In Vivo Bone Regeneration Induced by a Scaffold of Chitosan/Dicarboxylic Acid Seeded with Human Periodontal Ligament Cells
Abstract
1. Introduction
2. Results
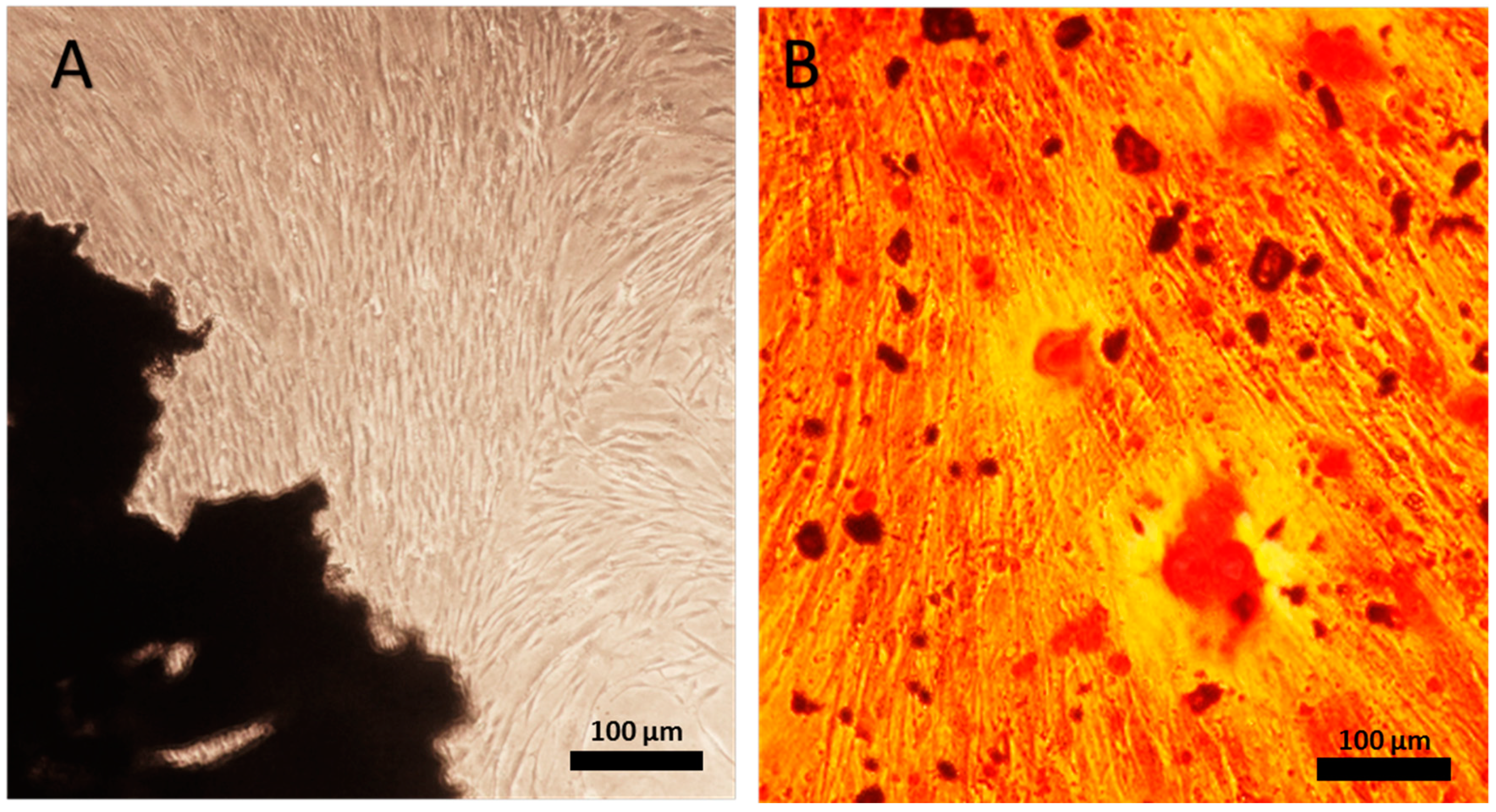
2.1. Human Periodontal Ligament Cell Differentiation
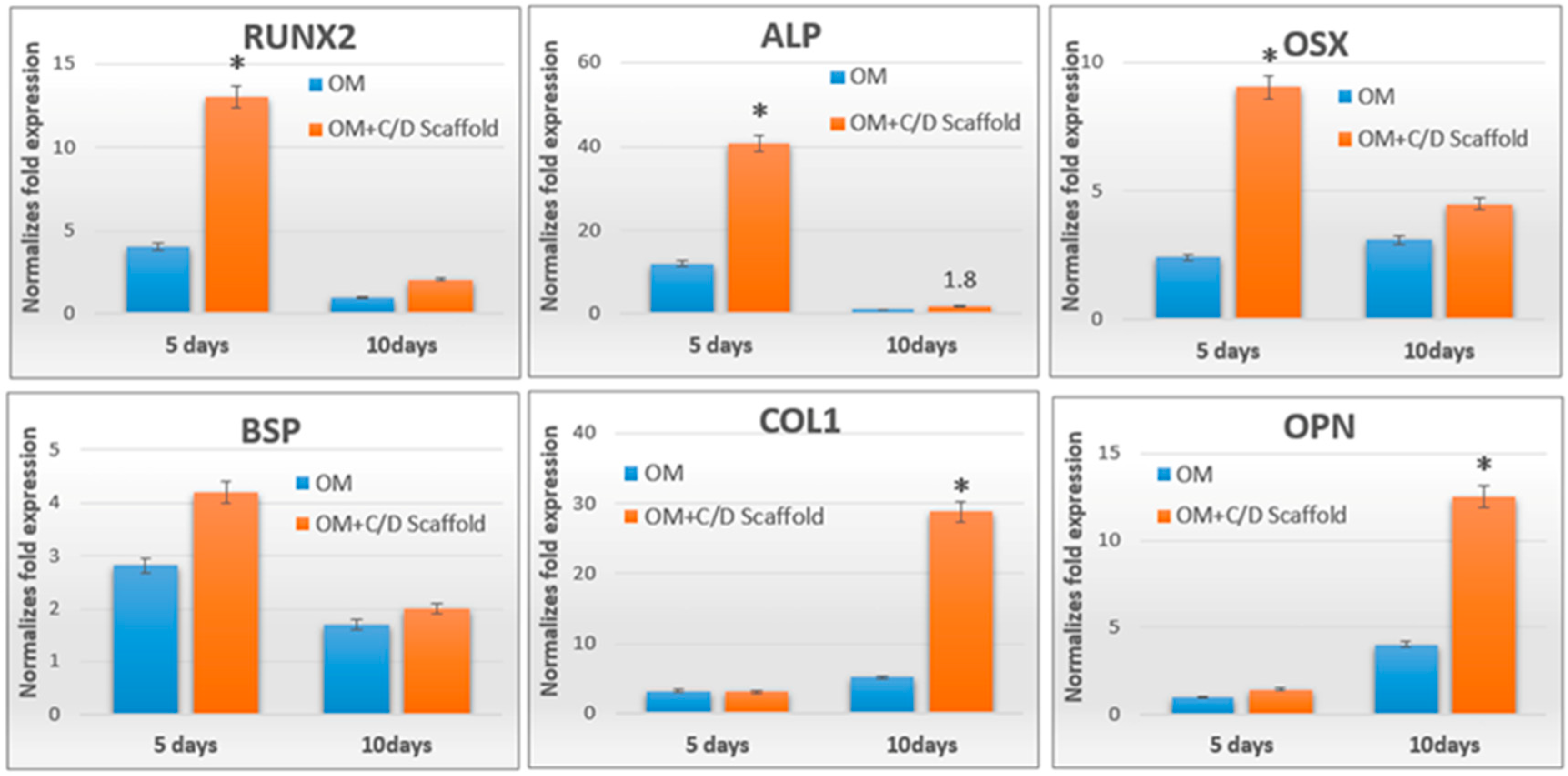
2.2. Chitosan/Dicarboxylic Acid (CS/DA) Scaffold Promoted Osteoblast-Related Gene Expression by hPDLCs
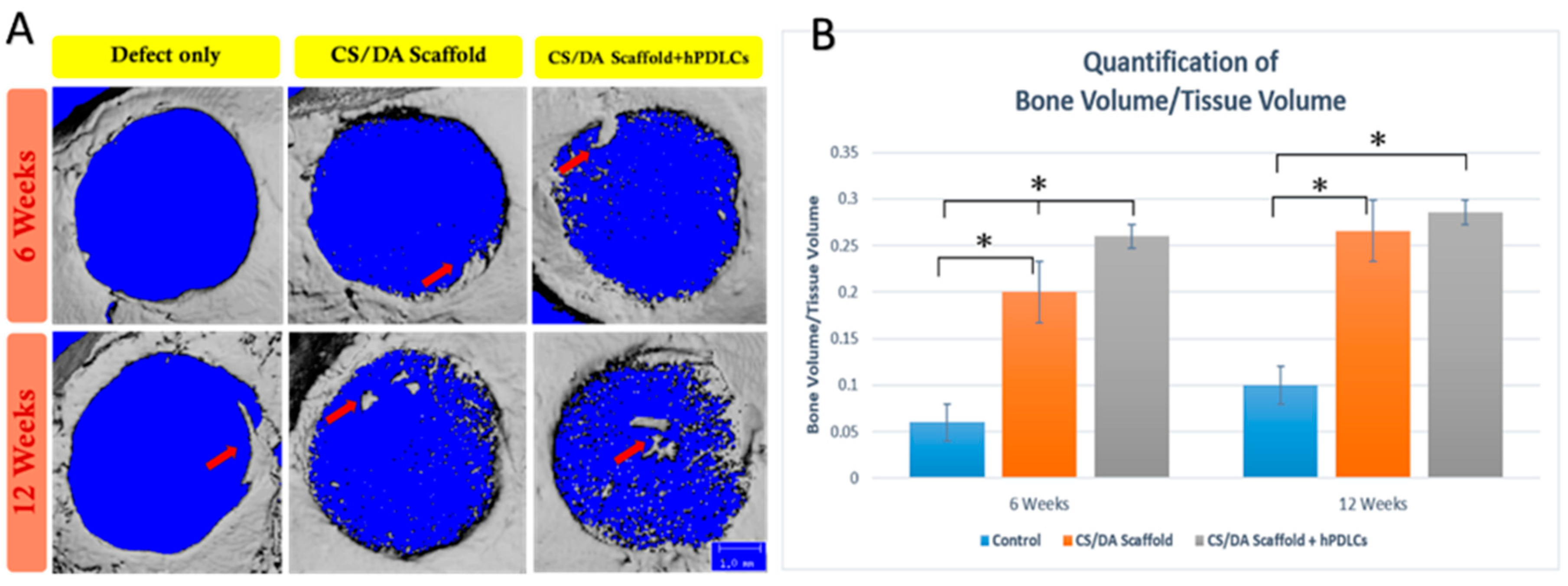
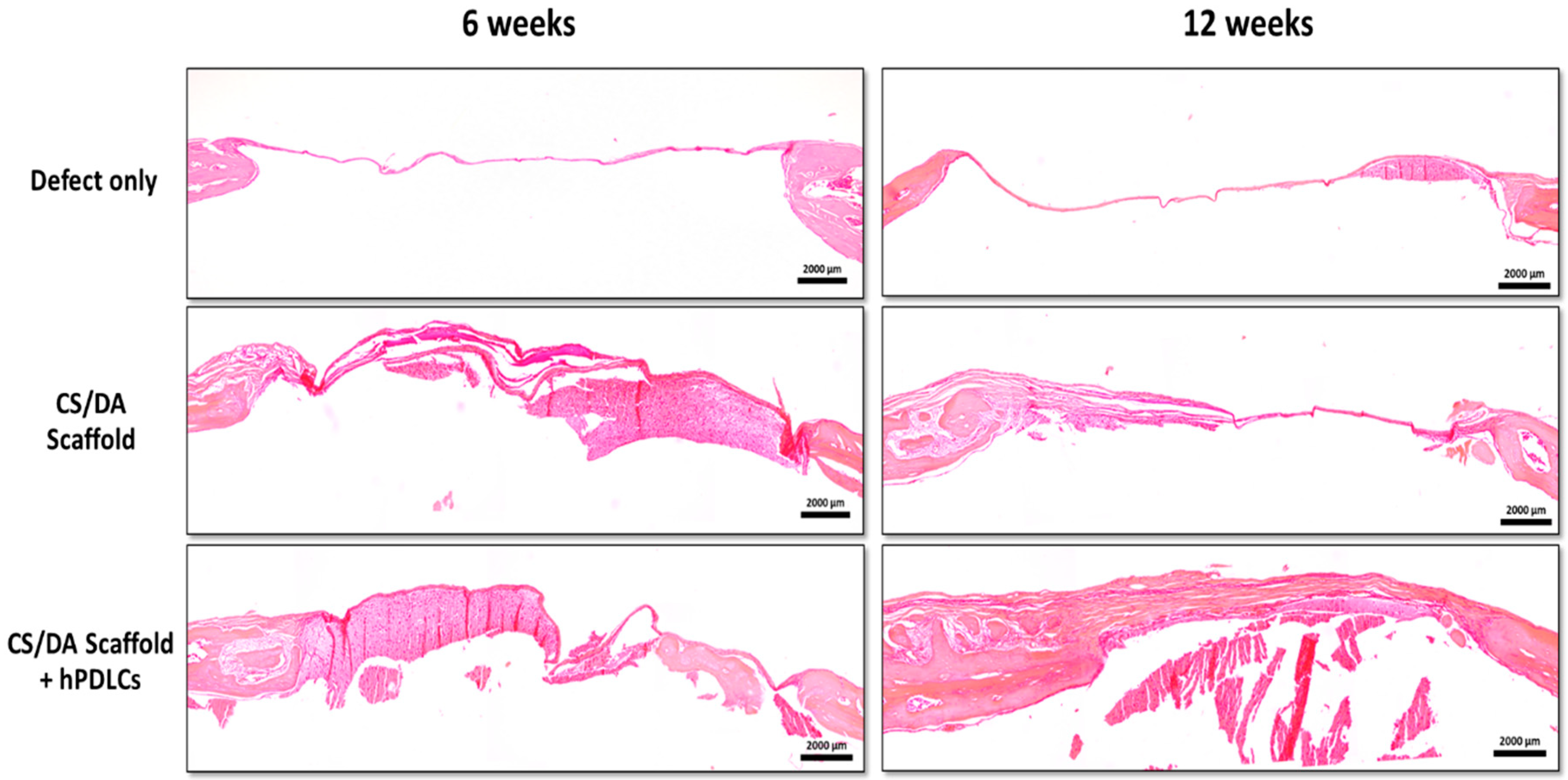
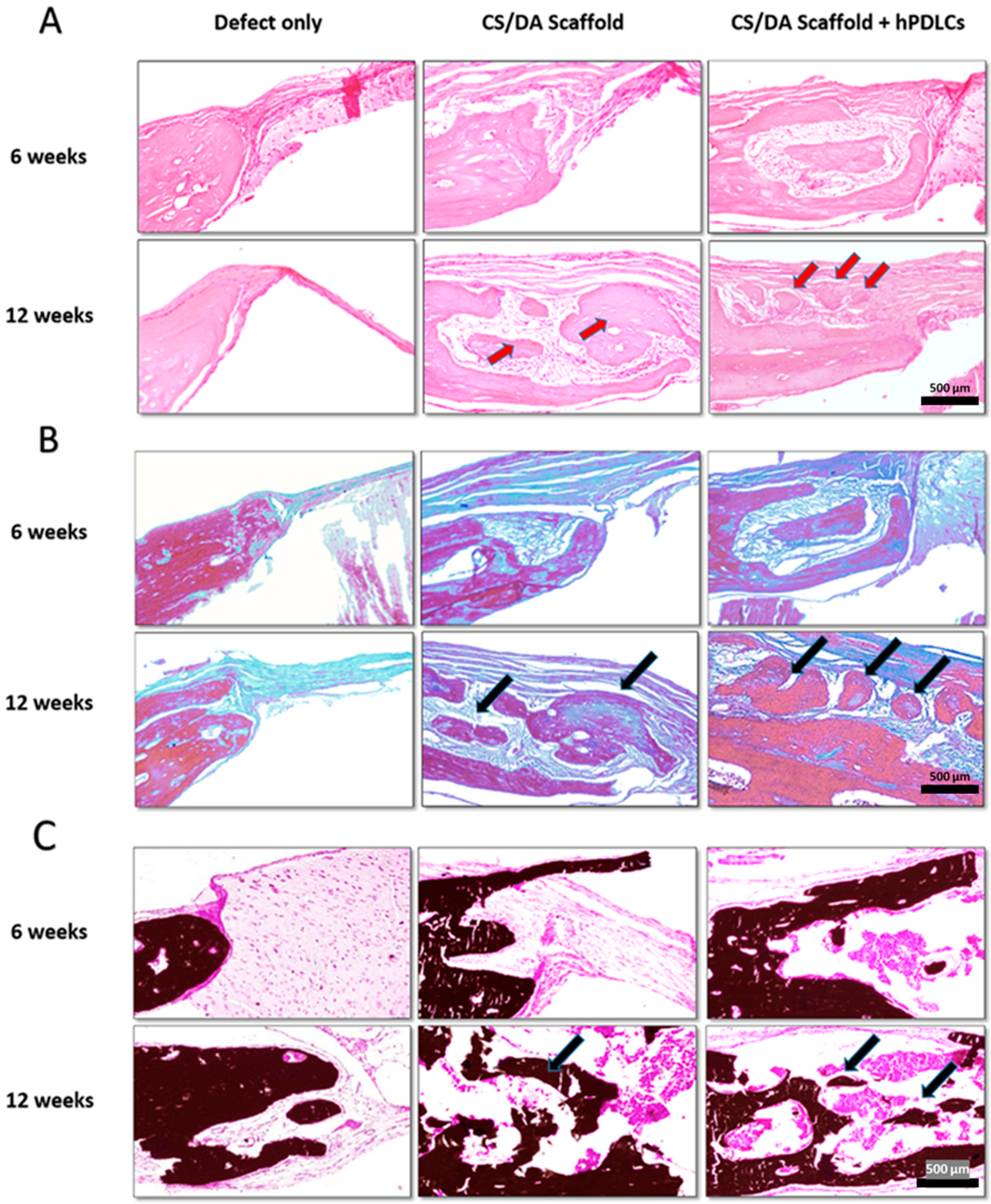
2.3. CS/DA Scaffold Enhanced Bone Regeneration in Calvariae
3. Discussion
4. Materials and Methods
4.1. Isolation of hPDLCs
4.2. In Vitro Differentiation Assay
4.3. RT-PCR of Osteogenesis-Related Gene Expression
4.4. Preparation of Chitosan/Dicarboxylic Acid (CS/DA) Scaffold
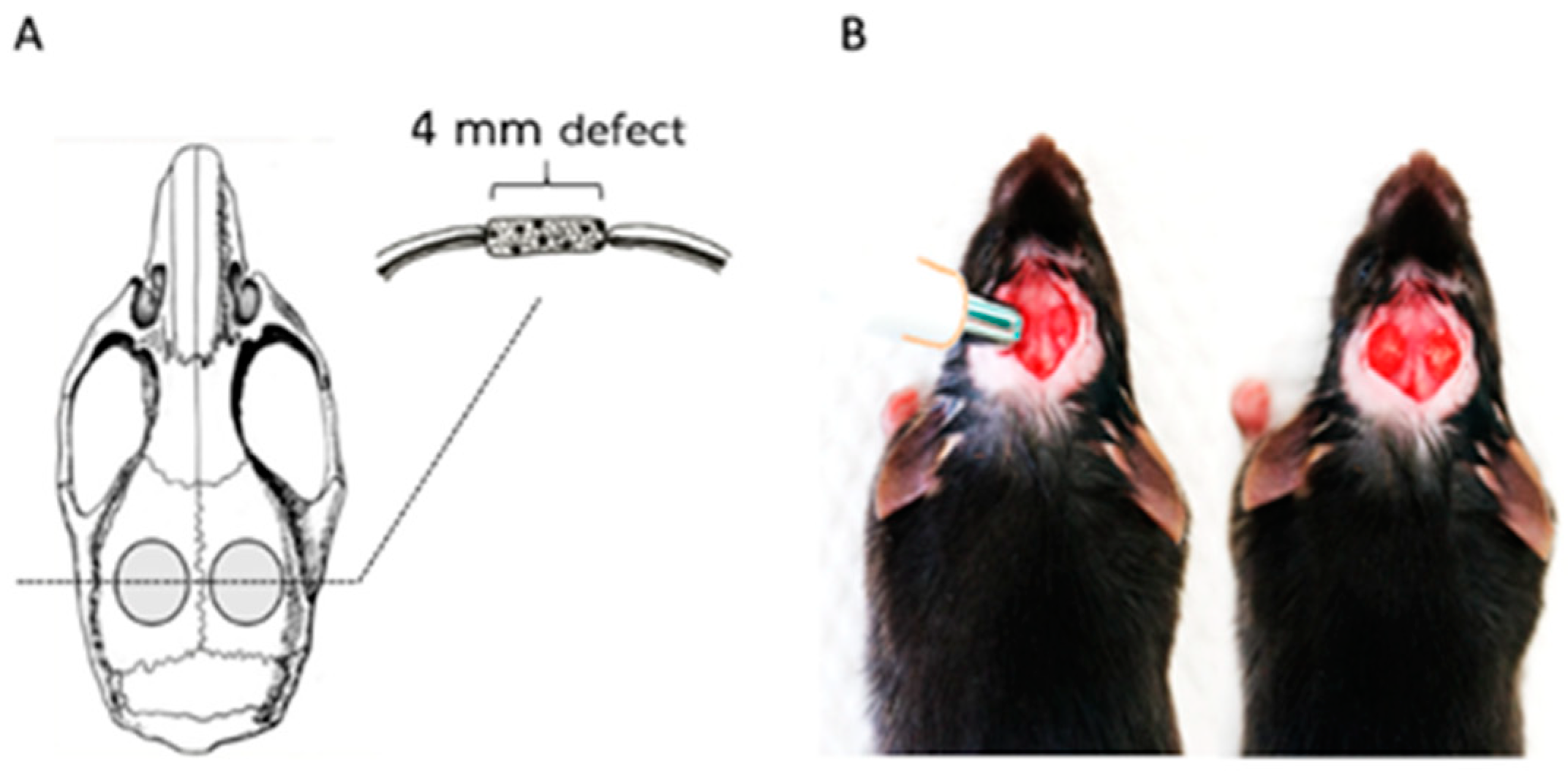
4.5. Mouse Calvaria Defect Model
4.6. Micro-Computed Tomography (Micro-CT)
4.7. Histologic Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| hPDLCs | Human Periodontal Ligament Cells |
| CS/DA | Chitosan/Dicarboxylic Acid |
References
