Association between Advanced Glycation End Products, Soluble RAGE Receptor, and Endothelium Dysfunction, Evaluated by Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Mild and Resistant Hypertension
Abstract
1. Introduction
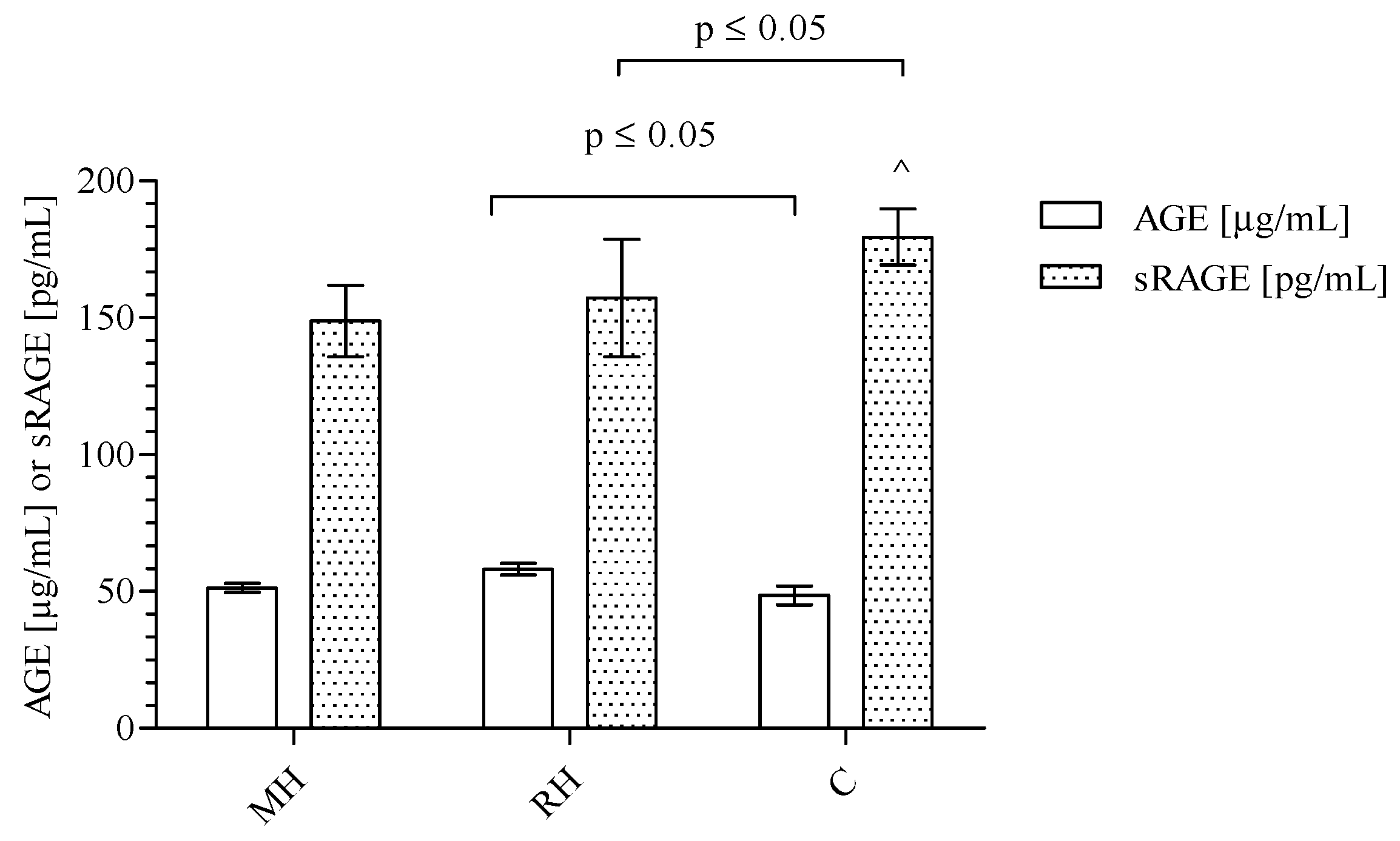
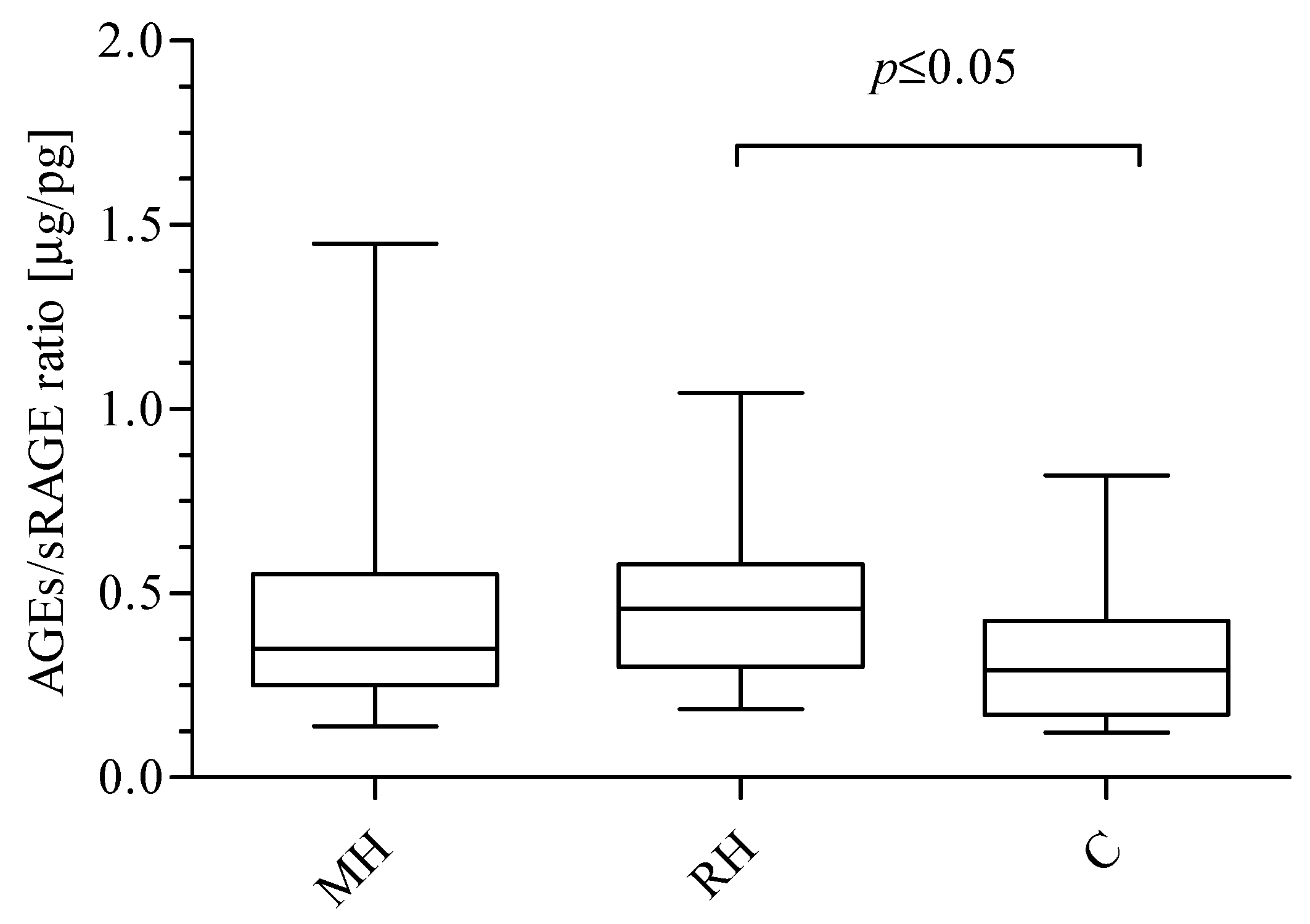
2. Results
3. Discussion
4. Material and Methods
4.1. Patients
4.2. Blood Pressure Measurements
4.3. Sample Collection
4.4. Biochemical Parameters
4.5. AGE Assay Kit (Cell Biolabs, Inc., San Diego, CA, USA)
4.6. sRAGE (RayBiotech, Norcross, Peachtree Corners, GA, USA)
4.7. Multicolor Flow Cytometry Analysis
4.8. Patient Exclusion Criteria
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gillis, E.E.; Sullivan, J.C. Sex differences in hypertension: Recent advances. Hypertension 2016, 68, 1322–1327. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Manson, J.E.; Gaziano, J.M.; Liu, S.; Cochrane, B.; Cook, N.R.; Ridger, P.M.; Rifai, N.; Sesso, H.D. Circulating inflammatory and endothelial markers and risk of hypertension in white and black postmenopausal women. Clin. Chem. 2011, 57, 729–736. [Google Scholar] [CrossRef] [PubMed]
- Sarafidis, P.A.; George, L. Resistant hypertension: An overview of evaluation and treatment. J. Am. Coll. Cardiol. 2008, 52, 1749–1757. [Google Scholar] [CrossRef] [PubMed]
- Yaxley, J.P.; Thambar, S.V. Resistant hypertension: An approach to management in primary care. J. Fam. Med. Prim. Care. 2015, 4, 193–199. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Clinical Guidelines for the Management of Hypertension; World Health Organization: Geneva, Switzerland, 2005. [Google Scholar]
- Guidelines Committee. 2003 European Society of Hypertension–European Society of Cardiology guidelines for the management of arterial hypertension. J. Hypertens. 2003, 21, 1011–1053. [Google Scholar] [CrossRef]
- Baradaran, A.; Nasri, H.; Rafieian-Kopaei, M. Oxidative stress and hypertension: Possibility of hypertension therapy with antioxidants. J. Res. Med. Sci. 2014, 19, 358–367. [Google Scholar] [PubMed]
- Rodrigo, R.; González, J.; Paoletto, F. The role of oxidative stress in the pathophysiology of hypertension. Hypertens. Res. 2011, 34, 431–440. [Google Scholar] [CrossRef]
- Henning, C.; Glomb, M.A. Pathways of the Maillard reaction under physiological conditions. Glycoconj. J. 2016, 33, 499–512. [Google Scholar] [CrossRef]
- Senatus, L.M.; Schmidt, A.M. The AGE-RAGE Axis: Implications for Age-Associated Arterial Diseases. Front. Genet. 2017, 8, 187. [Google Scholar] [CrossRef]
