Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans
Abstract
1. Introduction
2. Chemical Composition of RJ
2.1. Sugars
2.2. Lipids
2.3. Proteins
2.4. Phenols, Flavonoids, and Free Amino Acids
2.5. Vitamins, Minerals, and Bioactive Substances
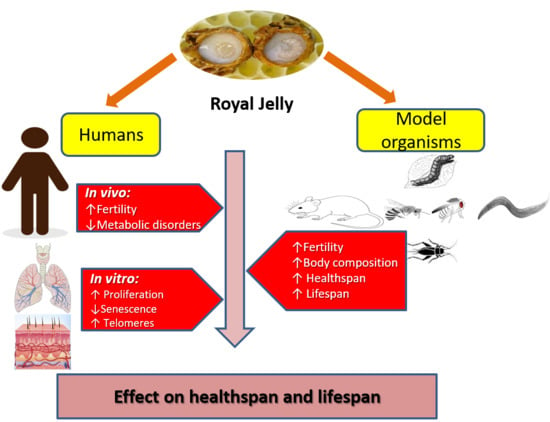
3. Healthspan and Longevity Effects of RJ in Various Species
3.1. RJ Enhances Fertility and Longevity in Population of the Beehive
3.2. RJ Enhances Healthspan and Longevity in Other Species
3.2.1. Drosophila Melanogaster (Fruit Fly)
3.2.2. Gryllus Bimaculatus Cricket and Silkworms
3.2.3. Caenorhabditis (C.) Elegans Nematodes
3.2.4. Mice
4. RJ Might Enhance Longevity in Humans by Promoting General Health
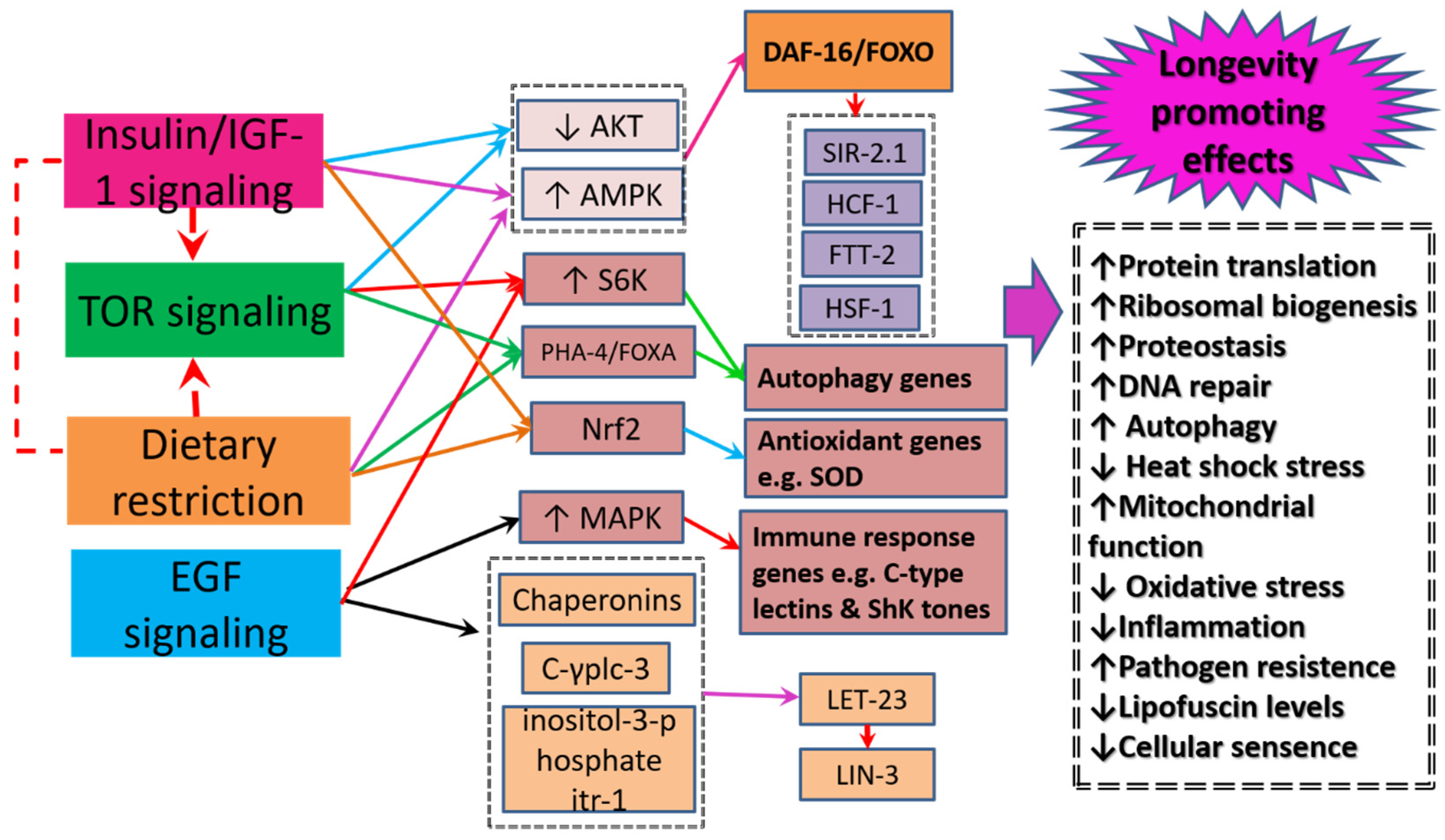
5. Healthspan and Longevity Enhancing Mechanisms
5.1. Insulin-Signaling/insulin like Growth Factor-1 Signaling
5.2. The Mechanistic Target of Rapamycin Signaling
5.3. Dietary Restriction Signaling
5.4. Epidermal Growth Factor Signaling
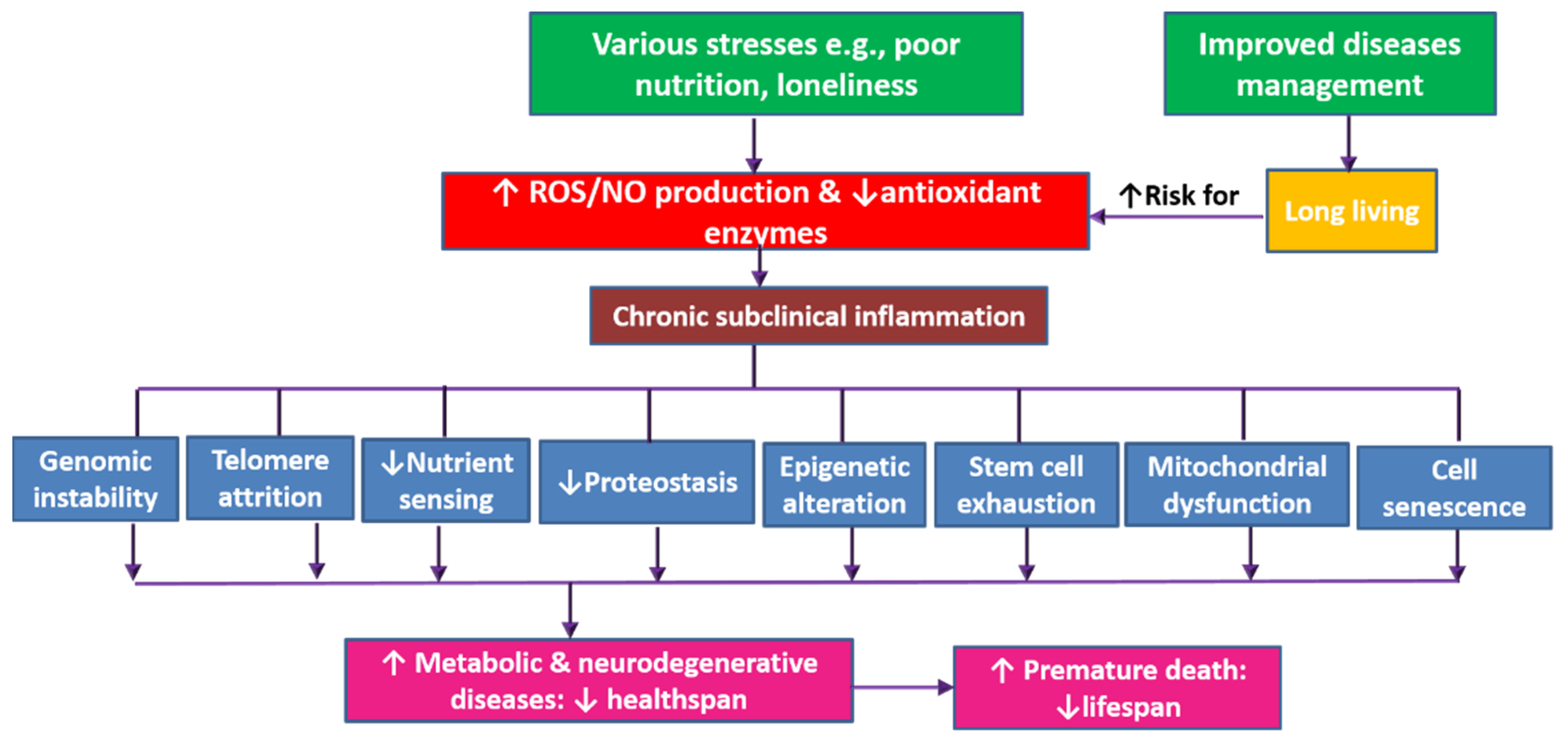
5.5. Oxidative Stress
6. Discussion
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| 4E-BP1 | Binding protein |
| 10-HDA | 10-hydroxydecanoic acid |
| 10H2DA | 10-hydroxy-2-decenoic acid |
| Ach | Acetylcholine |
| ATF4 | Activating transcription factor-4 |
| ADP | Adenosine diphosphate |
| AMP | Adenosine monophosphate |
| ATP | Adenosine triphosphate |
| EGFR | Epidermal growth factor receptor |
| eIF4E | Eukaryotic translation initiation factor |
| eNOs | Endothelial nitric oxide synthase |
| ERK | Extracellular signal-regulated kinase |
| ERs | Estrogen receptors |
| FDRJ | Freeze-dried RJ |
| FOXO | Forkhead box O |
| HCF-1 | Host cell factor-1 |
| HPO-DAEE | 4-Hydroperoxy-2-decenoic acid ethyl ester |
| IGFs | Insulin-like growth factors |
| IIS | Insulin/IGF-1 signaling |
| InR | Insulin receptor |
| MAPK | Mitogen-activated protein kinase |
| MDA | Malonaldehayde |
| MMPs | Matrix metalloproteinases |
| MRJPs | Major royal jelly proteins |
| mTOR | Mechanistic target of rapamycin |
| NRF2 | Nuclear factor erythroid 2 |
| pRJ | Protease-treated RJ |
| pRJ-Fr.5 | pRJ-Fraction 5 |
| RA | Rheumatoid arthritis |
| RAPTOR | Regulatory-associated protein of target of rapamycin |
| RJ | Royal jelly |
| ROS | Reactive oxygen species |
| S6K | Ribosomal proteins S6 kinase |
| SA | Sebacic acid |
| SIR-2.1 | Sirtuin homologue-2.1 |
| SNPs | Single-nucleotide polymorphisms |
| SOD | Superoxide dismutase |
| TNF | Tumor necrosis factor |
| TRP | Tyrosinase-related protein |
| w/w | Weight/weight |
| WJ | Worker jelly |
References
- Cao, B.; Bray, F.; Ilbawi, A.; Soerjomataram, I. Effect on longevity of one-third reduction in premature mortality from non-communicable diseases by 2030: A global analysis of the Sustainable Development Goal health target. Lancet Glob. Health 2018, 6, E1288–E1296. [Google Scholar] [CrossRef]
- Inoue, S.-i.; Koya-Miyata, S.; Ushio, S.; Iwaki, K.; Ikeda, M.; Kurimoto, M. Royal Jelly prolongs the life span of C3H/HeJ mice: Correlation with reduced DNA damage. Exp. Gerontol. 2003, 38, 965–969. [Google Scholar] [CrossRef]
- Hassanzadeh, K.; Rahimmi, A. Oxidative stress and neuroinflammation in the story of Parkinson’s disease: Could targeting these pathways write a good ending? J. Cell Physiol. 2019, 234, 23–32. [Google Scholar] [CrossRef]
- Weichhart, T. mTOR as Regulator of Lifespan, Aging, and Cellular Senescence: A Mini-Review. Gerontology 2018, 64, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Martins, R.; Lithgow, G.J.; Link, W. Long live FOXO: Unraveling the role of FOXO proteins in aging and longevity. Aging Cell 2016, 15, 196–207. [Google Scholar] [CrossRef]
- Ali, A.M.; Kunugi, H. Bee honey protects astrocytes against oxidative stress: A preliminary in vitro investigation. Neuropsychopharmacol. Rep. 2019, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Niraula, A.; Sheridan, J.F.; Godbout, J.P. Microglia Priming with Aging and Stress. Neuropsychopharmacology 2017, 42, 318–333. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Bae, H.; Sun, F.X.; Andersen, S.L.; Daw, E.W.; Malovini, A.; Kojima, T.; Hirose, N.; Schupf, N.; Puca, A.; et al. Meta-analysis of genetic variants associated with human exceptional longevity. Aging (Albany Ny) 2013, 5, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Sebastiani, P.; Solovieff, N.; Dewan, A.T.; Walsh, K.M.; Puca, A.; Hartley, S.W.; Melista, E.; Andersen, S.; Dworkis, D.A.; Wilk, J.B.; et al. Genetic signatures of exceptional longevity in humans. PLoS ONE 2012, 7, e29848. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Partridge, L. Promoting Health and Longevity through Diet: From Model Organisms to Humans. Cell 2015, 161, 106–118. [Google Scholar] [CrossRef]
