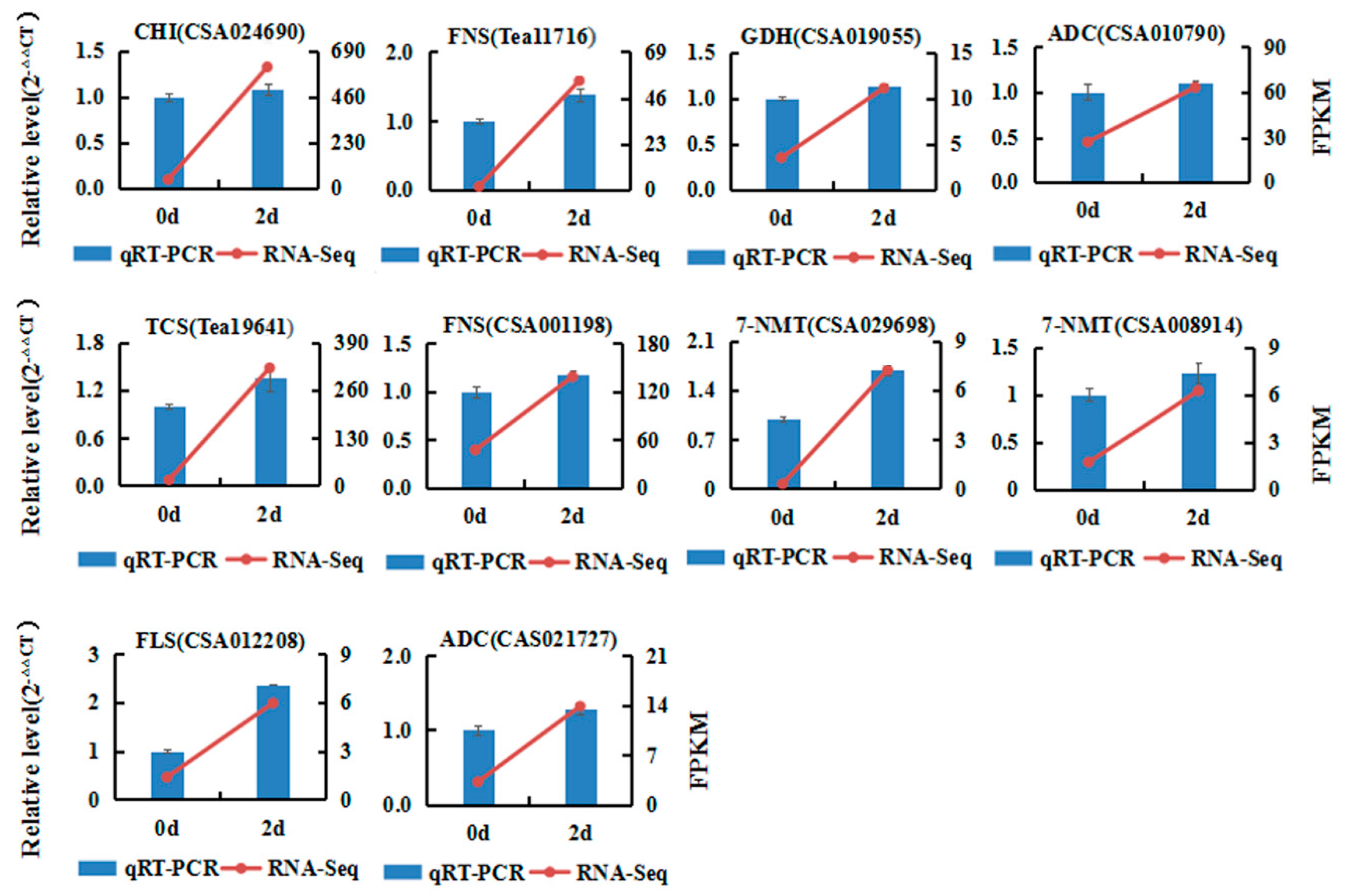
2.6. qRT-PCR Validation of DEGs from RNA-seq
3. Discussion
4. Materials and Methods
4.1. F treatment and Leaf Sampling
4.2. Extraction and Determination of F in Tea Samples
4.3. Extraction and Determination of Polyphenol, Flavonoid, and Amino Acid
4.4. Extraction and Determination of Theanine, Catechins, and Caffeine
4.5. Construction of RNA Library and RNA Sequencing
4.6. Identification and Analysis of DEGs Related to Amino Acid Metabolism and Secondary Metabolism
4.7. Quantitative Real-Time PCR (qRT-PCR) Analysis of the Selected DEGs
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| DEG | differentially expressed gene |
| HPLC | high performance liquid chromatography |
| qRT-PCR | quantitative real time PCR |
| RNA-Seq | RNA Sequencing |
| PAL | phenylalanine ammonia lyase |
| CHI | chalcone isomerase |
| F3′5′H | flavonoid 3′,5′-hydroxylase |
| FLS | flavonol synthase |
| FNS | flavone synthase |
| ANS | anthocyanidin synthase |
| UFGT | flavonoid 3-O-glucosyl transferase |
| 7-NMT | 7-methylxanthosine synthase |
| IMPDH | IMP dehydrogenase |
| TCS | tea caffeine synthase |
| GDH | glutamate dehydrogenase |
| ADC | arginine decarboxylase |
| GS | glutamine synthetase |
| NR | non-redundant |
| Swiss-Prot | swiss-prot database |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| COG | Cluster of Orthologous Groups of proteins |
| F | fluoride |
| ACAs | Ca2+ ATPase |
| GC | gallocatechin |
| EGC | epigallocatechin |
| EC | epicatechin |
| EGCG | epigallocatechin gallate |
| C | catechin |
| ECG | epicatechin gallate |
References
- Smith, G.E. Fluoride, the Environment, and Human Health. Perspect. Biol. Med. 1986, 29, 560–572. [Google Scholar] [CrossRef] [PubMed]
- Jin, C.; Yan, Z.; Yi, L.; Hui, J.D.; Juan, Y.; Ran, W.L. Fluoride levels in various black tea commodities: Measurement and safety evaluation. Food Chem. Toxicol. 2006, 44, 1131–1137. [Google Scholar]
- Sofuoglu, S.C.; Kavcar, P. An exposure and risk assessment for fluoride and trace metals in black tea. J. Hazard. Mater. 2008, 158, 392–400. [Google Scholar] [CrossRef]
- Fernandes, M.D.; Yanai, M.M.; Martins, G.M.; Iano, F.G.; Leite, A.L.; Cestari, T.M.; Taga, R.; Buzalaf, M.A.R.; de Oliveira, R.C. Effects of fluoride in bone repair: An evaluation of RANKL, OPG and TRAP expression. Odontology 2014, 102, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Heikens, A.; Sumarti, S.; van Bergen, M.; Widianarko, B.; Fokkert, L.; van Leeuwen, K.; Seinen, W. The impact of the hyperacid Ijen Crater Lake: Risks of excess fluoride to human health. Sci. Total Environ. 2005, 346, 56–69. [Google Scholar] [CrossRef]
