Activating Hippo Pathway via Rassf1 by Ursolic Acid Suppresses the Tumorigenesis of Gastric Cancer
Abstract
1. Introduction
2. Results
2.1. Cell Proliferation Inhibition by UA in Gastric Cancer Cells
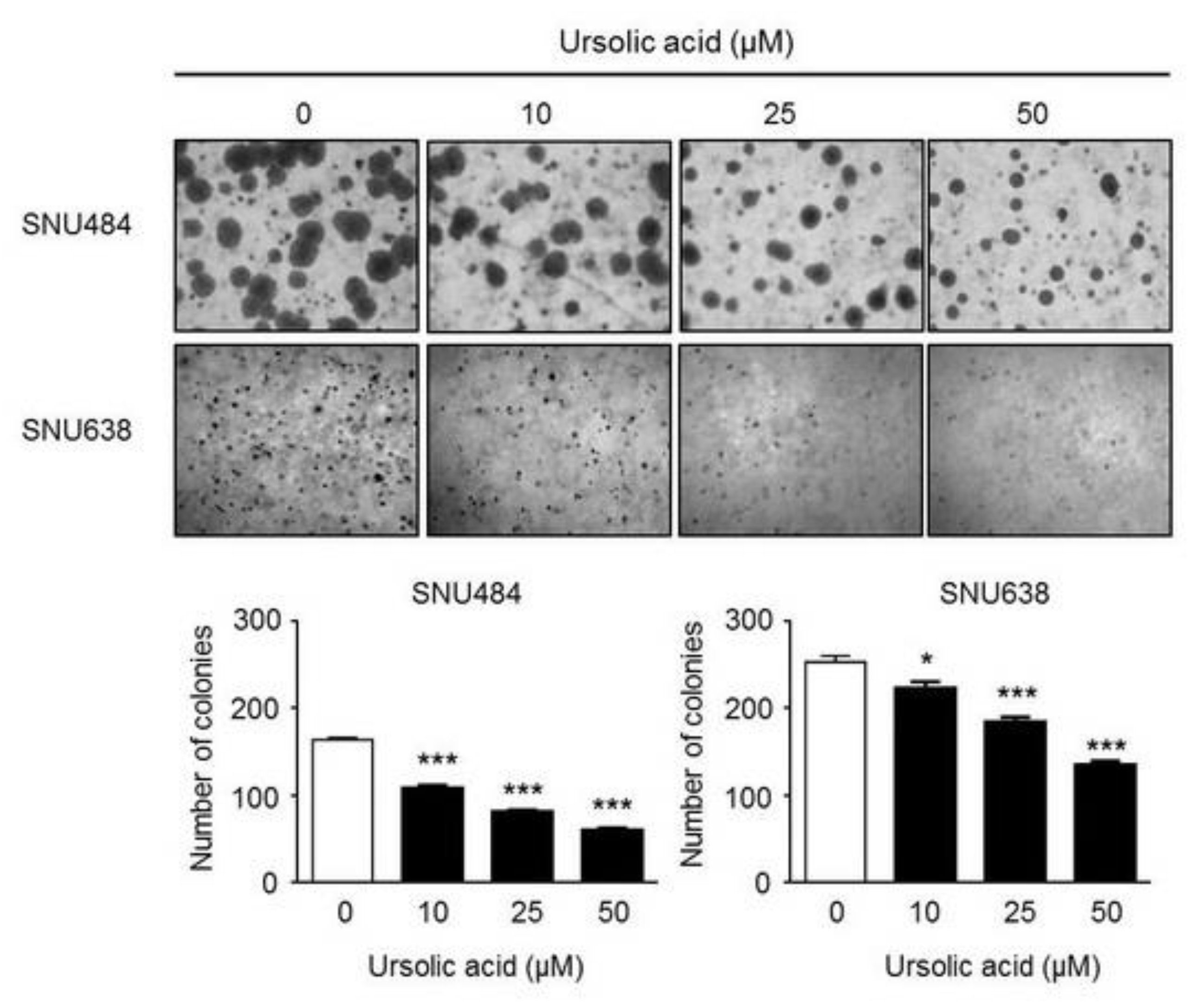
2.2. Inhibitory Effect of UA on Colony Formation in Gastric Cancer Cells
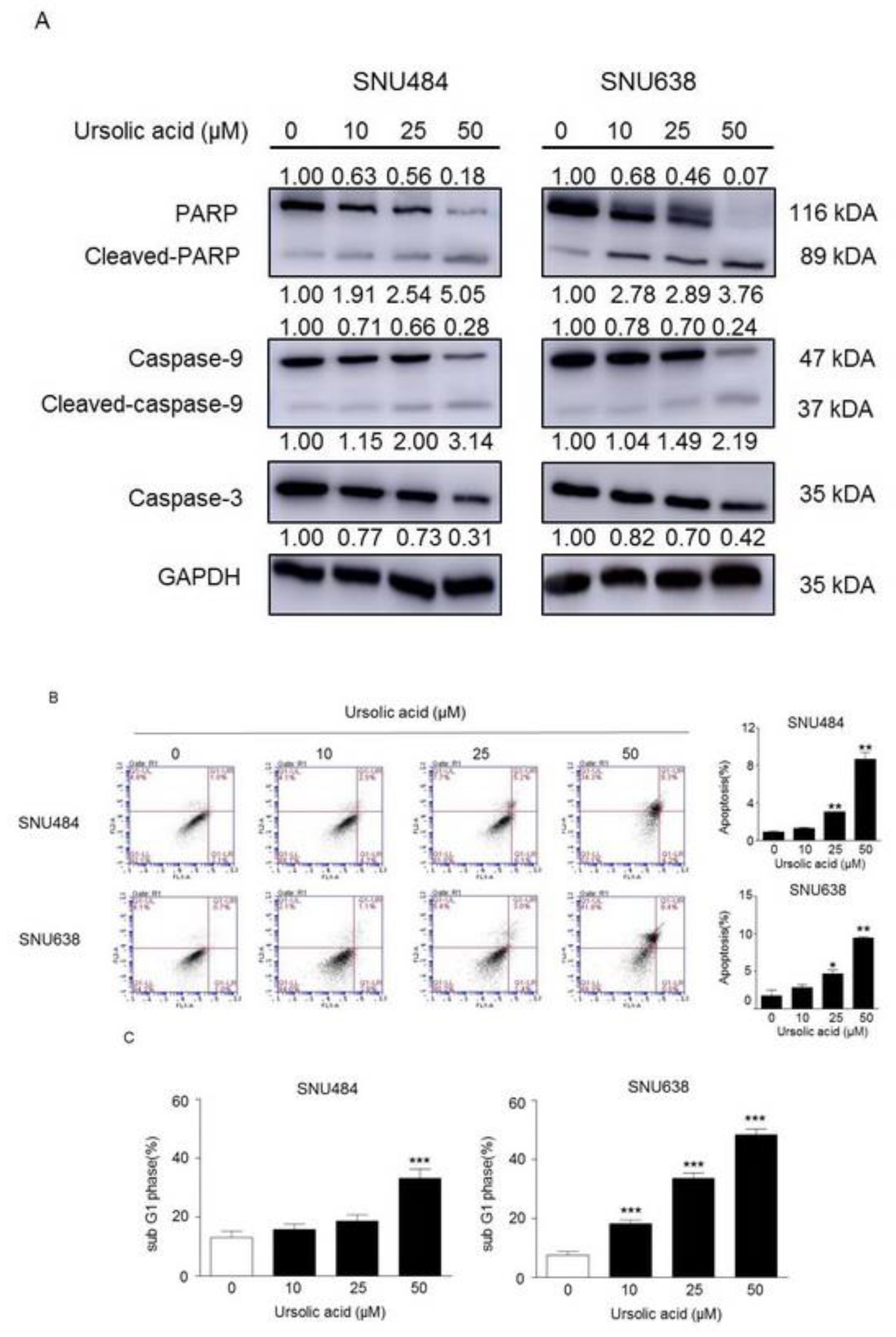
2.3. Induction of Apoptosis by UA in Gastric Cancer Cells
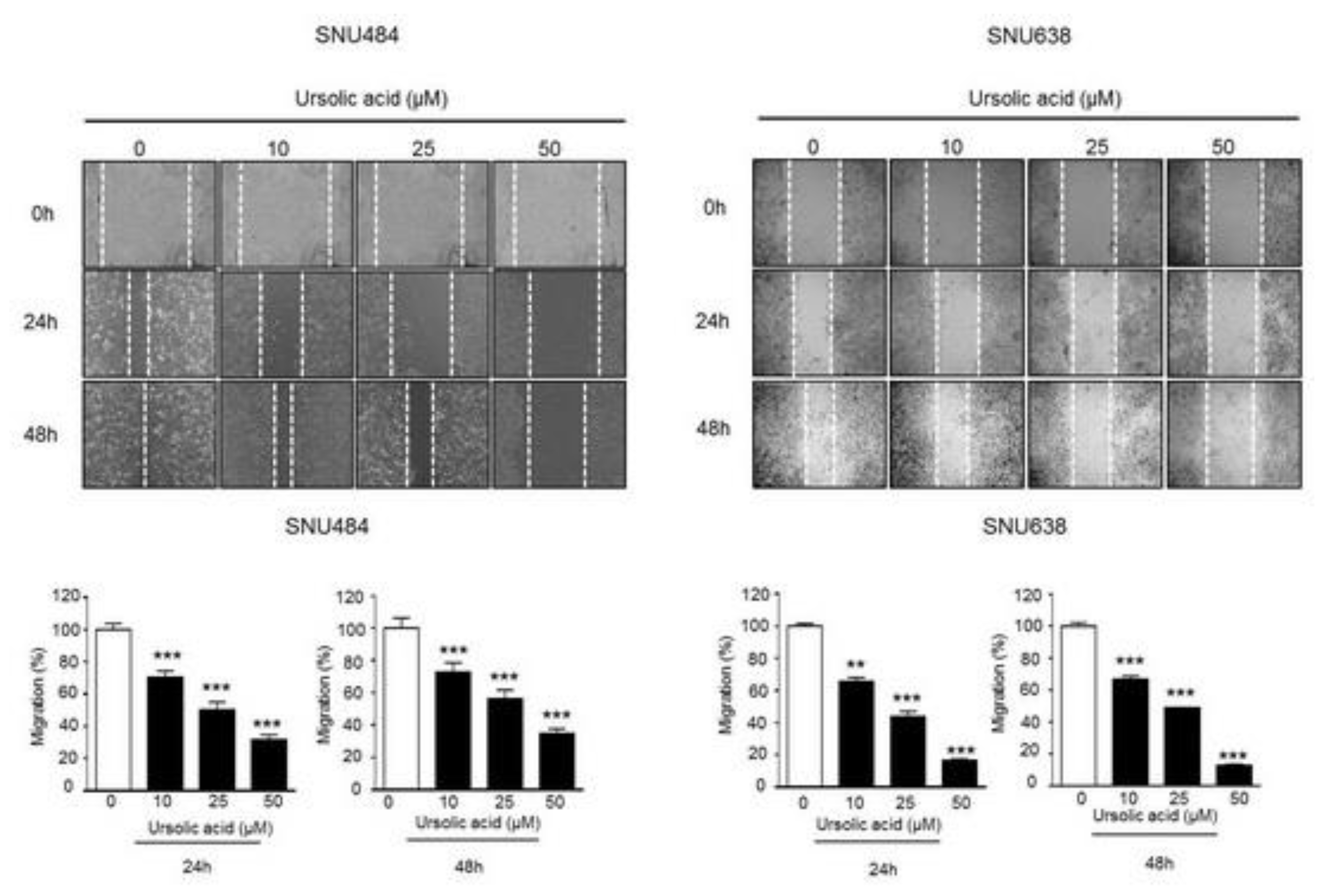
2.4. Inhibitory Effect of UA on Migration in Gastric Cancer Cells
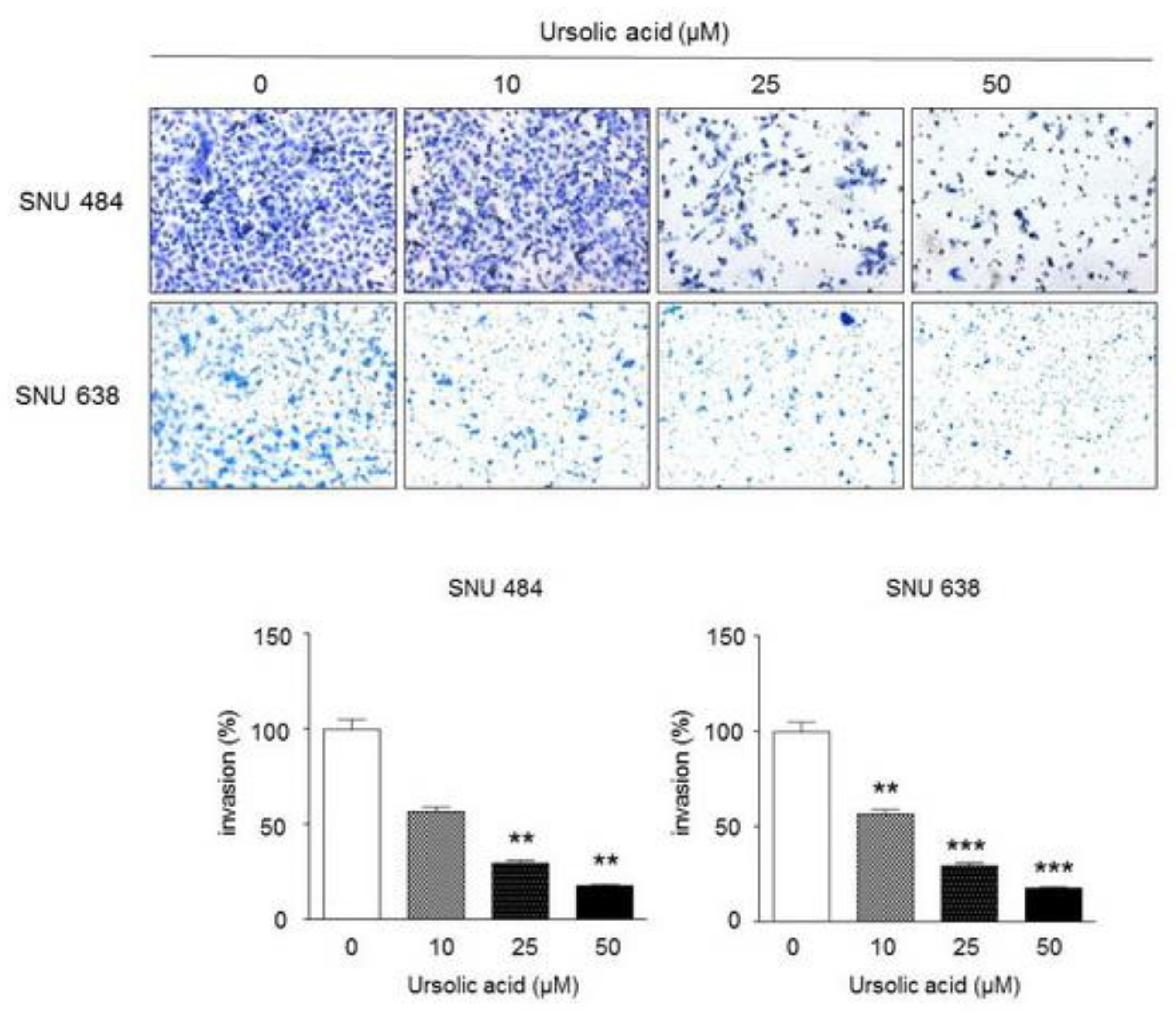
2.5. Inhibitory Effect of UA on Invasiveness in Gastric Cancer Cells
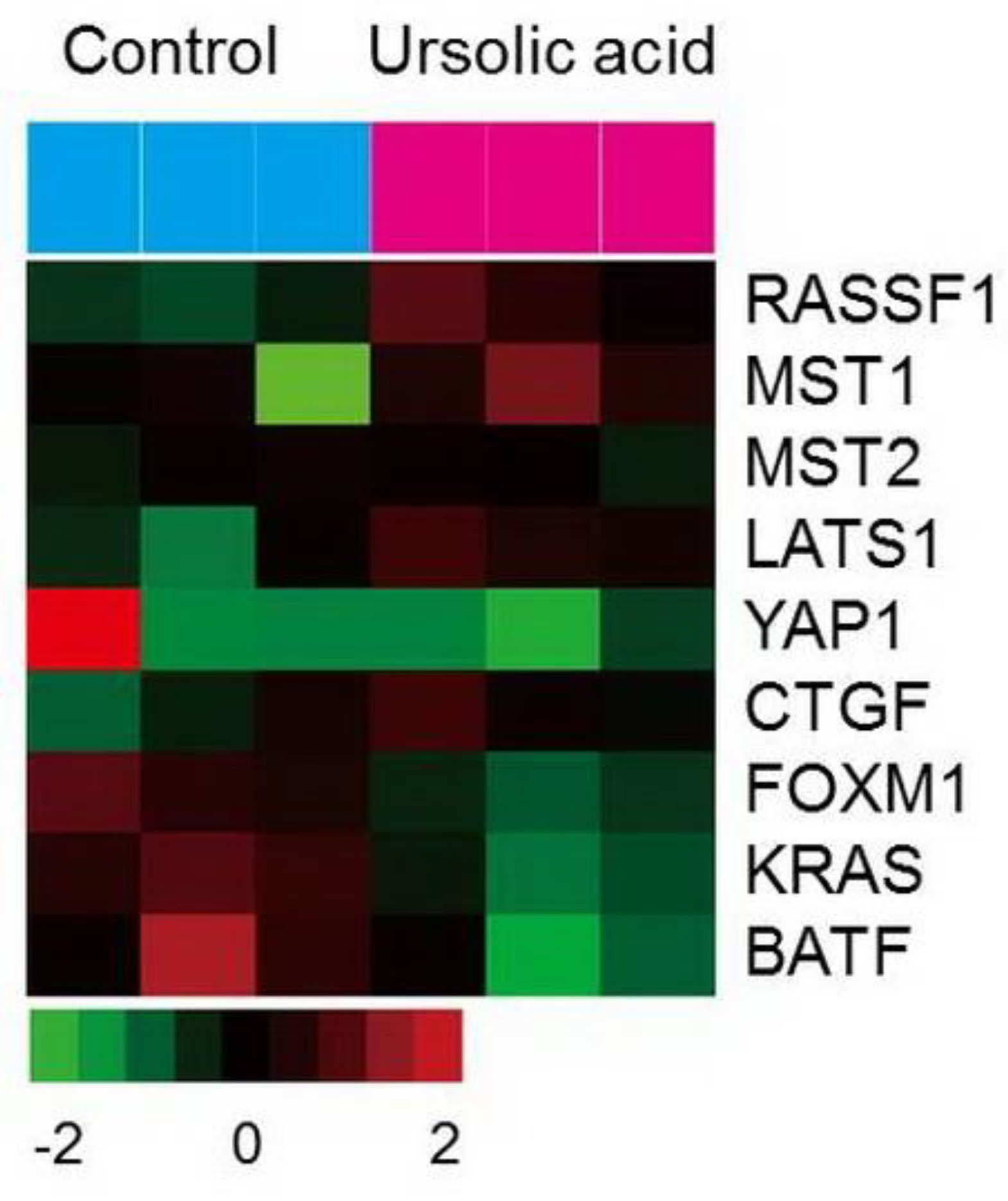
2.6. Gene Expression of the Hippo Pathway by UA in Gastric Cancer Cells
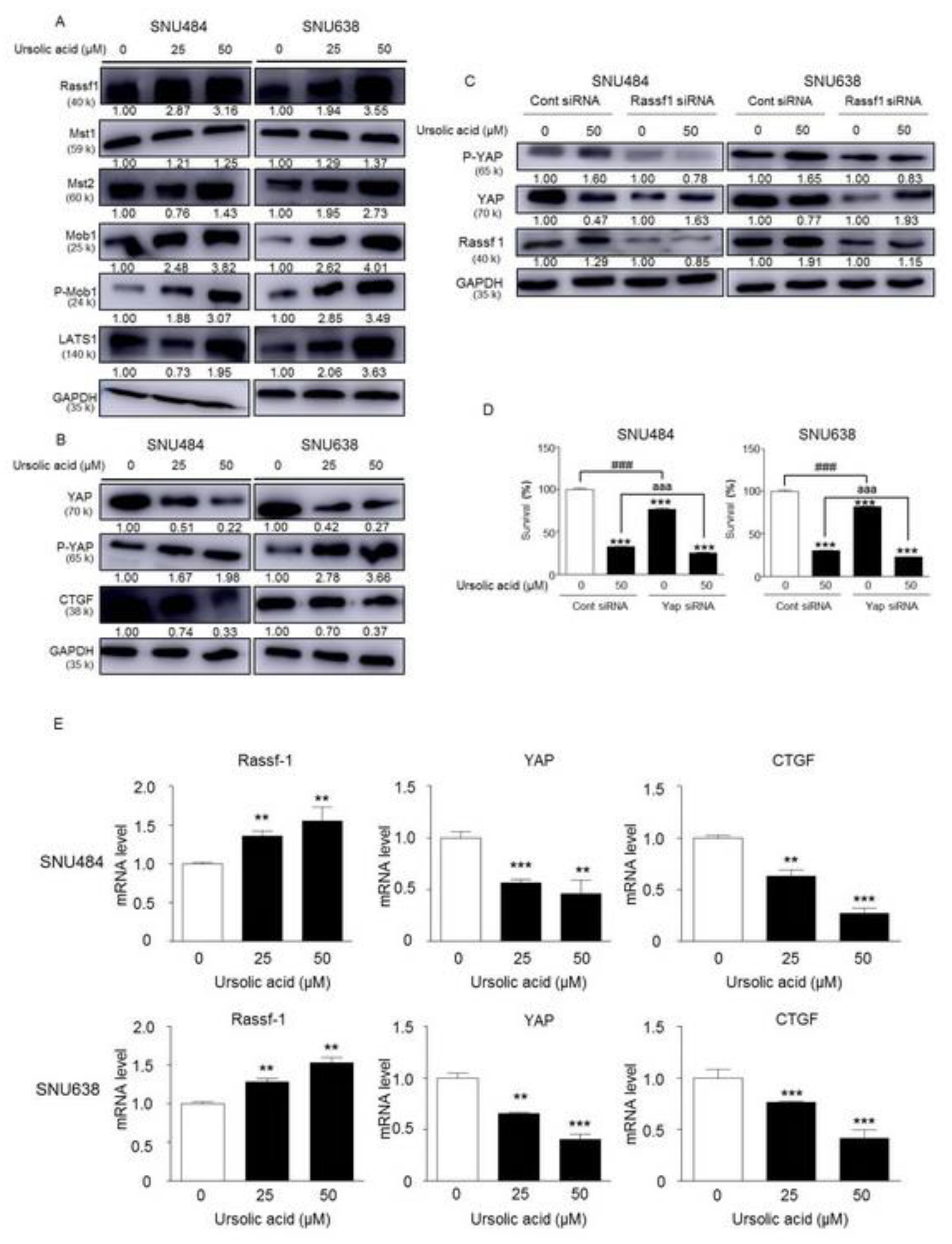
2.7. Activation of Hippo Signaling Pathway by UA in Gastric Cancer Cells
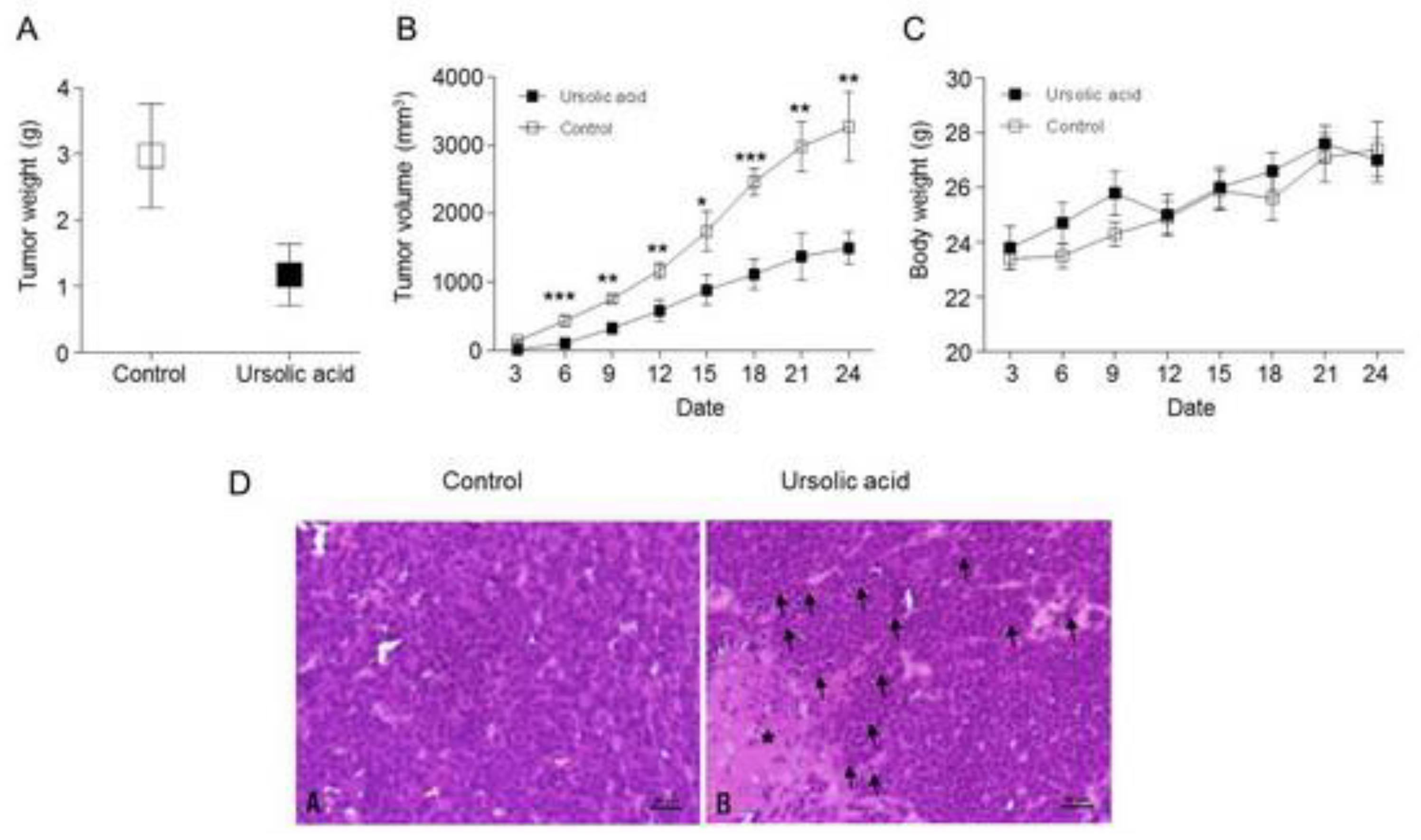
2.8. Inhibitory effect of UA on Tumor Growth in Xenograft Animal
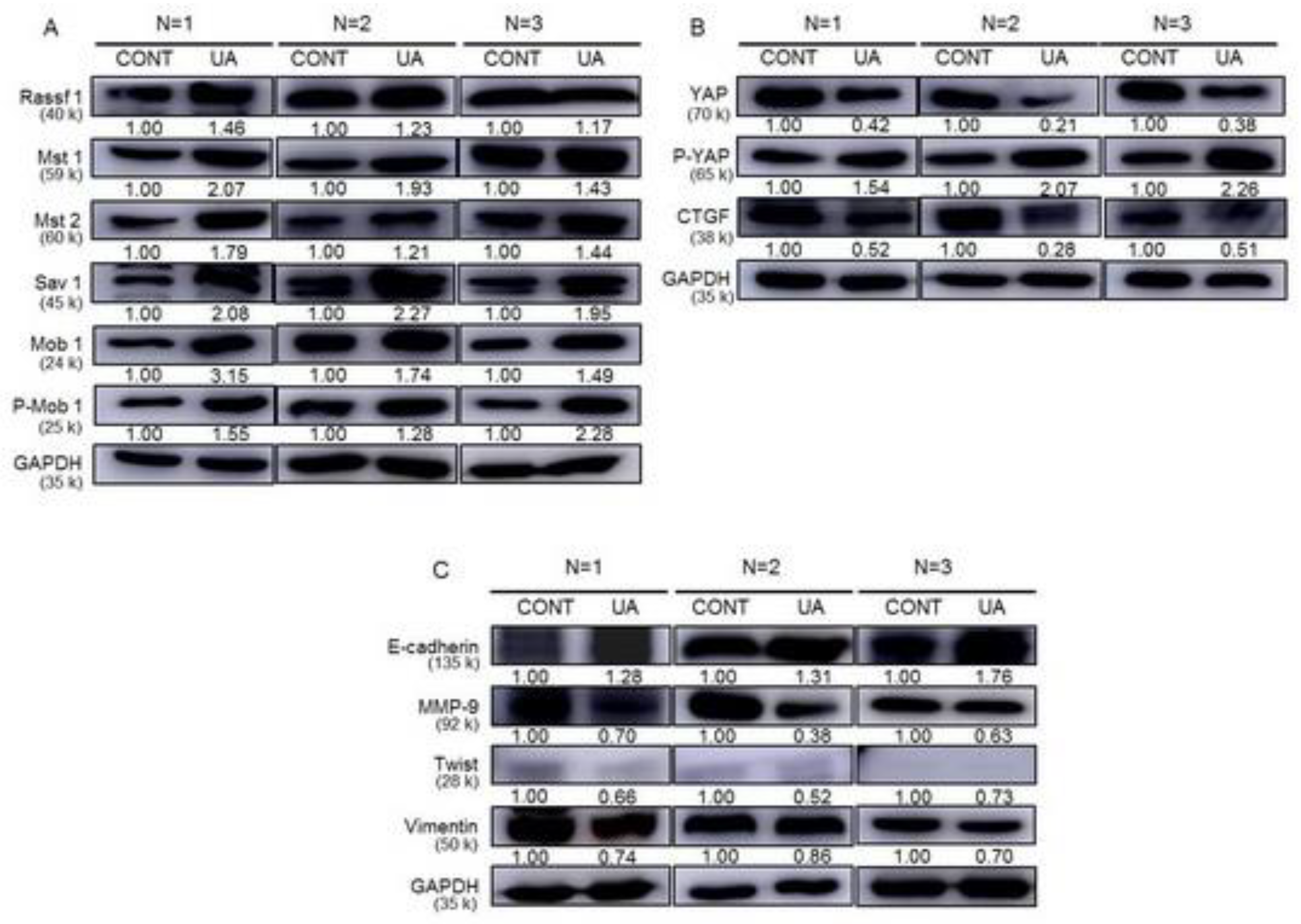
2.9. Activation of the Hippo Signaling Pathway by UA in the Tumor Tissues of Xenograft Animals
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Reagents
4.2. Cell Proliferation Assay
4.3. Western Blot Analysis
4.4. Soft Agar Colony Formation
4.5. Matrigel Invasion Assay
4.6. Wound Healing Assay
4.7. Microarray Experiment and Data Analysis
4.8. Cell Cycle Analysis
4.9. FITC Annexin V Staining
4.10. siRNA Transfection
4.11. RNA Isolation and Real-Time Polymerase Chain Reaction
4.12. In vivo Xenograft Animal Study
4.13. Histopathological Analysis
4.14. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Cho, J.Y.; Lim, J.Y.; Cheong, J.H.; Park, Y.Y.; Yoon, S.L.; Kim, S.M.; Kim, S.B.; Kim, H.; Hong, S.W.; Park, Y.N.; et al. Gene expression signature-based prognostic risk score in gastric cancer. Clin. Cancer Res. 2011, 17, 1850–1857. [Google Scholar] [CrossRef] [PubMed]
