Comparison between Fractionated Dose and Single Dose of Cu-64 Trastuzumab Therapy in the NCI-N87 Gastric Cancer Mouse Model
Abstract
1. Introduction
2. Results
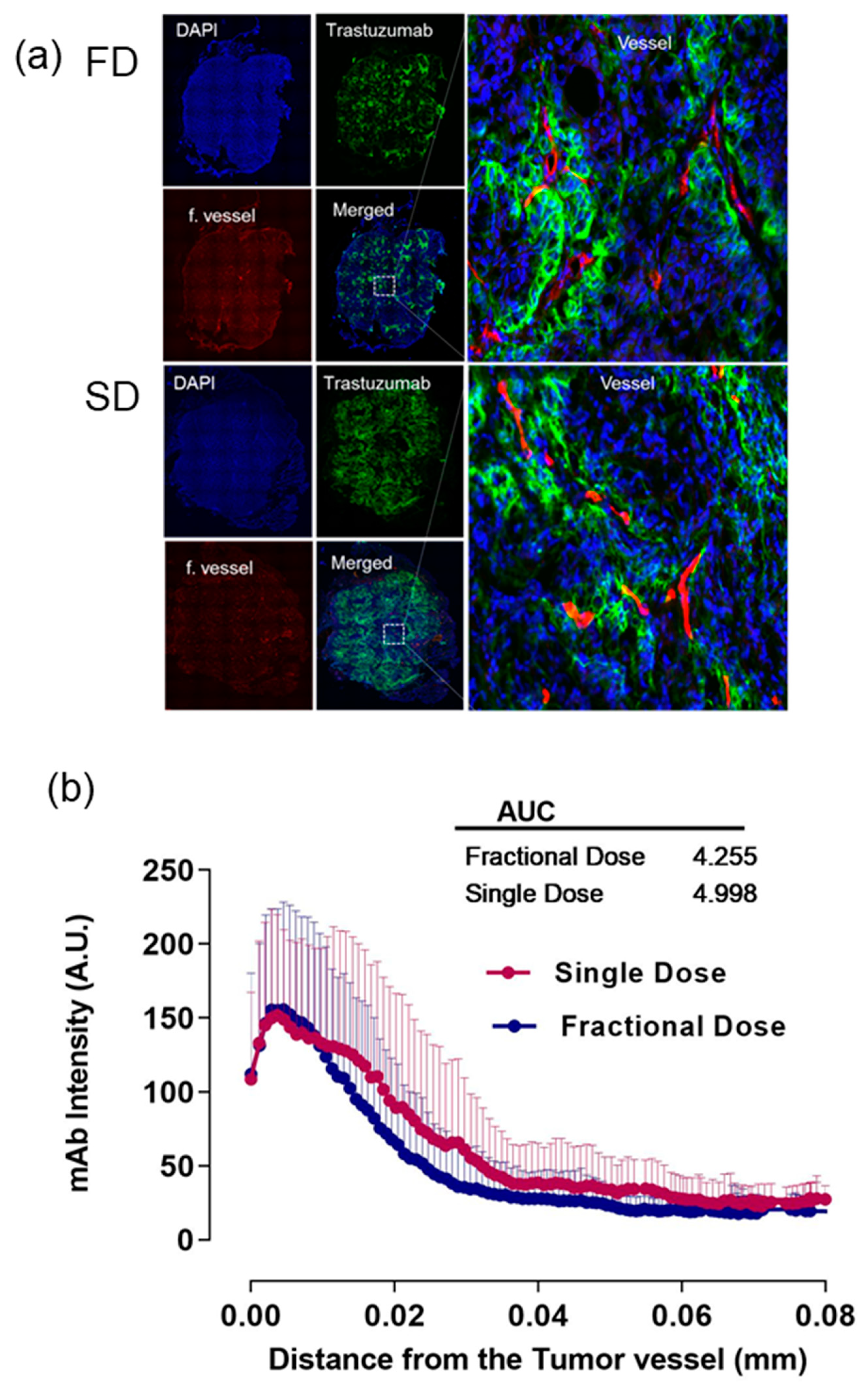
2.1. Alexa-647-Trastuzumab Penetration from the Tumor Vessel
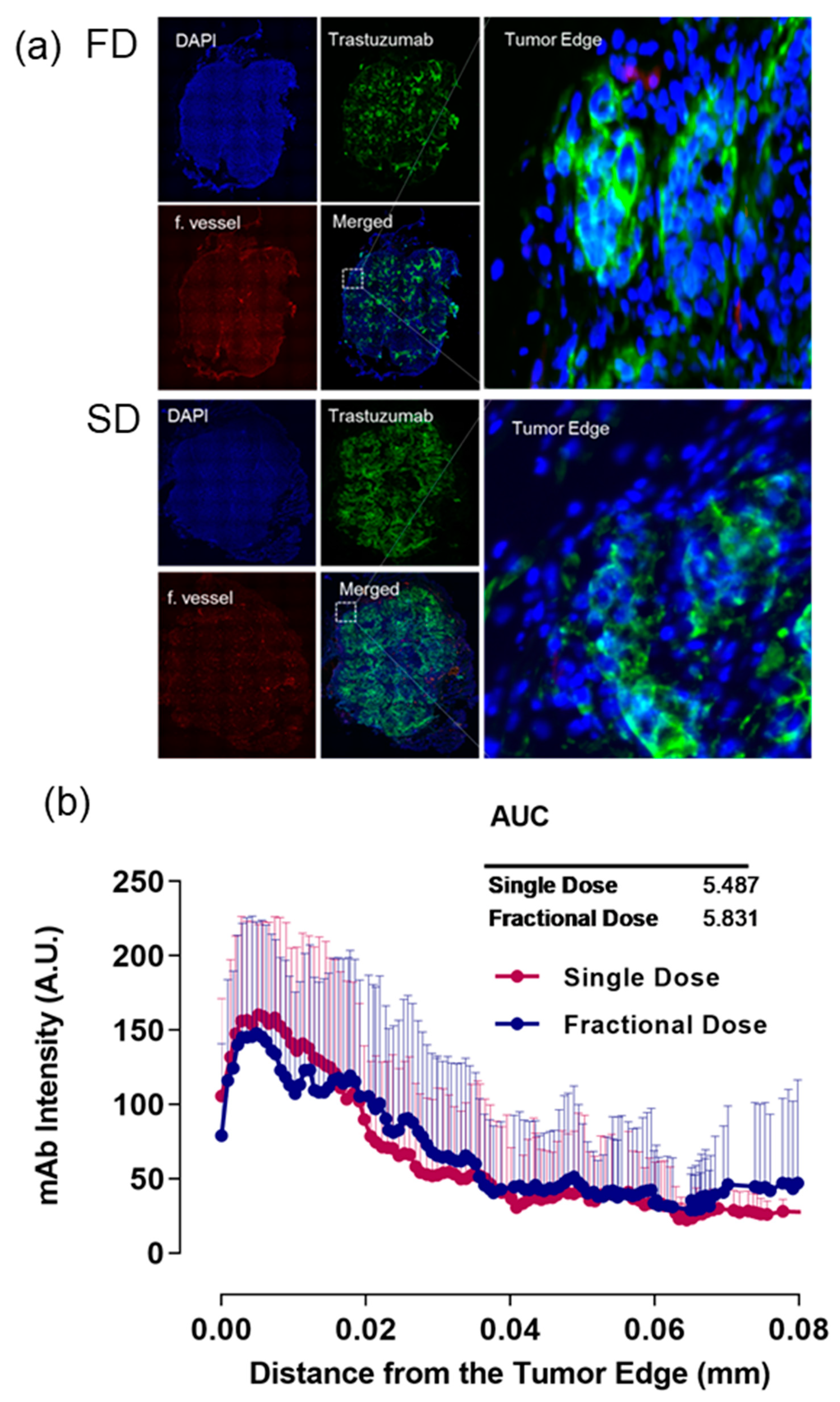
2.2. Alexa-647-Trastuzumab Penetration near the Tumor Edge
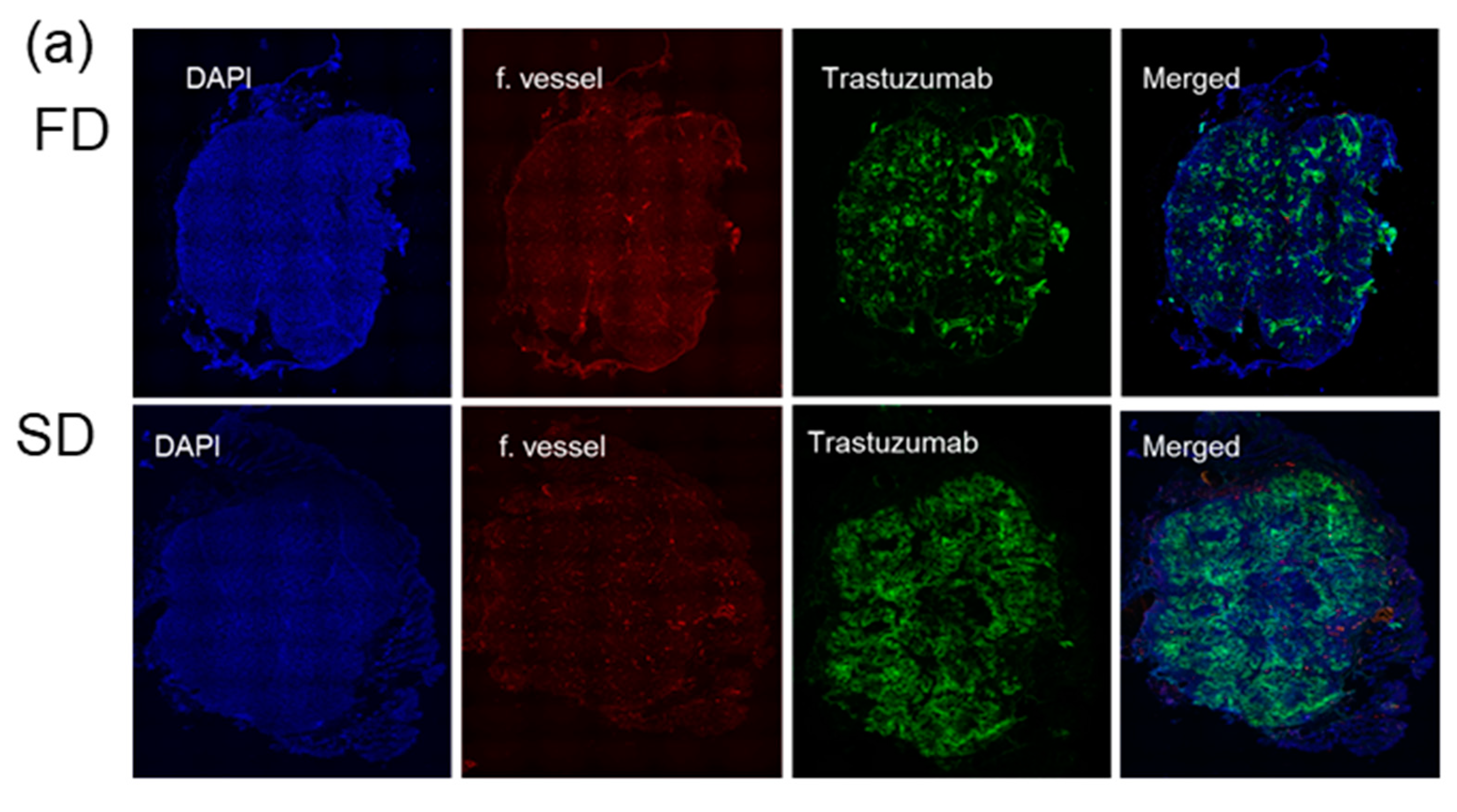
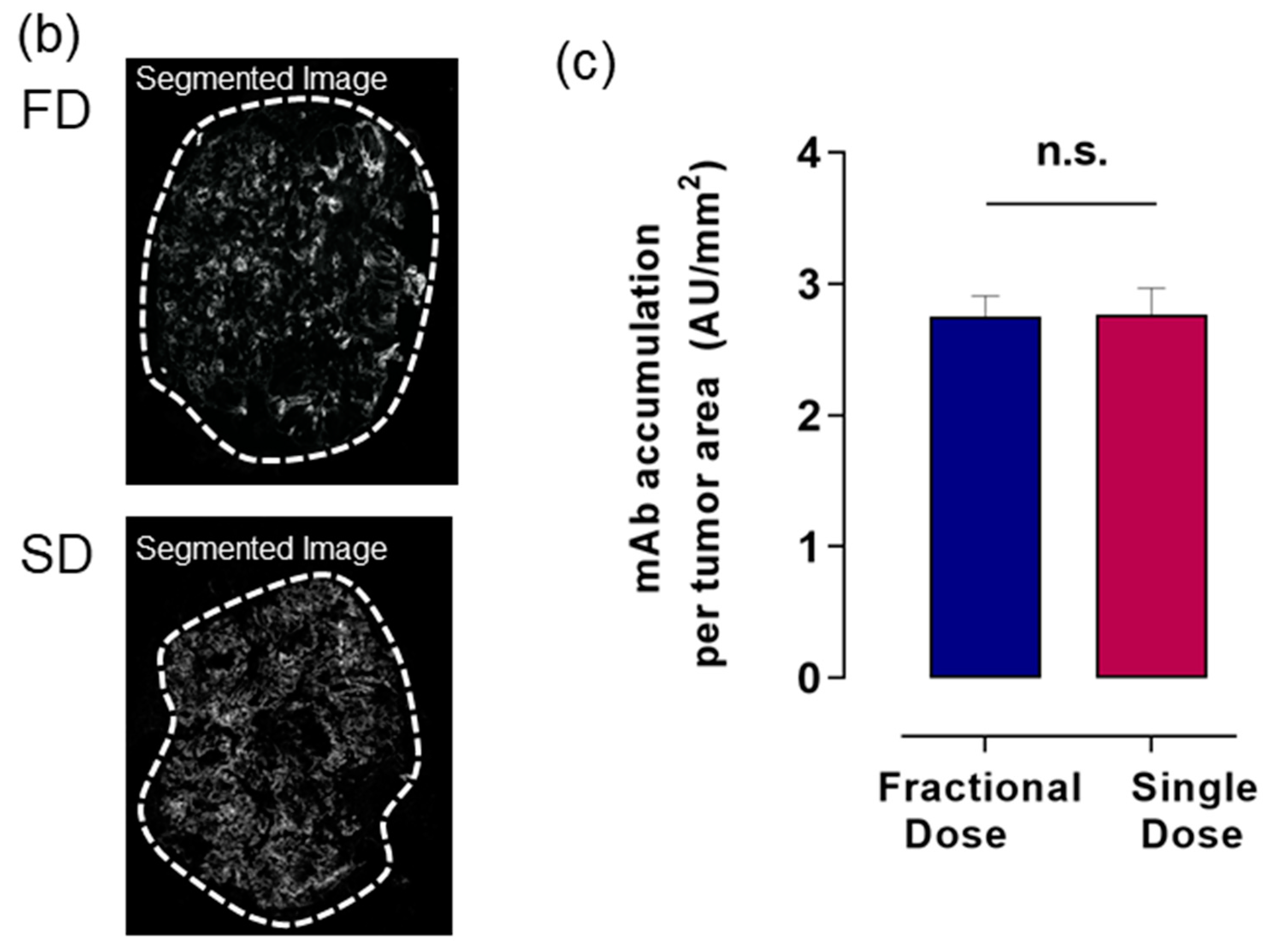
2.3. Alexa-647-Trastuzumab Accumulation across Whole Tumor
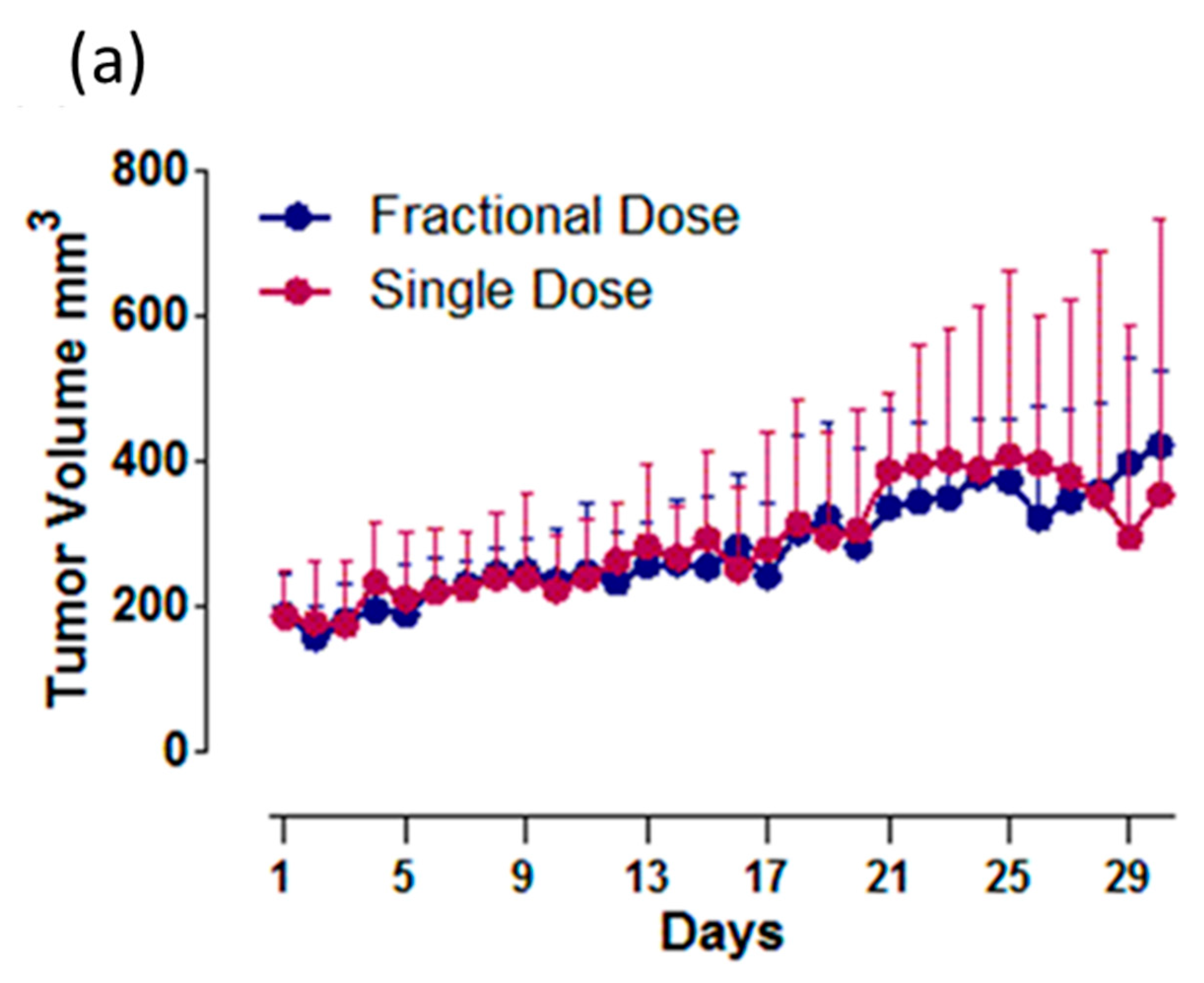
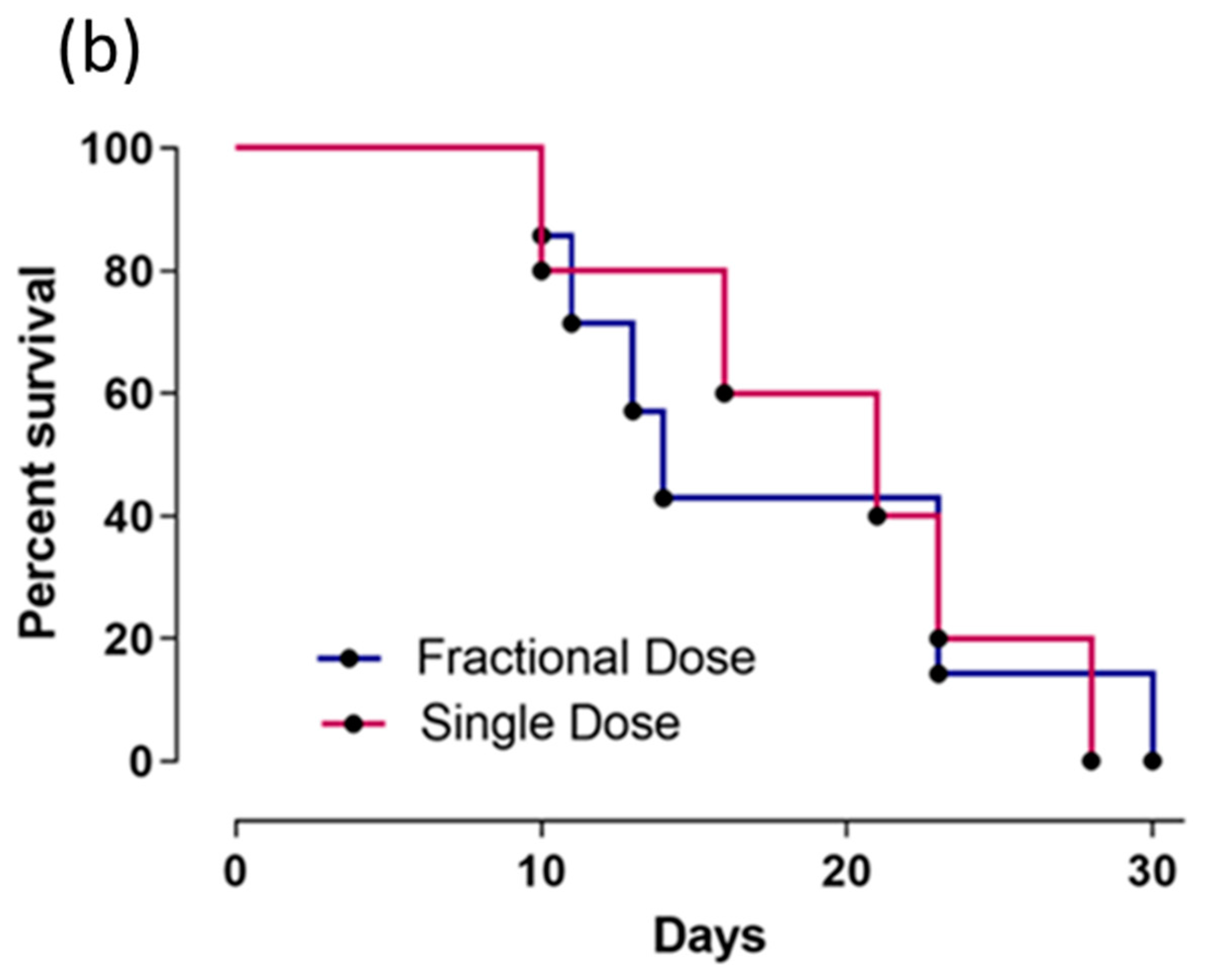
2.4. Cu-64-Labeled Trastuzumab Efficacy in Tumor Size Reduction
3. Discussion
4. Materials and Methods
4.1. Cell Culture
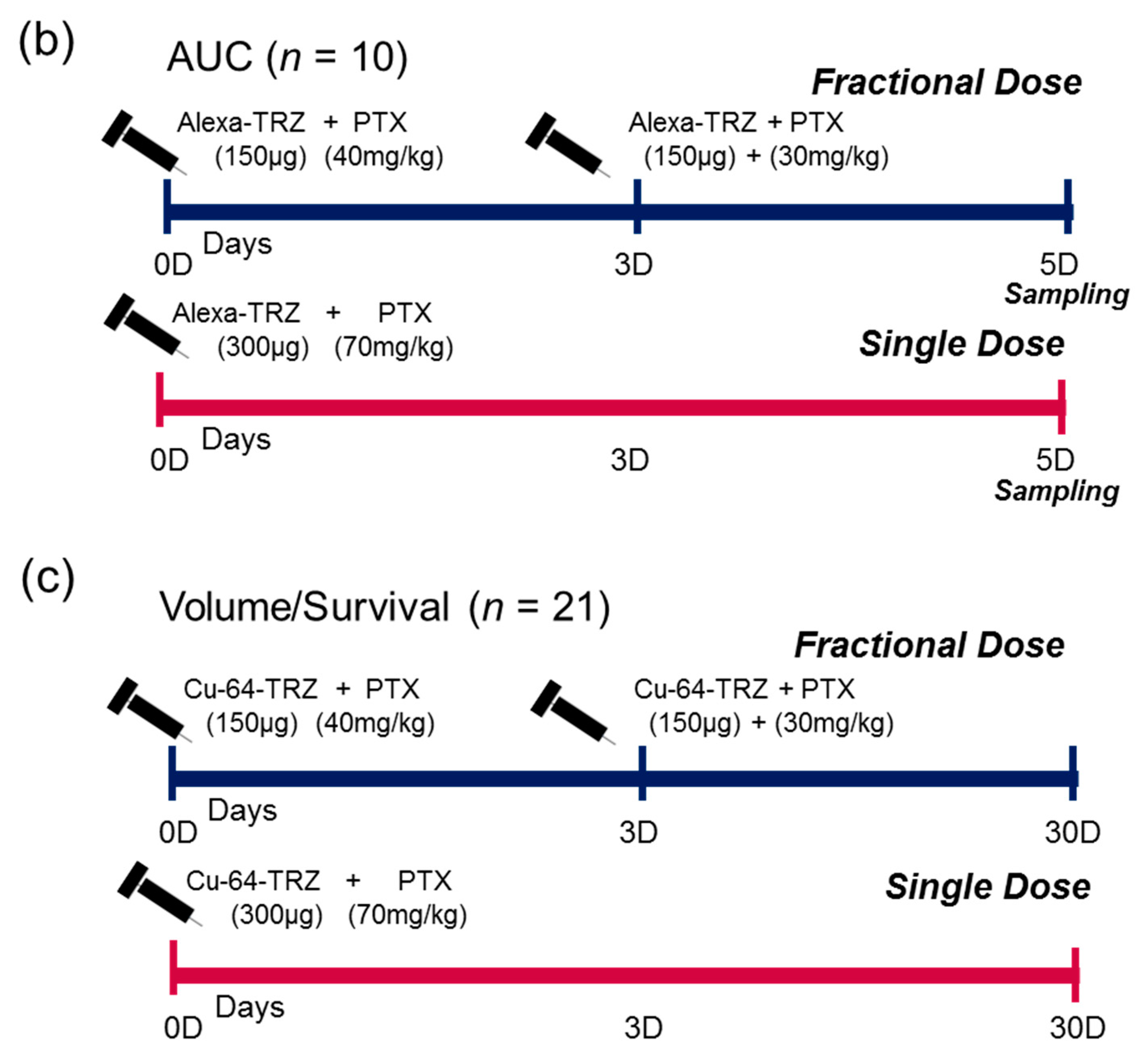
4.2. Animal Model and Treatment Plan
4.3. Conjugation of Alexa Fluor 647 to Trastuzumab
4.4. Labelling of Cu-64-Trastuzumab
4.5. Immunofluorescence Staining
4.6. Histological Image Acquisition
4.7. Penetration and Accumulation of Alexa-647-Trastuzumab
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Appendix A
Appendix A.1. Tumor Model
Appendix A.2. Antibody Penetration Studies
References
