Abstract
The plant hormone jasmonic acid (JA) has been recognized as an important promoter of leaf senescence in plants. However, upstream transcription factors (TFs) that control JA biosynthesis during JA-promoted leaf senescence remain unknown. In this study, we report the possible involvement of a TEOSINTE BRANCHED1/CYCLOIDEA/PCF (TCP) TF BrTCP7 in methyl jasmonate (MeJA)-promoted leaf senescence in Chinese flowering cabbage. Exogenous MeJA treatment reduced maximum quantum yield (Fv/Fm) and total chlorophyll content, accompanied by the increased expression of senescence marker and chlorophyll catabolic genes, and accelerated leaf senescence. To further understand the transcriptional regulation of MeJA-promoted leaf senescence, a class I member of TCP TFs BrTCP7 was examined. BrTCP7 is a nuclear protein and possesses trans-activation ability through subcellular localization and transcriptional activity assays. A higher level of BrTCP7 transcript was detected in senescing leaves, and its expression was up-regulated by MeJA. The electrophoretic mobility shift assay and transient expression assay showed that BrTCP7 binds to the promoter regions of a JA biosynthetic gene BrOPR3 encoding OPDA reductase3 (OPR3) and a chlorophyll catabolic gene BrRCCR encoding red chlorophyll catabolite reductase (RCCR), activating their transcriptions. Taken together, these findings reveal that BrTCP7 is associated with MeJA-promoted leaf senescence at least partly by activating JA biosynthesis and chlorophyll catabolism, thus expanding our knowledge of the transcriptional mechanism of JA-mediated leaf senescence.
1. Introduction
As one of the major leafy vegetables, Chinese flowering cabbage (Brassica rapa ssp. Parachinensis) is a popular in the Asian diet due to its health-promoting compounds and valuable anticancer properties [1,2,3]. Generally, flowering shoots, stems, and younger leaves of this cabbage are harvested for eating. However, harvested cabbage leaves senesce rapidly during transportation and storage, wilt, and yellow, reducing product quality and commercial value [4,5,6]. Thus, there is a demand for the elucidation of the molecular mechanisms of leaf senescence of Chinese flowering cabbage. Such knowledge is important for developing practical solutions to maintain the quality and extend the shelf-life of this vegetable.
Previous genetic and molecular studies have demonstrated that leaf senescence is an active biological process that is tightly regulated by various intrinsic and external factors, such as developmental stage, natural plant hormones, and stresses [7,8,9,10,11]. Among the various phytohormones, jasmonic acid (JA) and its derivate methyl jasmonate (MeJA), have been reported to accelerate leaf senescence [12,13,14]. In a variety of plants such as Arabidopsis, rice, and maize, endogenous JA is found to be significantly accumulated during leaf senescence [15,16,17]. Exogenous JA/MeJA application can accelerate leaf yellowing and senescence and enhance the expression of several senescence-associated genes (SAGs) including chlorophyll catabolic genes (CCGs), as well as genes involved in the JA biosynthesis and signaling pathways [18].
Evidence suggests that transcription factors (TFs) are important regulatory proteins that control the onset and progression of JA-induced leaf senescence by altering the expression of SAGs [11,14]. For example, Arabidopsis MYC2, 3, and 4 function redundantly to activate JA-induced leaf senescence by directly activating the expression of SAG29 and CCGs [18]. In contrast, bHLH subgroup IIId TFs bHLH03, 13, 14, and 1, target the SAG29 promoter and repress its expression [19]. WRKY57 directly suppresses the transcription of SEN4 and SAG12, acting as a negative regulator of JA-induced leaf senescence in Arabidopsis [20]. These findings highlight the complicated regulatory network of JA-promoted leaf senescence governed by a series of TFs, promoting the identification of other TFs associated with this process.
TEOSINTE BRANCHED1/CYCLOIDEA/PCF (TCP) proteins, with the conserved TCP domains, are plant-specific TFs that have been identified in many plant species including Arabidopsis [21], rice [22], maize [23], tomato [24], and Chinese cabbage [25]. TCP TFs have been well documented to participate in multiple biological processes such as cell proliferation and growth, and stress response [26]. Several TCP TFs have been shown to mediate hormone-induced changes in cell proliferation and act as modulators, or even as key players of hormone synthesis, transport, and signal transduction [26]. For instance, Arabidopsis TCP15 is required for the optimal balance between auxin levels and cytokinins responses in the developing carpel [27]. Rice OsTCP19 is strongly associated with abscisic acid (ABA)-mediated abiotic stress responses by binding and modulating the activity of OsABI4 (ABSCISIC ACID INSENSITIVE4), and overexpression of OsTCP19 in Arabidopsis affects not only ABA but also auxin and JA signaling [28]. GhTCP19 from gladiolus was shown to be a positive regulator of the corm dormancy release process by repressing the expression of an ABA biosynthesis gene expression (GhNCED, 9-cis-epoxycarotenoid dioxygenase), as well as promoting cytokinins biosynthesis (GhIPT, isopentenyl transferase) and signal transduction (GhARR, ARABIDOPSIS RESPONSE REGULATORS) [29]. Genome-wide transcriptional analyses revealed that JA-related global responses are affected by gain or loss of TCP activity [30]. Breeze et al. conducted transcriptome analysis and suggested the role of TCPs during leaf senescence in Arabidopsis [31]. Three Arabidopsis TCP TFs, TCP20, TCP9, and TCP4, were further found to target the promoter of a JA biosynthetic gene LOX2 (Lipoxygenase2), repressing and activating its transcription, thus acting antagonistically controlling JA-mediated leaf development and senescence [32]. The involvement of TCP members in JA-promoted leaf senescence as well as the associated regulatory mechanism remain to be elucidated.