- Yi, J.; Cao, J. Tea and fluorosis. J. Fluor. Chem. 2008, 129, 76–81. [Google Scholar] [CrossRef]
- Ruan, J.Y.; Wong, M.H. Accumulation of fluoride and aluminium related to different varieties of tea plant. Environ. Geochem. Health 2001, 23, 53–63. [Google Scholar] [CrossRef]
- Shu, W.S.; Zhang, Z.Q.; Lan, C.Y.; Wong, M.H. Fluoride and aluminium concentrations of tea plants and tea products from Sichuan Province, PR China. Chemosphere 2003, 52, 1475–1482. [Google Scholar] [CrossRef]
- Shyu, T.H.; Chen, J.H. Fluoride levels in different types and forms of tea drinks consumed in Taiwan and daily human exposure from tea drinks. J. Food Agric. Environ. 2013, 11, 178–183. [Google Scholar]
- Chan, L.; Mehra, A.; Saikat, S.; Lynch, P. Human exposure assessment of fluoride from tea (Camellia sinensis L.): A UK based issue? Food Res. Int. 2013, 51, 564–570. [Google Scholar] [CrossRef]
- Li, C.F.; Zhu, Y.; Yu, Y.; Zhao, Q.Y.; Wang, S.J.; Wang, X.C.; Yao, M.Z.; Luo, D.; Li, X.; Chen, L.; et al. Global transcriptome and gene regulation network for secondary metabolite biosynthesis of tea plant (Camellia sinensis). Bmc Genom. 2015, 16, 560. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.D.; Xin, H.H.; Wang, M.L.; Ma, Q.P.; Wang, L.; Kaleri, N.A.; Wang, Y.H.; Li, X.H. Transcriptomic Analysis Reveals the Molecular Mechanisms of Drought-Stress-Induced Decreases in Camellia sinensis Leaf Quality. Front. Plant Sci. 2016, 7, 385. [Google Scholar] [CrossRef]
- Li, X.; Aharnmed, G.J.; Li, Z.X.; Zhang, L.; Wei, J.P.; Yan, P.; Zhang, L.P.; Han, W.Y. Freezing stress deteriorates tea quality of new flush by inducing photosynthetic inhibition and oxidative stress in mature leaves. Sci. Hortic.-Amsterdam 2018, 230, 155–160. [Google Scholar] [CrossRef]
- Li, X.; Wei, J.P.; Ahammed, G.J.; Zhang, L.; Li, Y.; Yan, P.; Zhang, L.P.; Han, W.Y. Brassinosteroids Attenuate Moderate High Temperature-Caused Decline in Tea Quality by Enhancing Theanine Biosynthesis in Camellia sinensis L. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.H.; Hu, X.-F.; Chen, F.S.; Deng, Z.Y. Effects of Simulated Acid Rain and Aluminum Addition on Growth and Physiological and Biochemical Characteristics of Tea Plants. Acta Agriculture Universitatis Jiangxiensis 2012, 34, 304–310. [Google Scholar]
- Yang, X.; Yu, Z.; Zhang, B.B.; Huang, J.; Zhang, Y.H.; Fang, F.X.; Li, C.L.; Zhu, H.K.; Chen, Y.Q. Effect of fluoride on the biosynthesis of catechins in tea [Camellia sinensis (L.) O. Kuntze] leaves. Sci. Hortic.-Amsterdam 2015, 184, 78–84. [Google Scholar] [CrossRef]
- Li, C.L.; Ni, D.J. Effect of Fluoride on the Amino Acid Composition of Tea Leaves. Fluoride 2016, 49, 266–270. [Google Scholar]
- Li, C.L.; Ni, D.J. Effect of Fluoride on Chemical Constituents of Tea Leaves. Fluoride 2009, 42, 237–243. [Google Scholar]