- Linssen, P.B.C.; Henry, R.M.A.; Schalkwijk, C.G.; Dekker, J.M.; Nijpels, G.; Brunner-La Rocca, H.; Stehouwer, C.D. Serum advanced glycation endproducts are associated with left ventricular dysfunction in normal glucose metabolism but not in type 2 diabetes: The Hoorn Study. Diab. Vasc. Dis. Res. 2016, 13, 278–285. [Google Scholar] [CrossRef] [PubMed]
- Mudau, M.; Genis, A.; Lochner, A.; Strijdom, H. Endothelial dysfunction: The early predictor of atherosclerosis. Cardiovasc. J. Afr. 2012, 23, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Budzyń, M.; Gryszczyńska, B.; Majewski, W.; Krasiński, Z.; Kasprzak, M.P.; Formanowicz, D.; Strzyżewski, K.W.; Iskra, M. The Association of Serum Thrombomodulin with Endothelial Injuring Factors in Abdominal Aortic Aneurysm. Biomed. Res. Int. 2017. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.F.; Ramasamy, R.; Naka, Y.; Schmidt, A.M. Glycation, inflammation, and RAGE: A scaffold for the macrovascular complications of diabetes and beyond. Circ. Res. 2003, 93, 1159–1169. [Google Scholar] [CrossRef] [PubMed]
- Tahara, N.; Yamagishi, S.I.; Tahara, A.; Ishibashi, M.; Hayabuchi, N.; Takeuchi, M.; Imaizumi, T. Adiponectin is inversely associated with ratio of serum levels of AGEs to sRAGE and vascular inflammation. Int. J. Cardiol. 2012, 158, 461–462. [Google Scholar] [CrossRef]
- Kajikawa, M.; Nakashima, A.; Fujimura, N.; Maruhashi, T.; Iwamoto, Y.; Iwamoto, A.; Matsumoto, T.; Oda, N.; Hidaka, T.; Kihara, Y.; et al. Ratio of serum levels of AGEs to soluble form of RAGE is a predictor of endothelial function. Diabetes Care 2015, 38, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Gryszczyńska, B.; Formanowicz, D.; Budzyń, M.; Wanic-Kossowska, M.; Pawliczak, E.; Formanowicz, P.; Majewski, W.; Strzyżewski, K.W.; Kasprzak, M.P.; Iskra, M. Advanced Oxidation Protein Products and Carbonylated Proteins as Biomarkers of Oxidative Stress in Selected Atherosclerosis-Mediated Diseases. Biomed. Res. Int. 2017, 9, 4975264. [Google Scholar]
- Prasad, K.; Manish, M. Do advanced glycation end products and its receptor play a role in pathophysiology of hypertension? Int. J. Angiol. 2017, 26, 001–011. [Google Scholar] [CrossRef]
- Schmidt, A.M.; Yan, S.D.; Wautier, J.L.; Stern, D. Activation of receptor for advanced glycation end products: A mechanism for chronic vascular dysfunction in diabetic vasculopathy and atherosclerosis. Circ. Res. 1999, 84, 489–897. [Google Scholar] [CrossRef]
- Huang, Q.F.; Sheng, C.S.; Liu, M.; Li, F.H.; Li, Y.; Wang, J.G. Arterial stiffness and wave reflections in relation to plasma advanced glycation end products in a Chinese population. Am. J. Hypertens. 2013, 26, 754–761. [Google Scholar] [CrossRef]