- Erdi, A.K.; Erdi, Y.E.; Yorke, E.D.; Wessels, B.W. Treatment planning for radio-immunotherapy. Phys. Med. Biol. 1996, 41, 2009–2026. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M. Ligand-targeted therapeutics in anticancer therapy. Nat. Rev. Cancer 2002, 2, 750–763. [Google Scholar] [CrossRef] [PubMed]
- Jhanwar, Y.S.; Divgi, C. Current status of therapy of solid tumors. J. Nucl. Med. 2005, 46 (Suppl. 1), 141S–150S. [Google Scholar]
- Thurber, G.M.; Schmidt, M.M.; Wittrup, K.D. Factors determining antibody distribution in tumors. Trends Pharmacol. Sci. 2008, 29, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Heldin, C.H.; Rubin, K.; Pietras, K.; Ostman, A. High interstitial fluid pressure—An obstacle in cancer therapy. Nat. Rev. Cancer 2004, 4, 806–813. [Google Scholar] [CrossRef] [PubMed]
- Heine, M.; Freund, B.; Nielsen, P.; Jung, C.; Reimer, R.; Hohenberg, H.; Zangemeister-Wittke, U.; Wester, H.J.; Luers, G.H.; Schumacher, U. High interstitial fluid pressure is associated with low tumour penetration of diagnostic monoclonal antibodies applied for molecular imaging purposes. PLoS ONE 2012, 7, e36258. [Google Scholar] [CrossRef] [PubMed]