- De Risi, V.; Clementini, M.; Vittorini, G.; Mannocci, A.; De Sanctis, M. Alveolar ridge preservation techniques: A systematic review and meta-analysis of histological and histomorphometrical data. Clin. Oral Implants Res. 2015, 26, 50–68. [Google Scholar] [CrossRef] [PubMed]
- Pietrokovski, J.; Starinsky, R.; Arensburg, B.; Kaffe, I. Morphologic Characteristics of Bony Edentulous. Jaws. J. Prosthodont. 2007, 16, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Chanchareonsook, N.; Junker, R.; Jongpaiboonkit, L.; Jansen, J.A. Tissue-engineered mandibular bone reconstruction for continuity defects: A systematic approach to the literature. Tissue Eng. Part B Rev. 2014, 20, 147–162. [Google Scholar] [CrossRef] [PubMed]
- Vignoletti, F.; Matesanz, P.; Rodrigo, D.; Figuero, E.; Martin, C.; Sanz, M. Surgical protocols for ridge preservation after tooth extraction. A systematic review. Clin. Oral Implants Res. 2012, 23 (Suppl. 5), 22–38. [Google Scholar] [CrossRef] [PubMed]
- Marei, M.K.; Nouh, S.R.; Saad, M.M.; Ismail, N.S. Preservation and Regeneration of Alveolar Bone by Tissue-Engineered Implants. Tissue Eng. 2005, 11, 751–767. [Google Scholar] [CrossRef] [PubMed]