- Zieman, S.J.; Melenovsky, V.; Kass, D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 932–943. [Google Scholar] [CrossRef]
- Aronson, D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiffening of aging and diabetes. J. Hypertens. 2003, 21, 3–12. [Google Scholar] [CrossRef]
- Budzyń, M.; Gryszczyńka, B.; Boruczkowski, M.; Kaczmarek, M.; Begier-Krasińska, B.; Osińska, A.; Bukowska, A.; Kasprzak, M.P.; Iskra, M. The endothelial status reflected by circulating endothelial cells, circulating endothelial progenitor cells and soluble thrombomodulin in patients with mild and resistant hypertension. Vasc. Pharm. 2019, 113, 77–85. [Google Scholar] [CrossRef]
- Madian, A.G.; Regnier, F.E. Proteomic identification of carbonylated proteins and their oxidation sites. J. Proteome Res. 2010, 9, 3766–3780. [Google Scholar] [CrossRef]
- Geroldi, D.; Falcone, C.; Emanuele, E.; D’angelo, A.; Calcagnino, M.; Buzzi, M.P.; Scioli, G.A.; Fogari, R. Decreased plasma levels of soluble receptor for advanced glycation end-products in patients with essential hypertension. J. Hypertens. 2005, 23, 1725–1729. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; de Strihou, C.V.Y.; Ueda, Y.; Ichimori, K.; Inagi, R.; Onogi, H.; Ishikawa, N.; Nangaku, M.; Kurokawa, K. Angiotensin II receptor antagonists and angiotensin-converting enzyme inhibitors lower in vitro the formation of advanced glycation end products: Biochemical mechanisms. J. Am. Soc. Nephrol. 2002, 13, 2478–2487. [Google Scholar] [CrossRef] [PubMed]
- Susic, D.; Varagic, J.; Ahn, J.; Frohlich, E.D. Crosslink breakers: A new approach to cardiovascular therapy. Curr. Opin. Cardiol. 2004, 19, 336–340. [Google Scholar] [CrossRef]
- Gkogkolou, P.; Böhm, M. Advanced glycation end products: Key players in skin aging? Dermato-endocrinology 2012, 4, 259–270. [Google Scholar] [CrossRef]
- Uribarri, J.; Cai, W.; Sandu, O.; Peppa, M.; Goldberg, T.; Vlassara, H. Diet-derived advanced glycation end products are major contributors to the body’s AGE pool and induce inflammation in healthy subjects. Ann. N. Y. Acad. Sci. 2005, 1043, 461–466. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Adachi, H.; Nakamura, K.; Matsui, T.; Jinnouchi, Y.; Takenaka, K.; Takeuchi, M.; Enomoto, M.; Furuki, K.; Hino, A.; et al. Positive association between serum levels of advanced glycation end products and the soluble form of receptor for advanced glycation end products in nondiabetic subjects. Metabolism 2006, 55, 1227–1231. [Google Scholar] [CrossRef]