- Ocean, A.J.; Pennington, K.L.; Guarino, M.J.; Sheikh, A.; Bekaii-Saab, T.; Serafini, A.N.; Lee, D.; Sung, M.W.; Gulec, S.A.; Goldsmith, S.J.; et al. Fractionated radioimmunotherapy with 90Y-clivatuzumab tetraxetan and low-dose gemcitabine is active in advanced pancreatic cancer: A phase 1 trial. Cancer 2012, 118, 5497–5506. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, D.M.; Sharkey, R.M.; Paganelli, G.; Barbet, J.; Chatal, J.F. Antibody pretargeting advances cancer radioimmunodetection and radioimmunotherapy. J. Clin. Oncol. 2006, 24, 823–834. [Google Scholar] [CrossRef]
- Orlova, A.; Jonsson, A.; Rosik, D.; Lundqvist, H.; Lindborg, M.; Abrahmsen, L.; Ekblad, C.; Frejd, F.Y.; Tolmachev, V. Site-Specific Radiometal Labeling and Improved Biodistribution Using ABY-027, A Novel HER2-Targeting Affibody Molecule-Albumin-Binding Domain Fusion Protein. J. Nucl. Med. 2013, 54, 961–968. [Google Scholar] [CrossRef]
- Weldon, J.E.; Xiang, L.; Zhang, J.; Beers, R.; Walker, D.A.; Onda, M.; Hassan, R.; Pastan, I. A recombinant immunotoxin against the tumor-associated antigen mesothelin reengineered for high activity, low off-target toxicity, and reduced antigenicity. Mol. Cancer Ther. 2013, 12, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Honeychurch, J.; Glennie, M.; Johnson, P.; Illidge, T. Microscopic