Changes in Membrane Ceramide Pools in Rat Soleus Muscle in Response to Short-Term Disuse
Abstract
1. Introduction
2. Results
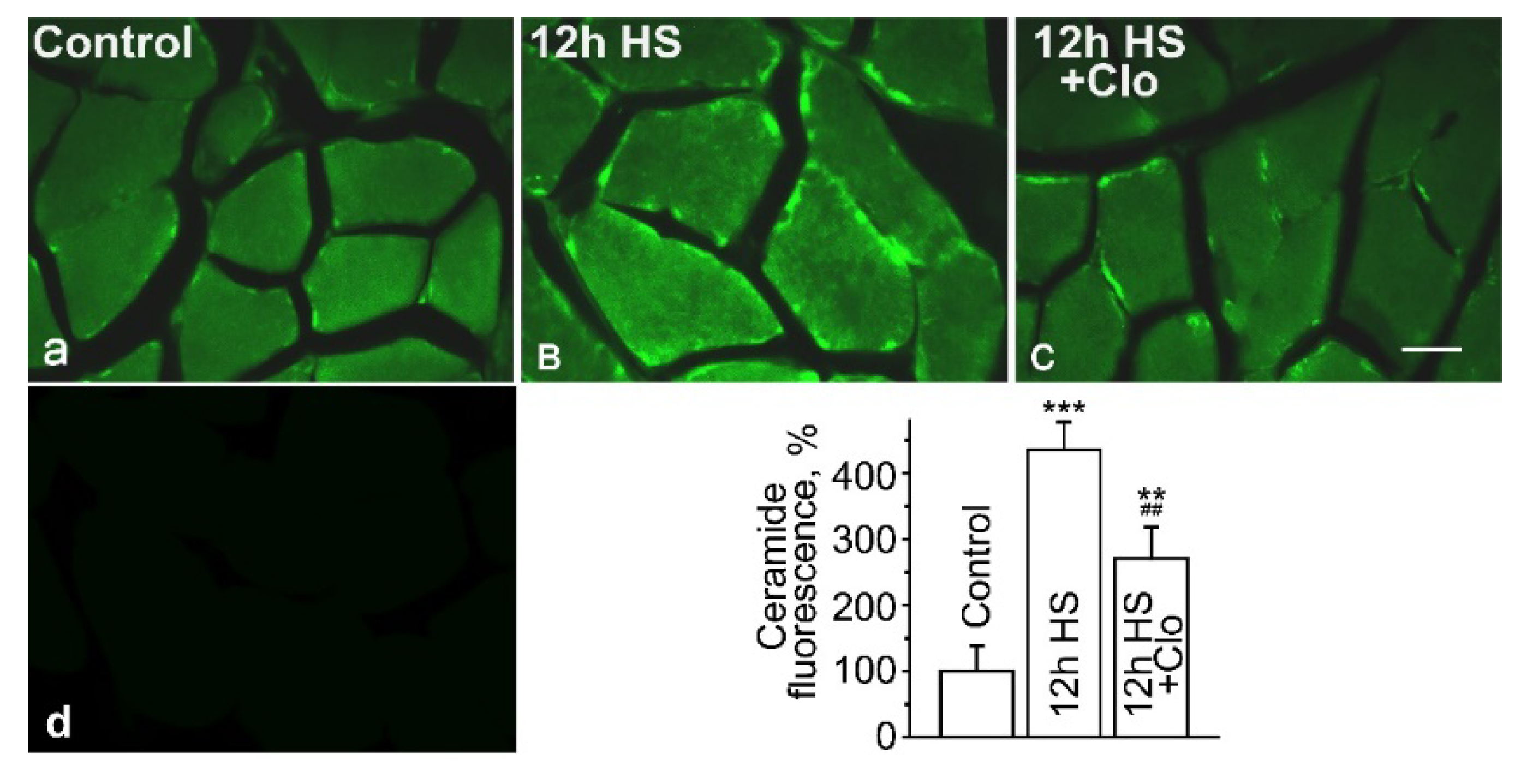
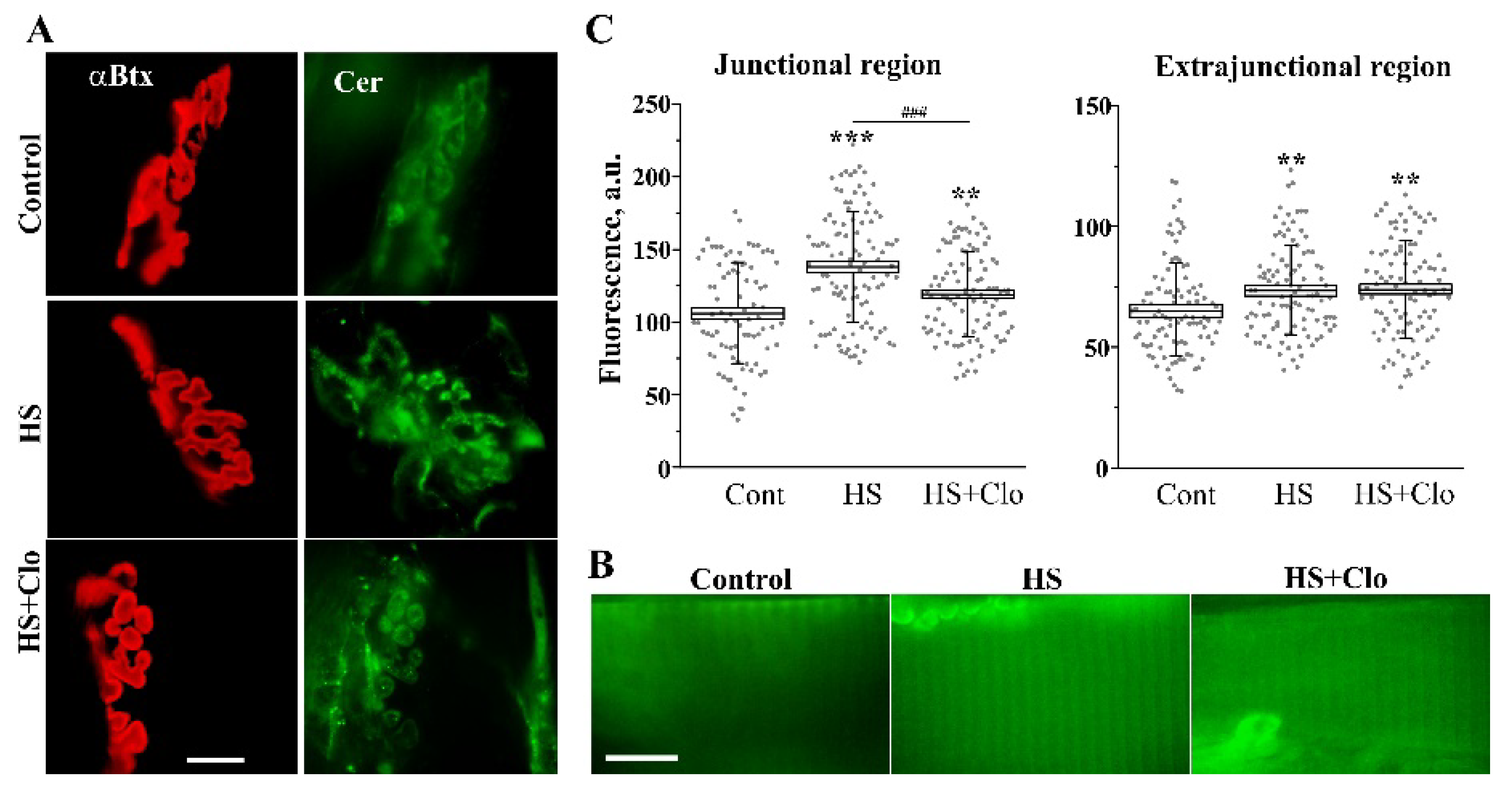
2.1. Immunofluorescent Detection of Ceramide Distribution on Transverse Sections
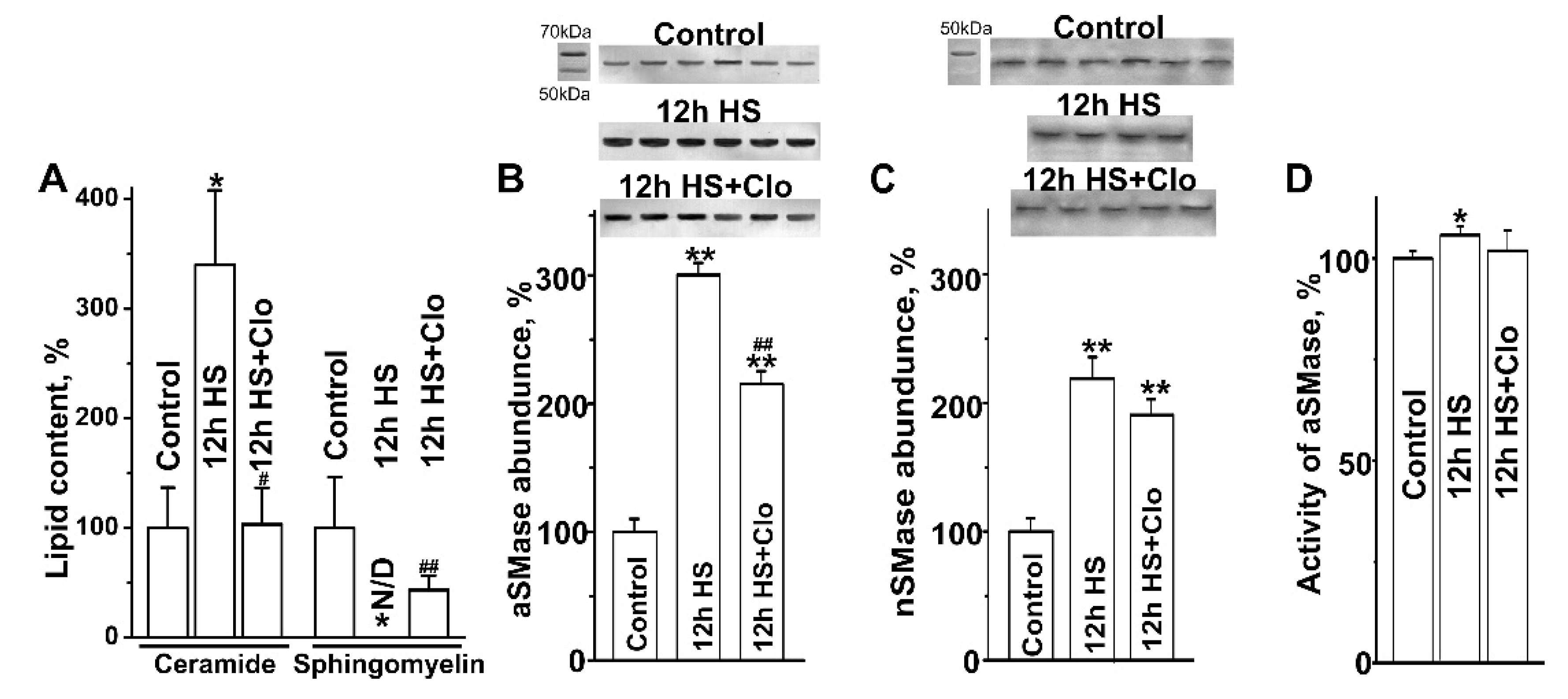
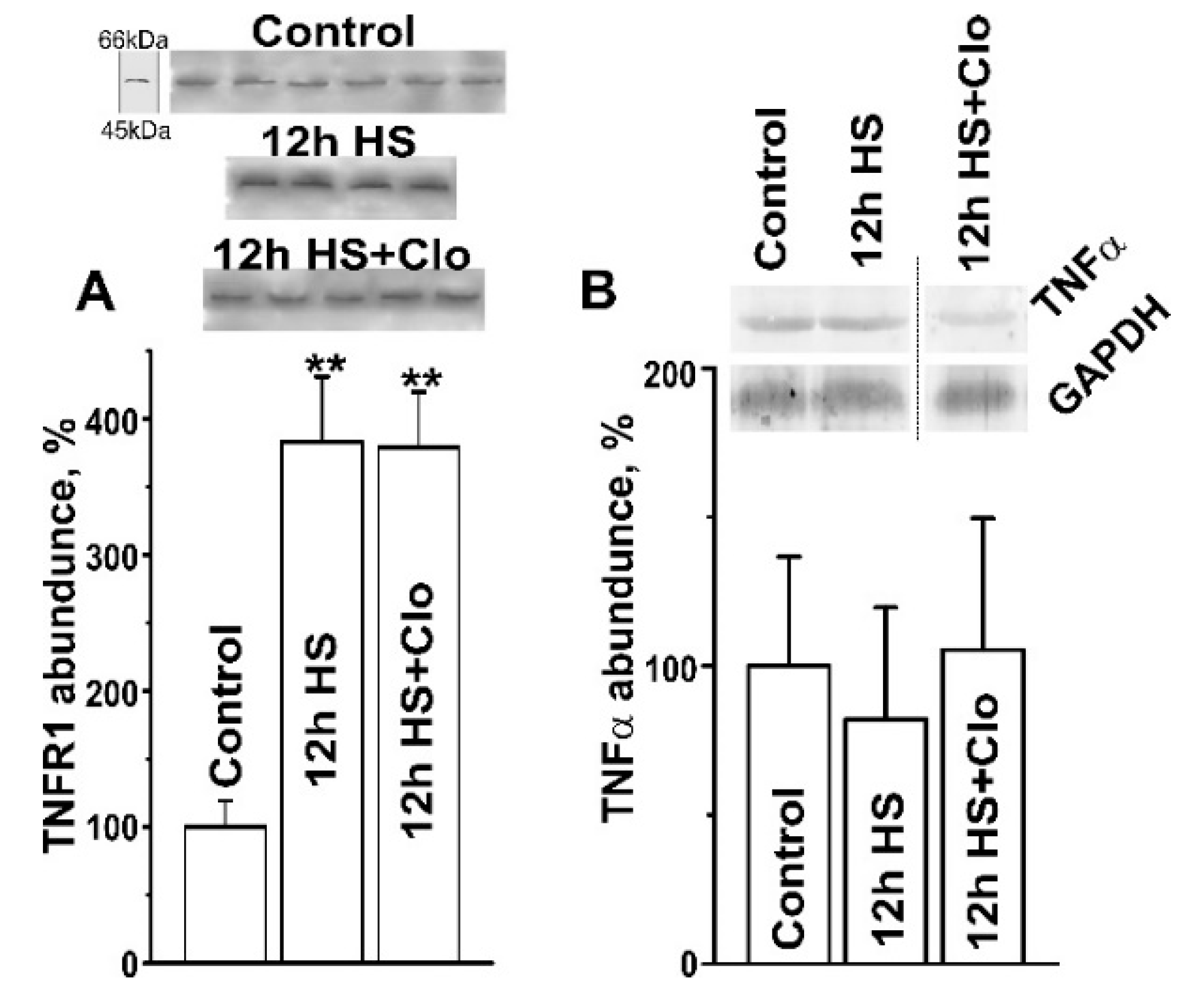
2.2. Biochemical Analysis of Isolated Lipid Raft Composition
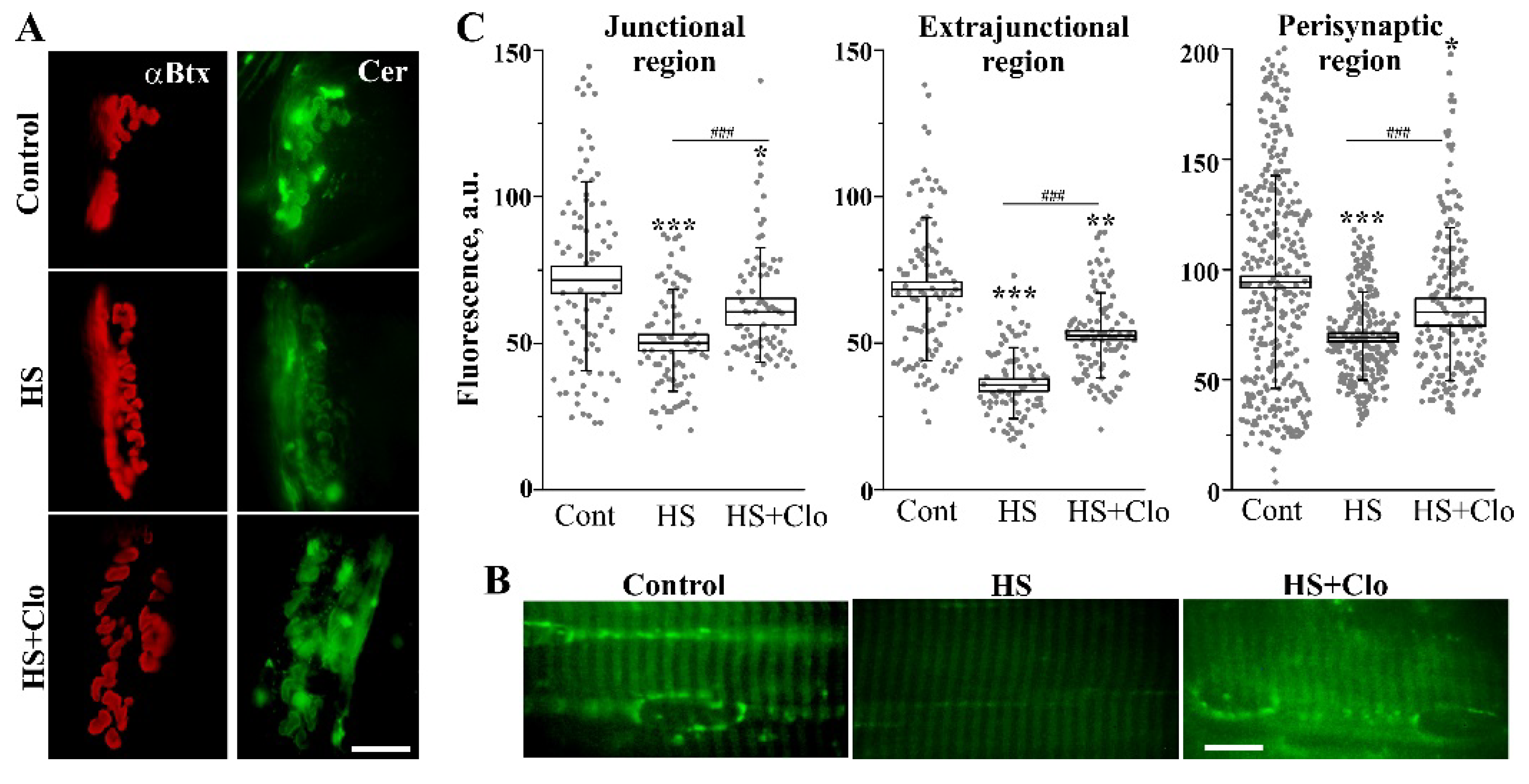
2.3. Fluorescent Analysis of Cer Distribution with Confocal Microscopy
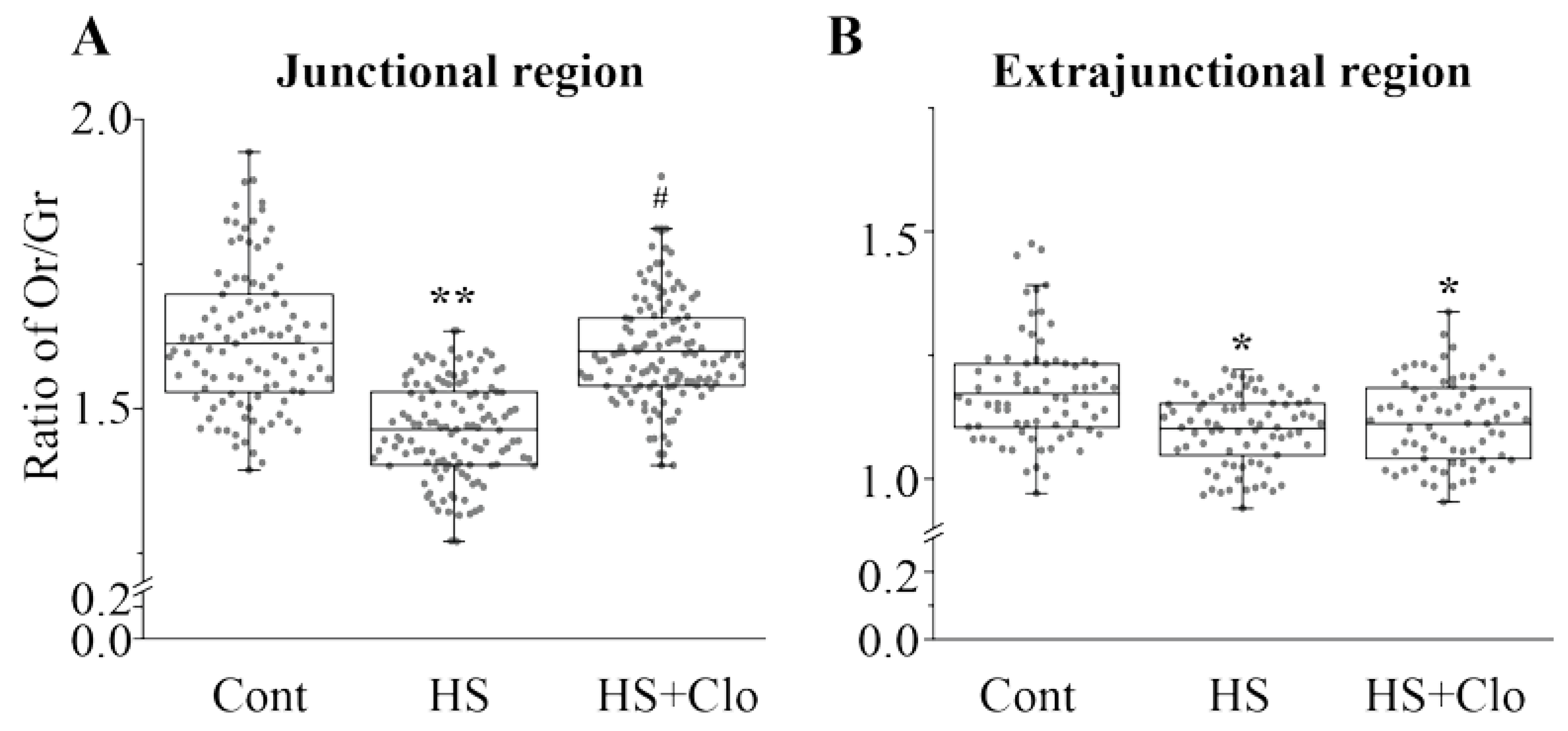
2.4. Estimation of Membrane Asymmetry Using F2N12S Probe
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Experimental Protocol
4.3. Ceramide, Sphingomyelin, Sphingomyelinase, TNFR1, and TNFα Assays
4.4. Immunohistochemistry
4.5. Fluorescence Microscopy
4.5.1. Staining with BODIPY-Cer
4.5.2. Immunolabeling of Plasma Membrane Cer
4.5.3. Monitoring Membrane Asymmetry
4.6. Statistics
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| α-Btx | rhodamine-conjugated α-bungarotoxin |
| aSMase | acid sphingomyelinase |
| Cer | ceramide |
| HS | hindlimb suspension |
| nAChR | nicotinic acetylcholine receptor |
| nSMase | neutral sphingomyelinase |
| TNFα | tumor necrosis factor α |
| TNFR1 | TNFα receptor 1 |
References
- Fitts, R.H.; Riley, D.R.; Widrick, J.J. Functional and Structural Adaptations of Skeletal Muscle to Microgravity. J. Exp. Biol. 2001, 204, 3201–3208. [Google Scholar] [PubMed]
- Bajotto, G.; Shimomura, Y. Determinants of Disuse-Induced Skeletal Muscle Atrophy: Exercise and Nutrition Countermeasures to Prevent Protein Loss. J. Nutr. Sci. Vitaminol. (Tokyo) 2006, 52, 233–247. [Google Scholar] [CrossRef] [PubMed]
- Chopard, A.; Hillock, S.; Jasmin, B.J. Molecular Events and Signalling Pathways Involved in Skeletal Muscle Disuse-Induced Atrophy and the Impact of Countermeasures. J. Cell Mol. Med. 2009, 13, 3032–3050. [Google Scholar] [CrossRef] [PubMed]
- Bodine, S.C. Disuse-induced muscle wasting. Int. J. Biochem. Cell Biol. 2013, 45, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Rudrappa, S.S.; Wilkinson, D.J.; Greenhaff, P.L.; Smith, K.; Idris, I.; Atherton, P.J. Human Skeletal Muscle Disuse Atrophy: Effects on Muscle Protein Synthesis, Breakdown, and Insulin Resistance-A Qualitative Review. Front. Physiol. 2016, 7, 361. [Google Scholar] [CrossRef]
- Gao, Y.; Arfat, Y.; Wang, H.; Goswami, N. Muscle Atrophy Induced by Mechanical Unloading: Mechanisms and Potential Countermeasures. Front. Physiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Il’in, E.A.; Novikov, V.E. Stand for Modelling the Physiological Effects of Weightlessness in Laboratory Experiments with Rats. Kosm. Biol. Aviakosm. Med. 1980, 14, 79–80. [Google Scholar]