- Hook, M.; Roy, S.; Williams, E.G.; Bou Sleiman, M.; Mozhui, K.; Nelson, J.F.; Lu, L.; Auwerx, J.; Williams, R.W. Genetic cartography of longevity in humans and mice: Current landscape and horizons. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2718–2732. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.-x.; Chen, Y.; Chen, D.; Xiao, F.; Parnell, L.D.; Zhao, J.; Liu, L.; Ordovas, J.M.; Lai, C.-Q.; Shen, L.-r. Supplementation with Major Royal-Jelly Proteins Increases Lifespan, Feeding, and Fecundity in Drosophila. J. Agric. Food Chem. 2016, 64, 5803–5812. [Google Scholar] [CrossRef] [PubMed]
- Maleki, V.; Jafari-Vayghan, H.; Saleh-Ghadimi, S.; Adibian, M.; Kheirouri, S.; Alizadeh, M. Effects of Royal jelly on metabolic variables in diabetes mellitus: A systematic review. Complement. Ther. Med. 2019, 43, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Pasupuleti, V.R.; Sammugam, L.; Ramesh, N.; Gan, S.H. Honey, Propolis, and Royal Jelly: A Comprehensive Review of Their Biological Actions and Health Benefits. Oxid. Med. Cell Longev. 2017, 2017, 1259510. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Tian, Y.; Han, M.; Miao, X. Longevity extension of worker honey bees (Apis mellifera) by royal jelly: Optimal dose and active ingredient. PeerJ 2017, 5, e3118. [Google Scholar] [CrossRef] [PubMed]
- Shorter, J.R.; Geisz, M.; Özsoy, E.; Magwire, M.M.; Carbone, M.A.; Mackay, T.F.C. The Effects of Royal Jelly on Fitness Traits and Gene Expression in Drosophila melanogaster. PLoS ONE 2015, 10, e0134612. [Google Scholar] [CrossRef] [PubMed]
- Kayashima, Y.; Yamanashi, K.; Sato, A.; Kumazawa, S.; Yamakawa-Kobayashi, K. Freeze-dried royal jelly maintains its developmental and physiological bioactivity in Drosophila melanogaster. Biosci. Biotechnol. Biochem. 2012, 76, 2107–2111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kocot, J.; Kielczykowska, M.; Luchowska-Kocot, D.; Kurzepa, J.; Musik, I. Antioxidant Potential of Propolis, Bee Pollen, and Royal Jelly: Possible Medical Application. Oxid. Med. Cell Longev. 2018, 2018, 7074209. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, M.; Dong, Y. Royal jelly promotes DAF-16-mediated proteostasis to tolerate β-amyloid toxicity in C. elegans model of Alzheimer’s disease. Oncotarget 2016, 7, 54183–54193. [Google Scholar] [CrossRef] [PubMed]
- You, M.; Pan, Y.; Liu, Y.; Chen, Y.; Wu, Y.; Si, J.; Wang, K.; Hu, F. Royal Jelly Alleviates Cognitive Deficits and β-Amyloid Accumulation in APP/PS1 Mouse Model Via Activation of the cAMP/PKA/CREB/BDNF Pathway and Inhibition of Neuronal Apoptosis. Front. Aging Neurosci. 2019, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Ramanathan, A.N.K.G.; Nair, A.J.; Sugunan, V.S. A review on Royal Jelly proteins and peptides. J. Funct. Foods 2018, 44, 255–264. [Google Scholar] [CrossRef]
- Xue, X.; Wu, L.; Wang, K. Chemical Composition of Royal Jelly. In Bee Products—Chemical and Biological Properties; Alvarez-Suarez, J.M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 181–190. [Google Scholar]
- Wytrychowski, M.; Chenavas, S.; Daniele, G.; Casabianca, H.; Batteau, M.; Guibert, S.; Brion, B. Physicochemical characterisation of French royal jelly: Comparison with commercial royal jellies and royal jellies produced through artificial bee-feeding. J. Food Compos. Anal. 2013, 29, 126–133. [Google Scholar] [CrossRef]
- Asencot, M.; Lensky, Y. The effect of sugars and Juvenile Hormone on the differentiation of the female honeybee larvae (Apis mellifera L.) to queens. Life Sci. 1976, 18, 693–699. [Google Scholar] [CrossRef]
- Buttstedt, A.; Ihling, C.H.; Pietzsch, M.; Moritz, R.F.A. Royalactin is not a royal making of a queen. Nature 2016, 537, E10. [Google Scholar] [CrossRef] [PubMed]
- Polsinelli, G.A.; Yu, H.D. Regulation of histone deacetylase 3 by metal cations and 10-hydroxy-2E-decenoic acid: Possible epigenetic mechanisms of queen-worker bee differentiation. PLoS ONE 2018, 13, e0204538. [Google Scholar] [CrossRef] [PubMed]
- Sediva, M.; Laho, M.; Kohutova, L.; Mojzisova, A.; Majtan, J.; Klaudiny, J. 10-HDA, A Major Fatty Acid of Royal Jelly, Exhibits pH Dependent Growth-Inhibitory Activity Against Different Strains of Paenibacillus larvae. Molecules 2018, 23, 3236. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-F.; You, M.-M.; Liu, Y.-C.; Shi, Y.-Z.; Wang, K.; Lu, Y.-Y.; Hu, F.-L. Potential protective effect of Trans-10-hydroxy-2-decenoic acid on the inflammation induced by Lipoteichoic acid. J. Funct. Foods 2018, 45, 491–498. [Google Scholar] [CrossRef]