- El-Saeed, G.S.; Fadel, F.; Elshamaa, M.F.; Galal, R.E.; Elghoroury, E.A.; Nasr, S.A.; Thabet, E.H.; Abdelrahman, S.M. Advanced glycation end products and soluble receptor as markers of oxidative stress in children on hemodialysis. Ren. Fail. 2015, 37, 1452–1456. [Google Scholar] [CrossRef]
- Yonekura, H.; Yamamoto, Y.; Sakurai, S.; Petrova, R.G.; Abedin, M.J.; Li, H.; Yasui, K.; Takeuchi, M.; Makita, Z.; Takasawa, S.; et al. Novel splice variants of the receptor for advanced glycation end-products expressed in human vascular endothelial cells and pericytes, and their putative roles in diabetes-induced vascular injury. Biochem. J. 2003, 370, 1097–1109. [Google Scholar] [CrossRef]
- Chen, J.; Jing, J.; Yu, S.; Song, M.; Tan, H.; Cui, B.; Huang, L. Advanced glycation end products induce apoptosis of endothelial progenitor cells by activating receptor RAGE and NADPH oxidase/JNK signaling axis. Am. J. Transl. Res. 2016, 8, 2169–2178. [Google Scholar]
- Dinh, Q.N.; Drummond, G.R.; Sobey, C.G.; Chrissobolis, S. Roles of Inflammation, Oxidative Stress, and Vascular Dysfunction in Hypertension. Biomed. Res. Int. 2014, 11, 406960. [Google Scholar] [CrossRef]
- Chae, C.U.; Lee, R.T.; Rifai, N.; Ridker, P.M. Blood pressure and inflammation in apparently healthy men. Hypertension 2001, 38, 399–403. [Google Scholar] [CrossRef]
- Mahmud, A.; Feely, J. Arterial stiffness is related to systemic inflammation in essential hypertension. Hypertension 2005, 46, 1118–1122. [Google Scholar] [CrossRef]
- McNair, E.D.; Wells, C.R.; Qureshi, A.M.; Basran, R.; Pearce, C.; Orvold, J.; Devilliers, J.; Prasad, K. Modulation of high sensitivity C-reactive protein by soluble receptor for advanced glycation end products. Mol. Cell Biochem. 2010, 341, 135–138. [Google Scholar] [CrossRef]
- Hamuaty, R.B.; Sukmawati, I.R.; Sandra, F. Relationship between sRAGE and hsCRP as Markers of Cardiovascular Disease Risk Factors in Diabetic and Non-Diabetic Men with Central Obesity. Mol. Cell. Biomed. Sci. 2017, 1, 70–74. [Google Scholar] [CrossRef][Green Version]
- Yan, S.D.; Bierhaus, A.; Nawroth, P.P.; Stern, D.M. Endothelial precursor cells and CRP on the RAGE: Activation or cell death? J. Cardiovasc. Pharmacol. 2009, 53, 349–351. [Google Scholar] [CrossRef]
- World Health Organization. Non Communicable Diseases Country Profiles: 2014; World Health Organization: Geneva, Switzerland, 2014; 201p. [Google Scholar]
- Chockalingam, A.; Campbell, N.R.; Fodor, J.G. Worldwide epidemic of hypertension. Can. J. Cardiol. 2006, 22, 553–555. [Google Scholar] [CrossRef]