- Thomason, D.B.; Booth, F.W. Atrophy of the Soleus Muscle by Hindlimb Unweighting. J. Appl. Physiol. 1990, 68, 1–12. [Google Scholar] [CrossRef]
- Morey-Holton, E.R.; Globus, R.K. Hindlimb Unloading Rodent Model: Technical Aspects. J. Appl. Physiol. 2002, 92, 1367–1377. [Google Scholar] [CrossRef]
- Cho, S.H.; Kim, J.H.; Song, W. In Vivo Rodent Models of Skeletal Muscle Adaptation to Decreased Use. Endocrinol. Metab. (Seoul) 2016, 31, 31–37. [Google Scholar] [CrossRef]
- Alford, E.K.; Roy, R.R.; Hodgson, J.A.; Edgerton, V.R. Electromyography of Rat Soleus, Medial Gastrocnemius, and Tibialis Anterior during Hind Limb Suspension. Exp. Neurol. 1987, 96, 635–649. [Google Scholar] [CrossRef]
- Henriksen, E.J.; Tischler, M.E. Glucose Uptake in Rat Soleus: Effect of Acute Unloading and Subsequent Reloading. J. Appl. Physiol. 1988, 64, 1428–1432. [Google Scholar] [CrossRef] [PubMed]
- Dupont-Versteegden, E.E.; Strotman, B.A.; Gurley, C.M.; Gaddy, D.; Knox, M.; Fluckey, J.D.; Peterson, C.A. Nuclear Translocation of EndoG at the Initiation of Disuse Muscle Atrophy and Apoptosis is Specific to Myonuclei. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1730–R1740. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, K.M.; Haddad, F.; Pandorf, C.E.; Roy, R.R.; Edgerton, V.R. Alterations in Muscle Mass and Contractile Phenotype in Response to Unloading Models: Role of Transcriptional/Pretranslational Mechanisms. Front. Physiol. 2013, 4, 284. [Google Scholar] [CrossRef] [PubMed]
- Kravtsova, V.V.; Petrov, A.M.; Matchkov, V.V.; Bouzinova, E.V.; Vasiliev, A.N.; Benziane, B.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Distinct α2 Na,K-ATPase Membrane Pools are Differently Involved in Early Skeletal Muscle Remodeling During Disuse. J. Gen. Physiol. 2016, 147, 175–188. [Google Scholar] [CrossRef]
- Mirzoev, T.; Tyganov, S.; Vilchinskaya, N.; Lomonosova, Y.; Shenkman, B. Key Markers of mTORC1-Dependent and mTORC1-Independent Signaling Pathways Regulating Protein Synthesis in Rat Soleus Muscle during Early Stages of Hindlimb Unloading. Cell Physiol. Biochem. 2016, 39, 1011–1020. [Google Scholar] [CrossRef] [PubMed]
- Petrov, A.M.; Kravtsova, V.V.; Matchkov, V.V.; Vasiliev, A.N.; Zefirov, A.L.; Chibalin, A.V.; Heiny, J.A.; Krivoi, I.I. Membrane Lipid Rafts are Disturbed in the Response of rat Skeletal Muscle to Short-Term Disuse. Am. J. Physiol. Cell Physiol. 2017, 312, C627–C637. [Google Scholar] [CrossRef] [PubMed]