- Yang, Y.C.; Chou, W.M.; Widowati, D.A.; Lin, I.P.; Peng, C.C. 10-hydroxy-2-decenoic acid of royal jelly exhibits bactericide and anti-inflammatory activity in human colon cancer cells. BMC Complement. Altern. Med. 2018, 18, 202. [Google Scholar] [CrossRef]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. Royal jelly-induced neurite outgrowth from rat pheochromocytoma PC12 cells requires integrin signal independent of activation of extracellular signal regulated kinases. Biomed. Res. 2007, 28, 139–146. [Google Scholar] [CrossRef]
- Weiser, M.J.; Grimshaw, V.; Wynalda, K.M.; Mohajeri, M.H.; Butt, C.M. Long-Term Administration of Queen Bee Acid (QBA) to Rodents Reduces Anxiety-Like Behavior, Promotes Neuronal Health and Improves Body Composition. Nutrients 2017, 10, 13. [Google Scholar] [CrossRef]
- Peng, C.C.; Sun, H.T.; Lin, I.P.; Kuo, P.C.; Li, J.C. The functional property of royal jelly 10-hydroxy-2-decenoic acid as a melanogenesis inhibitor. BMC Complement. Altern. Med. 2017, 17, 392. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Lai, W.; Zhu, G.; Wan, M.; Chen, J.; Tai, Y.; Lu, C. 10-Hydroxy-2-decenoic acid prevents ultraviolet A-induced damage and matrix metalloproteinases expression in human dermal fibroblasts. J. Eur. Acad. Derm. Venereol. 2013, 27, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.G.; Ruan, J.; Li, C.Y.; Wang, J.M.; Li, Y.; Zhai, W.T.; Zhang, W.; Ye, H.; Shen, N.H.; Lei, K.F.; et al. Connective tissue growth factor, a regulator related with 10-hydroxy-2-decenoic acid down-regulate MMPs in rheumatoid arthritis. Rheumatol. Int. 2012, 32, 2791–2799. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Wang, K.; Zhang, Y.Z.; Zheng, Y.F.; Hu, F.L. In Vitro Anti-Inflammatory Effects of Three Fatty Acids from Royal Jelly. Mediat. Inflamm. 2016, 2016, 3583684. [Google Scholar] [CrossRef] [PubMed]
- Moutsatsou, P.; Papoutsi, Z.; Kassi, E.; Heldring, N.; Zhao, C.; Tsiapara, A.; Melliou, E.; Chrousos, G.P.; Chinou, I.; Karshikoff, A.; et al. Fatty acids derived from royal jelly are modulators of estrogen receptor functions. PLoS ONE 2010, 5, e15594. [Google Scholar] [CrossRef] [PubMed]
- Inoue, Y.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Kamiya, T.; Itoh, A.; Adachi, T. 4-Hydroperoxy-2-decenoic acid ethyl ester protects against 6-hydroxydopamine-induced cell death via activation of Nrf2-ARE and eIF2 alpha-ATF4 pathways. Neurochem. Int. 2018, 112, 288–296. [Google Scholar] [CrossRef] [PubMed]
- Kamiya, T.; Watanabe, M.; Hara, H.; Mitsugi, Y.; Yamaguchi, E.; Itoh, A.; Adachi, T. Induction of Human-Lung-Cancer-A549-Cell Apoptosis by 4-Hydroperoxy-2-decenoic Acid Ethyl Ester through Intracellular ROS Accumulation and the Induction of Proapoptotic CHOP Expression. J. Agric. Food Chem. 2018, 66, 10741–10747. [Google Scholar] [CrossRef]
- Jiang, C.M.; Liu, X.; Li, C.X.; Qian, H.C.; Chen, D.; Lai, C.Q.; Shen, L.R. Anti-senescence effect and molecular mechanism of the major royal jelly proteins on human embryonic lung fibroblast (HFL-I) cell line. J. Zhejiang Univ. Sci. B 2018, 19, 960–972. [Google Scholar] [CrossRef]
- Moriyama, T.; Ito, A.; Omote, S.; Miura, Y.; Tsumoto, H. Heat Resistant Characteristics of Major Royal Jelly Protein 1 (MRJP1) Oligomer. PLoS ONE 2015, 10, e0119169. [Google Scholar] [CrossRef]
- Kamakura, M. Royalactin induces queen differentiation in honeybees. Nature 2011, 473, 478–483. [Google Scholar] [CrossRef]
- Wan, D.C.; Morgan, S.L.; Spencley, A.L.; Mariano, N.; Chang, E.Y.; Shankar, G.; Luo, Y.; Li, T.H.; Huh, D.; Huynh, S.K.; et al. Honey bee Royalactin unlocks conserved pluripotency pathway in mammals. Nat. Commun. 2018, 9, 5078. [Google Scholar] [CrossRef] [PubMed]
- Bilal, B.; Azim, M.K. Nematicidal activity of ‘major royal jelly protein’-containing glycoproteins from Acacia honey. Exp. Parasitol. 2018, 192, 52–59. [Google Scholar] [CrossRef] [PubMed]
- Abu-Serie, M.M.; Habashy, N.H. Two purified proteins from royal jelly with in vitro dual anti-hepatic damage potency: Major royal jelly protein 2 and its novel isoform X1. Int. J. Biol. Macromol. 2019, 128, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Fratini, F.; Cilia, G.; Mancini, S.; Felicioli, A. Royal Jelly: An ancient remedy with remarkable antibacterial properties. Microbiol. Res. 2016, 192, 130–141. [Google Scholar] [CrossRef] [PubMed]
- Hykollari, A.; Malzl, D.; Eckmair, B.; Vanbeselaere, J.; Scheidl, P.; Jin, C.; Karlsson, N.G.; Wilson, I.B.H.; Paschinger, K. Isomeric Separation and Recognition of Anionic and Zwitterionic N-glycans from Royal Jelly Glycoproteins. Mol. Cell Proteom. 2018, 17, 2177–2196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-R.; Yang, Y.-C.; Shi, L.-S.; Peng, C.-C. Antioxidant Properties of Royal Jelly Associated with Larval Age and Time of Harvest. J. Agric. Food Chem. 2008, 56, 11447–11452. [Google Scholar] [CrossRef]