- Lu, Y.; Guo, W.F.; Yang, X.Q. Fluoride content in tea and its relationship with tea quality. J. Agric. Food. Chem. 2004, 52, 4472–4476. [Google Scholar] [CrossRef]
- Li, Q.S.; Lin, X.M.; Qiao, R.Y.; Zheng, X.Q.; Lu, J.L.; Ye, J.H.; Liang, Y.R. Effect of fluoride treatment on gene expression in tea plant (Camellia sinensis). Sci. Rep. 2017, 7, 9847. [Google Scholar] [CrossRef]
- Li, C.; Zheng, Y.; Zhou, J.; Xu, J.; Ni, D. Changes of leaf antioxidant system, photosynthesis and ultrastructure in tea plant under the stress of fluorine. Biol. Plant. 2011, 55, 563–566. [Google Scholar] [CrossRef]
- Koblar, A.; Tavcar, G.; Ponikvar-Svet, M. Effects of airborne fluoride on soil and vegetation. J. Fluor. Chem. 2011, 132, 755–759. [Google Scholar] [CrossRef]
- Cai, H.M.; Dong, Y.Y.; Li, Y.Y.; Li, D.X.; Peng, C.Y.; Zhang, Z.Z.; Wan, X.C. Physiological and cellular responses to fluoride stress in tea (Camellia sinensis) leaves. Acta Physiol. Plant. 2016, 38, 144. [Google Scholar] [CrossRef]
- Li, C.L.; Yang, X.; Hu, J.H.; Ni, D.J. Effect of Fluoride on Aroma of Tea Leaves. Fluoride 2013, 46, 25–28. [Google Scholar]
- Wang, Y.X.; Li, Q.; Wang, Q.; Li, Y.J.; Ling, J.H.; Liu, L.L.; Chen, X.H.; Bi, K.S. Simultaneous Determination of Seven Bioactive Components in Oolong Tea Camellia sinensis: Quality Control by Chemical Composition and HPLC Fingerprints. J. Agric. Food. Chem. 2012, 60, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ahammed, G.J.; Li, Z.X.; Zhang, L.; Wei, J.P.; Shen, C.; Yan, P.; Zhang, L.P.; Han, W.Y. Brassinosteroids Improve Quality of Summer Tea (Camellia sinensis L.) by Balancing Biosynthesis of Polyphenols and Amino Acids. Front. Plant Sci. 2016, 7, 1304. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Grain, D.; Gourrierec, J.L.; Harscoët, E.; Berger, A.; Jauvion, V.; Scagnelli, A.; Berger, N.; Bidzinski, P.; Kelemen, Z.; et al. Regulation of flavonoid biosynthesis involves an unexpected complex transcriptional regulation of TT8 expression, in Arabidopsis. New Phytol. 2013, 198, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Ye, H.; Li, L.; Yin, Y. Recent advances in the regulation of brassinosteroid signaling and biosynthesis pathways. J. Integr. Plant Biol. 2011, 53, 455–468. [Google Scholar] [CrossRef]
- Khan, N.; Mukhtar, H.J.L.S. Tea polyphenols for health promotion. Life Sci. 2007, 81, 519–533. [Google Scholar] [CrossRef] [PubMed]
- Ruan, J.; Ma, L.; Yang, Y. Magnesium nutrition on accumulation and transport of amino acids in tea plants. J. Sci. Food. Agr. 2012, 92, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Winkel-Shirley, B. Biosynthesis of flavonoids and effects of stress. Curr. Opin. Plant Biol. 2002, 5, 218–223. [Google Scholar] [CrossRef]