- Bryndina, I.G.; Shalagina, M.N.; Sekunov, A.V.; Zefirov, A.L.; Petrov, A.M. Сlomipramine Counteracts Lipid Raft Disturbance due to Short-Term Muscle Disuse. Neurosci. Lett. 2018, 664, 1–6. [Google Scholar] [CrossRef]
- Chibalin, A.V.; Benziane, B.; Zakyrjanova, G.F.; Kravtsova, V.V.; Krivoi, I.I. Early Endplate Remodeling and Skeletal Muscle Signaling Events Following Rat Hindlimb Suspension. J. Cell Physiol. 2018, 233, 6329–6336. [Google Scholar] [CrossRef]
- Lechado, I.; Terradas, A.; Vitadello, M.; Traini, L.; Namuduri, A.V.; Gastaldello, S.; Gorza, L. Sarcolemmal Loss of active nNOS (Nos1) is an Oxidative Stress-Dependent, Early Event Driving Disuse Atrophy. J. Pathol. 2018, 246, 433–446. [Google Scholar] [CrossRef]
- Bruni, P.; Donati, C. Pleiotropic Effects of Sphingolipids in Skeletal Muscle. Cell Mol. Life Sci. 2008, 65, 3725–3736. [Google Scholar] [CrossRef] [PubMed]
- Nikolova-Karakashian, M.N.; Reid, M.B. Sphingolipid metabolism, oxidant signaling, and contractile function of skeletal muscle. J. Biol. Chem. 2011, 286, 32064–32073. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, O.M.; Discher, D.J.; Bishopric, N.H.; Webster, K.A. Rapid Activation of Neutral Sphingomyelinase by Hypoxia-Reoxygenation of Cardiac Myocytes. Circ. Res. 2000, 86, 198–204. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.L.; Kang, H.Y. Effects of Endurance Exercise and High-Fat Diet on Insulin Resistance and Ceramide Contents of Skeletal Muscle in Sprague-Dawley Rats. Korean Diabetes J. 2010, 34, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Zabielski, P.; Błachnio-Zabielska, A.U.; Wójcik, B.; Chabowski, A.; Górski, J. Effect of Plasma Free Fatty Acid Supply on the Rate of Ceramide Synthesis in Different Muscle Types in the Rat. PLoS ONE 2017, 12, e0187136. [Google Scholar] [CrossRef]
- Bryndina, I.G.; Shalagina, M.N.; Ovechkin, S.V.; Ovchinina, N.G. Sphingolipids in Skeletal Muscles of C57B1/6 Mice after Short-Term Simulated Microgravity. Ross. Fiziol. Zh. Im. I.M. Sechenova 2014, 100, 1280–1286. [Google Scholar] [PubMed]
- Kwon, O.S.; Tanner, R.E.; Barrows, K.M.; Runtsch, M.; Symons, J.D.; Jalili, T.; Bikman, B.T.; McClain, D.A.; O’Connell, R.M.; Drummond, M.J. MyD88 Regulates Physical Inactivity-Induced Skeletal Muscle Inflammation, Ceramide Biosynthesis Signaling, and Glucose Intolerance. Am. J. Physiol. Endocrinol. Metab. 2015, 309, E11–E21. [Google Scholar] [CrossRef] [PubMed]