- Salgado, A.J.; Coutinho, O.P.; Reis, R.L. Bone Tissue Engineering: State of the Art and Future Trends. Macromol. Biosci. 2004, 4, 743–765. [Google Scholar] [CrossRef]
- Velasco, M.A.; Narváez-Tovar, C.A.; Garzón-Alvarado, D.A. Design, Materials, and Mechanobiology of Biodegradable Scaffolds for Bone Tissue Engineering. BioMed Res. Int. 2015, 2015, 1–21. [Google Scholar] [CrossRef]
- Bose, S.; Roy, M.; Bandyopadhyay, A. Recent advances in bone tissue engineering scaffolds. Trends Biotechnol. 2012, 30, 546–554. [Google Scholar] [CrossRef]
- Burg, K.J.; Porter, S.; Kellam, J.F. Biomaterial developments for bone tissue engineering. Biomaterials 2000, 21, 2347–2359. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef]
- Marei, M.K.; El Backly, R.M. Dental Mesenchymal Stem Cell-Based Translational Regenerative Dentistry: From Artificial to Biological Replacement. Front. Bioeng. Biotechnol. 2018, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Mammana, S.; Gugliandolo, A.; Cavalli, E.; Diomede, F.; Iori, R.; Zappacosta, R.; Bramanti, P.; Conti, P.; Fontana, A.; Pizzicannella, J.; et al. Human gingival mesenchymal stem cells pretreated with vesicular moringin nanostructures as a new therapeutic approach in a mouse model of