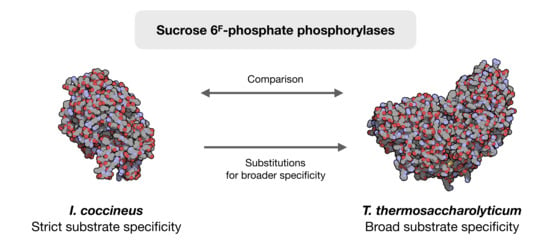
Structural Comparison of a Promiscuous and a Highly Specific Sucrose 6F-Phosphate Phosphorylase
Abstract
1. Introduction
2. Results and Discussion
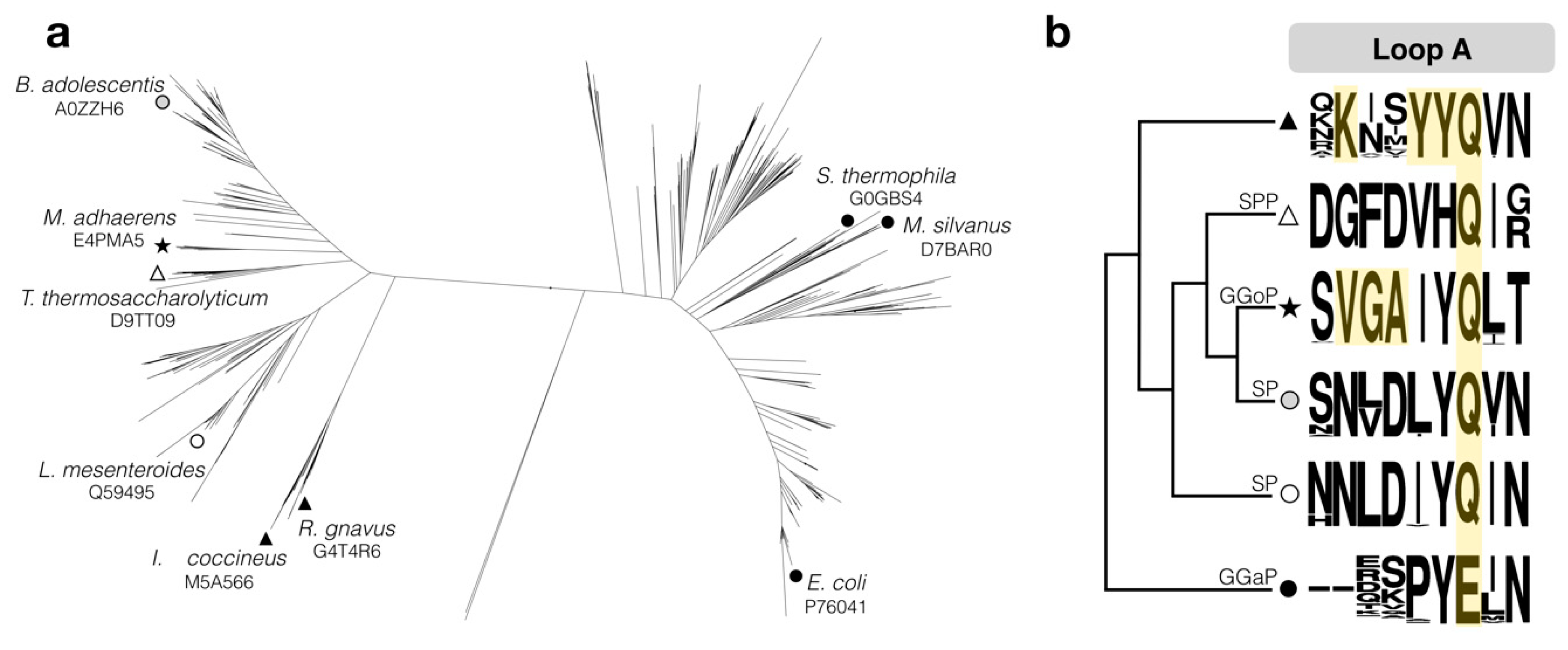
2.1. Choice of the Target Sequence
2.2. Expression and Characterization of IcSPP
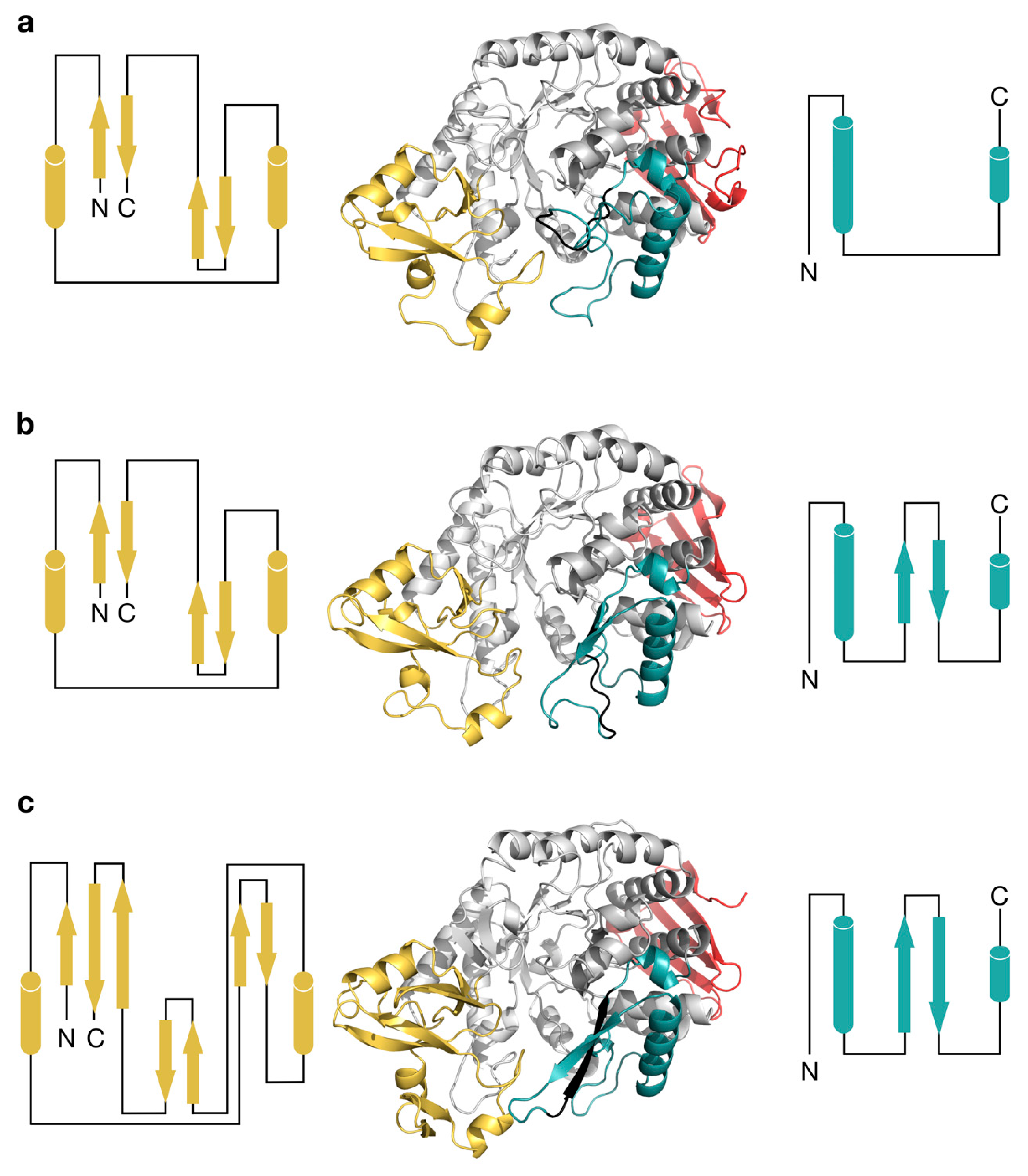
2.3. Structural Comparison of IcSPP and TtSPP
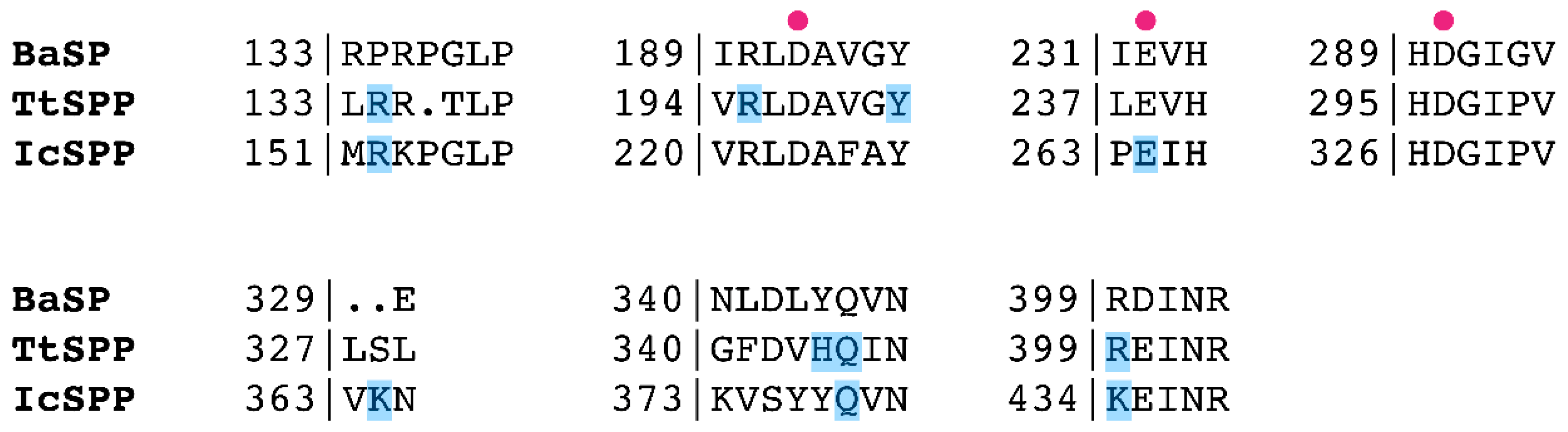
2.4. Mutagenesis
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Sequence Analysis
4.3. Gene Cloning and Transformation
4.4. Protein Expression and Purification
4.5. Site-Directed Mutagenesis
4.6. Colorimetric Assays
4.7. Characterization of IcSPP
4.8. Crystallography
4.9. Automated Docking and Protein Figures
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BaSP | Bifidobacterium adolescentis sucrose phosphorylase |
| Fru6P | Fructose 6-phosphate |
| GGaP | Glucosylglycerate phosphorylase |
| GGoP | Glucosylglycerol phosphorylase |
| GH13_18 | Subfamily 18 of glycoside hydrolase family 13 |
| Glc1P | α-d-glucose 1-phosphate |
| GOD-POD | Glucose oxidase—peroxidase |
| GP | Glycoside phosphorylase |
| IcSPP | Ilumatobacter coccineus sucrose 6F-phosphate phosphorylase |
| MES | 2-morpholinoethanesulfonic acid |
