NET4 Modulates the Compactness of Vacuoles in Arabidopsis thaliana
Abstract
1. Introduction
2. Results
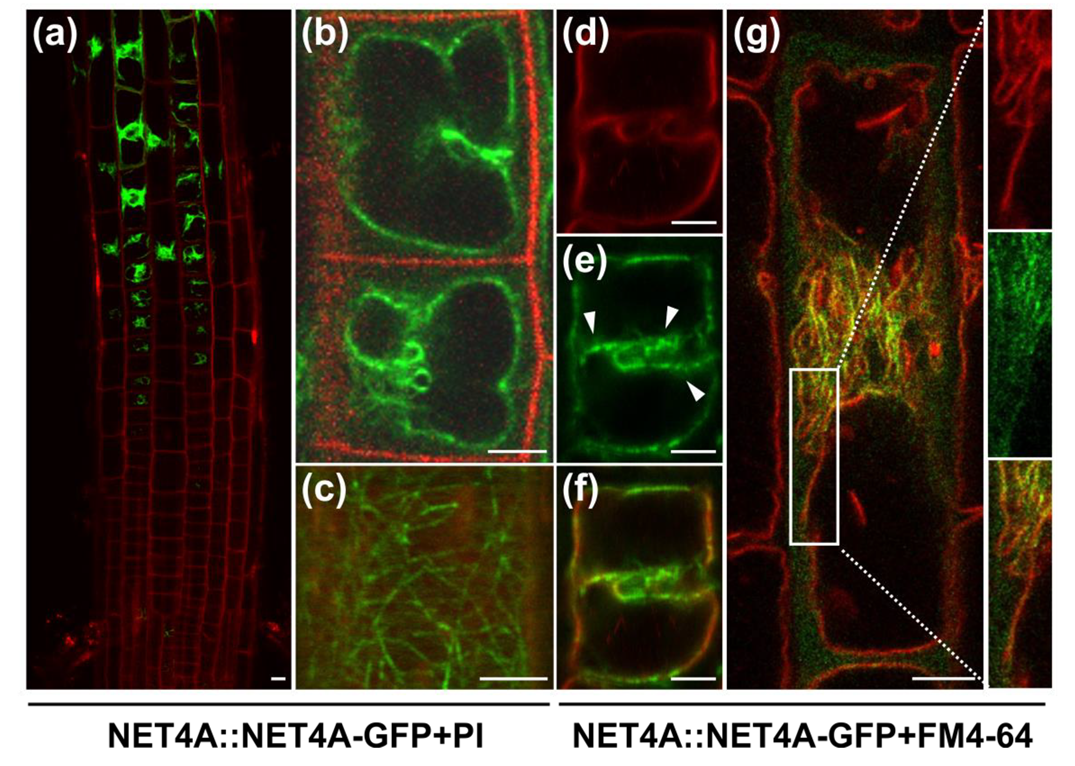
2.1. NET4A Shows a Bead-on-a-String Pattern at the Tonoplast
2.2. NET4A Localizes to Highly Constricted Vacuolar Membranes
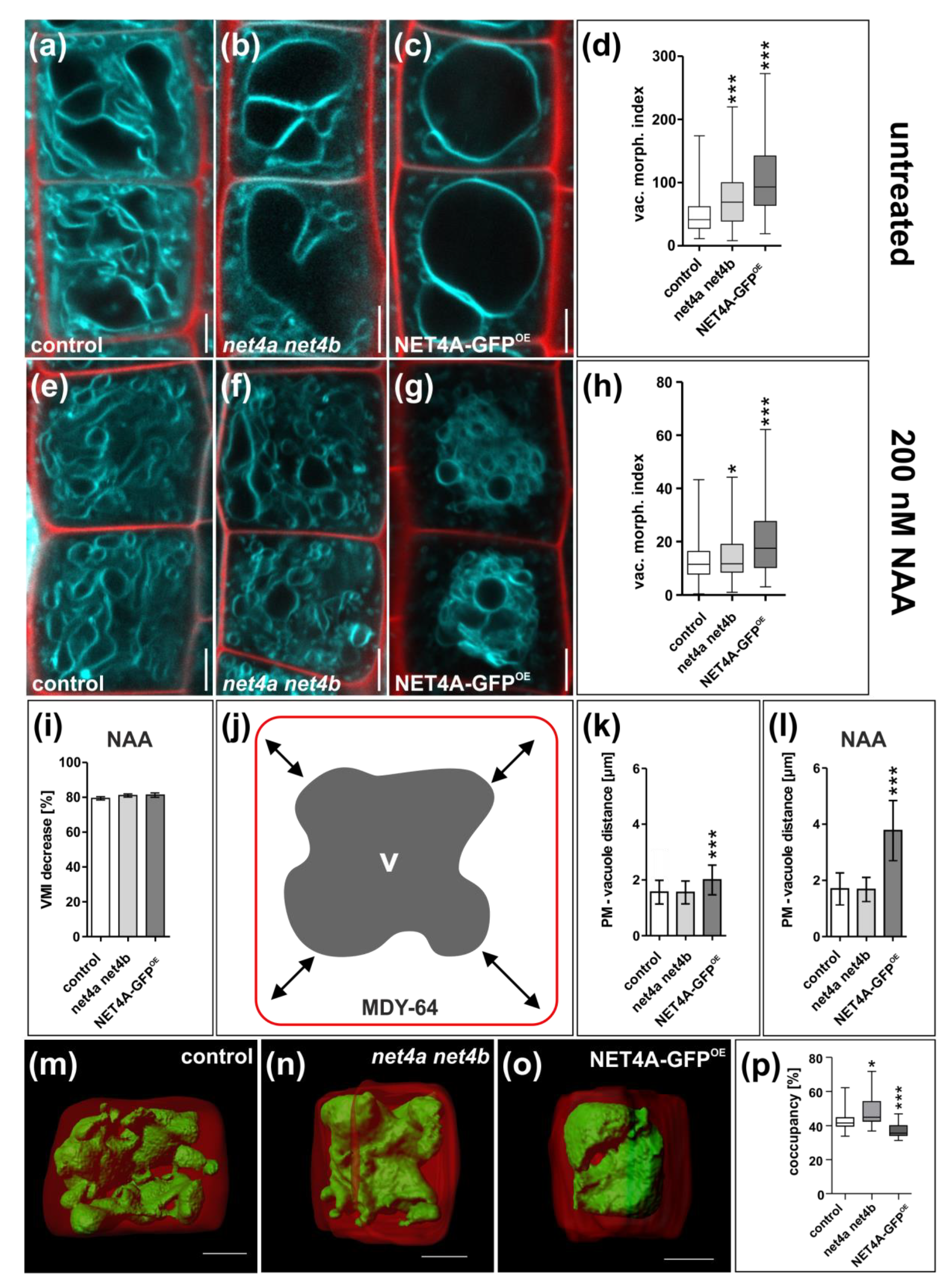
2.3. NET4A Impacts on Vacuolar Morphology
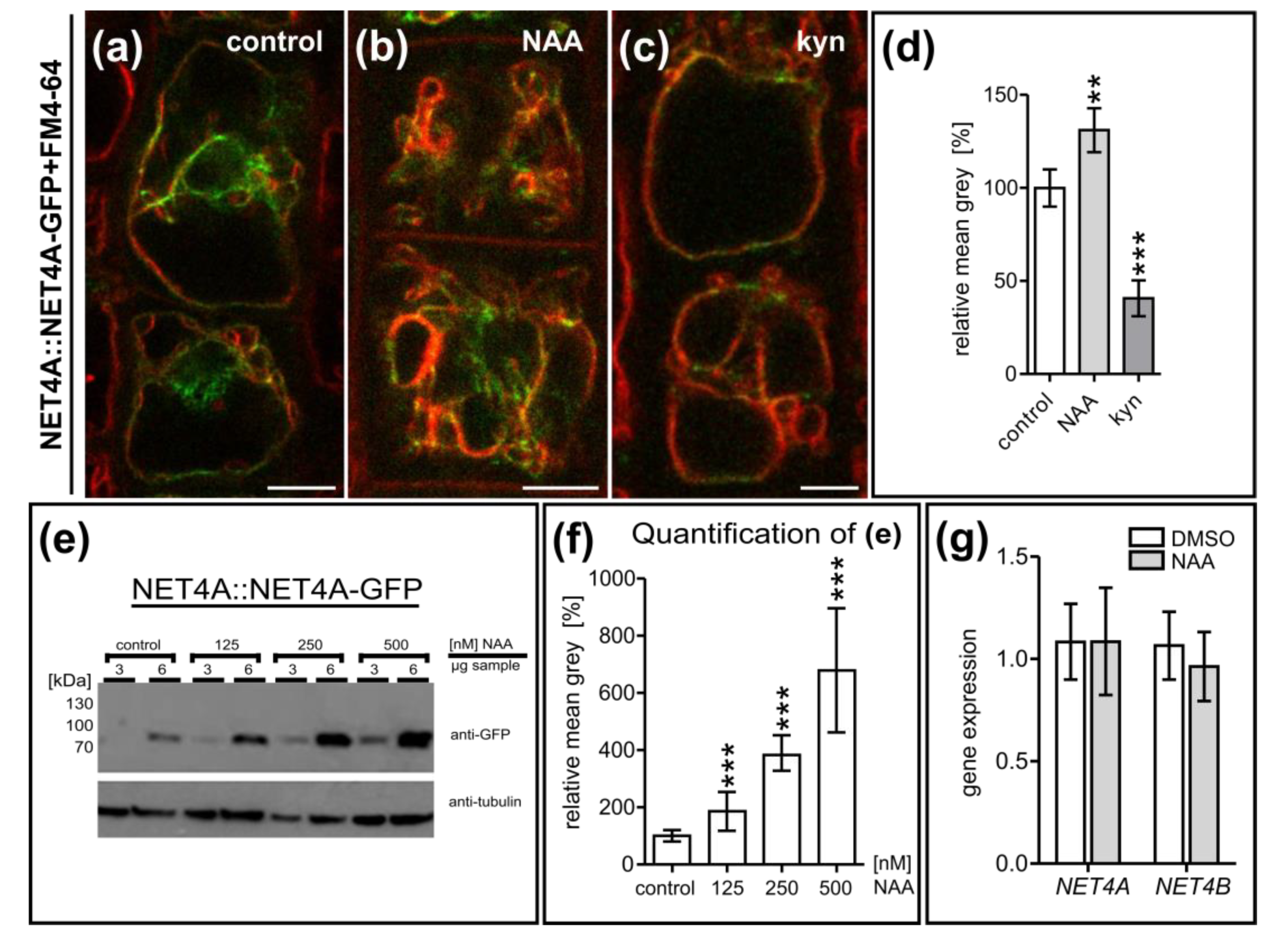
2.4. NET4 and Auxin Spatially Define Vacuolar Occupation within the Cell
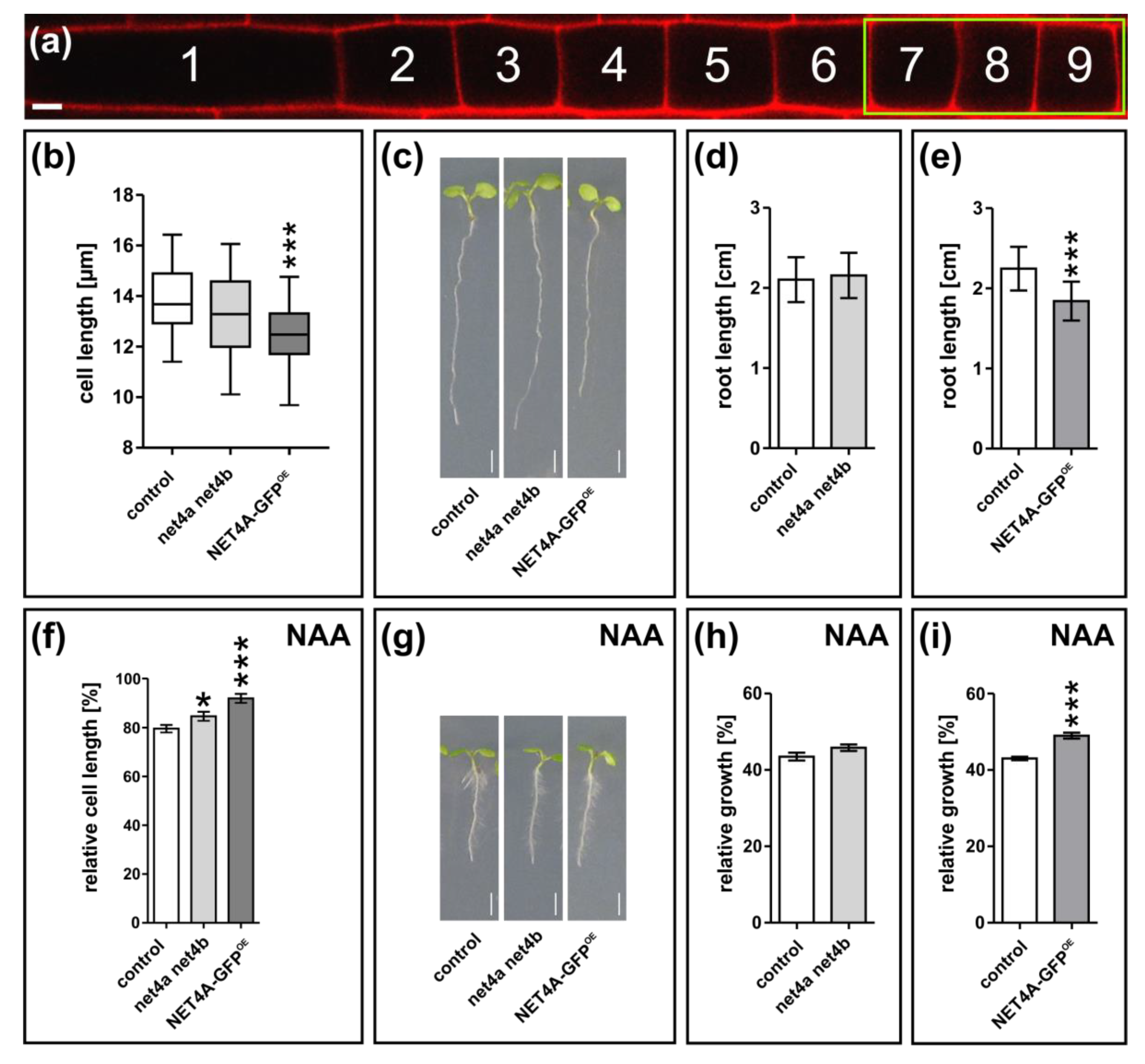
2.5. NET4A-Dependent Compacting of the Vacuole Correlates with Reduced Cell Size and Root Organ Growth
3. Discussion
4. Materials and Methods
4.1. NET4 Gene Accession Codes
4.2. Plant Material, Growth Conditions and DNA Constructs
4.3. Chemicals and Treatments
4.4. RNA Extraction and Quantitative Real Time PCR
4.5. Phenotype Analysis
4.6. 3D Surface Rendering
4.7. Western Blotting
4.8. Confocal Microscopy
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Löfke, C.; Dünser, K.; Scheuring, D.; Kleine-Vehn, J. Auxin regulates SNARE-dependent vacuolar morphology restricting cell size. Elife 2015, 4, e05868. [Google Scholar]
- Scheuring, D.; Löfke, C.; Krüger, F.; Kittelmann, M.; Eisa, A.; Hughes, L.; Smith, R.S.; Hawes, C.; Schumacher, K.; Kleine-Vehn, J. Actin-dependent vacuolar occupancy of the cell determines auxin-induced growth repression. Proc. Natl. Acad. Sci. USA 2016, 113, 452–457. [Google Scholar] [PubMed]