Previously, we identified and characterized several TFs including BrWRKY65 [1], BrWRKY6 [2], BrERF72 [5], and BrNAC055 [6] involved in leaf senescence in Chinese flowering cabbage, of which BrERF72 was shown to enhance JA accumulation by inducing the expression of three JA biosynthetic genes (BrLOX4, BrAOC3, and BrOPR3) during MeJA-promoted leaf senescence [5]. Leaf senescence is a complex biological process mediated by numerous TFs, and the underpinning regulatory mechanisms vary from species to species and with growing conditions. The current study revealed that a TCP, TF BrTCP7, is positively associated with MeJA-promoted leaf senescence by the direct transcriptional activation of a JA biosynthetic gene BrOPR3 and a CCG BrRCCR.
2. Results and Discussion
2.1. Leaf Senescence of Chinese Flowering Cabbage is Promoted by Exogenous MeJA Treatment
The phytohormone JA-induced leaf senescence has been observed in several plant species [14,15,16,17,18,19]. Our previous study showed that exogenous MeJA treatment promotes leaf senescence of Chinese flowering cabbage [5], which was further verified in the present work. As shown in Figure 1A, appearance and non-invasive chlorophyll fluorescence imaging with maximum quantum yield (Fv/Fm) default demonstrated that, in comparison to the control, MeJA-treated cabbage leaves exhibited a greater degree of yellowing on the fifth and seventh day of storage. As expected, the Fv/Fm value and total chlorophyll content were significantly lower in MeJA-treated leaves, which were 70.7% and 70.1% of the control leaves, respectively, on the fifth day of storage (Figure 1B). To investigate why MeJA promotes leaf senescence, we quantified the relative abundance of transcripts of two SAGs (BrSAG12 and BrSAG19) and six CCGs (BrPAO, BrPPH, BrNYC1, BrRCCR, BrSGR1, and BrSGR2) after MeJA treatment. The transcript levels of all these genes increased more dynamically upon treatment with MeJA, with a 2.89-, 1.44-, 2.03-, 1.81-, 1.47-, 2.62-, 1.93-, and 1.83-fold induction for BrNYC1, BrPPH, BrPAO, BrRCCR, BrSGR1, BrSGR2, BrSAG12, and BrSAG19, respectively on the fifth day of storage compared with the control (Figure 1C).
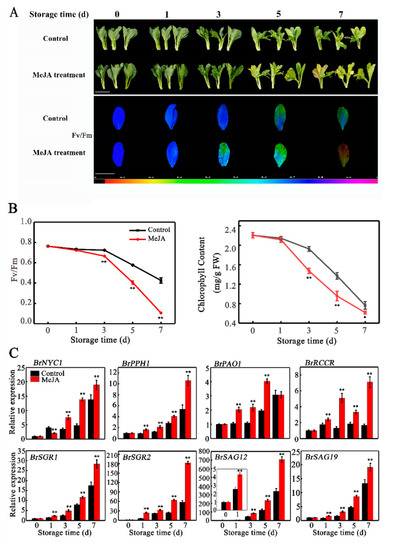
Figure 1.
Methyl jasmonate (MeJA) treatment promotes leaf senescence of Chinese flowering cabbage. (A) Appearance and chlorophyll fluorescence imaging (Fv/Fm) of control and MeJA-treated cabbage leaves during senescence. The false color code depicted at the bottom of the image ranges from 0 (black) to 1.0 (purple). Bar = 10 cm. (B) Changes in Fv/Fm and total chlorophyll content in control and MeJA-treated cabbage leaves during senescence. (C) Relative expression of six CCGs (BrNYC1, BrPPH1, BrPAO1, BrRCCR, BrSGR1 and BrSGR2) and two senescence-marker genes (BrSAG12 and BrSAG19) in control and MeJA-treated cabbage leaves during senescence. Data presented in (B) and (C) are the mean ± SD of three biological replicates. Asterisks indicate a significant difference in MeJA-treated leaves compared with control leaves (Student’s t-test: * p < 0.05 and ** p < 0.01).
2.2. BrTCP7 is A Member of Class I TCP
Transcriptional regulation mediated by TFs, such as MYCs, NACs, and WRKYs, plays an important role in JA-induced leaf senescence [14,33]. Numerous TCP TFs have been identified in plants; however, only few of them were reported to be involved in JA-mediated leaf development and senescence [26,32]. Thus, we focused on the identification of TCP TFs from our RNA-seq transcriptome database associated with Chinese flowering cabbage leaf senescence. Using a false discovery rate (FDR) of less than 0.05 and a fold change larger than 0.5 as thresholds, six genes annotated as TCP proteins were found to be up-regulated (unpublished data). Among these TCPs, the member (GenBank number, XM_009152613.2) that was most up-regulated during leaf senescence, was selected for further investigation.
The resulting amplified full-length gene was 753 bp, encoding a protein with 250 amino acid residues, with a calculated molecular weight of 26.97 kDa, and a pI value of 9.69. By blasting the NCBI database, we found that this gene shares high similarity (74.9%) with Arabidopsis AtTCP7, and thus was named BrTCP7. The most distinguished characteristic of TCP proteins is the presence of a ~59-amino-acid-long conserved region with a non-canonical basic helix-loop-helix (bHLH) structure (the TCP domain) in their N-terminal, which was proven to be responsible for nuclear targeting, DNA binding, and protein–protein interactions [25,34]. Multiple-alignment of BrTCP7 showed that, similar to other orthologous TCP proteins, BrTCP7 contains the conserved TCP domain (Figure 2A). On the basis of the TCP domain, the TCP members can be grouped into two distinct subfamilies: class I (PCF or TCP-P class) and class II (TCP-C class), among which class II can be further divided into subclades CIN and CYC/TB1 [26,35]. As shown in Figure 2B, the phylogenetic tree shows that BrTCP7 together with AtTCP7, AtTCP14, and SlTCP14 belong to class I.