| MOPS | 3-(N-morpholino)propanesulfonic acid |
| SP | Sucrose phosphorylase |
| SPP | Sucrose 6F-phosphate phosphorylase |
| TtSPP | Thermoanaerobacterium thermosaccharolyticum sucrose 6F-phosphate phosphorylase |
References
- Desmet, T.; Soetaert, W. Enzymatic glycosyl transfer: Mechanisms and applications. Biocatal. Biotransform. 2011, 29, 1–18. [Google Scholar] [CrossRef]
- Puchart, V. Glycoside phosphorylases: Structure, catalytic properties and biotechnological potential. Biotechnol. Adv. 2015, 33, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, M. Diversity of phosphorylases in glycoside hydrolase families. Appl. Microbiol. Biotechnol. 2015, 99, 8377–8390. [Google Scholar] [CrossRef] [PubMed]
- Kok, D.S.; Yilmaz, D.; Suir, E.; Pronk, J.T.; Daran, J.M.; Van Maris, A.J.A. Increasing free-energy (ATP) conservation in maltose-grown Saccharomyces cerevisiae by expression of a heterologous maltose phosphorylase. Metab. Eng. 2011, 13, 518–526. [Google Scholar] [CrossRef]
- Goedl, C.; Sawangwan, T.; Wildberger, P.; Nidetzky, B. Sucrose phosphorylase: A powerful transglucosylation catalyst for synthesis of α-D-glucosides as industrial fine chemicals. Biocatal. Biotransform. 2010, 28, 10–21. [Google Scholar] [CrossRef]