intratumoral dosimetry of radiolabeled antibodies is a critical determinant of successful radioimmunotherapy in B-cell lymphoma. Cancer Res. 2007, 67, 1335–1343. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Kim, H.; Kang, Y.-r.; Kim, H.; Kim, J.Y.; Lee, Y.-J.; Kim, J.M.; Kim, J.S. Selection Criteria for Determination of Optimal Reconstruction Method for Cu-64 Trastuzumab Dosimetry on Siemens Inveon PET Scanner. J. Clin. Med. 2019, 8, 512. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S.; Lee, S.-J.; Kim, I.; Yao, Z.; Lee, J.-H.; Pastan, I.; Paik, C. Effect of mAb B3 with paclitaxel doses on accumulation and penetration of B3 into tumor. J. Nucl. Med. 2013, 54, 1322. [Google Scholar]
- Larson, S.M.; Carrasquillo, J.A.; Cheung, N.-K.V.; Press, O.W. Radioimmunotherapy of human tumours. Nat. Rev. Cancer 2015, 15, 347–360. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Shin, I.S.; Hancock, H.; Jang, B.S.; Kim, H.S.; Lee, S.M.; Zderic, V.; Frenkel, V.; Pastan, I.; Paik, C.H.; et al. Pulsed high intensity focused ultrasound increases penetration and therapeutic efficacy of monoclonal antibodies in murine