- Olsen, K.M.; Lea, U.S.; Slimestad, R.; Verheul, M.; Lillo, C. Differential expression of four Arabidopsis PAL genes; PAL1 and PAL2 have functional specialization in abiotic environmental-triggered flavonoid synthesis. J. Plant Physiol. 2008, 165, 1491–1499. [Google Scholar] [CrossRef] [PubMed]
- Nangle, E.J.; Gardner, D.S.; Metzger, J.D.; Rodriguez-Saona, L.; Guisti, M.M.; Danneberger, T.K.; Petrella, D.P. Pigment Changes in Cool-Season Turfgrasses in Response to Ultraviolet-B Light Irradiance. Agron. J. 2015, 107, 41–50. [Google Scholar] [CrossRef]
- Camargo-Ramã-Rez, R.; Val-Torregrosa, B.; San, S.B.J.P.; Physiology, C. MiR858-Mediated Regulation of Flavonoid-Specific MYB Transcription Factor Genes Controls Resistance to Pathogen Infection in Arabidopsis. Plant Cell Physiol. 2018, 59, 190–204. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tian, H.L.; Wu, J.H.; Cao, R.R.; Wang, R.X.; Qi, X.H.; Xu, Q.; Chen, X.H. Erratum: Relationship between gene expression and the accumulation of catechin during spring and autumn in tea plants (Camellia sinensis L.). Hortic. Res. 2015, 2, 15023. [Google Scholar] [CrossRef] [PubMed]
- Graham, H.N. Green Tea Composition, Consumption, and Polyphenol Chemistry. Prev. Med. 1992, 21, 334–350. [Google Scholar] [CrossRef]
- Jeyaramraja, P.; Pius, P.; Kumar, R.R.; Jayakumar, D. Soil moisture stress-induced alterations in bioconstituents determining tea quality. J. Sci. Food Agric. 2003, 83, 1187–1191. [Google Scholar] [CrossRef]
- Wang, Y.S.; Gao, L.P.; Wang, Z.R.; Liu, Y.J.; Sun, M.L.; Yang, D.Q.; Wei, C.L.; Shan, Y.; Xia, T. Light-induced expression of genes involved in phenylpropanoid biosynthetic pathways in callus of tea (Camellia sinensis (L.) O. Kuntze). Sci. Hortic.-Amsterdam 2012, 133, 72–83. [Google Scholar] [CrossRef]
- Lillo, C.; Lea, U.S.; Ruoff, P. Nutrient depletion as a key factor for manipulating gene expression and product formation in different branches of the flavonoid pathway. Plant Cell Environ. 2008, 31, 587–601. [Google Scholar] [CrossRef]
- Ashihara, H.; Kubota, H.J.P.P. Patterns of adenine metabolism and caffeine biosynthesis in different parts of tea seedlings. Physiol. Plant. 2010, 68, 275–281. [Google Scholar] [CrossRef]
- Deng, W.W.; Wang, S.; Chen, Q.; Zhang, Z.Z.; Hu, X.Y. Effect of salt treatment on theanine biosynthesis in Camellia sinensis seedlings. Plant Physiol. Biochem. 2012, 56, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Ghanati, F.; Morita, A.; Yokota, H. Effects of aluminum on the growth of tea plant and activation of antioxidant system. Plant Soil 2005, 276, 133–141. [Google Scholar] [CrossRef]