- Dünser, K.; Gupta, S.; Herger, A.; Feraru, M.I.; Ringli, C.; Kleine-Vehn, J. Extracellular matrix sensing by FERONIA and Leucine-Rich Repeat Extensins controls vacuolar expansion during cellular elongation in Arabidopsis thaliana. EMBO J. 2019, 38, e100353. [Google Scholar] [PubMed]
- Faix, J.; Steinmetz, M.; Boves, H.; Kammerer, R.A.; Lottspeich, F.; Mintert, U.; Murphy, J.; Stock, A.; Aebi, U.; Gerisch, G. Cortexillins, major determinants of cell shape and size, are actin-bundling proteins with a parallel coiled-coil tail. Cell 1996, 86, 631–642. [Google Scholar] [PubMed]
- Fletcher, D.A.; Mullins, R.D. Cell mechanics and the cytoskeleton. Nature 2010, 463, 485–492. [Google Scholar]
- Uemura, T.; Yoshimura, S.H.; Takeyasu, K.; Sato, M.H. Vacuolar membrane dynamics revealed by GFP-AtVam3 fusion protein. Genes Cells 2002, 7, 743–753. [Google Scholar]
- Mathur, J.; Mathur, N.; Kernebeck, B.; Hülskamp, M. Mutations in Actin-Related Proteins 2 and 3 Affect Cell Shape Development in Arabidopsis. Plant. Cell 2003, 15, 1632–1645. [Google Scholar]
- Rahman, A.; Bannigan, A.; Sulaman, W.; Pechter, P.; Blancaflor, E.B.; Baskin, T.I. Auxin, actin and growth of the Arabidopsis thaliana primary root. Plant. J. 2007, 50, 514–528. [Google Scholar]
- Li, G.; Liang, W.; Zhang, X.; Ren, H.; Hu, J.; Bennett, M.J.; Zhang, D. Rice actin-binding protein RMD is a key link in the auxin-actin regulatory loop that controls cell growth. Proc. Natl. Acad. Sci. USA 2014, 111, 10377–10382. [Google Scholar]
