T and B Cells in Periodontal Disease: New Functions in A Complex Scenario
Abstract
1. Introduction
2. T and B Lymphocytes
2.1. T Lymphocytes
2.2. B Lymphocytes
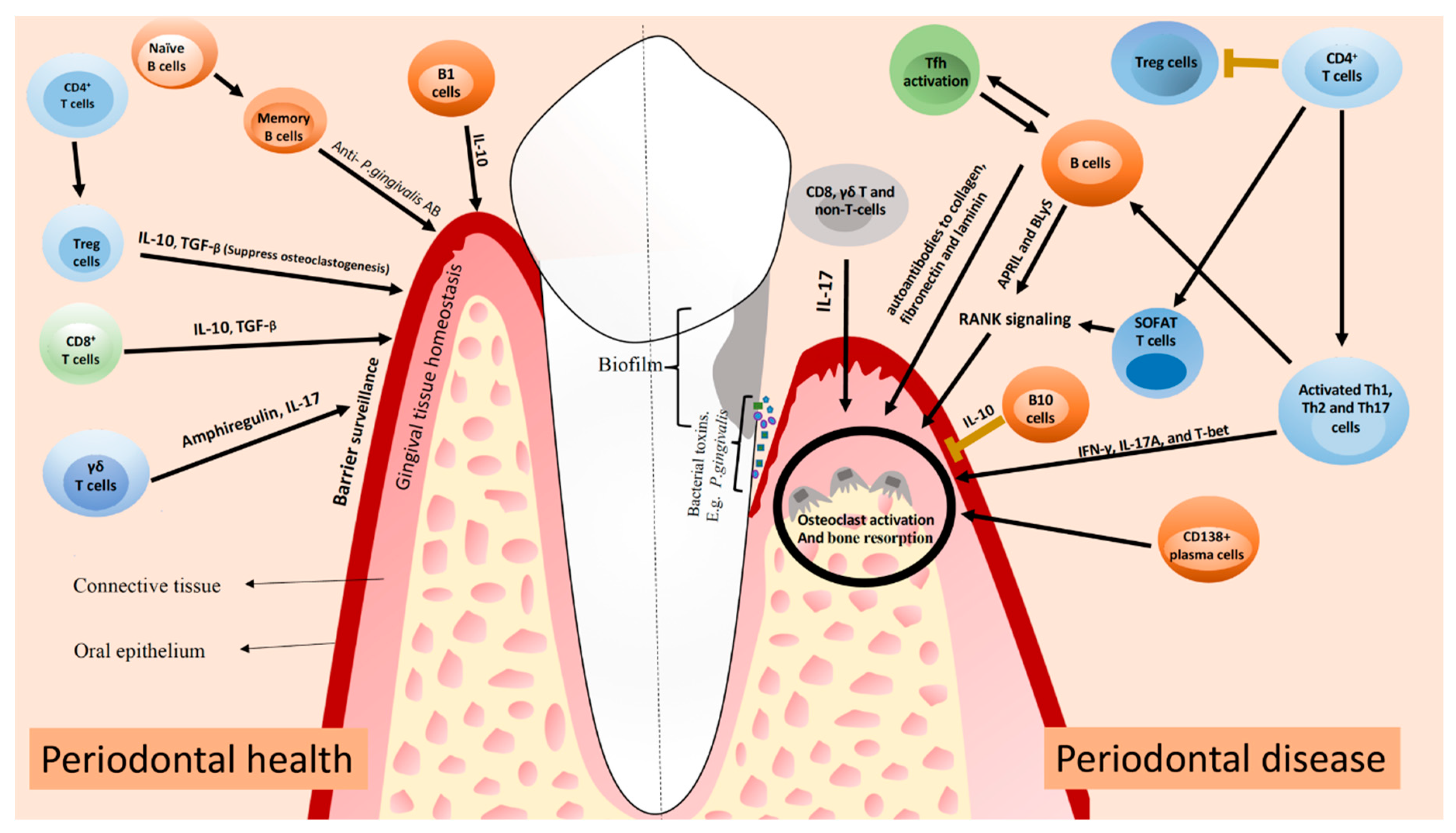
2.3. T and B Lymphocytes in Periodontal Homeostasis
2.3.1. T Lymphocytes
2.3.2. B Lymphocytes
2.4. Changes with Age
2.5. T and B Lymphocytes in Periodontal Inflammation
3. Conclusions
Funding
Conflicts of Interest
References
- Campbell, L.; Millhouse, E.; Malcolm, J.; Culshaw, S. T cells, teeth and tissue destruction—what do T cells do in periodontal disease? Mol. Oral. Microbiol. 2016, 31, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Ebersole, J.L.; Kirakodu, S.; Novak, M.J.; Stromberg, A.J.; Shen, S.; Orraca, L.; Orraca, L.; Gonzalez-Martinez, J.; Burgos, A.; Gonzalez, O.A. Cytokine gene expression profiles during initiation, progression and resolution of periodontitis. J Clin Periodontol. 2014, 41, 853–861. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, C.; Rojas, C.; Rojas, L.; Cafferata, E.A.; Monasterio, G.; Vernal, R. Regulatory T Lymphocytes in Periodontitis: A Translational View. Mediators Inflamm. 2018, 2018, 7806912. [Google Scholar] [CrossRef] [PubMed]
- Parachuru, V.P.B.; Coates, D.E.; Milne, T.J.; Rich, A.M.; Seymour, G.J. FoxP3(+) regulatory T cells, interleukin 17 and mast cells in chronic inflammatory periodontal disease. J. Periodontal Res. 2018, 53, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Liljenberg, B.; Tarkowski, A.; Lindhe, J. The presence of local and circulating autoreactive B cells in patients with advanced periodontitis. J. Clin. Periodontol. 2002, 29, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Dutzan, N.; Konkel, J.E.; Greenwell-Wild, T.; Moutsopoulos, N.M. Characterization of the human immune cell network at the gingival barrier. Mucosal Immunol. 2016, 9, 1163–1172. [Google Scholar] [CrossRef] [PubMed]
- Artese, L.; Simon, M.J.; Piattelli, A.; Ferrari, D.S.; Cardoso, L.A.; Faveri, M.; Onuma, T.; Piccirilli, M.; Perrotti, V.; Shibli, J.A. Immunohistochemical analysis of inflammatory infiltrate in aggressive and chronic periodontitis: A comparative study. Clin. Oral Investig. 2011, 15, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Jagannathan, M.; Hasturk, H.; Liang, Y.; Shin, H.; Hetzel, J.T.; Kantarci, A.; Rubin, D.; McDonnell, M.E.; Van Dyke, T.E.; Ganley-Leal, L.M.; et al. TLR cross-talk specifically regulates cytokine production by B cells from chronic inflammatory disease patients. J. Immunol. 2009, 183, 7461–7470. [Google Scholar] [CrossRef]
- Zundler, S.; Neurath, M.F. Interleukin-12: Functional activities and implications for disease. Cytokine Growth Factor Rev. 2015, 26, 559–568. [Google Scholar] [CrossRef]
- Han, Y.K.; Jin, Y.; Miao, Y.B.; Shi, T.; Lin, X.P. Improved RANKL production by memory B cells: A way for B cells promote alveolar bone destruction during periodontitis. Int. Immunopharmacol. 2018, 64, 232–237. [Google Scholar] [CrossRef]