- Parkin, D.M.; Bray, F.; Ferlay, J.; Pisani, P. Global cancer statistics, 2002. CA Cancer J. Clin. 2005, 55, 74–108. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Bray, F.; Siegel, R.L.; Ferlay, J.; Lortet-Tieulent, J.; Jemal, A. Global cancer statistics, 2012. CA Cancer J. Clin. 2015, 65, 87–108. [Google Scholar] [CrossRef] [PubMed]
- Torre, L.A.; Siegel, R.L.; Ward, E.M.; Jemal, A. Global Cancer Incidence and Mortality Rates and Trends—An Update. Cancer Epidemiol. Biomark. Prev. 2016, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.S.; Jiang, J. Induction of apoptosis and regulation of the MAPK pathway by ursolic acid in human leukemia K562 cells. Planta Med. 2007, 73, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Baek, J.H.; Yoo, M.A.; Chung, H.Y.; Kim, N.D.; Kim, K.W. Induction of apoptosis by ursolic acid through activation of caspases and down-regulation of c-IAPs in human prostate epithelial cells. Int. J. Oncol. 2000, 17, 565–571. [Google Scholar] [CrossRef]
- Hsu, Y.L.; Kuo, P.L.; Lin, C.C. Proliferative inhibition, cell-cycle dysregulation, and induction of apoptosis by ursolic acid in human non-small cell lung cancer A549 cells. Life Sci. 2004, 75, 2303–2316. [Google Scholar] [CrossRef]
- Manu, K.A.; Kuttan, G. Ursolic acid induces apoptosis by activating p53 and caspase-3 gene expressions and suppressing NF-κB mediated activation of bcl-2 in B16F-10 melanoma cells. Int. Immunopharmacol. 2008, 8, 974–981. [Google Scholar] [CrossRef]
- Achiwa, Y.; Hasegawa, K.; Komiya, T.; Udagawa, Y. Ursolic acid induces Bax-dependent apoptosis through the caspase-3 pathway in endometrial cancer SNG-II cells. Oncol. Rep. 2005, 13, 51–57. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, Y.H.; Song, G.Y.; Kim, D.E.; Jeong, Y.J.; Liu, K.H.; Chung, Y.H.; Oh, S. Ursolic acid and its natural derivative corosolic acid suppress the proliferation of APC-mutated colon cancer cells through promotion of beta-catenin degradation. Food Chem. Toxicol. 2014, 67, 87–95. [Google Scholar] [CrossRef]