- Gao, H.J.; Zhang, Z.Z.; Wan, X.C. Influences of charcoal and bamboo charcoal amendment on soil-fluoride fractions and bioaccumulation of fluoride in tea plants. Environ. Geochem. Health 2012, 34, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wasila, H.M.; Liu, L.W.; Yuan, T.; Gao, Z.M.; Zhao, B.T.; Ahmad, I.R. Physicochemical characteristics, polyphenol compositions and antioxidant potential of pomegranate juices from 10 Chinese cultivars and the environmental factors analysis. Food Chem. 2015, 175, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Zheng, M.Y.; Xia, Q.L.; Lu, S.M. Study on drying methods and their influences on effective components of loquat flower tea. Lwt-Food Sci. Technol. 2015, 63, 14–20. [Google Scholar] [CrossRef]
- Lin, J.Y.; Tang, C.Y. Determination of total phenolic and flavonoid contents in selected fruits and vegetables, as well as their stimulatory effects on mouse splenocyte proliferation. Food Chem. 2007, 101, 140–147. [Google Scholar] [CrossRef]
- Wan, J.Y.; Fan, Y.; Yu, Q.T.; Ge, Y.Z.; Yan, C.P.; Alolga, R.N.; Li, P.; Ma, Z.H.; Qi, L.W. Integrated evaluation of malonyl ginsenosides, amino acids and polysaccharides in fresh and processed ginseng. J. Pharmaceut. Biomed. 2015, 107, 89–97. [Google Scholar] [CrossRef]
- Tai, Y.L.; Wei, C.L.; Yang, H.; Zhang, L.; Chen, Q.; Deng, W.W.; Wei, S.; Zhang, J.; Fang, C.B.; Ho, C.T.; et al. Transcriptomic and phytochemical analysis of the biosynthesis of characteristic constituents in tea (Camellia sinensis) compared with oil tea (Camellia oleifera). Bmc Plant Biol. 2015, 15, 190. [Google Scholar] [CrossRef]
- Chen, P.A.; Lin, S.Y.; Liu, C.F.; Su, Y.S.; Cheng, H.Y.; Shiau, J.H.; Chen, I.Z. Correlation between nitrogen application to tea flushes and quality of green and black teas. Sci. Hortic.-Amsterdam 2015, 181, 102–107. [Google Scholar] [CrossRef]
- Ren, L.P.; Sun, J.; Chen, S.M.; Gao, J.J.; Dong, B.; Liu, Y.N.; Xia, X.L.; Wang, Y.J.; Liao, Y.; Teng, N.J.; et al. A transcriptomic analysis of Chrysanthemum nankingense provides insights into the basis of low temperature tolerance. Bmc Genom. 2014, 15, 844. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.D.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Sheng, X.; Shu, Z.; Li, D.; Pan, J.; Ye, X.; Chang, P.; Li, X.; Wang, Y. Combined Cytological and Transcriptomic Analysis Reveals a Nitric Oxide Signaling Pathway Involved in Cold-Inhibited Camellia sinensis Pollen Tube Growth. Front. Plant Sci. 2016, 7, 456. [Google Scholar] [CrossRef] [PubMed]
| Treatment Time (day) | GC | EGC | C | EC | EGCG | ECG | Caffeine |
|---|---|---|---|---|---|---|---|
| 0 | 21.01 ± 0.01a | 9.75 ± 0.40a | 3.65 ± 0.09a | 15.81 ± 0.16a | 12.40 ± 0.19a | 30.22 ± 0.60b | 9.39 ± 0.07c |