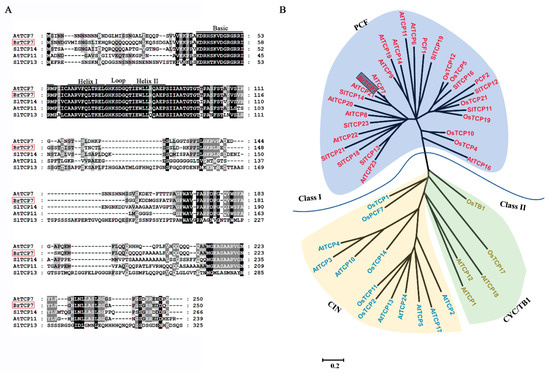
Figure 2.
Multiple sequence alignment and phylogenetic analysis of BrTCP7. (A) Multiple alignment of BrTCP7 with other TCP members. The following proteins were used for analysis: AtTCP7 (NP_197719.1), AtTCP11 (NP_196450.1), SlTCP13 (NP_001233966.1), and SlTCP14 (NP_001293140.1). Identical and similar amino acids are shaded in black and grey, respectively. Single underlining indicates the conserved non-canonical basic helix–loop–helix structure. (B) Phylogenetic analysis of TCPs. BrTCP7 is boxed. The phylogenetic tree was constructed using the maximum likelihood inference method using the MEGA program (version 5.0,Institute of Molecular Evolutionary Genetics, The Pennsylvania State University, PA, USA).
2.3. Molecular Properties of BrTCP7
To evaluate whether BrTCP7 is associated with MeJA-induced leaf senescence, we quantified its expression in Chinese flowering cabbage during leaf senescence. qRT-PCR analysis showed that, consistent with the RNA-seq data, the transcript level of BrTCP7 increases during leaf senescence. More pronounced elevation of the BrTCP7 transcript was observed in MeJA-treated leaves, which were ~0.85- and 0.51-fold higher than that of the control leaves on days 3 and 5 of storage, respectively (Figure 3A). Generally, TCP proteins are localized in nuclei [34,36,37]. Subcellular localization prediction also indicated that BrTCP7 is located in the nuclear region. To verify this prediction, BrTCP7 was fused to green fluorescent protein (GFP) and transiently expressed in Nicotiana benthamiana leaves. Compared with the fluorescence of control 35S-GFP that is distributed throughout the cell, BrTCP7-GFP fusion protein, like the nuclear marker (NLS-mCherry), was exclusively detected in the nucleus (Figure 3B). To provide evidence for potential roles of BrTCP7 in transcriptional regulation, we assessed the transcriptional activity of BrTCP7 in vivo using the dual-luciferase reporter system. As shown in Figure 3C, similar with the activator control VP16, co-infiltration of BrTCP7 with the reporter significantly increased the luciferase (LUC)/renilla luciferase (REN) ratio. These data reveal that BrTCP7 is a nuclear-localized transcriptional activator that is possibly associated with MeJA-induced leaf senescence of Chinese flowering cabbage.
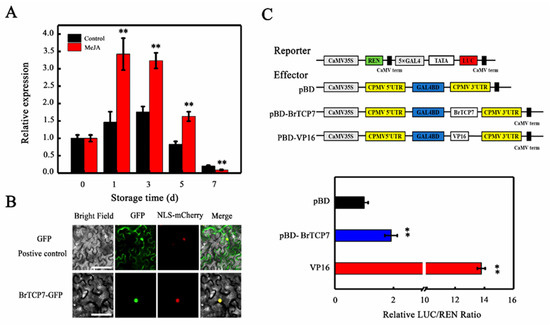
Figure 3.
Molecular properties of BrTCP7. (A) Relative expression of BrTCP7 in control and MeJA-treated cabbage leaves during senescence. Each value represents the mean ± SD of three biological replicates. Asterisks indicate a significant difference in MeJA-treated leaves compared with control leaves (Student’s t-test: * p < 0.05 and ** p < 0.01). (B) Subcellular localization of BrTCP7 in epidermal cells of Nicotiana benthamiana leaves. A plasmid harboring GFP or BrTCP7-GFP was transformed into N. benthamiana leaves by Agrobacterium tumefaciens strain EHA105. GFP signals were observed with a fluorescence microscope after two days of infiltration. Bars = 30 μm. (C) Trans-activation of BrTCP7 in N. benthamiana leaves. The trans-activation ability of BrTCP7 was indicated by the ratio of luciferase (LUC) to renilla luciferase (REN). The LUC/REN ratio of the empty pBD vector (negative control) was used as a calibrator (set to 1). pBD-VP16 was used as a positive control. Data are means ± SD of six independent biological replicates. Asterisks represent significant differences at the 0.01 level by Student’s t-test compared to pBD.
2.4. BrTCP7 Directly Binds to the Promoter of BrOPR3 and BrRCCR
We then intended to reveal the regulatory mechanism of BrTCP7 involved in MeJA-induced leaf senescence of Chinese flowering cabbage. For this purpose, it is vital to identify the potential targets of BrTCP7. Molecular analyses showed that the predicted consensus binding site of class I TCP TFs is GGNCCCAC, especially the core sequence GCCCR [26,32,34]. After surveying the promoter sequences of three JA biosynthetic genes (BrLOX4, BrAOC3, and BrOPR3) as well as six CCGs (BrPAO, BrPPH, BrNYC1, BrRCCR, BrSGR1, and BrSGR2) that were previously reported to be associated with leaf senescence in Chinese flowering cabbage [2,5,38], the TCP binding site was found in BrOPR3 and BrRCCR promoters (Text S1). To confirm the binding of BrTCP7 to BrOPR3 and BrRCCR promoters, an in vitro electrophoretic mobility shift assay (EMSA) was performed. Results showed that purified GST-BrTCP7 protein (Figure 4A), not the GST protein, binds to the TCP binding elements presented in BrOPR3 and BrRCCR promoters, causing a mobility shift band (Figure 4B). Adding excess amount (250-fold) of the cold probe (unlabeled wild-type DNA fragment) but not the mutant probe eliminated the binding complex (Figure 4B), indicating the specificity of the DNA-protein interaction. Expression analysis showed that BrOPR3 is significantly upregulated by exogenous MeJA treatment (Figure S1).