- Huang, M.T.; Ho, C.T.; Wang, Z.Y.; Ferraro, T.; Lou, Y.R.; Stauber, K.; Ma, W.; Georgiadis, C.; Laskin, J.D.; Conney, A.H. Inhibition of skin tumorigenesis by rosemary and its constituents carnosol and ursolic acid. Cancer Res. 1994, 54, 701–708. [Google Scholar]
- Ohigashi, H.; Takamura, H.; Koshimizu, K.; Tokuda, H.; Ito, Y. Search for possible antitumor promoters by inhibition of 12-O-tetradecanoylphorbol-13-acetate-induced Epstein-Barr virus activation; ursolic acid and oleanolic acid from an anti-inflammatory Chinese medicinal plant, Glechoma hederaceae L. Cancer Lett. 1986, 30, 143–151. [Google Scholar] [CrossRef]
- Sohn, K.H.; Lee, H.Y.; Chung, H.Y.; Young, H.S.; Yi, S.Y.; Kim, K.W. Anti-angiogenic activity of triterpene acids. Cancer Lett. 1995, 94, 213–218. [Google Scholar] [CrossRef]
- Choi, B.M.; Park, R.; Pae, H.O.; Yoo, J.C.; Kim, Y.C.; Jun, C.D.; Jung, B.H.; Oh, G.S.; So, H.S.; Kim, Y.M.; et al. Cyclic adenosine monophosphate inhibits ursolic acid-induced apoptosis via activation of protein kinase A in human leukaemic HL-60 cells. Pharmacol. Toxicol. 2000, 86, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.K.; Baek, J.H.; Kang, C.M.; Yoo, M.A.; Sung, J.W.; Chung, H.Y.; Kim, N.D.; Choi, Y.H.; Lee, S.H.; Kim, K.W. Apoptotic activity of ursolic acid may correlate with the inhibition of initiation of DNA replication. Int. J. Cancer 2000, 87, 629–636. [Google Scholar] [CrossRef]
- Harmand, P.O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Shishodia, S.; Majumdar, S.; Banerjee, S.; Aggarwal, B.B. Ursolic acid inhibits nuclear factor-κB activation induced by carcinogenic agents through suppression of IκBα kinase and p65 phosphorylation: Correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Res. 2003, 63, 4375–4383. [Google Scholar] [PubMed]
- Kassi, E.; Sourlingas, T.G.; Spiliotaki, M.; Papoutsi, Z.; Pratsinis, H.; Aligiannis, N.; Moutsatsou, P. Ursolic acid triggers apoptosis and Bcl-2 downregulation in MCF-7 breast cancer cells. Cancer Investig. 2009, 27, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Subbaramaiah, K.; Michaluart, P.; Sporn, M.B.; Dannenberg, A.J. Ursolic acid inhibits cyclooxygenase-2 transcription in human mammary epithelial cells. Cancer Res. 2000, 60, 2399–2404. [Google Scholar]
