Topographical Visualization of the Reciprocal Projection between the Medial Septum and the Hippocampus in the 5XFAD Mouse Model of Alzheimer’s Disease
Abstract
1. Introduction
2. Results
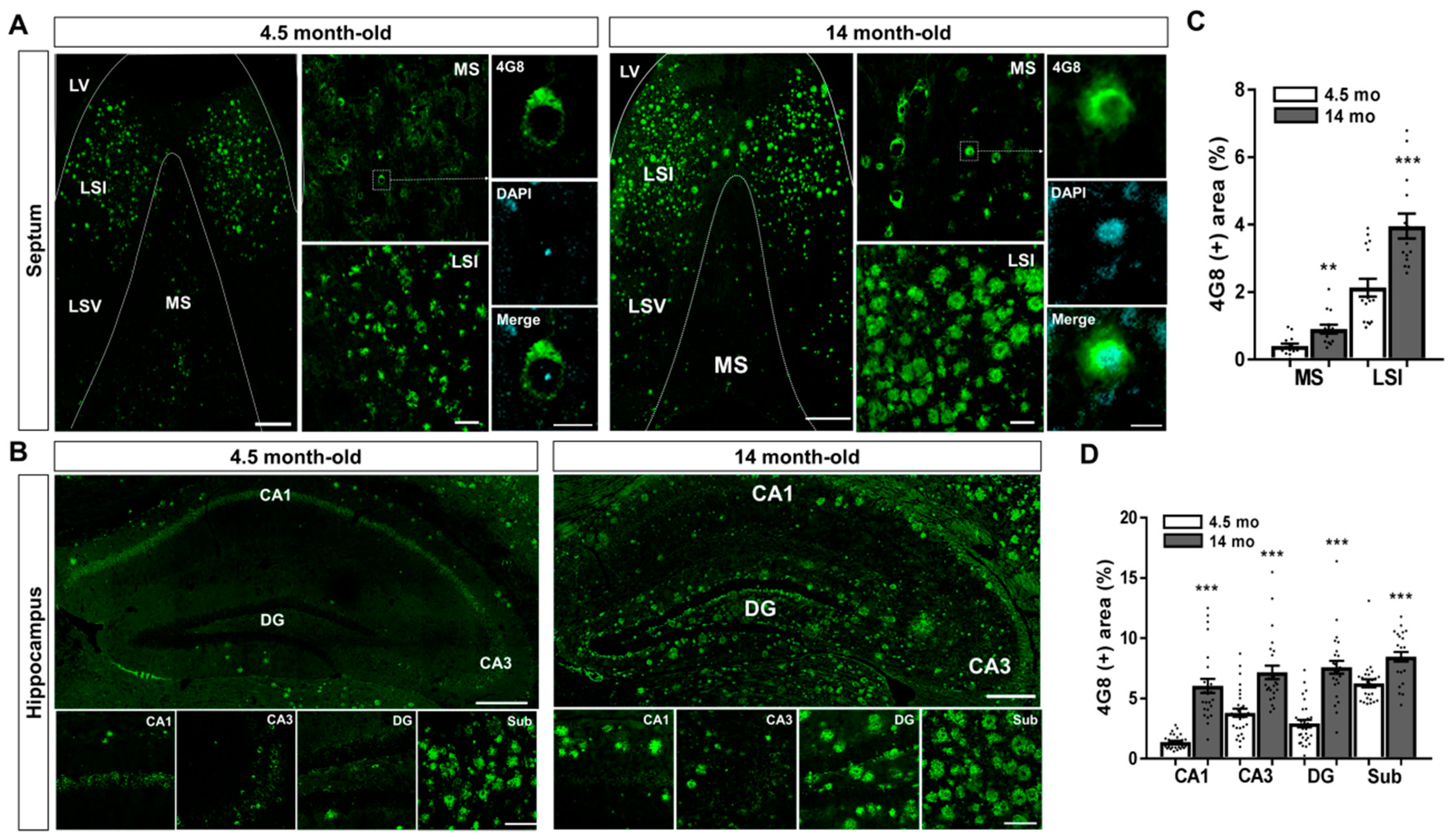
2.1. Aβ Accumulation in the MS and Hippocampal Formation of 5XFAD Mice
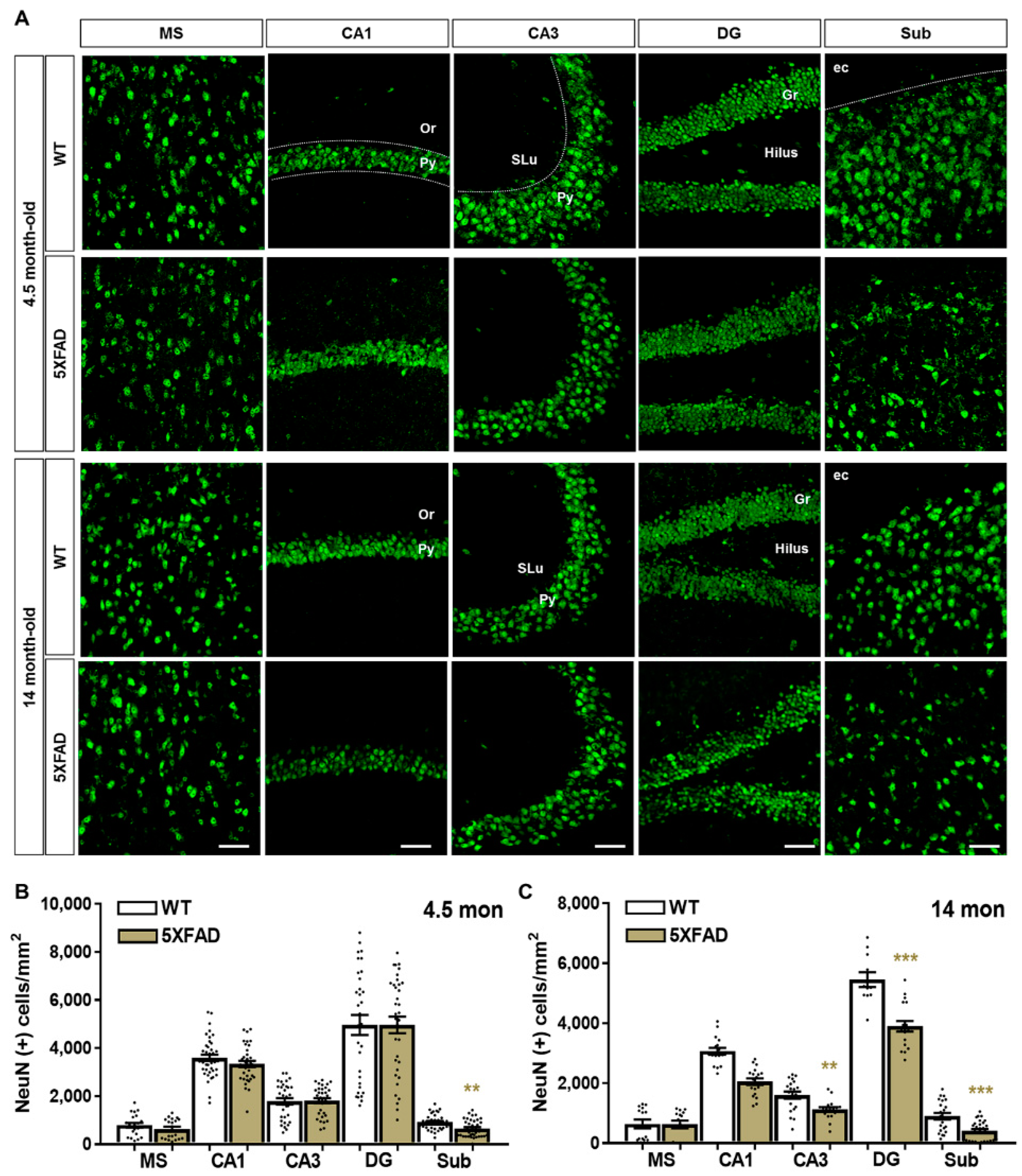
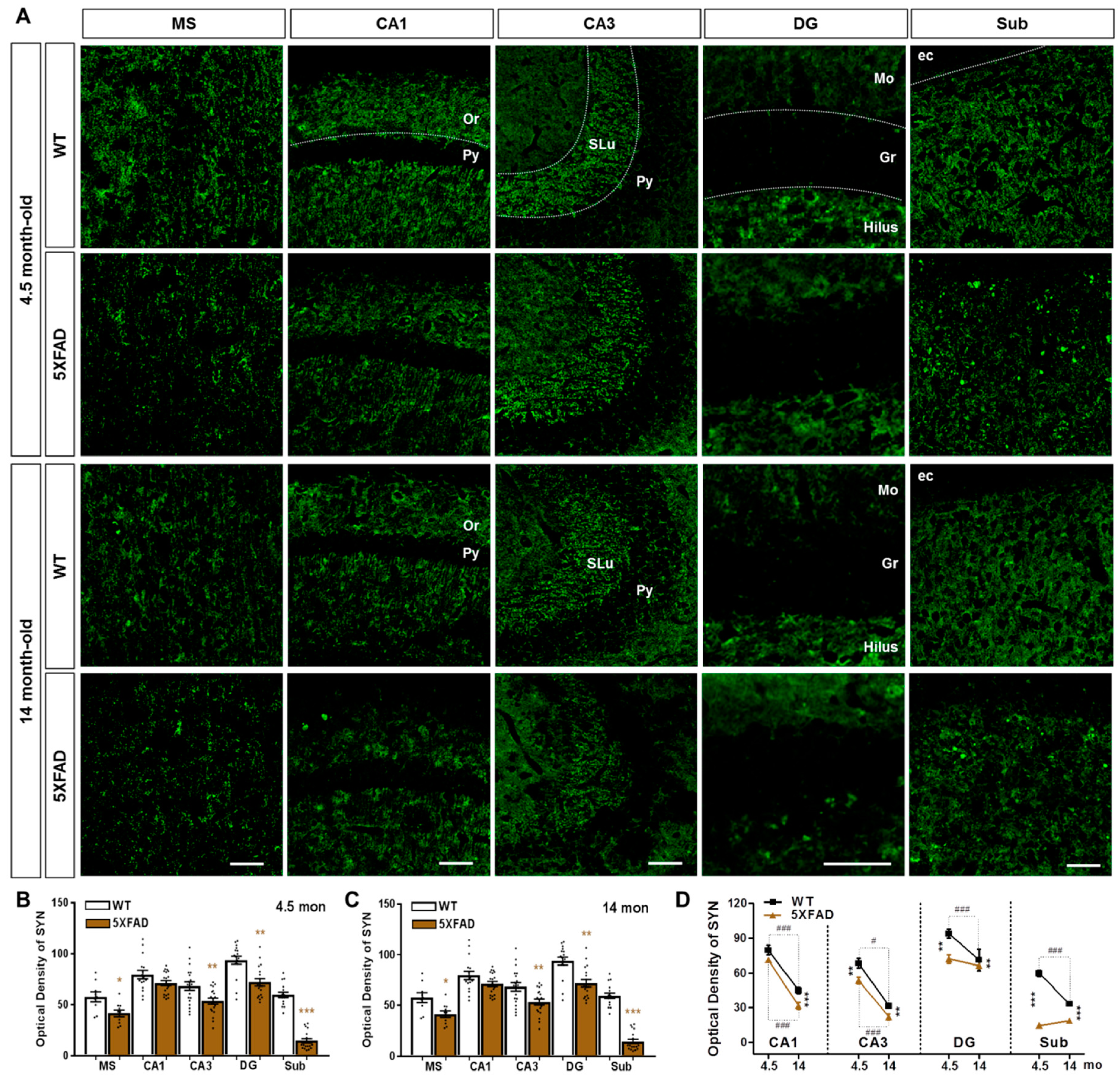
2.2. Neuronal and Synaptic Degeneration in the MS and Hippocampal Formation of 5XFAD Mice
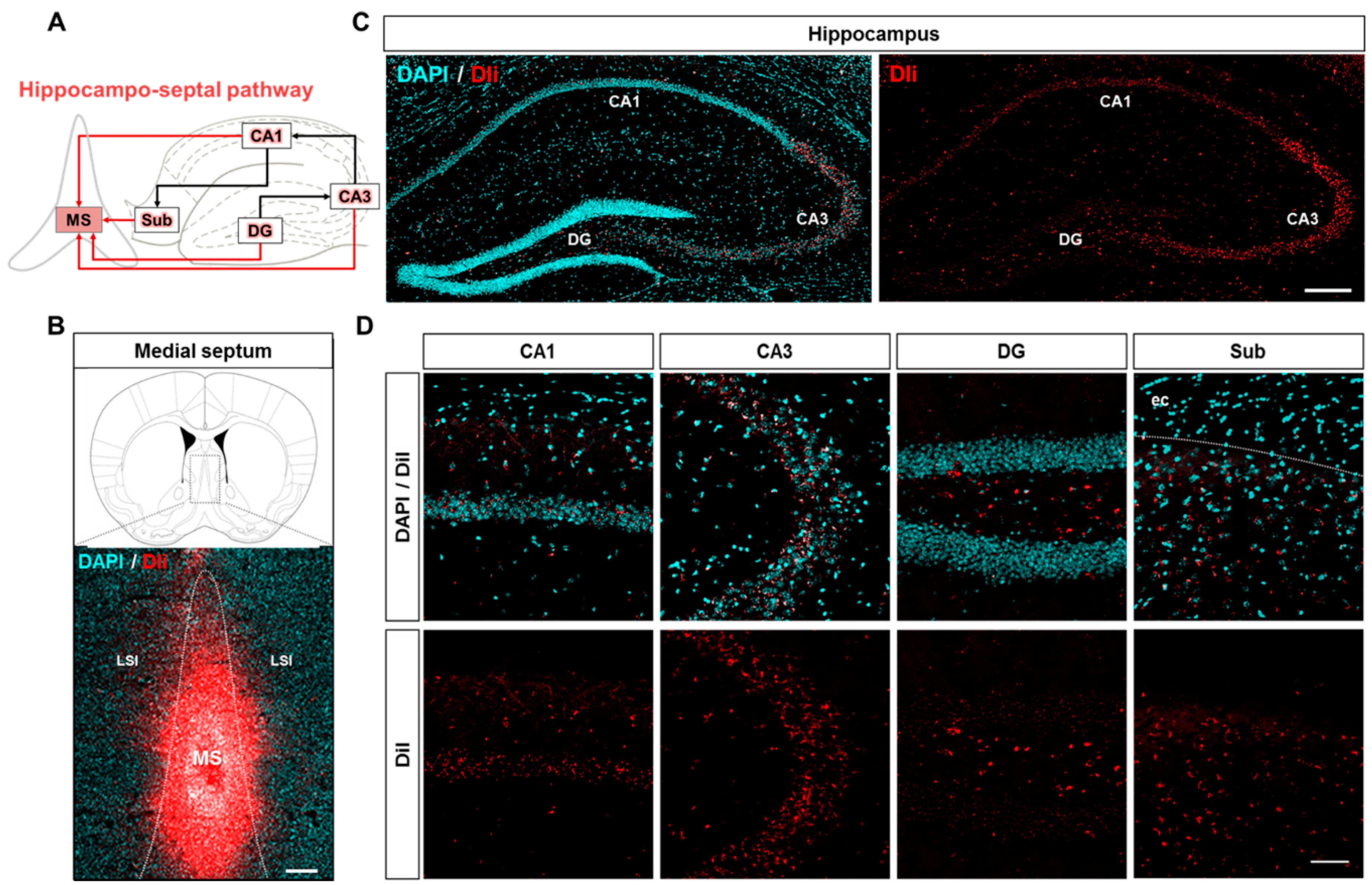
2.3. Neuroanatomical Tracing of the Hippocampo-Septal Pathway Using a Retrograde Tracer
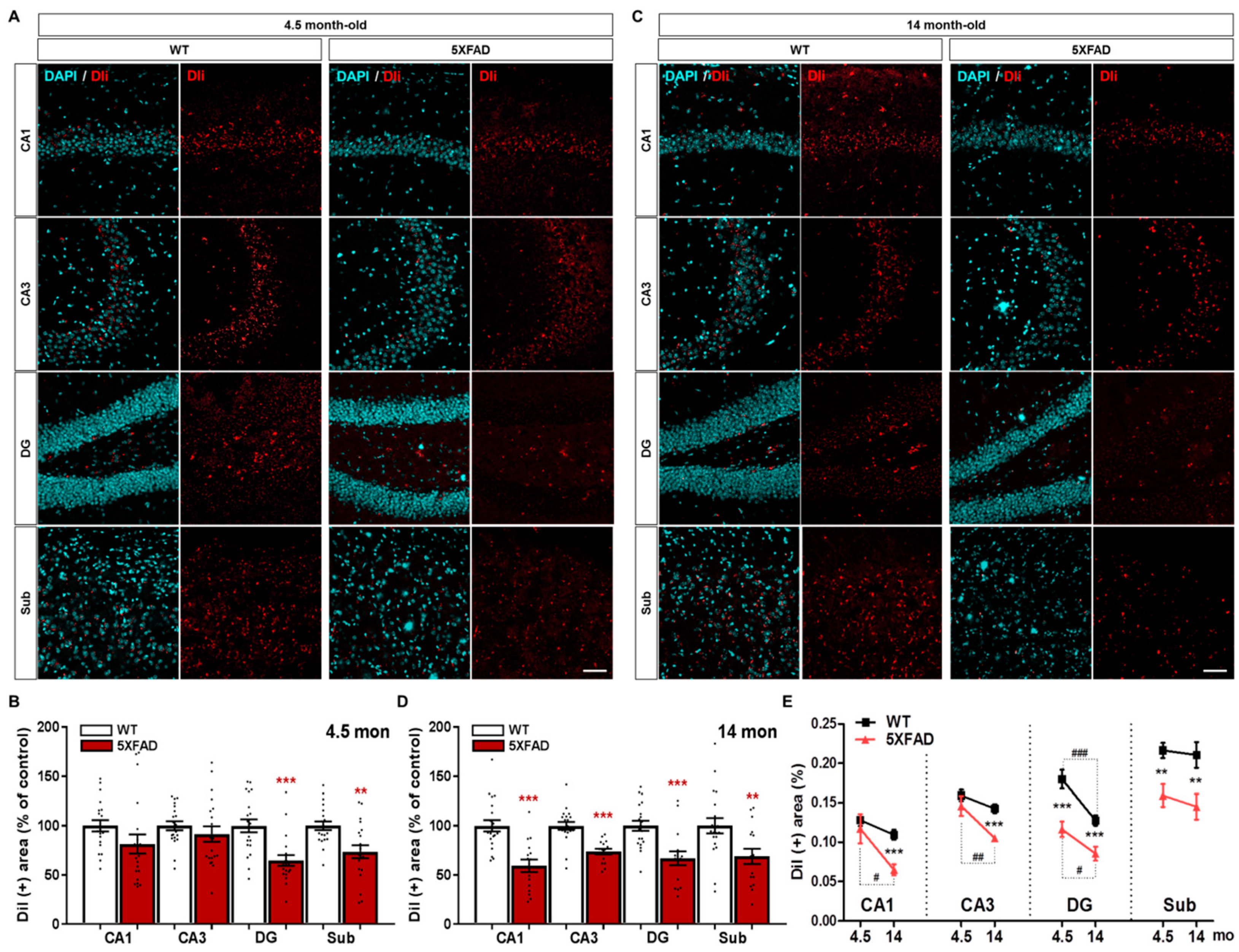
2.4. Disruption of the Hippocampo-Septal Pathway in 5XFAD Mice
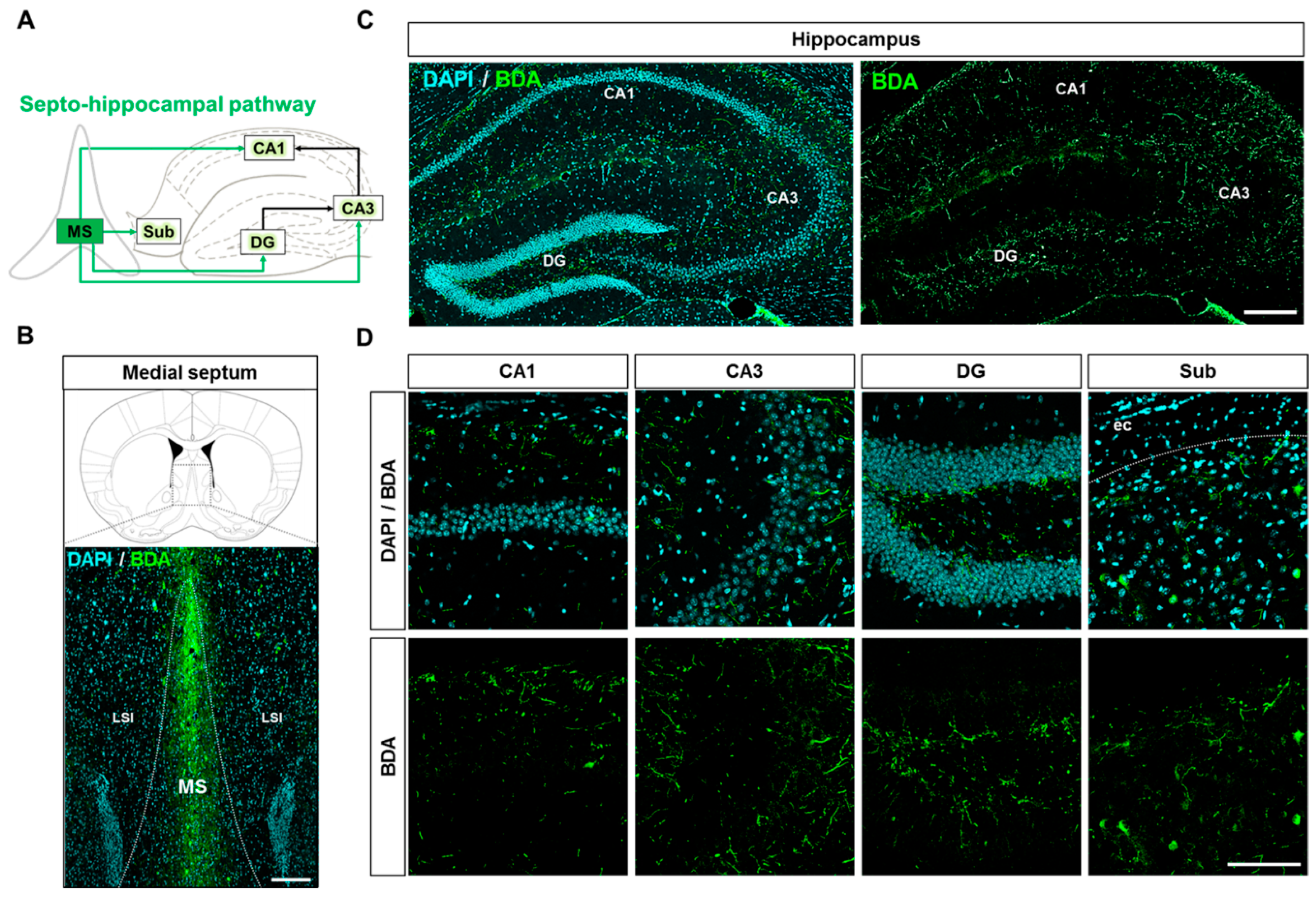
2.5. Neuroanatomical Tracing of the Septo-Hippocampal Pathway Using an Anterograde Tracer
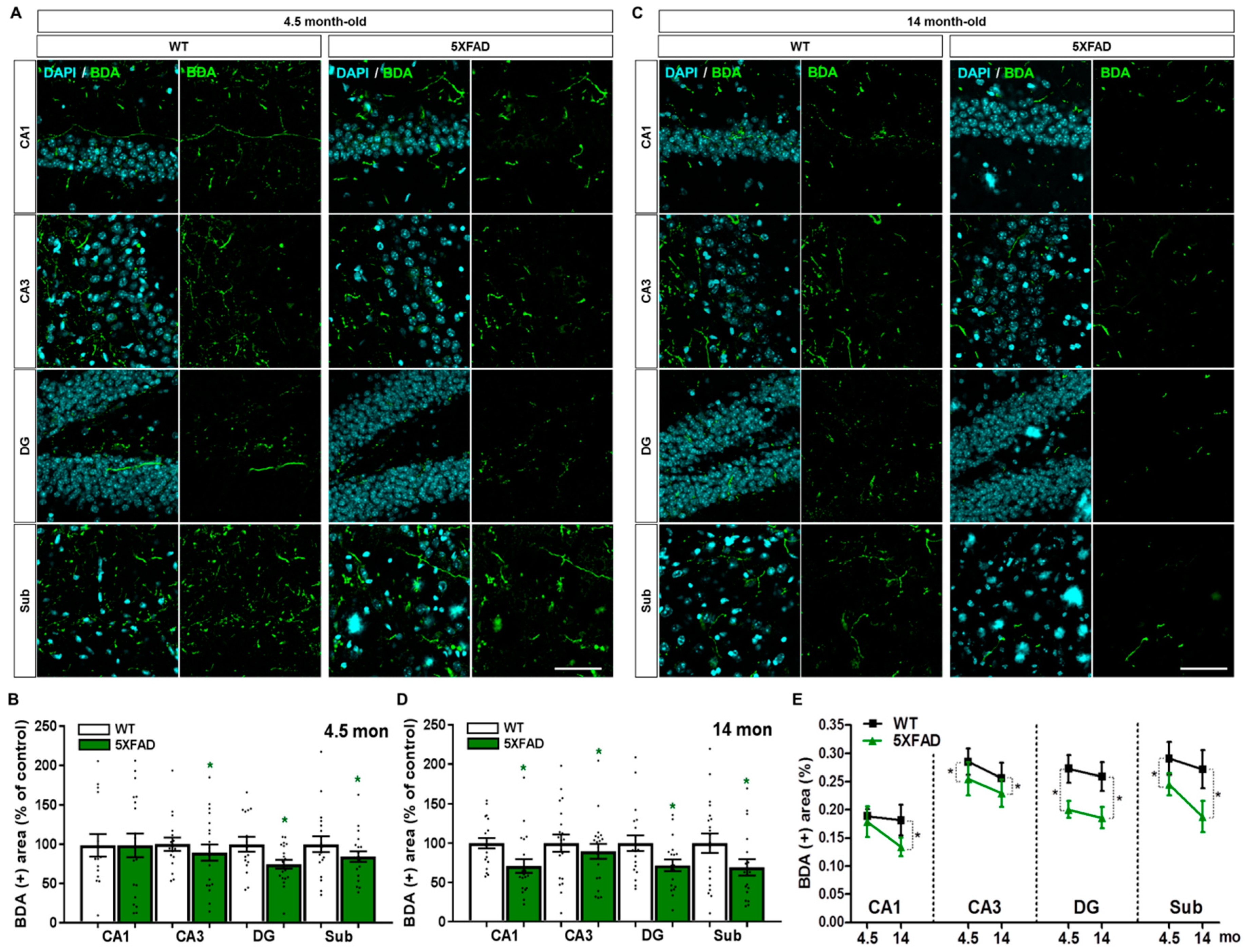
2.6. Disruption of the Septo-Hippocampal Pathway in 5XFAD Mice
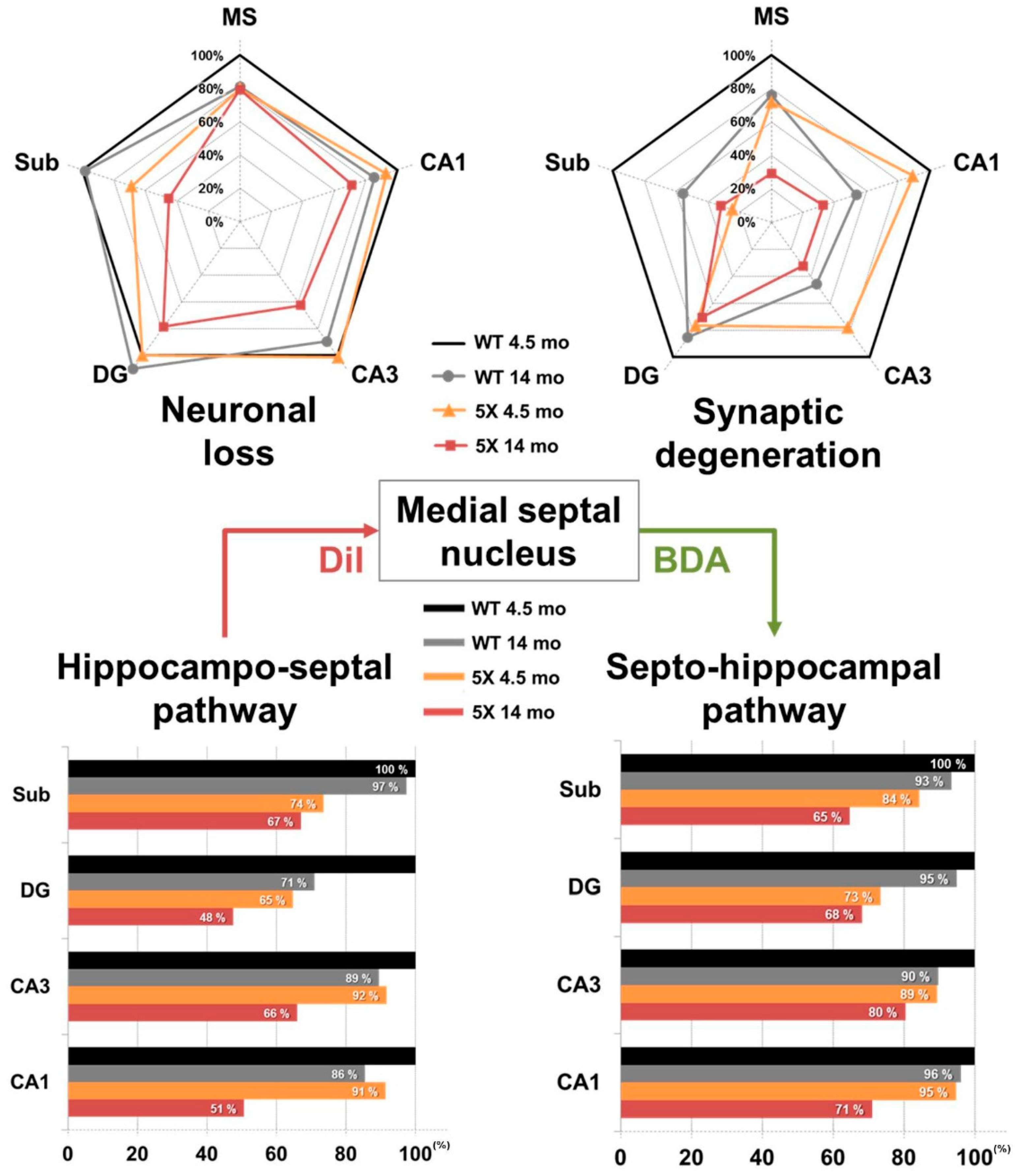
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Stereotaxic Injection of DiI for Retrograde Tracing
4.3. Stereotaxic Injection of BDA for Anterograde Tracing
4.4. Brain Tissue Preparation
4.5. Immunofluorescence Labeling
4.6. Image Acquisition and Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5XFAD | five familial AD mutations | |
| AchE | acetylcholine | |
| AD | Alzheimer’s disease | |
| Aβ | amyloid-β | |
| BDA | biotinylated dextran amine | |
| DB | diagonal band nucleus | |
| DG | dentate gyrus | |
| DiI | 1,1′-dioctadecyl-3,3,3′,3′-tetramethyl-indocarbocyanine perchlorate | |
| DMSO | dimethyl sulfoxide | |
| LSI | lateral septal nucleus intermediate part | |
| LSV | lateral septal nucleus ventral part oriens | |
| MS | medial septum | |
| nBM | nucleus basalis magnocellularis | |
| NeuN | neuronal nuclei | |
| PB | phosphate buffer | |
| PBS | phosphate-buffered saline | |
| SHS | septo-hippocampo-septal; | |
| Sub | subiculum | |
| SYN | synaptophysin | |
| WT | wild-type |
References
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Song, D.; Qing, H. Neural changes in alzheimer’s disease from circuit to molecule: Perspective of optogenetics. Neurosci. Biobehav. Rev. 2017, 79, 110–118. [Google Scholar] [CrossRef] [PubMed]