- Guo, H.; Kouzuma, Y.; Yonekura, M. Structures and properties of antioxidative peptides derived from royal jelly protein. Food Chem 2009, 113, 238–245. [Google Scholar] [CrossRef]
- Jie, H.; Li, P.M.; Zhao, G.J.; Feng, X.L.; Zeng, D.J.; Zhang, C.L.; Lei, M.Y.; Yu, M.; Chen, Q. Amino acid composition of royal jelly harvested at different times after larval transfer. Genet. Mol. Res. 2016, 15. [Google Scholar] [CrossRef]
- Pina, A.; Begou, O.; Kanelis, D.; Gika, H.; Kalogiannis, S.; Tananaki, C.; Theodoridis, G.; Zotou, A. Targeted profiling of hydrophilic constituents of royal jelly by hydrophilic interaction liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1531, 53–63. [Google Scholar] [CrossRef]
- Gu, H.; Song, I.-B.; Han, H.-J.; Lee, N.-Y.; Cha, J.-Y.; Son, Y.-K.; Kwon, J. Antioxidant Activity of Royal Jelly Hydrolysates Obtained by Enzymatic Treatment. Korean J. Food Sci. Anim. Resour. 2018, 38, 135–142. [Google Scholar] [CrossRef]
- Honda, Y.; Fujita, Y.; Maruyama, H.; Araki, Y.; Ichihara, K.; Sato, A.; Kojima, T.; Tanaka, M.; Nozawa, Y.; Ito, M.; et al. Lifespan-extending effects of royal jelly and its related substances on the nematode Caenorhabditis elegans. PLoS ONE 2011, 6, e23527. [Google Scholar] [CrossRef] [PubMed]
- D’Antona, G.; Ragni, M.; Cardile, A.; Tedesco, L.; Dossena, M.; Bruttini, F.; Caliaro, F.; Corsetti, G.; Bottinelli, R.; Carruba, M.O.; et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab. 2010, 12, 362–372. [Google Scholar] [CrossRef] [PubMed]
- Gardner, T.S. The use of Drosophila Melanogaster as a Screening Agent for Longevity FactorsII. The Effects of Biotin, Pyridoxine, Sodium Yeast Nucleate, and Pantothenic Acid on the Life Span of the Fruit Fly. J. Gerontol. 1948, 3, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Stocker, A.; Schramel, P.; Kettrup, A.; Bengsch, E. Trace and mineral elements in royal jelly and homeostatic effects. J. Trace Elem. Med. Biol. 2005, 19, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Wessler, I.; Gartner, H.A.; Michel-Schmidt, R.; Brochhausen, C.; Schmitz, L.; Anspach, L.; Grunewald, B.; Kirkpatrick, C.J. Honeybees Produce Millimolar Concentrations of Non-Neuronal Acetylcholine for Breeding: Possible Adverse Effects of Neonicotinoids. PLoS ONE 2016, 11, e0156886. [Google Scholar] [CrossRef] [PubMed]
- Zamani, Z.; Reisi, P.; Alaei, H.; Asghar Pilehvarian, A. Effect of Royal Jelly on spatial learning and memory in rat model of streptozotocin-induced sporadic Alzheimer’s disease. Adv. Biomed. Res. 2012, 1, 1–10. [Google Scholar] [CrossRef]
- Wu, L.; Chen, L.; Selvaraj, J.N.; Wei, Y.; Wang, Y.; Li, Y.; Zhao, J.; Xue, X. Identification of the distribution of adenosine phosphates, nucleosides and nucleobases in royal jelly. Food Chem. 2015, 173, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Hattori, N.; Nomoto, H.; Fukumitsu, H.; Mishima, S.; Furukawa, S. AMP N1-Oxide, a Unique Compound of Royal Jelly, Induces Neurite Outgrowth from PC12 Vells via Signaling by Protein Kinase A Independent of that by Mitogen-Activated Protein Kinase. Evid. Based Complement. Altern. Med. 2010, 7, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Winston, M.L. The role of nutrition and temperature in the ovarian development of the worker honey bee (Apis mellifera). Can. Entomol. 1998, 130, 883–891. [Google Scholar] [CrossRef]
- Matsuyama, S.; Nagao, T.; Sasaki, K. Consumption of tyrosine in royal jelly increases brain levels of dopamine and tyramine and promotes transition from normal to reproductive workers in queenless honey bee colonies. Gen. Comp. Endocrinol. 2015, 211, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.-l.; Liao, C.-h.; Wang, Z.-l.; Wu, X.-b. Effect of royal jelly on longevity and memory-related traits of Apis mellifera workers. J. Asia Pac. Entomol. 2018, 21, 1430–1433. [Google Scholar] [CrossRef]
- Altaye, S.Z.; Pirk, C.W.; Crewe, R.M.; Nicolson, S.W. Convergence of carbohydrate-biased intake targets in caged worker honeybees fed different protein sources. J. Exp. Biol. 2010, 213, 3311–3318. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-y.; Chen, X.-y.; Lin, C.-y.; Sun, L.-x. The Effects of Royal jelly on the Life Span and Antioxidative Enzyme Activity of Fruit fly. J. Bee 2009, 29, 3–5. [Google Scholar]