xenograft tumors. J. Control Release 2012, 162, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Jang, B.S.; Lee, S.M.; Kim, H.S.; Shin, I.S.; Razjouyan, F.; Wang, S.; Yao, Z.; Pastan, I.; Dreher, M.R.; Paik, C.H. Combined-modality radioimmunotherapy: synergistic effect of paclitaxel and additive effect of bevacizumab. Nucl. Med. Biol. 2012, 39, 472–483. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kosaka, N.; Ogawa, M.; Paik, D.S.; Paik, C.H.; Choyke, P.L.; Kobayashi, H. Semiquantitative assessment of the microdistribution of fluorescence-labeled monoclonal antibody in small peritoneal disseminations of ovarian cancer. Cancer Sci. 2010, 101, 820–825. [Google Scholar] [CrossRef]
- Khaibullina, A.; Jang, B.S.; Sun, H.; Le, N.; Yu, S.; Frenkel, V.; Carrasquillo, J.A.; Pastan, I.; Li, K.C.; Paik, C.H. Pulsed high-intensity focused ultrasound enhances uptake of radiolabeled monoclonal antibody to human epidermoid tumor in nude mice. J. Nucl. Med. 2008, 49, 295–302. [Google Scholar] [CrossRef]
- Huang, Y.; Goel, S.; Duda, D.G.; Fukumura, D.; Jain, R.K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 2013, 73, 2943–2948. [Google Scholar] [CrossRef]