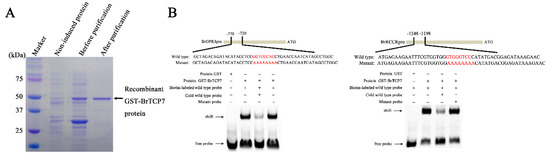
Figure 4.
BrTCP7 directly binds to BrOPR3 and BrRCCR promoters. (A) SDS-PAGE gel stained with Coomassie brilliant blue demonstrating affinity purification of the recombinant glutathione-S-transferases (GST)-tagged BrTCP7 protein used for EMSA. Recombinant GST-BrTCP7 protein is indicated by the arrow. (B) EMSA showing the binding of BrTCP7 to the TCP binding site of the BrOPR3 or BrRCCR promoter. Purified GST-BrTCP7 protein was incubated with the biotin-labeled wild-type probe containing the TCP binding site, and the DNA–protein complexes were separated on native polyacrylamide gels. Sequences of both the wild-type and mutant probes are shown at the top of the image (wild-type and mutant TCP binding site are marked with red letters). The mutant probe was used to test binding specificity. Shifted bands, suggesting the formation of DNA–protein complexes, are indicated by arrows. − represents absence, + represents presence. Competition experiments were conducted by adding 250-fold molar excess of cold probe (unlabeled wild-type DNA fragment) or mutant probe.
2.5. BrTCP7 Activates the Transcription of BrOPR3 and BrRCCR
After deciphering BrOPR3 and BrRCCR as the potential downstream targets of BrTCP7, and establishing BrTCP7 as a transcriptional activator, we hypothesized that BrTCP7 could act as a direct transcriptional activator of BrOPR3 and BrRCCR. To investigate this hypothesis, Nicotiana benthamiana leaves were co-transformed with transient over-expression effector construct containing 35S-BrTCP7, and a dual-luciferase reporter construct carrying BrOPR3 or BrRCCR promoter fused to LUC (Figure 5A). As illustrated in Figure 5B, expression of BrTCP7 resulted in 3.07- and 2.11-fold increases in LUC expression driven by BrOPR3 and BrRCCR, respectively, compared with the vector control, providing evidence for a positive trans-activation activity of BrTCP7. Collectively, BrTCP7 binds directly to the promoters of BrOPR3 and BrRCCR and activates their transcriptions, rendering them direct targets of BrTCP7 transcriptional regulation. Further experiments, such as chromatin immunoprecipitation (ChIP) assay or transient over-expression of BrTCP7 in Brassica protoplasts, are needed to confirm the binding of BrTCP7 to BrOPR3 and BrRCCR in vivo.
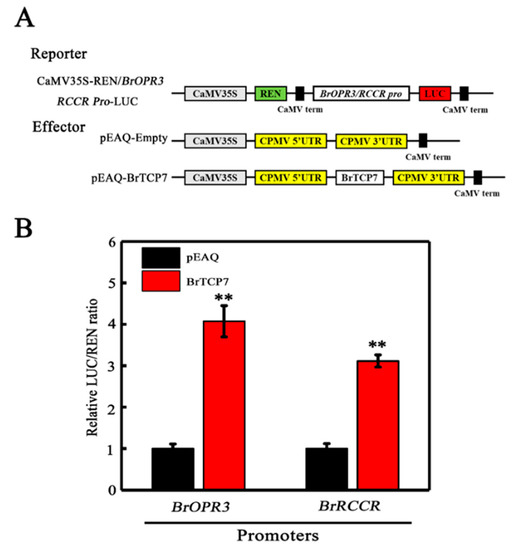
Figure 5.
BrTCP7 enhances BrOPR3 and BrRCCR transcriptions by transient transcription dual-luciferase assay in Nicotiana benthamiana leaves. (A) Diagrams of the reporter and effector vectors. (B) BrTCP7 activates BrOPR3 and BrRCCR promoters. Data are means ± S.D. of six independent biological replicates. Asterisks indicate significant differences by student’s t-test (** p < 0.01).
Similar to BrTCP7, Arabidopsis class II CINCINNATA (CIN)/TCP TFs, such as TCP4, positively influence JA biosynthesis by directly activating the transcription of a JA biosynthetic gene LOX2 [32]. Conversely, the class I TCP members TCP9 and TCP20 are negative regulators of leaf senescence via repressing LOX2 expression [32]. These findings highlight the complexity of TCPs in modulating JA-promoted leaf senescence. Auxin usually promotes leaf senescence and functions downstream of class I and CIN TCPs by regulating the same auxin-responsible TFs, indicating the possible involvement of TCPs in auxin-modulated leaf senescence [39,40]. A class I TCP TF GhTCP19 from gladiolus was reported to positively regulate corm dormancy release by suppressing the transcription of an ABA biosynthetic gene GhNCED while activating the transcription of cytokinin biosynthetic gene GhIPT and signal transduction gene GhARR [29]. As ABA and cytokinins have been implicated to accelerate and delay leaf senescence in Chinese flowering cabbage, respectively [4,37], investigating whether BrTCP7 or other TCP members like GhTCP19 have regulatory roles in ABA-, cytokinins-, and auxin-mediated leaf senescence will be required.
Transcriptional activity of TFs on their targets is finely controlled by many factors, such as homo-/hetero-dimerization, post-transcriptional/translational modification, and protein–protein interactions [41]. For example, miR319a/JAGGED targets class II TCPs and subsequently represses LOX2 expression, which attenuates JA-induced senescence [26,30]. A study in bananas showed that MaTCP20 is associated with MaTCP5 or MaTCP19 to form complexes, which influence the regulation of cell wall-modifying genes during fruit ripening [42]. An ERF TF BrERF72 was found to be associated with MeJA-promoted leaf senescence in Chinese flowering cabbage by enhancing JA accumulation through upregulating the expression of three JA biosynthetic genes: BrLOX4, BrAOC3, and BrOPR3 [5]. Thus, elucidating whether BrTCP7 and BrERF72 can form a complex to affect the expression of JA biosynthetic genes, as well as identifying other interaction proteins and regulatory factors, will provide more detailed information about the BrTCP7-mediated regulation of gene networks operating MeJA-promoted leaf senescence of Chinese flowering cabbage. Targeted transgenic research is required to unravel the biological function of BrTCP7 in regulating MeJA-promoted leaf senescence in Chinese flowering cabbage.