- Bonneville, M.; O’Brien, R.L.; Born, W.K. Gammadelta T cell effector functions: A blend of innate programming and acquired plasticity. Nat. Rev. Immunol. 2010, 10, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Valdor, R.; Macian, F. Induction and stability of the anergic phenotype in T cells. Semin Immunol. 2013, 25, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Luckheeram, R.V.; Zhou, R.; Verma, A.D.; Xia, B. CD4(+)T cells: Differentiation and functions. Clin. Dev. Immunol. 2012, 2012, 925135. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Wu, C. The biology and functions of Th22 cells. Adv. Exp. Med. Biol. 2014, 841, 209–230. [Google Scholar] [PubMed]
- Lee, N.; Kim, W.U. Microbiota in T-cell homeostasis and inflammatory diseases. Exp. Mol. Med. 2017, 49, e340. [Google Scholar] [CrossRef] [PubMed]
- Crotty, S. Follicular helper CD4 T cells (TFH). Annu. Rev. Immunol. 2011, 29, 621–663. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, M.H.; Hufford, M.M.; Olson, M.R. The development and in vivo function of T helper 9 cells. Nat. Rev. Immunol. 2015, 15, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Duhen, T.; Geiger, R.; Jarrossay, D.; Lanzavecchia, A.; Sallusto, F. Production of interleukin 22 but not interleukin 17 by a subset of human skin-homing memory T cells. Nat. Immunol. 2009, 10, 857–863. [Google Scholar] [CrossRef]
- Xu, Z.; Ho, S.; Chang, C.C.; Zhang, Q.Y.; Vasilescu, E.R.; Vlad, G.; Suciu-Foca, N. Molecular and Cellular Characterization of Human CD8 T Suppressor Cells. Front. Immunol. 2016, 7, 549. [Google Scholar] [CrossRef]
- Tilloy, F.; Treiner, E.; Park, S.H.; Garcia, C.; Lemonnier, F.; de la Salle, H.; Bendelac, A.; Bonneville, M.; Lantz, O. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J. Exp. Med. 1999, 189, 1907–1921. [Google Scholar] [CrossRef]
- Treiner, E.; Lantz, O. CD1d- and MR1-restricted invariant T cells: Of mice and men. Curr. Opin. Immunol. 2006, 18, 519–526. [Google Scholar] [CrossRef]
- Sobkowiak, M.J.; Davanian, H.; Heymann, R.; Gibbs, A.; Emgard, J.; Dias, J.; Aleman, S.; Krüger-Weiner, C.; Moll, M.; Tjernlund, A.; et al. Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur. J. Immunol. 2018. [Google Scholar] [CrossRef]
- Cerutti, A.; Cols, M.; Puga, I. Activation of B cells by non-canonical helper signals. EMBO Rep. 2012, 13, 798–810. [Google Scholar] [CrossRef]
- Cerutti, A.; Puga, I.; Cols, M. New helping friends for B cells. Eur. J. Immunol. 2012, 42, 1956–1968. [Google Scholar] [CrossRef]
- Bonilla, F.A.; Oettgen, H.C. Adaptive immunity. J. Allergy Clin. Immunol. 2010, 125, S33–S40. [Google Scholar] [CrossRef]
- Smith, F.L.; Baumgarth, N. B-1 cell responses to infections. Curr. Opin. Immunol. 2019, 5, 23–31. [Google Scholar] [CrossRef]
- Yang, M.; Rui, K.; Wang, S.; Lu, L. Regulatory B cells in autoimmune diseases. Cell Mol. Immunol. 2013, 10, 122–132. [Google Scholar] [CrossRef]