- Lanza, M.; Garcia-Ponce, B.; Castrillo, G.; Catarecha, P.; Sauer, M.; Rodriguez-Serrano, M.; Páez-García, A.; Sánchez-Bermejo, E.; Mohan, T.C.; del Puerto, Y.L.; et al. Role of actin cytoskeleton in brassinosteroid signaling and in its integration with the auxin response in plants. Dev. Cell 2012, 22, 1275–1285. [Google Scholar]
- Kutsuna, N.; Kumagai, F.; Sato, M.H.; Hasezawa, S. Three-dimensional reconstruction of tubular structure of vacuolar membrane throughout mitosis in living tobacco cells. Plant. Cell Physiol. 2003, 44, 1045–1054. [Google Scholar] [PubMed][Green Version]
- Deeks, M.J.; Calcutt, J.R.; Ingle, E.K.S.; Hawkins, T.J.; Chapman, S.; Richardson, A.C.; Mentlak, D.A.; Dixon, M.R.; Cartwright, F.; Smertenko, A.P.; et al. A superfamily of actin-binding proteins at the actin-membrane nexus of higher plants. Curr. Biol. 2012, 22, 1595–1600. [Google Scholar] [PubMed]
- Löfke, C.; Dünser, K.; Kleine-Vehn, J. Epidermal patterning genes impose non-cell autonomous cell size determination and have additional roles in root meristem size control. J. Integr. Plant. Biol. 2013, 55, 864–875. [Google Scholar] [PubMed]
- Scheuring, D.; Schöller, M.; Kleine-Vehn, J.; Löfke, C. Vacuolar staining methods in plant cells. Methods Mol. Biol. 2015, 1242, 83–92. [Google Scholar] [PubMed]
- Andrés, Z.; Pérez-Hormaeche, J.; Leidi, E.O.; Schlücking, K.; Steinhorst, L.; McLachlan, D.H.; Schumacher, K.; Hetherington, A.M.; Kudla, J.; Cubero, B.; et al. Control of vacuolar dynamics and regulation of stomatal aperture by tonoplast potassium uptake. Proc. Natl. Acad. Sci. USA 2014, 111, E1806–E1814. [Google Scholar] [PubMed]