- Bateman, R.J.; Xiong, C.; Benzinger, T.L.; Fagan, A.M.; Goate, A.; Fox, N.C.; Marcus, D.S.; Cairns, N.J.; Xie, X.; Blazey, T.M.; et al. Clinical and biomarker changes in dominantly inherited alzheimer’s disease. N. Engl. J. Med. 2012, 367, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Sheline, Y.I.; Raichle, M.E.; Snyder, A.Z.; Morris, J.C.; Head, D.; Wang, S.; Mintun, M.A. Amyloid plaques disrupt resting state default mode network connectivity in cognitively normal elderly. Biol. Psychiatry 2010, 67, 584–587. [Google Scholar] [CrossRef] [PubMed]
- Hedden, T.; Van Dijk, K.R.; Becker, J.A.; Mehta, A.; Sperling, R.A.; Johnson, K.A.; Buckner, R.L. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci. 2009, 29, 12686–12694. [Google Scholar] [CrossRef]
- Sperling, R.A.; Laviolette, P.S.; O’Keefe, K.; O’Brien, J.; Rentz, D.M.; Pihlajamaki, M.; Marshall, G.; Hyman, B.T.; Selkoe, D.J.; Hedden, T.; et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 2009, 63, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Selkoe, D.J. Alzheimer’s disease is a synaptic failure. Science 2002, 298, 789–791. [Google Scholar] [CrossRef]
- Lepeta, K.; Lourenco, M.V.; Schweitzer, B.C.; Martino Adami, P.V.; Banerjee, P.; Catuara-Solarz, S.; de La Fuente Revenga, M.; Guillem, A.M.; Haidar, M.; Ijomone, O.M.; et al. Synaptopathies: Synaptic dysfunction in neurological disorders—A review from students to students. J. Neurochem. 2016, 138, 785–805. [Google Scholar] [CrossRef]
- Palop, J.J.; Mucke, L. Amyloid-beta-induced neuronal dysfunction in alzheimer’s disease: From synapses toward neural networks. Nat. Neurosci. 2010, 13, 812–818. [Google Scholar] [CrossRef]
- Jeon, S.G.; Kim, Y.J.; Kim, K.A.; Mook-Jung, I.; Moon, M. Visualization of altered hippocampal connectivity in an animal model of alzheimer’s disease. Mol. Neurobiol. 2018, 55, 7886–7899. [Google Scholar] [CrossRef]
- Siskova, Z.; Justus, D.; Kaneko, H.; Friedrichs, D.; Henneberg, N.; Beutel, T.; Pitsch, J.; Schoch, S.; Becker, A.; von der Kammer, H.; et al. Dendritic structural degeneration is functionally linked to cellular hyperexcitability in a mouse model of alzheimer’s disease. Neuron 2014, 84, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Palop, J.J.; Chin, J.; Roberson, E.D.; Wang, J.; Thwin, M.T.; Bien-Ly, N.; Yoo, J.; Ho, K.O.; Yu, G.Q.; Kreitzer, A.; et al. Aberrant excitatory neuronal activity and compensatory remodeling of inhibitory hippocampal circuits in mouse models of alzheimer’s disease. Neuron 2007, 55, 697–711. [Google Scholar] [CrossRef] [PubMed]