- Salaun, E.; Lefeuvre-Orfila, L.; Cavey, T.; Martin, B.; Turlin, B.; Ropert, M.; Loreal, O.; Derbré, F. Myriocin Prevents Muscle Ceramide Accumulation but not Muscle Fiber Atrophy During Short-Term Mechanical Unloading. J. Appl. Physiol. 2016, 120, 178–187. [Google Scholar] [CrossRef]
- Reidy, P.T.; McKenzie, A.I.; Mahmassani, Z.; Morrow, V.R.; Yonemura, N.M.; Hopkins, P.N.; Marcus, R.L.; Rondina, M.T.; Lin, Y.K.; Drummond, M.J. Skeletal Muscle Ceramides and Relationship with Insulin Sensitivity after 2 Weeks of Simulated Sedentary Behaviour and Recovery in Healthy Older Adults. J. Physiol. 2018, 596, 5217–5236. [Google Scholar] [CrossRef]
- Gault, C.R.; Obeid, L.M.; Hannun, Y.A. An Overview of Sphingolipid Metabolism: From Synthesis to Breakdown. Adv. Exp. Med. Biol. 2010, 688, 1–23. [Google Scholar] [CrossRef]
- Bryndina, I.G.; Shalagina, M.N.; Ovechkin, S.V.; Iakovlev, A.A. Sphingolipid Metabolism in Mice Forelimb and Hindlimb Skeletal Muscles under Antiorthostatic Suspension of Different Durations. Aviakosm. i Ekolog. Med. 2017, 51, 94–98. [Google Scholar]
- Hannun, Y.A.; Obeid, L.M. Sphingolipids and their Metabolism in Pphysiology and Disease. Nat. Rev. Mol. Cell Biol. 2018, 19, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Cremesti, A.E.; Goni, F.M.; Kolesnick, R. Role of Sphingomyelinase and Ceramide in Modulating Rafts: Do Biophysical Properties Determine Biologic Outcome? FEBS Lett. 2002, 531, 47–53. [Google Scholar] [CrossRef]
- Catapano, E.R.; Natale, P.; Monroy, F.; López-Montero, I. The Enzymatic Sphingomyelin to Ceramide Conversion Increases the Shear Membrane Viscosity at the Air-Water Interface. Adv. Colloid Interface Sci. 2017, 247, 555–560. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Becker, K.A.; Gulbins, E. Ceramide-Enriched Membrane Domains--Structure and Function. Biochim. Biophys. Acta 2009, 1788, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Marchesini, N.; Hannun, Y.A. Acid and Neutral Sphingomyelinases: Roles and Mechanisms of Regulation. Biochem. Cell Biol. 2004, 82, 27–44. [Google Scholar] [CrossRef] [PubMed]
- Gulbins, E.; Grassmé, H. Ceramide and cell death receptor clustering. Biochim. Biophys. Acta 2002, 1585, 139–145. [Google Scholar] [CrossRef]
- Tchikov, V.; Bertsch, U.; Fritsch, J.; Edelmann, B.; Schütze, S. Subcellular compartmentalization of TNF receptor-1 and CD95 signaling pathways. Eur. J. Cell Biol. 2011, 90, 467–475. [Google Scholar] [CrossRef] [PubMed]
- De Larichaudy, J.; Zufferli, A.; Serra, F.; Isidori, A.M.; Naro, F.; Dessalle, K.; Desgeorges, M.; Piraud, M.; Cheillan, D.; Vidal, H.; et al. Némoz, G. TNF-α- and tumor-induced skeletal muscle atrophy involves sphingolipid metabolism. Skelet. Muscle 2012, 2, 2. [Google Scholar] [CrossRef]