spinal cord injury. J. Tissue Eng. Regen. Med. 2019, 13, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Vázquez, M.; Vega-Ruiz, B.; Ramos-Zuñiga, R.; Saldaña-Koppel, D.A.; Quiñones-Olvera, L.F. Chitosan and Its Potential Use as a Scaffold for Tissue Engineering in Regenerative Medicine. BioMed Res. Int. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Phongying, S.; Aiba, S.-I.; Chirachanchai, S. Direct chitosan nanoscaffold formation via chitin whiskers. Polymer 2007, 48, 393–400. [Google Scholar] [CrossRef]
- Chen, P.-H.; Kuo, T.-Y.; Liu, F.-H.; Hwang, Y.-H.; Ho, M.-H.; Wang, D.-M.; Lai, J.-Y.; Hsieh, H.-J. Use of Dicarboxylic Acids To Improve and Diversify the Material Properties of Porous Chitosan Membranes. J. Agric. Food Chem. 2008, 56, 9015–9021. [Google Scholar] [CrossRef]
- Mitra, T.; Sailakshmi, G.; Gnanamani, A.; Mandal, A.B. Studies on Cross-linking of succinic acid with chitosan/collagen. Mater. Res. 2013, 16, 755–765. [Google Scholar] [CrossRef]
- Bodnár, M.; Hartmann, J.F.; Borbély, J. Nanoparticles from Chitosan. Macromol. Symp. 2005, 1, 321–326. [Google Scholar]