- Masliah, E.; Terry, R.D.; DeTeresa, R.M.; Hansen, L.A. Immunohistochemical quantification of the synapse-related protein synaptophysin in alzheimer disease. Neurosci. Lett. 1989, 103, 234–239. [Google Scholar] [CrossRef]
- Busche, M.A.; Konnerth, A. Impairments of neural circuit function in alzheimer’s disease. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150429. [Google Scholar] [CrossRef] [PubMed]
- Terry, R.D.; Masliah, E.; Salmon, D.P.; Butters, N.; DeTeresa, R.; Hill, R.; Hansen, L.A.; Katzman, R. Physical basis of cognitive alterations in alzheimer’s disease: Synapse loss is the major correlate of cognitive impairment. Ann. Neurol. 1991, 30, 572–580. [Google Scholar] [CrossRef] [PubMed]
- DeKosky, S.T.; Scheff, S.W. Synapse loss in frontal cortex biopsies in alzheimer’s disease: Correlation with cognitive severity. Ann. Neurol. 1990, 27, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Canter, R.G.; Penney, J.; Tsai, L.H. The road to restoring neural circuits for the treatment of alzheimer’s disease. Nature 2016, 539, 187–196. [Google Scholar] [CrossRef]
- Roland, J.J.; Savage, L.M. The role of cholinergic and gabaergic medial septal/diagonal band cell populations in the emergence of diencephalic amnesia. Neuroscience 2009, 160, 32–41. [Google Scholar] [CrossRef]
- Vega-Flores, G.; Rubio, S.E.; Jurado-Parras, M.T.; Gomez-Climent, M.A.; Hampe, C.S.; Manto, M.; Soriano, E.; Pascual, M.; Gruart, A.; Delgado-Garcia, J.M. The gabaergic septohippocampal pathway is directly involved in internal processes related to operant reward learning. Cereb. Cortex 2014, 24, 2093–2107. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Blokland, A. The validity of scopolamine as a pharmacological model for cognitive impairment: A review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010, 34, 1307–1350. [Google Scholar] [CrossRef]
- Klinkenberg, I.; Sambeth, A.; Blokland, A. Acetylcholine and attention. Behav. Brain Res. 2011, 221, 430–442. [Google Scholar] [CrossRef] [PubMed]
- van der Zee, E.A.; Luiten, P.G. Muscarinic acetylcholine receptors in the hippocampus, neocortex and amygdala: A review of immunocytochemical localization in relation to learning and memory. Prog. Neurobiol. 1999, 58, 409–471. [Google Scholar] [CrossRef]
- Hasselmo, M.E.; Barkai, E. Cholinergic modulation of activity-dependent synaptic plasticity in the piriform cortex and associative memory function in a network biophysical simulation. J. Neurosci. 1995, 15, 6592–6604. [Google Scholar] [CrossRef] [PubMed]