- Miyashita, A.; Kizaki, H.; Sekimizu, K.; Kaito, C. Body-enlarging effect of royal jelly in a non-holometabolous insect species, Gryllus bimaculatus. Biol. Open 2016, 5, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Araki, Y.; Hata, T.; Ichihara, K.; Ito, M.; Tanaka, M.; Honda, S. 10-Hydroxy-2-decenoic Acid, the Major Lipid Component of Royal Jelly, Extends the Lifespan of Caenorhabditis elegans through Dietary Restriction and Target of Rapamycin Signaling. J. Aging Res. 2015, 2015, 425261. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cook, L.F.; Grasso, L.M.; Cao, M.; Dong, Y. Royal Jelly-Mediated Prolongevity and Stress Resistance in Caenorhabditis elegans Is Possibly Modulated by the Interplays of DAF-16, SIR-2.1, HCF-1, and 14-3-3 Proteins. J. Gerontol A Biol. Sci. Med. Sci. 2015, 70, 827–838. [Google Scholar] [CrossRef] [PubMed]
- Detienne, G.; De Haes, W.; Ernst, U.R.; Schoofs, L.; Temmerman, L. Royalactin extends lifespan of Caenorhabditis elegans through epidermal growth factor signaling. Exp. Gerontol. 2014, 60, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Ji, W.Z.; Zhang, C.P.; Wei, W.T.; Hu, F.L. The in vivo antiaging effect of enzymatic hydrolysate from royal jelly in d-galactose induced aging mouse. J. Chin. Inst. Food Sci. Technol. 2016, 16, 18–25. [Google Scholar]
- Okumura, N.; Toda, T.; Ozawa, Y.; Watanabe, K.; Ikuta, T.; Tatefuji, T.; Hashimoto, K.; Shimizu, T. Royal Jelly Delays Motor Functional Impairment During Aging in Genetically Heterogeneous Male Mice. Nutrients 2018, 10, 1191. [Google Scholar] [CrossRef]
- Alaraj, M.d. Royal Jelly Pretreatment Can Either Protect or Aggravate Brain Damage Induced by Hypoxia-Ischemia in Mice, Depending on its Dose. Int. J. Sci. Basic Appl. Res. 2015, 19, 338–346. [Google Scholar]
- Park, H.M.; Hwang, E.; Lee, K.G.; Han, S.M.; Cho, Y.; Kim, S.Y. Royal jelly protects against ultraviolet B-induced photoaging in human skin fibroblasts via enhancing collagen production. J. Med. Food 2011, 14, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Larson, M.G.; McCabe, E.L.; Murabito, J.M.; Rhee, E.P.; Ho, J.E.; Jacques, P.F.; Ghorbani, A.; Magnusson, M.; Souza, A.L.; et al. Distinct metabolomic signatures are associated with longevity in humans. Nat. Commun. 2015, 6, 6791. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Ikeda, T.; Kajita, K.; Fujioka, K.; Mori, I.; Okada, H.; Uno, Y.; Ishizuka, T. Effect of royal jelly ingestion for six months on healthy volunteers. Nutr. J. 2012, 11, 77. [Google Scholar] [CrossRef] [PubMed]
- Dalal, D.S.; Duran, J.; Brar, T.; Alqadi, R.; Halladay, C.; Lakhani, A.; Rudolph, J.L. Efficacy and safety of biological agents in the older rheumatoid arthritis patients compared to Young: A systematic review and meta-analysis. Semin. Arthritis Rheum. 2019, 48, 799–807. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Y.; Yang, D.S.; Wei, Z.; Wang, J.M.; Li, C.Y.; Hui, Y.; Lei, K.F.; Chen, X.F.; Shen, N.H.; Jin, L.Q.; et al. 10-Hydroxy-2-decenoic acid from Royal jelly: A potential medicine for RA. J. Ethnopharmacol. 2010, 128, 314–321. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Zou, H.; Lin, Y.; Lin, K.; Zhou, Z.; Qiang, J.; Lin, J.; Chuka, C.M.; Ge, R.; et al. 10-Hydroxy-2-decenoic acid inhibiting the proliferation of fibroblast-like synoviocytes by PI3K-AKT pathway. Int. Immunopharmacol. 2015, 28, 97–104. [Google Scholar] [CrossRef]
- Vitale, G.; Pellegrino, G.; Vollery, M.; Hofland, L.J. Role of IGF-1 system in the modulation of longevity: Controversies and new insights from a centenarians’ perspective. Front. Endocrinol. 2019, 10, 27. [Google Scholar] [CrossRef]
- Barbieri, M.; Bonafè, M.; Franceschi, C.; Paolisso, G. Insulin/IGF-I-signaling pathway: An evolutionarily conserved mechanism of longevity from yeast to humans. Am. J. Physiol. Endocrinol. Metab. 2003, 285, E1064–E1071. [Google Scholar] [CrossRef]
- Vella, V.; Malaguarnera, R. The Emerging Role of Insulin Receptor Isoforms in Thyroid Cancer: Clinical Implications and New Perspectives. Int. J. Mol. Sci. 2018, 19, 3814. [Google Scholar] [CrossRef]
- Wardelmann, K.; Blümel, S.; Rath, M.; Alfine, E.; Chudoba, C.; Schell, M.; Cai, W.; Hauffe, R.; Warnke, K.; Flore, T.; et al. Insulin action in the brain regulates mitochondrial stress responses and reduces diet-induced weight gain. Mol. Metab. 2019, 21, 68–81. [Google Scholar] [CrossRef]
- Bartke, A. Growth Hormone and Aging: Updated Review. World J. Mens. Health 2019, 37, 19–30. [Google Scholar] [CrossRef] [PubMed]
- De Medina, P. Deciphering the metabolic secret of longevity through the analysis of metabolic response to stress on long-lived species. Med. Hypotheses 2019, 122, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Kramer, K.J.; Childs, C.a.N.; Spiers, R.D.; Jacobs, R.M. Purification of insulin-like peptides from insect haemolymph and royal jelly. Insect Btochem. 1982, 12, 91–98. [Google Scholar] [CrossRef]
- Büyükipekçi, S.; Sarıtaş, N.; Soylu, M.; Mıstık, S.; Silici, S. Effects of royal jelly and honey mixture on some hormones in young males performing maximal strength workout. Phys. Educ. Stud. 2018, 22, 308–315. [Google Scholar] [CrossRef]