- Valderruten, N.; Valverde, J.; Zuluaga, F.; Ruiz-Durantez, E. Synthesis and characterization of chitosan hydrogels cross-linked with dicarboxylic acids. React. Funct. Polym. 2014, 84, 21–28. [Google Scholar] [CrossRef]
- Suwattanachai, P.; Pimkhaokham, A.; Chirachanchai, S. Multi-functional carboxylic acids for chitosan scaffold. Int. J. Boil. Macromol. 2019, 134, 156–164. [Google Scholar] [CrossRef]
- Yousefi, A.-M.; James, P.F.; Akbarzadeh, R.; Subramanian, A.; Flavin, C.; Oudadesse, H. Prospect of Stem Cells in Bone Tissue Engineering: A Review. Stem Cells Int. 2016, 2016, 1–13. [Google Scholar] [CrossRef]
- Huynh, N.C.-N.; Everts, V.; Nifuji, A.; Pavasant, P.; Ampornaramveth, R.S. Histone deacetylase inhibition enhances in-vivo bone regeneration induced by human periodontal ligament cells. Bone 2017, 95, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Seo, B.M.; Sonoyama, W.; Yamaza, T.; Coppe, C.; Kikuiri, T.; Akiyama, K.; Lee, J.S.; Shi, S. SHED repair critical-size calvarial defects in mice. Oral Dis. 2008, 14, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Transcriptional regulation of bone formation by the osteoblast-specific transcription factor Osx. J. Orthop. Surg. Res. 2010, 5, 37. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, S.; Leena, R.; Selvamurugan, N. Chitosan based biocomposite scaffolds for bone tissue engineering. Int. J. Boil. Macromol. 2016, 93, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De La Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef] [PubMed]
- Costa-Pinto, A.R.; Reis, R.L.; Neves, N.M. Scaffolds Based Bone Tissue Engineering: The Role of Chitosan. Tissue Eng. Part B Rev. 2011, 17, 331–347. [Google Scholar] [CrossRef]
- Pang, E.-K.; Paik, J.-W.; Kim, S.-K.; Jung, U.-W.; Kim, C.-S.; Cho, K.-S.; Kim, C.-K.; Choi, S.-H. Effects of Chitosan on Human Periodontal Ligament Fibroblasts In Vitro and on Bone Formation in Rat Calvarial Defects. J. Periodontol. 2005, 76, 1526–1533. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.K.; Kundu, B.; Basu, D. Protein growth factors loaded highly porous chitosan scaffold: A comparison of bone healing properties. Mater. Sci. Eng. C 2013, 33, 1267–1275. [Google Scholar] [CrossRef]
- Szymańska, E.; Winnicka, K. Stability of Chitosan—A Challenge for Pharmaceutical and Biomedical Applications. Mar. Drugs 2015, 13, 1819–1846. [Google Scholar] [CrossRef]