- Freund, T.F.; Antal, M. Gaba-containing neurons in the septum control inhibitory interneurons in the hippocampus. Nature 1988, 336, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Khakpai, F.; Zarrindast, M.R.; Nasehi, M.; Haeri-Rohani, A.; Eidi, A. The role of glutamatergic pathway between septum and hippocampus in the memory formation. EXCLI J. 2013, 12, 41–51. [Google Scholar] [PubMed]
- Muller, C.; Remy, S. Septo-hippocampal interaction. Cell Tissue Res. 2018, 373, 565–575. [Google Scholar] [CrossRef] [PubMed]
- Dragoi, G.; Carpi, D.; Recce, M.; Csicsvari, J.; Buzsaki, G. Interactions between hippocampus and medial septum during sharp waves and theta oscillation in the behaving rat. J. Neurosci. 1999, 19, 6191–6199. [Google Scholar] [CrossRef] [PubMed]
- Elvander-Tottie, E.; Eriksson, T.M.; Sandin, J.; Ogren, S.O. N-methyl-D-aspartate receptors in the medial septal area have a role in spatial and emotional learning in the rat. Neuroscience 2006, 142, 963–978. [Google Scholar] [CrossRef] [PubMed]
- Khakpai, F.; Nasehi, M.; Haeri-Rohani, A.; Eidi, A.; Zarrindast, M.R. Septo-hippocampo-septal loop and memory formation. Basic Clin. Neurosci. 2013, 4, 5–23. [Google Scholar]
- Rokers, B.; Mercado, E., 3rd; Allen, M.T.; Myers, C.E.; Gluck, M.A. A connectionist model of septohippocampal dynamics during conditioning: Closing the loop. Behav. Neurosci. 2002, 116, 48–62. [Google Scholar] [CrossRef]
- Lovett-Barron, M.; Kaifosh, P.; Kheirbek, M.A.; Danielson, N.; Zaremba, J.D.; Reardon, T.R.; Turi, G.F.; Hen, R.; Zemelman, B.V.; Losonczy, A. Dendritic inhibition in the hippocampus supports fear learning. Science 2014, 343, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Smythe, J.W.; Colom, L.V.; Bland, B.H. The extrinsic modulation of hippocampal theta depends on the coactivation of cholinergic and gaba-ergic medial septal inputs. Neurosci. Biobehav. Rev. 1992, 16, 289–308. [Google Scholar] [CrossRef]
- Mamad, O.; McNamara, H.M.; Reilly, R.B.; Tsanov, M. Medial septum regulates the hippocampal spatial representation. Front. Behav. Neurosci. 2015, 9, 166. [Google Scholar] [CrossRef] [PubMed]