- Yanaba, K.; Bouaziz, J.D.; Matsushita, T.; Magro, C.M.; St Clair, E.W.; Tedder, T.F. B-lymphocyte contributions to human autoimmune disease. Immunol. Rev. 2008, 223, 284–299. [Google Scholar] [CrossRef]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef]
- Cardoso, E.M.; Arosa, F.A. CD8(+) T Cells in Chronic Periodontitis: Roles and Rules. Front. Immunol. 2017, 8, 145. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. Gammadelta T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef]
- Krishnan, S.; Prise, I.E.; Wemyss, K.; Schenck, L.P.; Bridgeman, H.M.; McClure, F.A.; Zangerle-Murray, T.; O’Boyle, C.; Barbera, T.A.; Mahmood, F.; et al. Amphiregulin-producing gammadelta T cells are vital for safeguarding oral barrier immune homeostasis. Proc. Natl. Acad. Sci. USA 2018, 115, 10738–10743. [Google Scholar] [CrossRef]
- Wilharm, A.; Tabib, Y.; Nassar, M.; Reinhardt, A.; Mizraji, G.; Sandrock, I.; Heyman, O.; Barros-Martins, J.; Aizenbud, Y.; Khalaileh, A.; et al. Mutual interplay between IL-17-producing gammadeltaT cells and microbiota orchestrates oral mucosal homeostasis. Proc. Natl. Acad. Sci. USA 2019, 116, 2652–2661. [Google Scholar] [CrossRef]
- Arizon, M.; Nudel, I.; Segev, H.; Mizraji, G.; Elnekave, M.; Furmanov, K.; Eli-Berchoer, L.; Clausen, B.E.; Shapira, L.; Wilensky, A.; et al. Langerhans cells down-regulate inflammation-driven alveolar bone loss. Proc. Natl. Acad. Sci. USA 2012, 109, 7043–7048. [Google Scholar] [CrossRef]
- Dutzan, N.; Abusleme, L.; Konkel, J.E.; Moutsopoulos, N.M. Isolation, Characterization and Functional Examination of the Gingival Immune Cell Network. J. Vis. Exp. 2016, 108, 53736. [Google Scholar] [CrossRef]
- Sallusto, F.; Lenig, D.; Forster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef]
- Sheridan, B.S.; Lefrancois, L. Regional and mucosal memory T cells. Nat. Immunol. 2011, 12, 485–491. [Google Scholar] [CrossRef]
- Dutzan, N.; Abusleme, L.; Bridgeman, H.; Greenwell-Wild, T.; Zangerle-Murray, T.; Fife, M.E.; Bouladoux, N.; Linley, H.; Brenchley, L.; Wemyss, K.; et al. On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity 2017, 46, 133–147. [Google Scholar] [CrossRef]
- Mahanonda, R.; Champaiboon, C.; Subbalekha, K.; Sa-Ard-Iam, N.; Rattanathammatada, W.; Thawanaphong, S.; Rerkyen, P.; Yoshimura, F.; Nagano, K.; Lang, N.P.; et al. Human Memory B Cells in Healthy Gingiva, Gingivitis, and Periodontitis. J. Immunol. 2016, 197, 715–725. [Google Scholar] [CrossRef]
- Kim, Y.C.; Ko, Y.; Hong, S.D.; Kim, K.Y.; Lee, Y.H.; Chae, C.; Choi, Y. Presence of Porphyromonas gingivalis and plasma cell dominance in gingival tissues with periodontitis. Oral Dis. 2010, 16, 375–381. [Google Scholar] [CrossRef]
- Shelburne, C.E.; Shelburne, P.S.; Dhople, V.M.; Sweier, D.G.; Giannobile, W.V.; Kinney, J.S.; Coulter, W.A.; Mullally, B.H.; Lopatin, D.E. Serum antibodies to Porphyromonas gingivalis chaperone HtpG predict health in periodontitis susceptible patients. PLoS ONE 2008, 3, e1984. [Google Scholar] [CrossRef]
- Garlet, G.P. Destructive and protective roles of cytokines in periodontitis: A re-appraisal from host defense and tissue destruction viewpoints. J. Dent. Res. 2010, 89, 1349–1363. [Google Scholar] [CrossRef]