3. Materials and Methods
3.1. Plant Materials, MeJA Treatment, and Growth Conditions
Chinese flowering cabbages (Brassica rapa var. parachinensis) grown in a local commercial vegetable plantation near Guangzhou, Southern China, were harvested after 40 days of growth, and were immediately transported to laboratory under low temperature. Uniform size cabbages without appearance defects were selected and randomly divided into two groups for control and MeJA treatment. MeJA (100 μM) and distilled water (control treatment) were foliar-sprayed onto cabbages as described previously [5]. Subsequently, both control and MeJA-treated cabbages were stored at 15 °C in incubators with a 16-h light/8-h dark cycle for 5 days. On days 0, 1, 3, and 5 of storage, the third leaves from the bottom of 10 cabbages were collected for physiological and molecular analysis. All samples were immediately frozen in liquid nitrogen and stored at −80 °C for further assays.
Tobacco (Nicotiana benthamiana) plants were planted in a growth chamber set at 22 °C and a 16-h photoperiod. Leaves from four-week-old tobacco plants were used for Agrobacterium-tumefaciens-mediated transient expression assays.
3.2. Physiological Measurements of Leaf Senescence
Leaf total chlorophyll content and photochemical efficiency (Fv/Fm) are physiological parameters commonly used as indicators of leaf senescence. Total chlorophyll content was measured by extracting approximately 0.1 g of leaves in 10 mL in 80% acetone in the dark for 24 h and measuring the absorbance of extracts at 663 nm and 645 nm using a spectrophotometer as described earlier [5,6]. Fv/Fm was determined non-invasively after leaves were adapted to dark conditions for 30 min. A chlorophyll fluorometer (Imaging-PAM-M series, Heinz Walz GmbH, Effeltrich, Germany) equipped with a charge-coupled device (CCD) camera to capture high-resolution digital images of the emitted fluorescence from the dark-adapted leaves.
3.3. RNA Extraction, Gene Cloning, and Bioinformatic Analysis
A Quick RNA Isolation Kit (Huayueyang, Beijing, China) was used to extract total RNA from cabbage leaves. RNA was then treated with DNase I to remove contaminating DNA and reversely transcribed to cDNA using the reverse transcriptase M-MLV (TaKaRa, Shiga, Japan) using 1 μg RNA template, following the manufacturer’s protocol. The full-length BrTCP7 gene was isolated from our transcriptome database. Theoretical isoelectric points (pI) and mass values were calculated using the Compute pI/Mw tool (http://web.expasy.org/compute_pi/). Sequence alignment and phylogenetic analysis of TCP proteins were conducted with the CLUSTALW program (version 1.83, The Conway Institute of Biomolecular and Biomedical Research, University College Dublin, Dublin, Ireland). A phylogenetic tree was created with the MEGA5.0 program (Institute of Molecular Evolutionary Genetics, The Pennsylvania State University, PA, USA), using the maximum likelihood inference method.
3.4. Gene Expression via qRT-PCR
cDNA from each sample were quantified by quantitative real-time PCR (qRT-PCR) assays. qRT-PCR was performed using the step one plus a CFX96 Touch™ real-time PCR detection system (Bio-Rad, Hercules, CA, USA) and GoTaq qPCR master mix kit (Promega, Madison, WI, USA). The expression levels of target genes were normalized according to the cycle threshold (Ct) value using BrActin1 [43] as the reference gene.
3.5. Subcellular Localization Assay
The BrTCP7 coding sequence (CDS) fragment without the stop codon was amplified and inserted into the pEAQ-GFP vector [44] to produce the fusion protein BrTCP7-GFP. Then BrTCP7-GFP and the control pEAQ-GFP constructs were transformed to A. tumefaciens strain EHA105. Overnight cultures of Agrobacteria were collected by centrifugation, resuspended in MES buffer to 0.8–1.0 OD600, incubated at room temperature for 2 h before infiltration. Agrobacteria suspension collected in a 1-mL syringe (without the metal needle) was carefully press-infiltrated manually onto healthy leaves of 4-week-old Nicotiana benthamiana as described previously [45]. Two days after infiltration, the GFP fluorescent signals in the epidermal cells of leaves were directly observed and images were captured by using a Zeiss fluorescence microscope (Carl Zeiss AG, Oberkochen, Germany).
3.6. Recombinant Protein Induction, Purification, and EMSA Assay
For protein expression and purification, the BrTCP7 coding sequence was recombined into the vector pGEX-4T-1 and transferred to Escherichia coli strain Transetta (DE3). When the transformed DE3 cell density reached 0.6 (OD600), protein expression in 1-L cultures maintained at 37 °C was induced by the addition of 0.3 mM isopropyl thio-β-D-galactoside during a 3 h course. Cells were harvested by centrifugation. The cell pellet was resuspended in phosphate-buffered saline and incubated on ice for 30 min. Following sonication, the lysate was cleared by centrifugation. Glutathione-Superflow Resin (Clontech, Mountain View, CA, USA) was used for purification of GST-tagged protein by gravity-flow chromatography according to the manufacturer’s protocol, followed by SDS-PAGE and Coomassie Brilliant Blue staining to confirm protein size and purity.