- Moylan, J.S.; Smith, J.D.; Wolf Horrell, E.M.; McLean, J.B.; Deevska, G.M.; Bonnell, M.R.; Nikolova-Karakashian, M.N.; Reid, M.B. Neutral sphingomyelinase-3 mediates TNF-stimulated oxidant activity in skeletal muscle. Redox Biol. 2014, 2, 910–920. [Google Scholar] [CrossRef]
- Megha; London, E. Ceramide Selectively Displaces Cholesterol from Ordered Lipid Domains (Rafts): Implications for Lipid Raft Structure and Function. J. Biol. Chem. 2004, 279, 9997–10004. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, Y.; Ohba, T.; Miyata, H.; Ohki, K. Rapid Phase Change of Lipid Microdomains in Giant Vesicles Induced by Conversion of Sphingomyelin to Ceramide. Biochim. Biophys Acta 2006, 1758, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Tepper, A.D.; Ruurs, P.; Wiedmer, T.; Sims, P.J.; Borst, J.; van Blitterswijk, W.J. Sphingomyelin Hydrolysis to Ceramide during the Execution Phase of Apoptosis Results from Phospholipid Scrambling and Alters Cell-Surface Morphology. J. Cell Biol. 2000, 150, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.X.; Villar, A.V.; Alonso, A.; Kolesnick, R.N.; Goñi, F.M. Sphingomyelinase Activity Causes Transbilayer Lipid Translocation in Model and Cell Membranes. J. Biol. Chem. 2003, 278, 37169–37174. [Google Scholar] [CrossRef] [PubMed]
- Contreras, F.X.; Villar, A.V.; Alonso, A.; Goñi, F.M. Ceramide-Induced Transbilayer (Flip-Flop) Lipid Movement in Membranes. Methods Mol. Biol. 2009, 462, 155–165. [Google Scholar] [CrossRef]
- Ikeda, M.; Kihara, A.; Igarashi, Y. Lipid Asymmetry of the Eukaryotic Plasma Membrane: Functions and Related Enzymes. Biol. Pharm. Bull. 2006, 29, 1542–1546. [Google Scholar] [CrossRef] [PubMed]
- Bryndina, I.G.; Protopopov, V.A.; Sergeev, V.G.; Shalagina, M.N.; Ovechkin, S.V.; Yakovlev, A.A. Ceramide Enriched Membrane Domains in Rat Skeletal Muscle Exposed to Short-Term Hypogravitational Unloading. In Proceedings of the 39th ISGP & ESA Life Sciences Meeting, Noordwijk, The Netherlands, 18–22 June 2018. [Google Scholar]
- Yu, C.; Alterman, M.; Dobrowsky, R.T. Ceramide Displaces Cholesterol from Lipid Rafts and Decreases the Association of the Cholesterol Binding Protein Caveolin-1. J. Lipid Res. 2005, 46, 1678–1691. [Google Scholar] [CrossRef]
- Rohrbough, J.; Rushton, E.; Palanker, L.; Woodruff, E.; Matthies, H.J.; Acharya, U.; Acharya, J.K.; Broadie, K. Ceramidase Regulates Synaptic Vesicle Exocytosis and Trafficking. J. Neurosci. 2004, 24, 7789–7803. [Google Scholar] [CrossRef]