- Soler, H.; Dorca-Arevalo, J.; Gonzalez, M.; Rubio, S.E.; Avila, J.; Soriano, E.; Pascual, M. The gabaergic septohippocampal connection is impaired in a mouse model of tauopathy. Neurobiol. Aging 2017, 49, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rubio, S.E.; Vega-Flores, G.; Martinez, A.; Bosch, C.; Perez-Mediavilla, A.; del Rio, J.; Gruart, A.; Delgado-Garcia, J.M.; Soriano, E.; Pascual, M. Accelerated aging of the gabaergic septohippocampal pathway and decreased hippocampal rhythms in a mouse model of alzheimer’s disease. FASEB J. 2012, 26, 4458–4467. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, T.W.; Mur, M.; Aghourian, M.; Bedard, M.A.; Spreng, R.N.; Alzheimer’s Disease Neuroimaging Initiative. Longitudinal alzheimer’s degeneration reflects the spatial topography of cholinergic basal forebrain projections. Cell Rep. 2018, 24, 38–46. [Google Scholar] [CrossRef]
- Akil, H.; Martone, M.E.; Van Essen, D.C. Challenges and opportunities in mining neuroscience data. Science 2011, 331, 708–712. [Google Scholar] [CrossRef]
- Sotiropoulos, S.N.; Zalesky, A. Building connectomes using diffusion mri: Why, how and but. NMR Biomed. 2017, 32, e3752. [Google Scholar] [CrossRef]
- Zeng, H. Mesoscale connectomics. Curr. Opin. Neurobiol. 2018, 50, 154–162. [Google Scholar] [CrossRef]
- Oh, S.W.; Harris, J.A.; Ng, L.; Winslow, B.; Cain, N.; Mihalas, S.; Wang, Q.; Lau, C.; Kuan, L.; Henry, A.M.; et al. A mesoscale connectome of the mouse brain. Nature 2014, 508, 207–214. [Google Scholar] [CrossRef]
- Moon, M.; Cha, M.Y.; Mook-Jung, I. Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5xfad mice. J. Alzheimer’s Dis. 2014, 41, 233–241. [Google Scholar] [CrossRef]
- Oakley, H.; Cole, S.L.; Logan, S.; Maus, E.; Shao, P.; Craft, J.; Guillozet-Bongaarts, A.; Ohno, M.; Disterhoft, J.; Van Eldik, L.; et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci. 2006, 26, 10129–10140. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.; Hong, H.S.; Nam, D.W.; Baik, S.H.; Song, H.; Kook, S.Y.; Kim, Y.S.; Lee, J.; Mook-Jung, I. Intracellular amyloid-beta accumulation in calcium-binding protein-deficient neurons leads to amyloid-beta plaque formation in animal model of alzheimer’s disease. J. Alzheimer’s Dis. 2012, 29, 615–628. [Google Scholar] [CrossRef]
- Thal, D.R.; Rub, U.; Orantes, M.; Braak, H. Phases of a beta-deposition in the human brain and its relevance for the development of ad. Neurology 2002, 58, 1791–1800. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Acton, P.; Rao, V.; Ray, W.J. Functional and structural connectome properties in the 5xfad transgenic mouse model of alzheimer’s disease. Netw. Neurosci. 2018, 2, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Devi, L.; Ohno, M. Phospho-eif2alpha level is important for determining abilities of bace1 reduction to rescue cholinergic neurodegeneration and memory defects in 5xfad mice. PLoS ONE 2010, 5, e12974. [Google Scholar] [CrossRef]