| 2 | 17.06 ± 0.07b | 7.98 ± 0.13b | 3.45 ± 0.06b | 15.42 ± 0.25b | 8.89 ± 0.07b | 31.11 ± 010a | 12.18 ± 0.02b |
| 4 | 15.91 ± 0.24c | 6.36 ± 0.38c | 3.54 ± 0.12ab | 14.09 ± 0.04c | 5.12 ± 0.08c | 29.53 ± 0.23c | 12.35 ± 0.04a |
| Amino Acid | Treatment Times (day) | ||
|---|---|---|---|
| 0 | 2 | 4 | |
| The | 5.817 ± 0.228c | 6.655 ± 0.207a | 6.164 ± 0.069b |
| Asp | 1.369 ± 0.006a | 1.341 ± 0.013b | 1.327 ± 0.007c |
| Thr | 0.665 ± 0.003a | 0.661 ± 0.005a | 0.651 ± 0.008b |
| Ser | 0.703 ± 0.007a | 0.690 ± 0.003ab | 0.678 ± 0.008b |
| Glu | 1.917 ± 0.009a | 1.873 ± 0.005b | 1.826 ± 0.018c |
| Gly | 0.775 ± 0.003a | 0.774 ± 0.008a | 0.760 ± 0.009b |
| Ala | 0.778 ± 0.003a | 0.761 ± 0.006b | 0.750 ± 0.010b |
| Cys | 0.112 ± 0.001c | 0.119 ± 0.002b | 0.124 ± 0.002a |
| Val | 0.773 ± 0.003a | 0.762 ± 0.007ab | 0.747 ± 0.011b |
| Met | 0.153 ± 0.006a | 0.162 ± 0.003a | 0.165 ± 0.004a |
| Ile | 0.592 ± 0.004a | 0.584 ± 0.009a | 0.577 ± 0.007a |
| Leu | 1.192 ± 0.002a | 1.177 ± 0.010ab | 1.152 ± 0.020b |
| Tyr | 0.425 ± 0.022b | 0.451 ± 0.008ab | 0.461 ± 0.008a |
| Phe | 0.887 ± 0.010a | 0.869 ± 0.003ab | 0.851 ± 0.015b |
| Lys | 1.078 ± 0.008a | 1.060 ± 0.009ab | 1.048 ± 0.014b |
| His | 0.335 ± 0.001a | 0.324 ± 0.006b | 0.315 ± 0.002c |
| Arg | 1.233 ± 0.043a | 1.161 ± 0.008b | 0.992 ± 0.007c |
| Pro | 0.571 ± 0.007b | 0.588 ± 0.010a | 0.597 ± 0.011a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Pan, J.; Nong, S.; Ma, Y.; Xing, A.; Zhu, X.; Wen, B.; Fang, W.; Wang, Y. Transcriptome Analysis Reveals the Mechanism of Fluoride Treatment Affecting Biochemical Components in Camellia sinensis. Int. J. Mol. Sci. 2019, 20, 237. https://doi.org/10.3390/ijms20020237
Zhu J, Pan J, Nong S, Ma Y, Xing A, Zhu X, Wen B, Fang W, Wang Y. Transcriptome Analysis Reveals the Mechanism of Fluoride Treatment Affecting Biochemical Components in Camellia sinensis. International Journal of Molecular Sciences. 2019; 20(2):237. https://doi.org/10.3390/ijms20020237
Chicago/Turabian StyleZhu, Jiaojiao, Junting Pan, Shouhua Nong, Yuanchun Ma, Anqi Xing, Xujun Zhu, Bo Wen, Wanping Fang, and Yuhua Wang. 2019. "Transcriptome Analysis Reveals the Mechanism of Fluoride Treatment Affecting Biochemical Components in Camellia sinensis" International Journal of Molecular Sciences 20, no. 2: 237. https://doi.org/10.3390/ijms20020237
APA StyleZhu, J., Pan, J., Nong, S., Ma, Y., Xing, A., Zhu, X., Wen, B., Fang, W., & Wang, Y. (2019). Transcriptome Analysis Reveals the Mechanism of Fluoride Treatment Affecting Biochemical Components in Camellia sinensis. International Journal of Molecular Sciences, 20(2), 237. https://doi.org/10.3390/ijms20020237