- Willmann, R.; Pun, S.; Stallmach, L.; Sadasivam, G.; Santos, A.F.; Caroni, P.; Fuhrer, C. Cholesterol and Lipid Microdomains Stabilize the Postsynapse at the Neuromuscular Junction. EMBO J. 2006, 25, 4050–4060. [Google Scholar] [CrossRef]
- Petrov, A.M.; Kudryashova, K.E.; Odnoshivkina, Yu.G.; Zefirov, A.L. Cholesterol and Lipid Rafts in the Plasma Membrane of Nerve Terminal and Membrane of Synaptic Vesicles. Neurochem. J. 2011, 5, 13–19. [Google Scholar] [CrossRef]
- Mukhutdinova, K.A.; Kasimov, M.R.; Giniatullin, A.R.; Zakyrjanova, G.F.; Petrov, A.M. 24S-hydroxycholesterol Suppresses Neuromuscular Transmission in SOD1(G93A) mice: A possible Role of NO and Lipid Rafts. Mol. Cell Neurosci. 2018, 88, 308–318. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.; Balla, A.; Ma, H.; Knight, Z.A.; Shokat, K.M.; Balla, T. Phosphatidylinositol 4-Kinase IIIbeta Regulates the Transport of Ceramide Between the Endoplasmic Reticulum and Golgi. J. Biol. Chem. 2006, 281, 36369–36377. [Google Scholar] [CrossRef] [PubMed]
- Chazotte, B. Labeling the Golgi Apparatus with BODIPY-FL-Ceramide (C5-DMB-Ceramide) for Imaging. CSH Protoc. 2008, 2008, pdb.prot4931. [Google Scholar] [CrossRef] [PubMed]
- Merianda, T.T.; Lin, A.C.; Lam, J.S.; Vuppalanchi, D.; Willis, D.E.; Karin, N.; Holt, C.E.; Twiss, J.L. A Functional Equivalent of Endoplasmic Reticulum and Golgi in Axons for Secretion of Locally Synthesized Proteins. Mol. Cell Neurosci. 2009, 40, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Pollet, H.; Conrard, L.; Cloos, A.S.; Tyteca, D. Plasma Membrane Lipid Domains as Platforms for Vesicle Biogenesis and Shedding? Biomolecules 2018, 8, 94. [Google Scholar] [CrossRef] [PubMed]
- Lauwers, E.; Goodchild, R.; Verstreken, P. Membrane Lipids in Presynaptic Function and Disease. Neuron. 2016, 90, 11–25. [Google Scholar] [CrossRef]
- Kornhuber, J.; Tripal, P.; Reichel, M.; Mühle, C.; Rhein, C.; Muehlbacher, M.; Groemer, T.W.; Gulbins, E. Functional Inhibitors of Acid Sphingomyelinase (FIASMAs): A Novel Pharmacological Group of Drugs with Broad Clinical Applications. Cell Physiol. Biochem. 2010, 26, 9–20. [Google Scholar] [CrossRef]
- Hurwitz, R.; Ferlinz, K.; Sandhoff, K. The Tricyclic Antidepressant Desipramine Causes Proteolytic Degradation of Lysosomal Sphingomyelinase in Human Fibroblasts. Biol. Chem. Hoppe-Seyler 1994, 375, 447–450. [Google Scholar] [CrossRef]
- Kölzer, M.; Werth, N.; Sandhoff, K. Interactions of Acid Sphingomyelinase and Lipid Bilayers in the Presence of the Tricyclic Antidepressant Desipramine. FEBS Lett. 2004, 559, 96–98. [Google Scholar] [CrossRef]