- Ohno, M. Failures to reconsolidate memory in a mouse model of alzheimer’s disease. Neurobiol. Learn. Mem. 2009, 92, 455–459. [Google Scholar] [CrossRef]
- Onos, K.D.; Sukoff Rizzo, S.J.; Howell, G.R.; Sasner, M. Toward more predictive genetic mouse models of alzheimer’s disease. Brain Res. Bull. 2016, 122, 1–11. [Google Scholar] [CrossRef]
- Terry, R.D. Cell death or synaptic loss in alzheimer disease. J. Neuropathol. Exp. Neurol. 2000, 59, 1118–1119. [Google Scholar] [CrossRef]
- Yuki, D.; Sugiura, Y.; Zaima, N.; Akatsu, H.; Takei, S.; Yao, I.; Maesako, M.; Kinoshita, A.; Yamamoto, T.; Kon, R.; et al. Dha-pc and psd-95 decrease after loss of synaptophysin and before neuronal loss in patients with alzheimer’s disease. Sci. Rep. 2014, 4, 7130. [Google Scholar] [CrossRef]
- Khakpai, F.; Nasehi, M.; Zarrindast, M.R. The role of nmda receptors of the medial septum and dorsal hippocampus on memory acquisition. Pharmacol. Biochem. Behav. 2016, 143, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Ohno, M. Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5xfad alzheimer mouse model. Neurobiol. Dis. 2009, 33, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Perez, A.M.; Arnal-Vicente, I.; Santos, F.N.; Pereira, C.W.; ElMlili, N.; Sanjuan, J.; Ma, S.; Gundlach, A.L.; Olucha-Bordonau, F.E. Septal projections to nucleus incertus in the rat: Bidirectional pathways for modulation of hippocampal function. J. Comp. Neurol. 2015, 523, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Garcia, R.; Jaffard, R. The hippocampo-septal projection in mice: Long-term potentiation in the lateral septum. Neuroreport 1992, 3, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Francis, P.T.; Palmer, A.M.; Snape, M.; Wilcock, G.K. The cholinergic hypothesis of alzheimer’s disease: A review of progress. J. Neurol. Neurosurg. Psychiatry 1999, 66, 137–147. [Google Scholar] [CrossRef] [PubMed]
- Ballinger, E.C.; Ananth, M.; Talmage, D.A.; Role, L.W. Basal forebrain cholinergic circuits and signaling in cognition and cognitive decline. Neuron 2016, 91, 1199–1218. [Google Scholar] [CrossRef]