- Page, R.C.; Lantz, M.S.; Darveau, R.; Jeffcoat, M.; Mancl, L.; Houston, L.; Braham, P.; Persson, G.R. Immunization of Macaca fascicularis against experimental periodontitis using a vaccine containing cysteine proteases purified from Porphyromonas gingivalis. Oral Microbiol. Immunol. 2007, 22, 162–168. [Google Scholar] [CrossRef]
- Ebersole, J.L.; Kirakodu, S.S.; Novak, M.J.; Orraca, L.; Martinez, J.G.; Cunningham, L.L.; Thomas, M.V.; Stromberg, A.; Pandruvada, S.N.; Gonzalez, O.A. Transcriptome Analysis of B Cell Immune Functions in Periodontitis: Mucosal Tissue Responses to the Oral Microbiome in Aging. Front. Immunol. 2016, 7, 272. [Google Scholar] [CrossRef]
- Witkowski, J.M.; Mikosik, A.; Bryl, E.; Fulop, T. Proteodynamics in aging human T cells—The need for its comprehensive study to understand the fine regulation of T lymphocyte functions. Exp. Gerontol. 2018, 107, 161–168. [Google Scholar] [CrossRef]
- Okada, H.; Kida, T.; Yamagami, H. Identification and distribution of immunocompetent cells in inflamed gingiva of human chronic periodontitis. Infect. Immun. 1983, 41, 365–374. [Google Scholar]
- Okada, H.; Kassai, Y.; Kida, T. T lymphocyte subsets in the inflamed gingiva of human adult periodontitis. J. Periodontal Res. 1984, 19, 595–598. [Google Scholar] [CrossRef]
- Chen, M.L.; Sundrud, M.S. Cytokine Networks and T-Cell Subsets in Inflammatory Bowel Diseases. Inflamm. Bowel Dis. 2016, 22, 1157–1167. [Google Scholar] [CrossRef]
- Dutzan, N.K.T.; Abusleme, L.; Greenwell-Wild, T.; Zuazo, C.E.; Ikeuchi, T.; Brenchley, L.; Abe, T.; Hurabielle, C.; Martin, D.; Morell, R.J.; et al. A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci. Transl. Med. 2018, 10, eaat0797. [Google Scholar] [CrossRef]
- Jarry, C.R.; Duarte, P.M.; Freitas, F.F.; de Macedo, C.G.; Clemente-Napimoga, J.T.; Saba-Chujfi, E.; Passador-Santos, F.; de Araújo, V.C.; Napimoga, M.H. Secreted osteoclastogenic factor of activated T cells (SOFAT), a novel osteoclast activator, in chronic periodontitis. Hum Immunol. 2013, 74, 861–866. [Google Scholar] [CrossRef]
- Jarry, C.R.; Martinez, E.F.; Peruzzo, D.C.; Carregaro, V.; Sacramento, L.A.; Araujo, V.C.; Weitzmann, M.N.; Napimoga, M.H. Expression of SOFAT by T- and B-lineage cells may contribute to bone loss. Mol. Med. Rep. 2016, 13, 4252–4258. [Google Scholar] [CrossRef]
- Bittner-Eddy, P.D.; Fischer, L.A.; Tu, A.A.; Allman, D.A.; Costalonga, M. Discriminating between Interstitial and Circulating Leukocytes in Tissues of the Murine Oral Mucosa Avoiding Nasal-Associated Lymphoid Tissue Contamination. Front. Immunol. 2017, 8, 1398. [Google Scholar] [CrossRef]
- Seymour, G.J.; Powell, R.N.; Davies, W.I. The immunopathogenesis of progressive chronic inflammatory periodontal disease. J. Oral Pathol. 1979, 8, 249–265. [Google Scholar] [CrossRef]
- Zouali, M. The emerging roles of B cells as partners and targets in periodontitis. Autoimmunity. 2017, 50, 61–70. [Google Scholar] [CrossRef]