Electrophoretic mobility shift assay (EMSA) was performed as described previously [46]. The synthetic nucleotides (~60 bp) derived from the 5′ UTR of BrOPR3 and BrRCCR oligonucleotides, which contain the consensus binding site (GGNCCCAC) of TCPs, were biotin-labeled at the 5′ end. The purified recombinant BrTCP7 protein (1 μg) was incubated with biotin-labeled probe (2 × 10−6 μmol) in binding buffer for 25 min at 30 °C. Competitions were conducted by adding excess amount (250-fold) of cold probe (unlabeled DNA fragment) or mutant probe. The reaction mixture was electrophoresed on a 6% native polyacrylamide gels, and then transferred onto a positively charged nylon membrane, followed by cross-linking though illumination under an ultraviolet lamp. The signals from the labeled DNA were detected by using the LightShift chemiluminescent EMSA kit (Thermo Scientific, Rockford, IL, USA) in a ChemiDoc™ MP Imaging System (Bio-Rad, Hercules, CA, USA).
3.7. Transient Transcription Dual-Luciferase (Dual-LUC) Assays
To investigate the transcriptional ability of BrTCP7 in vivo, its full length was inserted into pBD [45] to construct pBD-BrTCP7 as an effector. The positive control (pBD-VP16) was constructed by fusing VP16, a herpes simplex virus-encoded transcriptional activator, to pBD. pBD itself was used as a negative control. The GAL4 plasmid with the firefly luciferase (LUC) gene was used as a reporter [45], and the renilla luciferase (REN) gene in the same plasmid was used as an internal control.
To determine the activation of BrOPR3 and BrRCCR by BrTCP7, the promoter fragments of BrOPR3 and BrRCCR were amplified and cloned into the transient dual luciferase expression vector pGreenII 0800-LUC [47] as reporter constructs. To generate the 35S::BrTCP7 effector construct, the BrTCP7 coding sequence was amplified by PCR and inserted into pEAQ vector [44]. The empty vector was included as a control.
Transient transcription dual-LUC assays were performed using Nicotiana benthamiana plants as described [46]. The reporter and effector constructs mentioned above were co-infiltrated into tobacco leaves. After 2 days of infiltration, the luciferase activity of tobacco leaf extract was quantified by a Luminoskan Ascent Microplate Luminometer (Thermo Fisher Scientific, Rockford, IL, USA), using commercial dual-luciferase reporter assay kit according to the manufacturer’s instruction (Promega, Madison, WI, USA). The trans-activation ability of BrTCP7 was indicated by the LUC/REN ratio.
3.8. Statistical Analysis
Data are represented as means ± SD of three or six biological replicates. Statistical differences of two treatments were examined using the Student’s t-test. Data are considered significant as follows: * p < 0.05, ** p < 0.01
3.9. Primers
All primers used in this research are listed in Table S1.
4. Conclusions
In summary, exogenous MeJA treatment promotes leaf senescence of Chinese flowering cabbage. A class I TCP member BrTCP7, which is MeJA-upregulated and acts as a nuclear-localized transcriptional activator, was identified. BrTCP7 targets the promoters of a JA biosynthetic gene BrOPR3 and a CCG gene BrRCCR, leading to their transcriptional activation. These findings expand our understanding of TCP TFs’ functions and shed light on the transcriptional mechanism operating JA-mediated leaf senescence, and also the molecular mechanism(s) involved in maintaining postharvest quality of an important leafy vegetable, Chinese flowering cabbage.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/20/16/3963/s1.
Author Contributions
J.-w.C. and J.-y.C. conceived and supervised the study; Y.-m.X, X.-m.X., Z.-x.Z., and X.-l.T. conducted the experiments; Y.-m.X, X.-m.X., Z.-x. Z., X.-l.T., Z.-l.L., J.-w.C., X.-g.S., and J.-y.C. analyzed data; Y.-m.X, J.-w.C., and J.-y.C. wrote the manuscript. All authors read and approved the manuscript.
Funding
This study was funded by a grant from the National Natural Science Foundation of China (31671897) and Natural Science Foundation of Guangdong Province, China (2018A030313457).
Acknowledgments
We are grateful to George P. Lomonossoff (Department of Biological Chemistry, John Innes Centre, Norwich Research Park) for providing the pEAQ vectors. We are grateful to Prakash Lakshmanan (Sugarcane Research Institute, Guangxi Academy of Agricultural Sciences) for his critical English language editing of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Fan, Z.Q.; Tan, X.L.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. BrWRKY65, a WRKY transcription factor, is involved in regulating three leaf senescence-associated genes in Chinese flowering cabbage. Int. J. Mol. Sci. 2017, 18, 1228. [Google Scholar]