- Beckmann, N.; Sharma, D.; Gulbins, E.; Becker, K.A.; Edelmann, B. Inhibition of Acid Sphingomyelinase by Tricyclic Antidepressants and Analogons. Front. Physiol. 2014, 5, 331. [Google Scholar] [CrossRef]
- Hunter, R.B.; Stevenson, E.; Koncarevic, A.; Mitchell-Felton, H.; Essig, D.A.; Kandarian, S.C. Activation of an Alternative NF-κB Pathway in Skeletal Muscle during Disuse Atrophy. FASEB J. 2002, 16, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Hirose, T.; Nakazato, K.; Song, H.; Ishii, N. TGF-beta1 and TNF-alpha are Involved in the Transcription of Type I Collagen alpha2 Gene in Soleus Muscle Atrophied by Mechanical Unloading. J. Appl. Physiol. 2008, 104, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Al-Nassan, S.; Fujita, N.; Kondo, H.; Murakami, S.; Fujino, H. Chronic Exercise Training Down-Regulates TNF-α and Atrogin-1/MAFbx in Mouse Gastrocnemius Muscle Atrophy Induced by Hindlimb Unloading. Acta Histochem. Cytochem. 2012, 45, 343–349. [Google Scholar] [CrossRef] [PubMed]
- Dutt, V.; Saini, V.; Gupta, P.; Kaur, N.; Bala, M.; Gujar, R.; Grewal, A.; Gupta, S.; Dua, A.; Mittal, A. S-allyl cysteine inhibits TNFα-induced skeletal muscle wasting through suppressing proteolysis and expression of inflammatory molecules. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 895–906. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Ogiso, H.; Takeuchi, T.; Kitatani, K.; Umehara, H.; Okazaki, T. Lysosomal Ceramide Generated by Acid Sphingomyelinase Triggers Cytosolic Cathepsin B-mediated Degradation of X-linked Inhibitor of Apoptosis Protein in Natural Killer/T Lymphoma Cell Apoptosis. Cell Death Dis 2015, 6, e1717. [Google Scholar] [CrossRef] [PubMed]
- Laurier-Laurin, M.E.; De Montigny, A.; Attiori Essis, S.; Cyr, M.; Massicotte, G. Blockade of lysosomal acid ceramidase induces glun2b-dependent Tau phosphorylation in rat hippocampal slices. Neural Plast. 2014, 2, 196812. [Google Scholar] [CrossRef] [PubMed]
- Dobrzyń, A.; Górski, J. Ceramides and Sphingomyelins in Skeletal Muscles of the Rat: Content and Composition. Effect of Prolonged Exercise. Am. J. Physiol. Endocrinol. Metab. 2002, 282, E277–E285. [Google Scholar] [CrossRef]
- Helge, J.W.; Dobrzyn, A.; Saltin, B.; Gorski, J. Exercise and Training Effects on Ceramide Metabolism in Human Skeletal Muscle. Exp. Physiol. 2004, 89, 119–127. [Google Scholar] [CrossRef]
- Shynkar, V.V.; Klymchenko, A.S.; Kunzelmann, C.; Duportail, G.; Muller, C.D.; Demchenko, A.P.; Freyssinet, J.M.; Mely, Y. Fluorescent Biomembrane Probe for Ratiometric Detection of Apoptosis. J. Am. Chem. Soc. 2007, 129, 2187–2193. [Google Scholar] [CrossRef]