- Schliebs, R.; Arendt, T. The cholinergic system in aging and neuronal degeneration. Behav. Brain Res. 2011, 221, 555–563. [Google Scholar] [CrossRef]
- Zadori, D.; Veres, G.; Szalardy, L.; Klivenyi, P.; Toldi, J.; Vecsei, L. Glutamatergic dysfunctioning in Alzheimer’s disease and related therapeutic targets. J. Alzheimer’s Dis. 2014, 42, S177–S187. [Google Scholar] [CrossRef]
- Colom, L.V.; Castaneda, M.T.; Banuelos, C.; Puras, G.; Garcia-Hernandez, A.; Hernandez, S.; Mounsey, S.; Benavidez, J.; Lehker, C. Medial septal beta-amyloid 1–40 injections alter septo-hippocampal anatomy and function. Neurobiol. Aging 2010, 31, 46–57. [Google Scholar] [CrossRef]
- Lacor, P.N.; Buniel, M.C.; Furlow, P.W.; Clemente, A.S.; Velasco, P.T.; Wood, M.; Viola, K.L.; Klein, W.L. Abeta oligomer-induced aberrations in synapse composition, shape, and density provide a molecular basis for loss of connectivity in alzheimer’s disease. J. Neurosci. 2007, 27, 796–807. [Google Scholar] [CrossRef]
- Leao, R.N.; Colom, L.V.; Borgius, L.; Kiehn, O.; Fisahn, A. Medial septal dysfunction by abeta-induced kcnq channel-block in glutamatergic neurons. Neurobiol. Aging 2012, 33, 2046–2061. [Google Scholar] [CrossRef] [PubMed]
- Solari, N.; Hangya, B. Cholinergic modulation of spatial learning, memory and navigation. Eur. J. Neurosci. 2018, 48, 2199–2230. [Google Scholar] [CrossRef] [PubMed]
- Benn, A.; Barker, G.R.; Stuart, S.A.; Roloff, E.V.; Teschemacher, A.G.; Warburton, E.C.; Robinson, E.S. Optogenetic stimulation of prefrontal glutamatergic neurons enhances recognition memory. J. Neurosci. 2016, 36, 4930–4939. [Google Scholar] [CrossRef] [PubMed]
- Combs, H.L.; Folley, B.S.; Berry, D.T.; Segerstrom, S.C.; Han, D.Y.; Anderson-Mooney, A.J.; Walls, B.D.; van Horne, C. Cognition and depression following deep brain stimulation of the subthalamic nucleus and globus pallidus pars internus in parkinson’s disease: A meta-analysis. Neuropsychol. Rev. 2015, 25, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Summers, J.J.; Kang, N.; Cauraugh, J.H. Does transcranial direct current stimulation enhance cognitive and motor functions in the ageing brain? A systematic review and meta- analysis. Ageing Res. Rev. 2016, 25, 42–54. [Google Scholar] [CrossRef] [PubMed]
- Vucic, S.; Kiernan, M.C. Transcranial magnetic stimulation for the assessment of neurodegenerative disease. Neurotherapeutics 2017, 14, 91–106. [Google Scholar] [CrossRef]