- Tesfaye, F.; Nawi, N.G.; Van Minh, H.; Byass, P.; Berhane, Y.; Bonita, R.; Wall, S. Association between body mass index and blood pressure across three populations in Africa and Asia. J. Hum. Hypertens. 2007, 21, 28–37. [Google Scholar] [CrossRef]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Wiinberg, N.; Høegholm, A.; Christensen, H.R.; Bang, L.E.; Mikkelsen, K.L.; Nielsen, P.E.; Svendsen, T.L.; Kampmann, J.P.; Madsen, N.H.; Bentzon, M.W. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am. J. Hypertens. 1995, 8, 978–986. [Google Scholar] [CrossRef]
- Luevano-Contreras, C.; Chapman-Novakofski, K. Dietary advanced glycation end products and aging. Nutrients 2010, 2, 1247–1265. [Google Scholar] [CrossRef]
- Cruz-Sanchez, F.F.; Girones, X.; Ortega, A.; Alameda, F.; Lafuente, J.V. Oxidative stress in Alzheimer’s disease hippocampus: A topographical study. J. Neurol. Sci. 2010, 299, 163–167. [Google Scholar] [CrossRef]
- Semba, R.D.; Nicklett, E.J.; Ferrucci, L. Does accumulation of advanced glycation end products contribute to the aging phenotype? J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 963–975. [Google Scholar] [CrossRef]
- Vlassara, H.; Fuh, H.; Makita, Z.; Krungkrai, S.; Cerami, A.; Bucala, R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: A model for diabetic and aging complications. Proc. Natl. Acad. Sci. USA 1992, 89, 12043–12047. [Google Scholar] [CrossRef]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced glycation end products: Sparking the development of diabetic vascular injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef]
- Soldatos, G.; Cooper, M.E. Advanced glycation end products and vascular structure and function. Curr. Hypertens. Rep. 2006, 8, 472–478. [Google Scholar] [CrossRef]
- Shapiro, B.P.; Owan, T.E.; Mohammed, S.F.; Meyer, D.M.; Mills, L.D.; Schwalkwijk, C.G.; Redfield, M.M. Advanced glycation end products accumulate in vascular smooth muscle and modify vascular but not ventricular properties in elderly hypertensive canines. Circulation 2008, 118, 1002–1010. [Google Scholar] [CrossRef]
- Takahashi, M.; Oikawa, M.; Nagano, A. Effect of age and menopause on serum concentrations of pentosidine, an advanced glycation end product. J. Gerontol. A Biol. Sci. Med. Sci. 2000, 55, M137–M140. [Google Scholar] [CrossRef]
- Szpera-Goździewicz, A.; Majcherek, M.; Boruczkowski, M.; Goździewicz, T.; Dworacki, G.; Wicherek, L.; Bręborowicz, G.H. Circulating endothelial cells, circulating endothelial progenitor cells, and von Willebrand factor in pregnancies complicated by hypertensive disorders. Am. J. Reprod. Immunol. 2017, 77, e12625. [Google Scholar] [CrossRef]