- Cha, H.J.; Park, M.T.; Chung, H.Y.; Kim, N.D.; Sato, H.; Seiki, M.; Kim, K.W. Ursolic acid-induced down-regulation of MMP-9 gene is mediated through the nuclear translocation of glucocorticoid receptor in HT1080 human fibrosarcoma cells. Oncogene 1998, 16, 771–778. [Google Scholar] [CrossRef]
- Pathak, A.K.; Bhutani, M.; Nair, A.S.; Ahn, K.S.; Chakraborty, A.; Kadara, H.; Guha, S.; Sethi, G.; Aggarwal, B.B. Ursolic acid inhibits STAT3 activation pathway leading to suppression of proliferation and chemosensitization of human multiple myeloma cells. Mol. Cancer Res. 2007, 5, 943–955. [Google Scholar] [CrossRef] [PubMed]
- Xiang, F.; Pan, C.; Kong, Q.; Wu, R.; Jiang, J.; Zhan, Y.; Xu, J.; Gu, X.; Kang, X. Ursolic Acid Inhibits the Proliferation of Gastric Cancer Cells by Targeting miR-133a. Oncol. Res. 2014, 22, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Moon, A. Ursolic acid inhibits the invasive phenotype of SNU-484 human gastric cancer cells. Oncol. Lett. 2015, 9, 897–902. [Google Scholar] [CrossRef] [PubMed]
- Halder, G.; Johnson, R.L. Hippo signaling: Growth control and beyond. Development 2011, 138, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Pan, D. Hippo signaling in organ size control. Genes Dev. 2007, 21, 886–897. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Feldmann, G.; Huang, J.; Wu, S.; Zhang, N.; Comerford, S.A.; Gayyed, M.F.; Anders, R.A.; Maitra, A.; Pan, D. Elucidation of a universal size-control mechanism in Drosophila and mammals. Cell 2007, 130, 1120–1133. [Google Scholar] [CrossRef] [PubMed]
- Saucedo, L.J.; Edgar, B.A. Filling out the Hippo pathway. Nat. Rev. Mol. Cell Biol. 2007, 8, 613–621. [Google Scholar] [CrossRef]
- Sohn, B.H.; Shim, J.J.; Kim, S.B.; Jang, K.Y.; Kim, S.M.; Kim, J.H.; Hwang, J.E.; Jang, H.J.; Lee, H.S.; Kim, S.C.; et al. Inactivation of Hippo Pathway Is Significantly Associated with Poor Prognosis in Hepatocellular Carcinoma. Clin. Cancer Res. 2016, 22, 1256–1264. [Google Scholar] [CrossRef]