- Münstedt, K.; Bargello, M.; Hauenschild, A. Royal Jelly Reduces the Serum Glucose Levels in Healthy Subjects. J. Med. Food 2009, 12, 1170–1172. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, K.M.; Isohama, Y.; Maruyama, H.; Yamada, Y.; Narita, Y.; Ohta, S.; Araki, Y.; Miyata, T.; Mishima, S. Estrogenic activities of Fatty acids and a sterol isolated from royal jelly. Evid. Based Complement. Altern. Med. 2008, 5, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Van Raamsdonk, J.M. Mechanisms underlying longevity: A genetic switch model of aging. Exp. Gerontol. 2018, 107, 136–139. [Google Scholar] [CrossRef]
- Zárate, S.; Stevnsner, T.; Gredilla, R. Role of Estrogen and Other Sex Hormones in Brain Aging. Neuroprotection and DNA Repair. Front. Aging Neurosci. 2017, 9, 430. [Google Scholar] [CrossRef] [PubMed]
- Mishima, S.; Suzuki, K.-M.; Isohama, Y.; Kuratsu, N.; Araki, Y.; Inoue, M.; Miyata, T. Royal jelly has estrogenic effects in vitro and in vivo. J. Ethnopharmacol. 2005, 101, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Lephart, E.D. A review of the role of estrogen in dermal aging and facial attractiveness in women. J. Cosmet. Derm. 2018, 17, 282–288. [Google Scholar] [CrossRef] [PubMed]
- Lambrinoudaki, I.; Augoulea, A.; Rizos, D.; Politi, M.; Tsoltos, N.; Moros, M.; Chinou, I.; Graikou, K.; Kouskouni, E.; Kambani, S.; et al. Greek-origin royal jelly improves the lipid profile of postmenopausal women. Gynecol. Endocrinol. 2016, 32, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Asama, T.; Matsuzaki, H.; Fukushima, S.; Tatefuji, T.; Hashimoto, K.; Takeda, T. Royal Jelly Supplementation Improves Menopausal Symptoms Such as Backache, Low Back Pain, and Anxiety in Postmenopausal Japanese Women. Evid. Based Complement. Altern. Med. 2018, 2018, 4868412. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, S.; Matsushita, H.; Minami, A.; Kanazawa, H.; Suzuki, T.; Watanabe, K.; Wakatsuki, A. Royal jelly does not prevent bone loss but improves bone strength in ovariectomized rats. Climacteric 2018, 21, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Hidaka, S.; Okamoto, Y.; Uchiyama, S.; Nakatsuma, A.; Hashimoto, K.; Ohnishi, S.T.; Yamaguchi, M. Royal jelly prevents osteoporosis in rats: Beneficial effects in ovariectomy model and in bone tissue culture model. Evid. Based Complement. Altern. Med. 2006, 3, 339–348. [Google Scholar] [CrossRef] [PubMed]
- Narita, Y.; Nomura, J.; Ohta, S.; Inoh, Y.; Suzuki, K.M.; Araki, Y.; Okada, S.; Matsumoto, I.; Isohama, Y.; Abe, K.; et al. Royal jelly stimulates bone formation: Physiologic and nutrigenomic studies with mice and cell lines. Biosci. Biotechnol. Biochem. 2006, 70, 2508–2514. [Google Scholar] [CrossRef] [PubMed]
- Kaku, M.; Rocabado, J.M.R.; Kitami, M.; Ida, T.; Uoshima, K. Royal jelly affects collagen crosslinking in bone of ovariectomized rats. J. Funct. Foods 2014, 7, 398–406. [Google Scholar] [CrossRef]
- Munstedt, K.; Henschel, M.; Hauenschild, A.; von Georgi, R. Royal jelly increases high density lipoprotein levels but in older patients only. J. Altern. Complement. Med. 2009, 15, 329–330. [Google Scholar] [CrossRef]
- Yakoot, M.; Salem, A.; Helmy, S. Effect of Memo®, a natural formula combination, on Mini-Mental State Examination scores in patients with mild cognitive impairment. Clin. Interv. Aging 2013, 8, 975–981. [Google Scholar] [CrossRef]
- Pan, Y.; Xu, J.; Chen, C.; Chen, F.; Jin, P.; Zhu, K.; Hu, C.W.; You, M.; Chen, M.; Hu, F. Royal Jelly Reduces Cholesterol Levels, Ameliorates Aβ Pathology and Enhances Neuronal Metabolic Activities in a Rabbit Model of Alzheimer’s Disease. Front. Aging Neurosci. 2018, 10, 50. [Google Scholar] [CrossRef]
- Donlon, T.A.; Morris, B.J.; He, Q.; Chen, R.; Masaki, K.H.; Allsopp, R.C.; Willcox, D.C.; Tranah, G.J.; Parimi, N.; Evans, D.S.; et al. Association of Polymorphisms in Connective Tissue Growth Factor and Epidermal Growth Factor Receptor Genes With Human Longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1038–1044. [Google Scholar] [CrossRef]
- Ewald, C.Y.; Castillo-Quan, J.I.; Blackwell, T.K. Untangling Longevity, Dauer, and Healthspan in Caenorhabditis elegans Insulin/IGF-1-Signalling. Gerontology 2018, 64, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Bartke, A. Healthspan and longevity can be extended by suppression of growth hormone signaling. Mamm. Genome 2016, 27, 289–299. [Google Scholar] [CrossRef] [PubMed]
- Altintas, O.; Park, S.; Lee, S.-J.V. The role of insulin/IGF-1 signaling in the longevity of model invertebrates, C. elegans and D. melanogaster. BMB Rep. 2016, 49, 81–92. [Google Scholar] [CrossRef] [PubMed]