- Oliver-Bell, J.; Butcher, J.P.; Malcolm, J.; MacLeod, M.K.; Adrados Planell, A.; Campbell, L.; Nibbs, R.J.; Garside, P.; McInnes, I.B.; Culshaw, S. Periodontitis in the absence of B cells and specific anti-bacterial antibody. Mol. Oral Microbiol. 2015, 30, 160–169. [Google Scholar] [CrossRef]
- Abe, T.; AlSarhan, M.; Benakanakere, M.R.; Maekawa, T.; Kinane, D.F.; Cancro, M.P.; Korostoff, J.M.; Hajishengallis, G. The B Cell-Stimulatory Cytokines BLyS and APRIL Are Elevated in Human Periodontitis and Are Required for B Cell-Dependent Bone Loss in Experimental Murine Periodontitis. J. Immunol. 2015, 195, 1427–1435. [Google Scholar] [CrossRef]
- Kawai, T.; Matsuyama, T.; Hosokawa, Y.; Makihira, S.; Seki, M.; Karimbux, N.Y.; Goncalves, R.B.; Valverde, P.; Dibart, S.; Li, Y.P.; et al. B and T lymphocytes are the primary sources of RANKL in the bone resorptive lesion of periodontal disease. Am. J. Pathol. 2006, 169, 987–998. [Google Scholar] [CrossRef]
- Malcolm, J.; Awang, R.A.; Oliver-Bell, J.; Butcher, J.P.; Campbell, L.; Adrados Planell, A.; Lappin, D.F.; Fukada, S.Y.; Nile, C.J.; Liew, F.Y.; et al. IL-33 Exacerbates Periodontal Disease through Induction of RANKL. J. Dent. Res. 2015, 94, 968–975. [Google Scholar] [CrossRef]
- Kanzaki, H.; Makihira, S.; Suzuki, M.; Ishii, T.; Movila, A.; Hirschfeld, J.; Mawardi, H.; Lin, X.; Han, X.; Taubman, M.A.; et al. Soluble RANKL Cleaved from Activated Lymphocytes by TNF-alpha-Converting Enzyme Contributes to Osteoclastogenesis in Periodontitis. J. Immunol. 2016, 197, 3871–3883. [Google Scholar] [CrossRef]
- Demoersman, J.; Pochard, P.; Framery, C.; Simon, Q.; Boisrame, S.; Soueidan, A.; Pers, J.O. B cell subset distribution is altered in patients with severe periodontitis. PLoS ONE. 2018, 13, e0192986. [Google Scholar] [CrossRef]
- Coat, J.; Demoersman, J.; Beuzit, S.; Cornec, D.; Devauchelle-Pensec, V.; Saraux, A.; Pers, J.O. Anti-B lymphocyte immunotherapy is associated with improvement of periodontal status in subjects with rheumatoid arthritis. J. Clin. Periodontol. 2015, 42, 817–823. [Google Scholar] [CrossRef]
- Kalampokis, I.; Yoshizaki, A.; Tedder, T.F. IL-10-producing regulatory B cells (B10 cells) in autoimmune disease. Arthritis Res. Ther. 2013, 15, S1. [Google Scholar] [CrossRef]
- Yanaba, K.; Yoshizaki, A.; Asano, Y.; Kadono, T.; Tedder, T.F.; Sato, S. IL-10-producing regulatory B10 cells inhibit intestinal injury in a mouse model. Am. J. Pathol. 2011, 178, 735–743. [Google Scholar] [CrossRef]
- Dai, J.; Bi, L.; Lin, J.; Qi, F. Evaluation of interleukin-10 producing CD19(+) B cells in human gingival tissue. Arch. Oral Biol. 2017, 84, 112–117. [Google Scholar] [CrossRef]
- Hu, Y.; Yu, P.; Yu, X.; Hu, X.; Kawai, T.; Han, X. IL-21/anti-Tim1/CD40 ligand promotes B10 activity in vitro and alleviates bone loss in experimental periodontitis in vivo. Biochim. Biophys Acta Mol. Basis Dis. 2017, 1863, 2149–2157. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, X.; Lin, J.; Hu, Y.; Zhao, Q.; Kawai, T.; Taubman, M.A.; Han, X. B10 Cells Alleviate Periodontal Bone Loss in Experimental Periodontitis. Infect Immun. 2017, 85, e00335-17. [Google Scholar] [CrossRef]