- Kim, S.M.; Ye, S.; Rah, S.Y.; Park, B.H.; Wang, H.; Kim, J.R.; Kim, S.H.; Jang, K.Y.; Lee, K.B. RhBMP-2 Activates Hippo Signaling through RASSF1 in Esophageal Cancer Cells. Sci. Rep. 2016, 6, 26821. [Google Scholar] [CrossRef]
- Uttamchandani, M.; Wang, J.; Yao, S.Q. Protein and small molecule microarrays: Powerful tools for high-throughput proteomics. Mol. Biosyst. 2006, 2, 58–68. [Google Scholar] [CrossRef]
- Uttamchandani, M.; Walsh, D.P.; Yao, S.Q.; Chang, Y.T. Small molecule microarrays: Recent advances and applications. Curr. Opin. Chem. Biol. 2005, 9, 4–13. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.N.; Sabatini, D.M.; Stockwell, B.R. Microarrays of small molecules embedded in biodegradable polymers for use in mammalian cell-based screens. Proc. Natl. Acad. Sci. USA 2004, 101, 16144–16149. [Google Scholar] [CrossRef] [PubMed]
- Deng, N.; Goh, L.K.; Wang, H.; Das, K.; Tao, J.; Tan, I.B.; Zhang, S.; Lee, M.; Wu, J.; Lim, K.H.; et al. A comprehensive survey of genomic alterations in gastric cancer reveals systematic patterns of molecular exclusivity and co-occurrence among distinct therapeutic targets. Gut 2012, 61, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, S.; Heallen, T.; Martin, J.F. The Hippo pathway in the heart: Pivotal roles in development, disease, and regeneration. Nat. Rev. Cardiol. 2018, 15, 672–684. [Google Scholar] [CrossRef] [PubMed]
- Abdi, K.; Kuo, C.T. Laminating the mammalian cortex during development: Cell polarity protein function and Hippo signaling. Genes Dev. 2018, 32, 740–741. [Google Scholar] [CrossRef] [PubMed]
- Hilman, D.; Gat, U. The evolutionary history of YAP and the hippo/YAP pathway. Mol. Biol. Evol. 2011, 28, 2403–2417. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, Y.K.; Shin, D.H.; Lee, H.J.; Shin, N.; Kim, A.; Lee, J.H.; Choi, K.U.; Kim, J.Y.; Lee, C.H.; et al. Yes associated protein is a poor prognostic factor in well-differentiated lung adenocarcinoma. Int. J. Clin. Exp. Pathol. 2015, 8, 15933–15939. [Google Scholar] [PubMed]