- Lechler, M.C.; Crawford, E.D.; Groh, N.; Widmaier, K.; Jung, R.; Kirstein, J.; Trinidad, J.C.; Burlingame, A.L.; David, D.C. Reduced Insulin/IGF-1 Signaling Restores the Dynamic Properties of Key Stress Granule Proteins during Aging. Cell Rep. 2017, 18, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Albert, V.; Hall, M.N. mTOR signaling in cellular and organismal energetics. Curr. Opin. Cell Biol. 2015, 33, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Hindupur, S.K.; González, A.; Hall, M.N. The opposing actions of target of rapamycin and AMP-activated protein kinase in cell growth control. Cold Spring Harb. Perspect. Biol. 2015, 7, a019141. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, B.K.; Lamming, D.W. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab. 2016, 23, 990–1003. [Google Scholar] [CrossRef]
- Nakamura, S.; Yoshimori, T. Autophagy and Longevity. Mol. Cells 2018, 41, 65–72. [Google Scholar] [CrossRef]
- Bok, E.; Jo, M.; Lee, S.; Lee, B.R.; Kim, J.; Kim, H.J. Dietary Restriction and Neuroinflammation: A Potential Mechanistic Link. Int. J. Mol. Sci. 2019, 20, 464. [Google Scholar] [CrossRef]
- Yamada, Y.; Kemnitz, J.W.; Weindruch, R.; Anderson, R.M.; Schoeller, D.A.; Colman, R.J. Caloric Restriction and Healthy Life Span: Frail Phenotype of Nonhuman Primates in the Wisconsin National Primate Research Center Caloric Restriction Study. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 273–278. [Google Scholar] [CrossRef]
- Vasconcelos, A.R.; Santos, N.B.d.; Scavone1, C.; Munhoz, C.D. Nrf2/ARE Pathway Modulation by Dietary Energy Regulation in Neurological Disorders. Front. Pharm. 2019, 10, 33. [Google Scholar] [CrossRef] [PubMed]
- Detienne, G.; Walle, P.V.d.; Haes, W.D.; Cockx, B.; Braeckman, B.P.; Schoofs, L.; Temmerman, L. Royalactin induces copious longevity via increased translation and proteasome activity in C. elegans. bioRxiv 2018, 2018, 421818. [Google Scholar] [CrossRef]
- Troemel, E.R.; Chu, S.W.; Reinke, V.; Lee, S.S.; Ausubel, F.M.; Kim, D.H. p38 MAPK regulates expression of immune response genes and contributes to longevity in C. elegans. PLoS Genet. 2006, 2, e183. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Driscoll, M. EGF signaling comes of age: Promotion of healthy aging in C. elegans. Exp. Gerontol. 2011, 46, 129–134. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, H.; Yu, S.; Xue, J.; Driscoll, M. Novel EGF pathway regulators modulate C. elegans healthspan and lifespan via EGF receptor, PLC-gamma, and IP3R activation. Aging Cell 2010, 9, 490–505. [Google Scholar] [CrossRef] [PubMed]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of antioxidants supplementation on aging and longevity. Biomed. Res. Int. 2014, 2014, 404680. [Google Scholar] [CrossRef] [PubMed]
- Haddad, L.S.; Kelbert, L.; Hulbert, A.J. Extended longevity of queen honey bees compared to workers is associated with peroxidation-resistant membranes. Exp. Gerontol. 2007, 42, 601–609. [Google Scholar] [CrossRef]
- Anderson, K.E.; Ricigliano, V.A.; Mott, B.M.; Copeland, D.C.; Floyd, A.S.; Maes, P. The queen’s gut refines with age: Longevity phenotypes in a social insect model. Microbiome 2018, 6, 108. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the biological properties and toxicity of bee propolis (propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Henkel, R.; Sandhu, I.S.; Agarwal, A. The excessive use of antioxidant therapy: A possible cause of male infertility? Andrologia 2019, 51, e13162. [Google Scholar] [CrossRef]
- Codling, G.; Al Naggar, Y.; Giesy, J.P.; Robertson, A.J. Concentrations of neonicotinoid insecticides in honey, pollen and honey bees (Apis mellifera L.) in central Saskatchewan, Canada. Chemosphere 2016, 144, 2321–2328. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, R.C.; Queiroz, S.; da Luz, C.F.P.; Porto, R.S.; Rath, S. Bee pollen as a bioindicator of environmental pesticide contamination. Chemosphere 2016, 163, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Tosi, S.; Costa, C.; Vesco, U.; Quaglia, G.; Guido, G. A 3-year survey of Italian honey bee-collected pollen reveals widespread contamination by agricultural pesticides. Sci. Total Env. 2018, 615, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Böhme, F.; Bischoff, G.; Zebitz, C.P.W.; Rosenkranz, P.; Wallner, K. From field to food—will pesticide-contaminated pollen diet lead to a contamination of royal jelly? Apidologie 2018, 49, 112–119. [Google Scholar] [CrossRef]
- Giroud, B.; Bruckner, S.; Straub, L.; Neumann, P.; Williams, G.R.; Vulliet, E. Trace-level determination of two neonicotinoid insecticide residues in honey bee royal jelly using ultra-sound assisted salting-out liquid liquid extraction followed by ultra-high-performance liquid chromatography-tandem mass spectrometry. Microchem. J. 2019, 151, 104249. [Google Scholar] [CrossRef]