| Parameter | Quartile I | Quartile II and III | Quartile IV | |
|---|---|---|---|---|
| MH | ||||
| AGEs/sRAGE < 0.2567 | AGEs/sRAGE 0.2567–0.5501 | AGEs/sRAGE > 0.5501 | p value | |
| CECs | 198 (163–284) | 126 (66–152) | 74 (34–150) ^ | 0.0252 a |
| CEPCs | 413 (202–523) | 140 (96–359) | 83 (70–357) ^ | 0.0321 a |
| C | ||||
| AGEs < 38.28 | AGEs 38.28–60.98 | AGEs > 60.98 | p value | |
| sRAGE | 185.50 ± 48.30 | 180.10 ± 47.20 | 126.30 ± 25.88 ^ | 0.0425 b |
| AGEs * | ||
| r | Group of patients | |
| sRAGE | −0.5169 | C |
| sRAGE * | ||
| r | Group of patients | |
| SBP1 | −0.3735 | MH |
| CECs | 0.5591 | MH |
| CEPCs | 0.5703 | MH |
| AGEs | −0.5169 | C |
| AGEs/sRAGE | ||
| r | Group of patients | |
| CECs | −0.5983 | MH |
| CEPCs | −0.6375 | MH |
| CECs | ||
| r | Group of patients | |
| sRAGE | 0.5591 | MH |
| CEPCs | 0.5997 | MH |
| hsCRP | 0.4598 | RH |
| CEPCs | 0.6715 | RH |
| CEPCs | ||
| r | Group of patients | |
| CECs | 0.5997 | MH |
| sRAGE | 0.5703 | MH |
| CECs | 0.5997 | MH |
| CECs | 0.6715 | RH |
| Group | hsCRP (mg/L) | AGEs (μg/mL) | sRAGE (pg/mL) | AGEs/RAGEs (μg/pg) | SBP1 (mmHg) | r |
|---|---|---|---|---|---|---|
| MH | <1 (n = 8) | 54.39 ± 10.16 | 149.9 ± 75.1 | 0.325 (0.232–0.832) | 141.8 ± 17.9 | |
| 1 ≤ hsCRP < 3 (n = 12) | 50.70 ± 7.08 | 169.30 ± 86.46 | 0.318 (0.199–0.494) | 144.8 ± 16.5 | −0.6197 (sRAGE) 0.6363 (AGEs/sRAGE) | |
| ≥3 (n = 8) | 53.55 ± 10.24 | 140.0 ± 67.7 | 0.414 (0.265–0.540) | 141.9 ± 16.1 | ||
| RH | <1 (n = 5) | 59.35 ± 8.00 | 184.3 ± 71.5 | 0.283 (0.258–0.586) | 193.5 ± 21.8 * | |
| 1 ≤ hsCRP < 3 (n = 9) | 48.64 ± 14.00 | 142.60 ± 64.43 | 0.418 (0.310–0.532) | 178.3 ± 21.9 ^ | ||
| ≥3 (n = 11) | 62.60 ± 14.89 a | 114.4 ± 33.2 b | 0.554 (0.390–0.852) | 167.7 ± 21.0#b |
| Group | Gender | Age (years) | AGEs (μg/mL) | sRAGE (pg/mL) | AGEs/RAGEs (μg/pg) | hsCRP (mg/L) | SBP1 (mmHg) |
|---|---|---|---|---|---|---|---|
| MH | Female (n = 10) | 54.0 ± 13.5 | 52.88 ±6.97 | 160.90 ± 87.32 | 0.44 ± 0.27 | 2.7 (0.8–6.4) | 149.10 ± 17.03 |
| Male (n = 20) | 53.15 ± 14.18 | 50.36 ± 10.12 | 141.5 ± 59.2 | 0.46 ± 0.32 | 1.5 (0.9–3.53) | 141.90 ± 15.53 | |
| RH | Female (n = 11) | 58.82 ± 11.63 | 58.19 ± 6.13 ^ | 146.4 ± 53.9 | 0.44 ± 0.14 | 1.55 (0.73–4.9) | 180.8 ± 24.3 ^ |
| Male (n = 19) | 57.95 ± 12.28 | 57.26 ± 14.49 # | 165.3 ± 140.5 | 0.49 ± 0.29 | 6.1 (2.55−11.43) *# | 168.5 ± 21.2 # |
| Group | Age | AGEs (μg/mL) | sRAGE (pg/mL) | AGEs/RAGEs (μg/pg) | hsCRP (mg/L) | SBP1 (mmHg) |