- Fan, Z.Q.; Tan, X.L.; Shan, W.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Characterization of a transcriptional regulator, BrWRKY6, associated with gibberellin-suppressed leaf senescence of Chinese flowering cabbage. J. Agric. Food Chem. 2018, 66, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Ombra, M.N.; Cozzolino, A.; Nazzaro, F.; d’Acierno, A.; Tremonte, P.; Coppola, R.; Fratianni, F. Biochemical and biological characterization of two Brassicaceae after their commercial expiry date. Food Chem. 2017, 218, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, Z.; Li, J.; Wu, L.; Guo, J.; Ouyang, L.; Xia, Y.; Huang, X.; Pang, X. Correlation of leaf senescence and gene expression/activities of chlorophyll degradation enzymes in harvested Chinese flowering cabbage (Brassica rapa var. parachinensis). J. Plant Physiol. 2011, 168, 2081–2087. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.L.; Fan, Z.Q.; Shan, W.; Yin, X.R.; Kuang, J.F.; Lu, W.J.; Chen, J.Y. Association of BrERF72 with methyl jasmonate-induced leaf senescence of Chinese flowering cabbage through activating JA biosynthesis-related genes. Hortic. Res. 2018, 5, 22. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Tan, X.L.; Chen, J.W.; Liu, Z.L.; Kuang, J.F.; Lu, W.J.; Shan, W.; Chen, J.Y. BrNAC055, a novel transcriptional activator, regulates leaf senescence in Chinese flowering cabbage by modulating reactive oxygen species production and chlorophyll degradation. J. Agric. Food Chem. 2018, 66, 9399–9408. [Google Scholar] [CrossRef]
- Jibran, R.; Hunter, D.; Dijkwel, P. Hormonal regulation of leaf senescence through integration of developmental and stress signals. Plant Mol. Biol. 2013, 82, 547–561. [Google Scholar] [CrossRef]
- Schippers, J.H.; Schmidt, R.; Wagstaff, C.; Jing, H.C. Living to die and dying to live: The survival strategy behind leaf senescence. Plant Physiol. 2015, 169, 914–930. [Google Scholar] [CrossRef]
- Schippers, J.H. Transcriptional networks in leaf senescence. Curr. Opin. Plant Biol. 2015, 27, 77–83. [Google Scholar] [CrossRef]
- Kim, J.; Woo, H.R.; Nam, H.G. Toward systems understanding of leaf senescence: An integrated multi-omics perspective on leaf senescence research. Mol. Plant 2016, 9, 813–825. [Google Scholar] [CrossRef]
- Woo, H.R.; Kim, H.J.; Lim, P.O.; Nam, H.G. Leaf senescence: Systems and dynamics aspects. Annu. Rev. Plant Biol. 2019, 70, 347–376. [Google Scholar] [CrossRef]
- Zhang, H.; Zhou, C. Signal transduction in leaf senescence. Plant Mol. Biol. 2013, 82, 539–545. [Google Scholar] [CrossRef]
- Huang, H.; Liu, H.; Liu, L.; Song, S. Jasmonate action in plant growth and development. J. Exp. Bot. 2017, 68, 1349–1359. [Google Scholar] [CrossRef]
- Hu, Y.; Jiang, Y.; Han, X.; Wang, H.; Pan, J.; Yu, D. Jasmonate regulates leaf senescence and tolerance to cold stress: Crosstalk with other phytohormones. J. Exp. Bot. 2017, 68, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Fukushige, H.; Hildebrand, D.F.; Gan, S. Evidence supporting a role of jasmonic acid in Arabidopsis leaf senescence. Plant Physiol. 2002, 128, 876–884. [Google Scholar] [CrossRef]
- Yan, Y.; Christensen, S.; Isakeit, T.; Engelberth, J.; Meeley, R.; Hayward, A.; Emery, R.J.; Kolomiets, M.V. Disruption of OPR7 and OPR8 reveals the versatile functions of jasmonic acid in maize development and defense. Plant Cell 2012, 24, 1420–1436. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Sakuraba, Y.; Lee, T.; Kim, K.W.; An, G.; Lee, H.Y.; Paek, N.C. Mutation of Oryza sativa CORONATINE INSENSITIVE 1b (OsCOI1b) delays leaf senescence. J. Integr. Plant Biol. 2015, 57, 562–576. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chen, J.; Xie, Z.; Gao, J.; Ren, G.; Gao, S.; Zhou, X.; Kuai, B. Jasmonic acid promotes degreening via MYC2/3/4- and ANAC019/055/072-mediated regulation of major chlorophyll catabolic genes. Plant J. 2015, 84, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Qi, T.; Wang, J.; Huang, H.; Liu, B.; Gao, H.; Liu, Y.; Song, S.; Xie, D. Regulation of jasmonate-induced leaf senescence by sntagonism between bHLH subgroup IIIe and IIId factors in Arabidopsis. Plant Cell 2015, 27, 1634–1649. [Google Scholar] [CrossRef]
- Jiang, Y.; Liang, G.; Yang, S.; Yu, D. Arabidopsis WRKY57 functions as a node of convergence for jasmonic acid- and auxin-mediated signaling in jasmonic acid-induced leaf senescence. Plant Cell 2014, 26, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Cubas, P.; Lauter, N.; Doebley, J.; Coen, E. The TCP domain: A motif found in proteins regulating plant growth and development. Plant J. Cell Mol. Biol. 1999, 18, 215–222. [Google Scholar] [CrossRef]
- Kosugi, S.; Ohashi, Y. PCF1 and PCF2 specifically bind to cis elements in the rice proliferating cell nuclear antigen gene. Plant Cell 1997, 9, 1607–1619. [Google Scholar] [PubMed]
- Doebley, J.; Stec, A.; Hubbard, L. The evolution of apical dominance in maize. Nature 1997, 386, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Parapunova, V.; Busscher, M.; Busscher-Lange, J.; Lammers, M.; Karlova, R.; Bovy, A.G.; Angenent, G.C.; de Maagd, R.A. Identification, cloning and characterization of the tomato TCP transcription factor family. BMC Plant Biol. 2014, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Guan, X.; Liu, S.; Yang, M.; Ren, J.; Guo, M.; Huang, Z.; Zhang, Y. Genome-wide identification and analysis of TCP transcription factors involved in the formation of leafy head in Chinese cabbage. Int. J. Mol. Sci. 2018, 19, 847. [Google Scholar] [CrossRef]
- Nicolas, M.; Cubas, P. TCP factors: New kids on the signaling block. Curr. Opin. Plant Biol. 2016, 33, 33–41. [Google Scholar] [CrossRef]