- Cooper, G.M.; Mooney, M.P.; Gosain, A.K.; Campbell, P.G.; Losee, J.E.; Huard, J. Testing the critical size in calvarial bone defects: Revisiting the concept of a critical-size defect. Plast. Reconstr. Surg. 2010, 125, 1685–1692. [Google Scholar] [CrossRef]
- Huynh, N.C.; Everts, V.; Pavasant, P.; Ampornaramveth, R.S. Inhibition of Histone Deacetylases Enhances the Osteogenic Differentiation of Human Periodontal Ligament Cells. J. Cell. Biochem. 2016, 117, 1384–1395. [Google Scholar] [CrossRef] [PubMed]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]

| Genes | Upstream Primers | Downstream Primers |
|---|---|---|
| COL-1 | GTGCTAAAGGTGCCAATGGT | ACCAGGTTCACCGCTGTTAC |
| RUNX2 | CCCCACGACAACCGCACCAT | CACTCCGGCCCACAAATC |
| OSX ALP BSP OPN | GCCAGAAGCTGTGAAACCTC CGAGATACAAGCACTCCCACTTC ATGGCCTGTGCTTTCTCAATG AGGAGGAGGCAGAGCACA | GACAGCAGGGGACAGAAAAG CTGTTCAGCTCGTACTGCATGTC AGGATAAAAGTAGGCATGCTTG CTGGTATGGCACAGGTGATG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sukpaita, T.; Chirachanchai, S.; Suwattanachai, P.; Everts, V.; Pimkhaokham, A.; Ampornaramveth, R.S. In Vivo Bone Regeneration Induced by a Scaffold of Chitosan/Dicarboxylic Acid Seeded with Human Periodontal Ligament Cells. Int. J. Mol. Sci. 2019, 20, 4883. https://doi.org/10.3390/ijms20194883