- Aerts, D.; Verhaeghe, T.F.; Roman, B.I.; Stevens, C.V.; Desmet, T.; Soetaert, W. Transglucosylation potential of six sucrose phosphorylases toward different classes of acceptors. Carbohydr. Res. 2011, 346, 1860–1867. [Google Scholar] [CrossRef]
- Gudiminchi, R.K.; Nidetzky, B. Walking a fine line with sucrose phosphorylase: Efficient single-step biocatalytic production of l-ascorbic acid 2-glucoside from sucrose. ChemBioChem 2017, 18, 1387–1390. [Google Scholar] [CrossRef]
- De Winter, K.; Desmet, T. Biphasic catalysis with disaccharide phosphorylases: Chemoenzymatic synthesis of α-d-glucosides using sucrose phosphorylase. Org. Process Res. Dev. 2014, 18, 781–787. [Google Scholar] [CrossRef]
- Luley-Goedl, C.; Sawangwan, T.; Brecker, L.; Wildberger, P.; Nidetzky, B. Regioselective O-glucosylation by sucrose phosphorylase: A promising route for functional diversification of a range of 1,2-propanediols. Carbohydr. Res. 2010, 345, 1736–1740. [Google Scholar] [CrossRef]
- Sawangwan, T.; Goedl, C.; Nidetzky, B. Single-step enzymatic synthesis of (R)-2-O-α-D-glucopyranosyl glycerate, a compatible solute from micro-organisms that functions as a protein stabiliser. Org. Biomol. Chem. 2009, 7, 4267–4270. [Google Scholar] [CrossRef]