- Hawes, C.; Saint-Jore, C.M.; Brandizzi, F.; Zheng, H.; Andreeva, A.V.; Boevink, P. Cytoplasmic illuminations: In planta targeting of fluorescent proteins to cellular organelles. Protoplasma 2001, 215, 77–88. [Google Scholar] [PubMed]
- Singh, M.K.; Krüger, F.; Beckmann, H.; Brumm, S.; Vermeer, J.E.M.; Munnik, T.; Mayer, U.; Stierhof, Y.-D.; Grefen, C.; Schumacher, K.; et al. Protein delivery to vacuole requires SAND protein-dependent Rab GTPase conversion for MVB-vacuole fusion. Curr. Biol. 2014, 24, 1383–1389. [Google Scholar] [PubMed]
- Barbez, E.; Dünser, K.; Gaidora, A.; Lendl, T.; Busch, W. Auxin steers root cell expansion via apoplastic pH regulation in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2017, 114, E4884–E4893. [Google Scholar] [PubMed]
- Rojo, E.; Gillmor, C.S.; Kovaleva, V.; Somerville, C.R.; Raikhel, N.V. VACUOLELESS1 is an essential gene required for vacuole formation and morphogenesis in Arabidopsis. Dev. Cell 2001, 1, 303–310. [Google Scholar]
- Schumacher, K.; Vafeados, D.; McCarthy, M.; Sze, H.; Wilkins, T.; Chory, J. The Arabidopsis det3 mutant reveals a central role for the vacuolar H+–ATPase in plant growth and development. Genes Dev. 1999, 13, 3259–3270. [Google Scholar]
- Owens, T.; Poole, R.J. Regulation of cytoplasmic and vacuolar volumes by plant cells in suspension culture. Plant. Physiol. 1979, 64, 900–904. [Google Scholar] [PubMed]