Sukpaita T, Chirachanchai S, Suwattanachai P, Everts V, Pimkhaokham A, Ampornaramveth RS. In Vivo Bone Regeneration Induced by a Scaffold of Chitosan/Dicarboxylic Acid Seeded with Human Periodontal Ligament Cells. International Journal of Molecular Sciences. 2019; 20(19):4883. https://doi.org/10.3390/ijms20194883
Chicago/Turabian StyleSukpaita, Teerawat, Suwabun Chirachanchai, Pornchanok Suwattanachai, Vincent Everts, Atiphan Pimkhaokham, and Ruchanee Salingcarnboriboon Ampornaramveth. 2019. "In Vivo Bone Regeneration Induced by a Scaffold of Chitosan/Dicarboxylic Acid Seeded with Human Periodontal Ligament Cells" International Journal of Molecular Sciences 20, no. 19: 4883. https://doi.org/10.3390/ijms20194883
APA StyleSukpaita, T., Chirachanchai, S., Suwattanachai, P., Everts, V., Pimkhaokham, A., & Ampornaramveth, R. S. (2019). In Vivo Bone Regeneration Induced by a Scaffold of Chitosan/Dicarboxylic Acid Seeded with Human Periodontal Ligament Cells. International Journal of Molecular Sciences, 20(19), 4883. https://doi.org/10.3390/ijms20194883