- Deschenes, M.R.; Adan, M.A.; Kapral, M.C.; Kressin, K.A.; Leathrum, C.M.; Seo, A.; Li, S.; Schaffrey, E.C. Neuromuscular adaptability of male rats to muscle unloading. J. Neurosci. Res. 2018, 96, 284–296. [Google Scholar] [CrossRef]
- Radeva, G.; Sharom, F.J. Isolation and Characterization of Lipid Rafts with Different Properties from RBL-2H3 (Rat Basophilic Leukaemia) Cells. Biochem. J. 2004, 380, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.A. Biological Membranes: A Practical Approach; Findlay, J.B.C., Evans, W.H., Eds.; IRL Press: Washington, DC, USA, 1987; Chapter 4; pp. 103–137. ISBN 978-0-9479-4683-8. [Google Scholar]
- Folch, J.; Lees, M.; Sloane-Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [PubMed]
- Boath, A.; Graf, C.; Lidome, E.; Ullrich, T.; Nussbaumer, P.; Bornancin, F. Regulation and Traffic of Ceramide 1-Phosphate Produced by Ceramide Kinase: Comparative Analysis to Glucosylceramide and Sphingomyelin. J. Biol. Chem. 2008, 283, 8517–8526. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of Structural Proteins during the Assembly of the Head of Bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrov, A.M.; Shalagina, M.N.; Protopopov, V.A.; Sergeev, V.G.; Ovechkin, S.V.; Ovchinina, N.G.; Sekunov, A.V.; Zefirov, A.L.; Zakirjanova, G.F.; Bryndina, I.G. Changes in Membrane Ceramide Pools in Rat Soleus Muscle in Response to Short-Term Disuse. Int. J. Mol. Sci. 2019, 20, 4860. https://doi.org/10.3390/ijms20194860
Petrov AM, Shalagina MN, Protopopov VA, Sergeev VG, Ovechkin SV, Ovchinina NG, Sekunov AV, Zefirov AL, Zakirjanova GF, Bryndina IG. Changes in Membrane Ceramide Pools in Rat Soleus Muscle in Response to Short-Term Disuse. International Journal of Molecular Sciences. 2019; 20(19):4860. https://doi.org/10.3390/ijms20194860
Chicago/Turabian StylePetrov, Alexey M., Maria N. Shalagina, Vladimir A. Protopopov, Valeriy G. Sergeev, Sergey V. Ovechkin, Natalia G. Ovchinina, Alexey V. Sekunov, Andrey L. Zefirov, Guzalia F. Zakirjanova, and Irina G. Bryndina. 2019. "Changes in Membrane Ceramide Pools in Rat Soleus Muscle in Response to Short-Term Disuse" International Journal of Molecular Sciences 20, no. 19: 4860. https://doi.org/10.3390/ijms20194860
APA StylePetrov, A. M., Shalagina, M. N., Protopopov, V. A., Sergeev, V. G., Ovechkin, S. V., Ovchinina, N. G., Sekunov, A. V., Zefirov, A. L., Zakirjanova, G. F., & Bryndina, I. G. (2019). Changes in Membrane Ceramide Pools in Rat Soleus Muscle in Response to Short-Term Disuse. International Journal of Molecular Sciences, 20(19), 4860. https://doi.org/10.3390/ijms20194860