- Sun, L.; Chen, F.; Shi, W.; Qi, L.; Zhao, Z.; Zhang, J. Prognostic impact of TAZ and beta-catenin expression in adenocarcinoma of the esophagogastric junction. Diagn. Pathol. 2014, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Yagi, H.; Asanoma, K.; Ohgami, T.; Ichinoe, A.; Sonoda, K.; Kato, K. GEP oncogene promotes cell proliferation through YAP activation in ovarian cancer. Oncogene 2016, 35, 4471–4480. [Google Scholar] [CrossRef]
- Liu, J.Y.; Li, Y.H.; Lin, H.X.; Liao, Y.J.; Mai, S.J.; Liu, Z.W.; Zhang, Z.L.; Jiang, L.J.; Zhang, J.X.; Kung, H.F.; et al. Overexpression of YAP 1 contributes to progressive features and poor prognosis of human urothelial carcinoma of the bladder. BMC Cancer 2013, 13, 349. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.; Wang, G.; Zhang, Z.; Wu, X.; Zhang, T.; Fu, B.; Chen, G. Yes-associated protein expression is a predictive marker for recurrence of hepatocellular carcinoma after liver transplantation. Dig. Surg. 2014, 31, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.X.; Zhao, B.; Panupinthu, N.; Jewell, J.L.; Lian, I.; Wang, L.H.; Zhao, J.; Yuan, H.; Tumaneng, K.; Li, H.; et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 2012, 150, 780–791. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Yang, Z.; Xie, C.; Zhu, Y.; Shu, X.; Zhang, Z.; Li, N.; Chai, N.; Zhang, S.; Wu, K.; et al. PTEN lipid phosphatase inactivation links the hippo and PI3K/Akt pathways to induce gastric tumorigenesis. J. Exp. Clin. Cancer Res. 2018, 37, 198. [Google Scholar] [CrossRef] [PubMed]
- Qiao, Y.; Lin, S.J.; Chen, Y.; Voon, D.C.; Zhu, F.; Chuang, L.S.; Wang, T.; Tan, P.; Lee, S.C.; Yeoh, K.G.; et al. RUNX3 is a novel negative regulator of oncogenic TEAD-YAP complex in gastric cancer. Oncogene 2016, 35, 2664–2674. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Tong, J.H.; Chan, A.W.; Lee, T.L.; Lung, R.W.; Leung, P.P.; So, K.K.; Wu, K.; Fan, D.; Yu, J.; et al. Yes-associated protein 1 exhibits oncogenic property in gastric cancer and its nuclear accumulation associates with poor prognosis. Clin. Cancer Res. 2011, 17, 2130–2139. [Google Scholar] [CrossRef] [PubMed]