- Lucero, L.E.; Uberti-Manassero, N.G.; Arce, A.L.; Colombatti, F.; Alemano, S.G.; Gonzalez, D.H. TCP15 modulates cytokinin and auxin responses during gynoecium development in Arabidopsis. Plant J. 2015, 84, 267–282. [Google Scholar] [CrossRef]
- Mukhopadhyay, P.; Tyagi, A.K. OsTCP19 influences developmental and abiotic stress signaling by modulating ABI4-mediated pathways. Sci. Rep. 2015, 5, 9998. [Google Scholar] [CrossRef]
- Wu, J.; Wu, W.; Liang, J.; Jin, Y.; Gazzarrini, S.; He, J.; Yi, M. GhTCP19 transcription factor regulates corm dormancy release by repressing GhNCED expression in gladiolus. Plant Cell Physiol. 2019, 60, 52–62. [Google Scholar] [CrossRef]
- Schommer, C.; Palatnik, J.F.; Aggarwal, P.; Chételat, A.; Cubas, P.; Farmer, E.E.; Nath, U.; Weigel, D. Control of jasmonate biosynthesis and senescence by miR319 targets. PLoS Biol. 2008, 6, e230. [Google Scholar] [CrossRef]
- Breeze, E.; Harrison, E.; McHattie, S.; Hughes, L.; Hickman, R.; Hill, C.; Kiddle, S.; Kim, Y.S.; Penfold, C.A.; Jenkins, D.; et al. High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 2011, 23, 873–894. [Google Scholar] [CrossRef] [PubMed]
- Danisman, S.; van der Wal, F.; Dhondt, S.; Waites, R.; de Folter, S.; Bimbo, A.; van Dijk, A.; Muino, J.; Cutri, L.; Dornelas, M.; et al. Arabidopsis class I and class II TCP transcription factors regulate jasmonic acid metabolism and leaf development antagonistically. Plant Physiol. 2012, 159, 1511–1523. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Kim, J.H.; Lyu, J.I.; Woo, H.R.; Lim, P.O. New insights into the regulation of leaf senescence in Arabidopsis. J. Exp. Bot. 2018, 69, 787–799. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Yang, J.; Lou, T.; Zhang, J.; Yu, W.; Wen, C. Transcriptome profile analysis reveals that CsTCP14 induces susceptibility to foliage diseases in cucumber. Int. J. Mol. Sci. 2019, 20, 2582. [Google Scholar] [CrossRef] [PubMed]
- Martin-Trillo, M.; Cubas, P. TCP genes: A family snapshot ten years later. Trends Plant Sci. 2010, 15, 31–39. [Google Scholar] [CrossRef]
- Du, J.; Hu, S.; Yu, Q.; Wang, C.; Yang, Y.; Sun, H.; Yang, Y.; Sun, X. Genome-wide identification and characterization of BrrTCP transcription factors in Brassica rapa ssp. rapa. Front Plant Sci. 2017, 8, 1588. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.M.; Sun, C.H.; Wen, L.Z.; Liu, B.W.; Ren, H.; Sun, X.; Ma, F.F.; Zheng, C.S. CmTCP20 plays a key role in nitrate and auxin signaling-regulated lateral root development in Chrysanthemum. Plant Cell Physiol. 2019, 60, 1581–1594. [Google Scholar] [CrossRef]
- Tan, X.L.; Fan, Z.Q.; Kuang, J.F.; Lu, W.J.; Reiter, R.J.; Lakshmanan, P.; Su, X.G.; Zhou, J.; Chen, J.Y.; Shan, W. Melatonin delays leaf senescence of Chinese flowering cabbage by suppressing ABFs-mediated abscisic acid biosynthesis and chlorophyll degradation. J. Pineal. Res. 2019, 67, e12570. [Google Scholar] [CrossRef]
- Uberti-Manassero, N.G.; Lucero, L.E.; Viola, I.L.; Vegetti, A.C.; Gonzalez, D.H. The class I protein AtTCP15 modulates plant development through a pathway that overlaps with the one affected by CIN-like TCP proteins. J. Exp. Bot. 2012, 63, 809–823. [Google Scholar] [CrossRef]
- Koyama, T.; Sato, F.; Ohme-Takagi, M. Roles of miR319 and TCP transcription factors in leaf development. Plant Physiol. 2017, 175, 874–885. [Google Scholar] [CrossRef]
- Shaikhali, J. GIP1 protein is a novel cofactor that regulates DNA-binding affinity of redox-regulated members of bZIP transcription factors involved in the early stages of Arabidopsis development. Protoplasma 2015, 252, 867–883. [Google Scholar] [CrossRef] [PubMed]
- Song, C.B.; Shan, W.; Yang, Y.Y.; Tan, X.L.; Fan, Z.Q.; Chen, J.Y.; Lu, W.J.; Kuang, J.F. Heterodimerization of MaTCP proteins modulates the transcription of MaXTH10/11 genes during banana fruit ripening. BBA-Gene Regul. Mech. 2018, 1861, 613–622. [Google Scholar] [CrossRef] [PubMed]
- Qi, J.N.; Yu, S.C.; Zhang, F.L.; Shen, X.Q.; Zhang, X.Z.; Yu, Y.J.; Zhang, D.S. Reference gene selection for real-time quantitative polymerase chain reaction of mRNA transcript levels in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). Plant Mol. Biol. Rep. 2010, 28, 597–604. [Google Scholar] [CrossRef]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Cheng, M.N.; Ba, L.J.; Zeng, R.X.; Luo, D.L.; Qin, Y.H.; Liu, Z.L.; Kuang, J.F.; Lu, W.J.; Chen, J.Y.; et al. Pitaya HpWRKY3 is associated with fruit sugar accumulation by transcriptionally modulating sucrose metabolic genes HpINV2 and HpSuSy1. Int. J. Mol. Sci. 2019, 20, 1890. [Google Scholar] [CrossRef]
- Fan, Z.Q.; Ba, L.J.; Shan, W.; Xiao, Y.Y.; Lu, W.J.; Kuang, J.F.; Chen, J.Y. A banana R2R3-MYB transcription factor MaMYB3 is involved in fruit ripening through modulation of starch degradation by repressing starch degradation-related genes and MabHLH6. Plant J. 2018, 96, 1191–1205. [Google Scholar] [CrossRef] [PubMed]
- Hellens, R.P.; Allan, A.C.; Friel, E.N.; Bolitho, K.; Grafton, K.; Templeton, M.D.; Karunairetnam, S.; Gleave, A.P.; Laing, W.A. Transient expression vectors for functional genomics, quantification of promoter activity and RNA silencing in plants. Plant Methods 2005, 1, 13. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