- Berger, F.; Hung, C.Y.; Dolan, L.; Schiefelbein, J. Control of cell division in the root epidermis of Arabidopsis thaliana. Dev. Biol. 1998, 194, 235–245. [Google Scholar] [PubMed]
- Krüger, F.; Schumacher, K. Pumping up the volume-vacuole biogenesis in Arabidopsis thaliana. Semin. Cell Dev. Biol. 2018, 80, 106–112. [Google Scholar] [PubMed]
- Higaki, T.; Kutsuna, N.; Okubo, E.; Sano, T.; Hasezawa, S. Actin microfilaments regulate vacuolar structures and dynamics: Dual observation of actin microfilaments and vacuolar membrane in living tobacco BY-2 Cells. Plant. Cell Physiol. 2006, 47, 839–852. [Google Scholar] [PubMed]
- Eitzen, G.; Wang, L.; Thorngren, N.; Wickner, W. Remodeling of organelle-bound actin is required for yeast vacuole fusion. J. Cell Biol. 2002, 158, 669–679. [Google Scholar] [PubMed]
- Zhang, C.; Hicks, G.R.; Raikhel, N.V. Plant vacuole morphology and vacuolar trafficking. Front. Plant Sci. 2014, 5, 476. [Google Scholar] [PubMed]
- Staiger, C.J.; Yuan, M.; Valenta, R.; Shaw, P.J.; Warn, R.M.; Lloyd, C.W. Microinjected profilin affects cytoplasmic streaming in plant cells by rapidly depolymerizing actin microfilaments. Curr. Biol. 1994, 4, 215–219. [Google Scholar]
- Li, L.-J.; Ren, F.; Gao, X.-Q.; Wei, P.-C.; Wang, X.-C. The reorganization of actin filaments is required for vacuolar fusion of guard cells during stomatal opening in Arabidopsis. Plant. Cell Environ. 2013, 36, 484–497. [Google Scholar]