- Song, M.; Cheong, J.H.; Kim, H.; Noh, S.H.; Kim, H. Nuclear expression of Yes-associated protein 1 correlates with poor prognosis in intestinal type gastric cancer. Anticancer Res. 2012, 32, 3827–3834. [Google Scholar] [PubMed]
- Yu, L.; Gao, C.; Feng, B.; Wang, L.; Tian, X.; Wang, H.; Ma, D. Distinct prognostic values of YAP1 in gastric cancer. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, J.S.; Xu, Z.P. RNA interference mediated YAP gene silencing inhibits invasion and metastasis of human gastric cancer cell line SGC-7901. Hepatogastroenterology 2011, 58, 2156–2161. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhu, J.S.; Gao, C.P.; Li, L.P.; Zhou, C.; Wang, H.; Liu, X.G. siRNA targeting YAP gene inhibits gastric carcinoma growth and tumor metastasis in SCID mice. Oncol. Lett. 2016, 11, 2806–2814. [Google Scholar] [CrossRef]
- Khan, M.S.A.; Khundmiri, S.U.K.; Khundmiri, S.R.; Al-Sanea, M.M.; Mok, P.L. Fruit-Derived Polysaccharides and Terpenoids: Recent Update on the Gastroprotective Effects and Mechanisms. Front. Pharmacol. 2018, 9, 569. [Google Scholar] [CrossRef]
- Xu, X.; Zhu, G.Q.; Zhang, K.; Zhou, Y.C.; Li, X.L.; Xu, W.; Zhang, H.; Shao, Y.; Zhang, Z.Y.; Sun, W.H. Cyclooxygenase-2 mediated synergistic effect of ursolic acid in combination with paclitaxel against human gastric carcinoma. Oncotarget 2017, 8, 92770–92777. [Google Scholar] [CrossRef]
- Nakajima, K.; Oiso, S.; Uto, T.; Morinaga, O.; Shoyama, Y.; Kariyazono, H. Triterpenes suppress octanoylated ghrelin production in ghrelin-expressing human gastric carcinoma cells. Biomed. Res. 2016, 37, 343–349. [Google Scholar] [CrossRef]