- Muresan, C.I.; Schierhorn, A.; Buttstedt, A. The Fate of Major Royal Jelly Proteins during Proteolytic Digestion in the Human Gastrointestinal Tract. J. Agric. Food Chem. 2018, 66, 4164–4170. [Google Scholar] [CrossRef]
- Ostan, R.; Monti, D.; Gueresi, P.; Bussolotto, M.; Franceschi, C.; Baggio, G. Gender, aging and longevity in humans: An update of an intriguing/neglected scenario paving the way to a gender-specific medicine. Clin. Sci. 2016, 130, 1711–1725. [Google Scholar] [CrossRef]

| RJ/RJ Components | Species | Effect on Lifespan | Effect on Healthspan | Main Mechanism of Action | References |
|---|---|---|---|---|---|
| RJ | Apis mellifera (worker) | No effect on lifespan | ↑ Ovarian activation | NI—Inability to defecate in 100% RJ-fed bees | [60] |
| RJ | Apis mellifera (worker) | ↑ Mean lifespan | ↑ Ovarian activation | NI | [63] |
| RJ | Apis mellifera (worker) | -- | ↑ Ovarian activation | ↑ Brain levels of tyrosine, dopamine, and tyramine | [61] |
| RJ + Ach | Apis mellifera (larvae) | ↑ Mean lifespan | -- | NI—possibly the trophic effects of Ach mediated via muscarinic or nicotinic receptors | [56] |
| RJ RJP60 | Apis mellifera (worker) | ↑ Mean and maximum lifespan | -- | NI Lower DNA methylation levels | [15] |
| RJ | Apis mellifera (worker) | ↑ Mean lifespan | ↑ Expression of memory genes | NI | [62] |
| RJ, heat-treated RJ, pKRJ, RJ plus MRJP1 vs RJ plus MRJPs2,3,5 | Apis mellifera (larvae) | ↑ Mean lifespan (except MRJP1) | ↑ Ovarian activation | NI | [25] |
| Dehydrated RJ RJ pantothenic acid RJ organic acids | Drosophila M. | ↑ Mean lifespan | -- | NI Synergizing action of other vitamins | [54] |
| RJ | Drosophila M. | ↑ Mean lifespan (both sexes) | -- | ↑ Anti-oxidation capacity--SOD and CAT levels | [64] |
| RJ, royalactin | Drosophila Canton-S Apis mellifera (larvae) | ↑ Mean lifespan | ↑ Body size ↑ Cell size ↑ Eggs laying ↓ Developmental time | ↑ EGFR-mediated signaling pathway, S6K, MAPK, juvenile hormone titre, 20E titre | [41] |
| Freeze-dried RJ | Drosophila M. | ↑ Mean lifespan (males only) | ↓ Developmental time ↑ Eggs laying | ↓ Insulin/IGF-1 (dilp5) signaling ↓ Target of Rapamycin signaling | [17] |
| RJ | Drosophila Canton S | No effect on lifespan | ↑ Body size | ↑ Gene expression related to oxidative stress and catabolism | [16] |
| MRJPs | Drosophila M. | ↑ Mean and maximum lifespan (both sexes) | ↑ Feeding ↑ Eggs laying | ↑ Anti-oxidation capacity—CuZn-SOD signaling ↑ EGFR-mediated signaling | [12] |
| RJ | Gryllus bimaculatus crickets silkworms | ↑ Mean lifespan in crickets (both sexes) | ↑ Eggs size (silkworms) ↑ Body size ↓ Developmental time, | NI | [65] |
| RJ, pRJ, pRJ-Fr.5, 10-HDA | C. elegans | ↑ Mean lifespan | -- | ↓ Insulin/IGF-1 and ins-9 signaling | [52] |
| Royalactin | C. elegans | ↑ Mean lifespan | ↑ Locomotion in early and mid-adulthood | ↑ EGFR-mediated signaling pathway | [68] |
| 10-HDA | C. elegans | ↑ Mean lifespan | ↑ Stress resistance | ↓ Insulin/IGF-1 signaling ↓ Target of Rapamycin signaling ↑ Dietary Restriction | [66] |
| RJ, pRJ | C. elegans | ↑ Mean lifespan | ↑ Stress resistance | ↓ Insulin/IGF-1 signaling | [67] |
| Powdered RJ | C3H/HeJ mice | ↑ Mean lifespan | -- | ↓ Oxidative stress and DNA damage | [2] |
| RJ | Male Swiss albino mice | ↑ Survival time after NaNO2 IP injection | -- | NI | [71] |
| RJ, pRJ | D-galactose induced aging mice model | -- | ↓ Atrophy of thymus ↓ Weight loss ↓ Locomotor decline ↑ Learning and memory | ↓ Oxidative stress | [69] |
| RJ, pRJ | HET mice | No effect on lifespan | ↓ Muscle atrophy ↓ Age-related motor impairment | ↑ Muscle satellite cell (muscle stem cell) markers Suppression of catabolic genes | [70] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kunugi, H.; Mohammed Ali, A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. Int. J. Mol. Sci. 2019, 20, 4662. https://doi.org/10.3390/ijms20194662
Kunugi H, Mohammed Ali A. Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. International Journal of Molecular Sciences. 2019; 20(19):4662. https://doi.org/10.3390/ijms20194662
Chicago/Turabian StyleKunugi, Hiroshi, and Amira Mohammed Ali. 2019. "Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans" International Journal of Molecular Sciences 20, no. 19: 4662. https://doi.org/10.3390/ijms20194662
APA StyleKunugi, H., & Mohammed Ali, A. (2019). Royal Jelly and Its Components Promote Healthy Aging and Longevity: From Animal Models to Humans. International Journal of Molecular Sciences, 20(19), 4662. https://doi.org/10.3390/ijms20194662