- Joseph, A.; O’Donoghue, G.S.; Chaitanya, R.D.; John, L.H. Single-Dose Versus Fractionated Radioimmunotherapy: Model Comparisons for Uniform Tumor Dosimetry. J. Nucl. Med. 2000, 41, 538–547. [Google Scholar]
- DeNardo, G.L.; Schlom, J.; Buchsbaum, D.J.; Meredith, R.F.; O’Donoghue, J.A.; Sgouros, G.; Humm, J.L.; DeNardo, S.J. Rationales, evidence, and design considerations for fractionated radioimmunotherapy. Cancer 2002, 94, 1332–1348. [Google Scholar] [CrossRef] [PubMed]
- Buchsbaum, D.; Khazaeli, M.B.; Liu, T.; Bright, S.; Richardson, K.; Jones, M.; Meredith, R. Fractionated Radioimmunotherapy of Human Colon Carcinoma Xenografts with 131I-labeled Monoclonal Antibody CC49. Cancer Res. 1995, 55, 5881s–5887s. [Google Scholar] [PubMed]
- Kim, H.; Choi, H.S.; Kim, S.-K.; Lee, B.I.; Choi, Y. Antigen-responsive molecular sensor enables real-time tumor-specific imaging. Theranostics 2017, 7, 952–961. [Google Scholar] [CrossRef]
- Schjoeth-Eskesen, C.; Nielsen, C.H.; Heissel, S.; Højrup, P.; Hansen, P.R.; Gillings, N.; Kjaer, A. 64Cu-labelled trastuzumab: optimisation of labelling by DOTA and NODAGA conjugation and initial evaluation in mice. J. Labelled Comp. Radiopharm. 2015, 58, 227–233. [Google Scholar] [CrossRef]