- Yang, Y.; Jiang, M.; Hu, J.; Lv, X.; Yu, L.; Qian, X.; Liu, B. Enhancement of Radiation Effects by Ursolic Acid in BGC-823 Human Adenocarcinoma Gastric Cancer Cell Line. PLoS ONE 2015, 10, e0133169. [Google Scholar] [CrossRef]
- Li, R.; Wang, X.; Zhang, X.H.; Chen, H.H.; Liu, Y.D. Ursolic acid promotes apoptosis of SGC-7901 gastric cancer cells through ROCK/PTEN mediated mitochondrial translocation of cofilin-1. Asian Pac. J. Cancer Prev. 2014, 15, 9593–9597. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.; Ding, J.; Xu, H.; Dai, X.; Hou, Z.; Zhang, K.; Sun, K.; Sun, W. Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int. J. Pharm. 2013, 441, 261–268. [Google Scholar] [CrossRef]
- Eisen, M.B.; Spellman, P.T.; Brown, P.O.; Botstein, D. Cluster analysis and display of genome-wide expression patterns. Proc. Natl. Acad. Sci. USA 1998, 95, 14863–14868. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.-H.; Jin, H.; Meng, R.Y.; Kim, D.-Y.; Liu, Y.C.; Chai, O.H.; Park, B.H.; Kim, S.M. Activating Hippo Pathway via Rassf1 by Ursolic Acid Suppresses the Tumorigenesis of Gastric Cancer. Int. J. Mol. Sci. 2019, 20, 4709. https://doi.org/10.3390/ijms20194709
Kim S-H, Jin H, Meng RY, Kim D-Y, Liu YC, Chai OH, Park BH, Kim SM. Activating Hippo Pathway via Rassf1 by Ursolic Acid Suppresses the Tumorigenesis of Gastric Cancer. International Journal of Molecular Sciences. 2019; 20(19):4709. https://doi.org/10.3390/ijms20194709
Chicago/Turabian StyleKim, Seong-Hun, Hua Jin, Ruo Yu Meng, Da-Yeah Kim, Yu Chuan Liu, Ok Hee Chai, Byung Hyun Park, and Soo Mi Kim. 2019. "Activating Hippo Pathway via Rassf1 by Ursolic Acid Suppresses the Tumorigenesis of Gastric Cancer" International Journal of Molecular Sciences 20, no. 19: 4709. https://doi.org/10.3390/ijms20194709
APA StyleKim, S.-H., Jin, H., Meng, R. Y., Kim, D.-Y., Liu, Y. C., Chai, O. H., Park, B. H., & Kim, S. M. (2019). Activating Hippo Pathway via Rassf1 by Ursolic Acid Suppresses the Tumorigenesis of Gastric Cancer. International Journal of Molecular Sciences, 20(19), 4709. https://doi.org/10.3390/ijms20194709