|---|---|---|---|---|---|---|
| MH | ≤56 (n = 15) | 50.61 ± 7.90 | 170.6 ± 74.2 | 0.29 (0.24–0.48) | 1.5 (1.1–3.5) | 140.7 ± 17.5 |
| > 56 (n = 15) | 52.08 ± 10.30 | 126.6 ± 64.0 | 0.43 (0.27–0.84) | 1.90 (0.85–5.65) | 147.90 ± 15.05 | |
| RH | ≤60 (n = 14) | 58.01 ± 13.15 | 171.3 ± 151.6 | 0.43 (0.29–0.61) | 5.05 (1.43–8.13) | 176.6 ± 26.1 |
| > 60 (n = 16) | 54.85 ± 14.25 | 152.9 ± 69.3 | 0.46 (0.26–0.58) | 2.2 (1.2–4.9) | 172.4 ± 19.5 |
| Model | CEPCs | CECs | CEPCs/CECs | |||
|---|---|---|---|---|---|---|
| Coefficient | p | Coefficient | p | Coefficient | p | |
| Model 1 | ||||||
| AGEs | −5.679 | 0.4110 | −1.638 | 0.3159 | −0.0300 | 0.4875 |
| sRAGE | 0.03253 | 0.9747 | 0.2171 | 0.3723 | −0.0025 | 0.7009 |
| AGEs/sRAGE | −276.26 | 0.5130 | −0.6247 | 0.9950 | −0.6646 | 0.8017 |
| Model 2 | ||||||
| AGEs | −5.744 | 0.4197 | −1.688 | 0.3158 | −0.03187 | 0.4753 |
| sRAGE | 0.03323 | 0.9744 | 0.2176 | 0.3755 | −0.00245 | 0.7056 |
| AGEs/sRAGE | −274.61 | 0.5209 | 0.6357 | 0.9950 | −0.6197 | 0.8171 |
| Model 3 | ||||||
| AGEs | −7.069 | 0.3037 | −1.573 | 0.3432 | −0.03957 | 0.3564 |
| sRAGE | −0.1109 | 0.9130 | 0.2239 | 0.3636 | −0.003458 | 0.5864 |
| AGEs/sRAGE | −337.00 | 0.4204 | 2.233 | 0.9823 | −1.080 | 0.6789 |
| Model 4 | ||||||
| AGEs | −11.355 | 0.1902 | −4.027 | 0.0451 | 0.006681 | 0.8919 |
| sRAGE | 0.6907 | 0.7381 | 0.5898 | 0.2158 | −0.004173 | 0.7243 |
| AGEs/sRAGE | −18.047 | 0.9751 | 96.908 | 0.4657 | −0.9481 | 0.7749 |
| Model 5 | ||||||
| AGEs | −4.773 | 0.4956 | −1.757 | 0.2917 | −0.007762 | 0.8444 |
| sRAGE | −0.4750 | 0.6913 | 0.2836 | 0.3193 | −0.01498 | 0.0302 |
| AGEs/sRAGE | −408.23 | 0.3674 | 16.674 | 0.8762 | −3.915 | 0.1291 |
| Parameters | MH | RH | C | p Value |
|---|---|---|---|---|
| Age (years) | 52.87 ± 13.55 * | 58.27 ± 11.85 ** | 32.80 ± 9.20 | 0.001 a |
| Gender F/M (n) | 10/20 | 12/18 | 8/15 | NS b |
| BMI (kg/m2) | 28.53 ± 5.14 | 30.09± 5.73 | 26.90 ± 4.80 | NS a |
| SBP1 [mm Hg] | 144.5 ± 16.5 * | 173.0 ± 23.0 ** | 121.4 ± 2.7 | <0.0001 a |
| Red blood cells (RBC) [1012/L] | 4.82 (4.46–5.13) * | 4.60 (4.34–5.07) ** | 5.01 (4.89–5.33) | 0.026 c |
| White blood cells (WBC) [109/L] | 7.22 (5.68–8.95) * | 6.95 (5.69–8.60) ** | 6.00 (5.20–7.05) | 0.009 c |
| Platelets (PLT) [109/L] | 226.0 ± 56.2 | 221.0 ± 51.8 | 236.0 ± 54.0 | NS a |
| Neutrophils (NEUT) [109/L] | 4.48 (3.41–5.72) * | 4.42 (3.55–5.85) ** | 3.16 (2.52–3.53) | <0.0001 c |
| Lymphocytes (LYMPH) [109/L] | 1.63 (1.36–2.36) | 1.82 (1.37–2.15) | 2.16 (1.77–2.50) | NS c |