- Wang, P.; Hawkins, T.J.; Richardson, C.; Cummins, I.; Deeks, M.J.; Sparkes, I.; Hawes, C.; Hussey, P.J. The plant cytoskeleton, NET3C, and VAP27 mediate the link between the plasma membrane and endoplasmic reticulum. Curr. Biol. 2014, 24, 1397–1405. [Google Scholar]
- Karimi, M.; De Meyer, B.; Hilson, P. Modular cloning in plant cells. Trends Plant Sci. 2005, 10, 103–105. [Google Scholar]
- Barbez, E.; Kubeš, M.; Rolčík, J.; Béziat, C.; Pěnčík, A.; Wang, B.; Rosquete, M.R.; Zhu, J.; Dobrev, P.I.; Lee, Y.; et al. A novel putative auxin carrier family regulates intracellular auxin homeostasis in plants. Nature 2012, 485, 119–122. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaiser, S.; Eisa, A.; Kleine-Vehn, J.; Scheuring, D. NET4 Modulates the Compactness of Vacuoles in Arabidopsis thaliana. Int. J. Mol. Sci. 2019, 20, 4752. https://doi.org/10.3390/ijms20194752
Kaiser S, Eisa A, Kleine-Vehn J, Scheuring D. NET4 Modulates the Compactness of Vacuoles in Arabidopsis thaliana. International Journal of Molecular Sciences. 2019; 20(19):4752. https://doi.org/10.3390/ijms20194752
Chicago/Turabian StyleKaiser, Sabrina, Ahmed Eisa, Jürgen Kleine-Vehn, and David Scheuring. 2019. "NET4 Modulates the Compactness of Vacuoles in Arabidopsis thaliana" International Journal of Molecular Sciences 20, no. 19: 4752. https://doi.org/10.3390/ijms20194752
APA StyleKaiser, S., Eisa, A., Kleine-Vehn, J., & Scheuring, D. (2019). NET4 Modulates the Compactness of Vacuoles in Arabidopsis thaliana. International Journal of Molecular Sciences, 20(19), 4752. https://doi.org/10.3390/ijms20194752