- Schulze, L.; Wheeler, S.; McAndrews, M.P.; Solomon, C.J.; Giacobbe, P.; Downar, J. Cognitive safety of dorsomedial prefrontal repetitive transcranial magnetic stimulation in major depression. Eur. Neuropsychopharmacol. 2016, 26, 1213–1226. [Google Scholar] [CrossRef] [PubMed]
- Titiz, A.S.; Hill, M.R.; Mankin, E.A.; Aghajan, Z.M.; Eliashiv, D.; Tchemodanov, N.; Maoz, U.; Stern, J.; Tran, M.E.; Schuette, P.; et al. Theta-burst microstimulation in the human entorhinal area improves memory specificity. Elife 2017, 6, e29515. [Google Scholar] [CrossRef]
- Paxinos, G.; Franklin, K.B. The Mouse Brain in Stereotaxic Coordinates, 4th ed.; Gulf Professional Publishing: Houston, TX, USA, 2012. [Google Scholar]
- Lee, W.; Moon, M.; Kim, H.G.; Lee, T.H.; Oh, M.S. Heat stress-induced memory impairment is associated with neuroinflammation in mice. J. NeuroInflamm. 2015, 12, 102. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Nam, Y.; Jeong, Y.-o.; Park, H.H.; Lee, S.-k.; Shin, S.J.; Jung, H.; Kim, B.-H.; Hong, S.B.; Park, Y.H.; et al. Topographical Visualization of the Reciprocal Projection between the Medial Septum and the Hippocampus in the 5XFAD Mouse Model of Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3992. https://doi.org/10.3390/ijms20163992
Kim S, Nam Y, Jeong Y-o, Park HH, Lee S-k, Shin SJ, Jung H, Kim B-H, Hong SB, Park YH, et al. Topographical Visualization of the Reciprocal Projection between the Medial Septum and the Hippocampus in the 5XFAD Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences. 2019; 20(16):3992. https://doi.org/10.3390/ijms20163992
Chicago/Turabian StyleKim, Sujin, Yunkwon Nam, Yu-on Jeong, Hyun Ha Park, Seong-kyung Lee, Soo Jung Shin, Haram Jung, Byeong-Hyeon Kim, Sang Bum Hong, Yong Ho Park, and et al. 2019. "Topographical Visualization of the Reciprocal Projection between the Medial Septum and the Hippocampus in the 5XFAD Mouse Model of Alzheimer’s Disease" International Journal of Molecular Sciences 20, no. 16: 3992. https://doi.org/10.3390/ijms20163992
APA StyleKim, S., Nam, Y., Jeong, Y.-o., Park, H. H., Lee, S.-k., Shin, S. J., Jung, H., Kim, B.-H., Hong, S. B., Park, Y. H., Kim, J., Yu, J., Yoo, D.-H., Park, S.-H., Jeon, S. G., & Moon, M. (2019). Topographical Visualization of the Reciprocal Projection between the Medial Septum and the Hippocampus in the 5XFAD Mouse Model of Alzheimer’s Disease. International Journal of Molecular Sciences, 20(16), 3992. https://doi.org/10.3390/ijms20163992