- Lira-Junior, R.; Figueredo, C.M. Periodontal and inflammatory bowel diseases: Is there evidence of complex pathogenic interactions? World J. Gastroenterol. 2016, 22, 7963. [Google Scholar] [CrossRef]
| Cell | Subtype | Function in the Periodontal Tissues |
|---|---|---|
| T cells | Treg | Periodontal homeostasis by producing IL-10 and TGF-β. |
| MAIT | Largely unknown. | |
| Th | Specific immunological challenges lead to distinct cells’ subsets; Th1, Th2, and Th17 cells can produce a variety of pro-inflammatory cytokines that activate other immune cells such as dendritic cells, neutrophils, and B cells. Th17 can also be produced in response to biological barrier damage in healthy tissue. | |
| Tissue-resident epithelial γδ | Barrier surveillance, tissue homeostasis, and epithelial repair; Major source of IL-17 in homeostasis. | |
| CD8+ | Downregulate inflammation and suppress osteoclastogenesis. IL-10 and TGF-β production. | |
| Tfh | Activation of B cells; IL-21 production. | |
| SOFAT | Induce osteoclastogenesis in a RANKL-independent manner. | |
| B cells | Activated | Activation and expression of RANKL in the gingiva; promote osteoclastogenesis. |
| Memory | To prevent bone loss due to subclinical inflammation in clinically healthy periodontium. Production of antibodies against periodontal pathogens. | |
| Immunoglobulin-bearing lymphocytes; plasma cells | Clinical progression of the periodontal lesion; Stimulate the expression of RANKL in the gingiva. | |
| B1 | Associated with regulatory functions and the numbers might be decreased in periodontitis patients; produces antibodies against antigens and act as antigen-presenting cells. | |
| Breg/B10 | Negatively regulates the inflammatory responses via IL-10. | |
| CD138+ plasma cells | Association with the advancing front of the periodontal lesion. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Figueredo, C.M.; Lira-Junior, R.; Love, R.M. T and B Cells in Periodontal Disease: New Functions in A Complex Scenario. Int. J. Mol. Sci. 2019, 20, 3949. https://doi.org/10.3390/ijms20163949
Figueredo CM, Lira-Junior R, Love RM. T and B Cells in Periodontal Disease: New Functions in A Complex Scenario. International Journal of Molecular Sciences. 2019; 20(16):3949. https://doi.org/10.3390/ijms20163949
Chicago/Turabian StyleFigueredo, C.M., R. Lira-Junior, and R.M. Love. 2019. "T and B Cells in Periodontal Disease: New Functions in A Complex Scenario" International Journal of Molecular Sciences 20, no. 16: 3949. https://doi.org/10.3390/ijms20163949
APA StyleFigueredo, C. M., Lira-Junior, R., & Love, R. M. (2019). T and B Cells in Periodontal Disease: New Functions in A Complex Scenario. International Journal of Molecular Sciences, 20(16), 3949. https://doi.org/10.3390/ijms20163949