- Woo, S.-K.; Jang, S.J.; Seo, M.-J.; Park, J.H.; Kim, B.S.; Kim, E.J.; Lee, Y.J.; Lee, T.S.; An, G.I.; Song, I.H.; et al. Development of 64Cu-NOTA-Trastuzumab for HER2 Targeting: A Radiopharmaceutical with Improved Pharmacokinetics for Human Studies. J. Nucl. Med. 2019, 60, 26–33. [Google Scholar] [CrossRef]
- Lee, Y.S.; Kim, J.S.; Cho, K.D.; Kang, J.H.; Lim, S.M. Tumor dosimetry for 131I trastuzumab therapy in a Her2+ NCI N87 xenograft mouse model using the Siemens SYMBIA E gamma camera with a pinhole collimator. J. Instrum. 2015, 10, P07001. [Google Scholar] [CrossRef]
- Kurihara, H.; Hamada, A.; Yoshida, M.; Shimma, S.; Hashimoto, J.; Yonemori, K.; Tani, H.; Miyakita, Y.; Kanayama, Y.; Wada, Y.; et al. 64Cu-DOTA-trastuzumab PET imaging and HER2 specificity of brain metastases in HER2-positive breast cancer patients. EJNMMI Res. 2015, 5, 8. [Google Scholar] [CrossRef]
- De Brabander, M.; Geuens, G.; Nuydens, R.; Willebrords, R.; De Mey, J. Taxol induces the assembly of free microtubules in living cells and blocks the organizing capacity of the centrosomes and kinetochores. Proc. Natl. Acad. Sci. USA 1981, 78, 5608–5612. [Google Scholar] [CrossRef]

© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zaheer, J.; Kim, H.; Lee, Y.-J.; Lim, S.M.; Kim, J.S. Comparison between Fractionated Dose and Single Dose of Cu-64 Trastuzumab Therapy in the NCI-N87 Gastric Cancer Mouse Model. Int. J. Mol. Sci. 2019, 20, 4708. https://doi.org/10.3390/ijms20194708
Zaheer J, Kim H, Lee Y-J, Lim SM, Kim JS. Comparison between Fractionated Dose and Single Dose of Cu-64 Trastuzumab Therapy in the NCI-N87 Gastric Cancer Mouse Model. International Journal of Molecular Sciences. 2019; 20(19):4708. https://doi.org/10.3390/ijms20194708
Chicago/Turabian StyleZaheer, Javeria, Hyeongi Kim, Yong-Jin Lee, Sang Moo Lim, and Jin Su Kim. 2019. "Comparison between Fractionated Dose and Single Dose of Cu-64 Trastuzumab Therapy in the NCI-N87 Gastric Cancer Mouse Model" International Journal of Molecular Sciences 20, no. 19: 4708. https://doi.org/10.3390/ijms20194708
APA StyleZaheer, J., Kim, H., Lee, Y.-J., Lim, S. M., & Kim, J. S. (2019). Comparison between Fractionated Dose and Single Dose of Cu-64 Trastuzumab Therapy in the NCI-N87 Gastric Cancer Mouse Model. International Journal of Molecular Sciences, 20(19), 4708. https://doi.org/10.3390/ijms20194708