| Monocytes (MONO) [109/L] | 0.47 (0.34–0.53) * | 0.45 (0.28–0.67) | 0.56 (0.43–0.63) | 0.036 c |
| Hemoglobin (HGB) [mmol/L] | 9.04 ± 0.86 * | 8.84 ± 0.86 ** | 14.90 ± 1.26 | <0.0001 a |
| Creatinine (Cr) [μmol/L] | 85.0 (66.0–92.4) | 83.45 (70.58–112.10) | 70.20 (62.1–94.90) | NS c |
| Glucose (G) [mmol/L] | 5.59 (5.03–6.30) | 5.58 (5.20–6.38) | 4.98 (4.12–5.10) | NS c |
| hsCRP [mg/L] | 1.75 (0.95–3.58) * | 4.0 (1.47–8.03) ** | 1.0 (0.80–1.20) | 0.0001 c |
| CECs | 126 (67–198) * | 113 (64–233) ** | 50 (17–78) | <0.0001 c |
| CEPCs | 167(106–411) | 164 (101−320) | 153 (102–232) | NS c |
| CEPCs/CECs ratio | 1.60 (1.01–2.25) * | 1.35 (1.09–1.98) ** | 3.25 (2.03–14.11) | 0.012 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gryszczyńska, B.; Budzyń, M.; Begier-Krasińska, B.; Osińska, A.; Boruczkowski, M.; Kaczmarek, M.; Bukowska, A.; Iskra, M.; Kasprzak, M.P. Association between Advanced Glycation End Products, Soluble RAGE Receptor, and Endothelium Dysfunction, Evaluated by Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Mild and Resistant Hypertension. Int. J. Mol. Sci. 2019, 20, 3942. https://doi.org/10.3390/ijms20163942
Gryszczyńska B, Budzyń M, Begier-Krasińska B, Osińska A, Boruczkowski M, Kaczmarek M, Bukowska A, Iskra M, Kasprzak MP. Association between Advanced Glycation End Products, Soluble RAGE Receptor, and Endothelium Dysfunction, Evaluated by Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Mild and Resistant Hypertension. International Journal of Molecular Sciences. 2019; 20(16):3942. https://doi.org/10.3390/ijms20163942
Chicago/Turabian StyleGryszczyńska, Bogna, Magdalena Budzyń, Beata Begier-Krasińska, Angelika Osińska, Maciej Boruczkowski, Mariusz Kaczmarek, Alicja Bukowska, Maria Iskra, and Magdalena Paulina Kasprzak. 2019. "Association between Advanced Glycation End Products, Soluble RAGE Receptor, and Endothelium Dysfunction, Evaluated by Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Mild and Resistant Hypertension" International Journal of Molecular Sciences 20, no. 16: 3942. https://doi.org/10.3390/ijms20163942
APA StyleGryszczyńska, B., Budzyń, M., Begier-Krasińska, B., Osińska, A., Boruczkowski, M., Kaczmarek, M., Bukowska, A., Iskra, M., & Kasprzak, M. P. (2019). Association between Advanced Glycation End Products, Soluble RAGE Receptor, and Endothelium Dysfunction, Evaluated by Circulating Endothelial Cells and Endothelial Progenitor Cells in Patients with Mild and Resistant Hypertension. International Journal of Molecular Sciences, 20(16), 3942. https://doi.org/10.3390/ijms20163942