- Cerdobbel, A.; De Winter, K.; Aerts, D.; Kuipers, R.; Joosten, H.J.; Soetaert, W.; Desmet, T. Increasing the thermostability of sucrose phosphorylase by a combination of sequence- and structure-based mutagenesis. Protein Eng. Des. Sel. 2011, 24, 829–834. [Google Scholar] [CrossRef]
- Verhaeghe, T.; De Winter, K.; Berland, M.; De Vreese, R.; D’hooghe, M.; Offmann, B.; Desmet, T. Converting bulk sugars into prebiotics: Semi-rational design of a transglucosylase with controlled selectivity. Chem. Commun. 2016, 52, 3687–3689. [Google Scholar] [CrossRef]
- Kraus, M.; Grimm, C.; Seibel, J. Redesign of the active site of sucrose phosphorylase through a clash-induced cascade of loop shifts. ChemBioChem 2016, 17, 33–36. [Google Scholar] [CrossRef]
- Kraus, M.; Grimm, C.; Seibel, J. Switching enzyme specificity from phosphate to resveratrol glucosylation. Chem. Commun. 2017, 53, 12181–12184. [Google Scholar] [CrossRef]
- Franceus, J.; Dhaene, S.; Decadt, H.; Vandepitte, J.; Caroen, J.; Van der Eycken, J.; Beerens, K.; Desmet, T. Rational design of an improved transglucosylase for production of the rare sugar nigerose. Chem. Commun. 2019, 55, 4531–4533. [Google Scholar] [CrossRef]
- Stam, M.R.; Danchin, E.G.J.; Rancurel, C.; Coutinho, P.M.; Henrissat, B. Dividing the large glycoside hydrolase family 13 into subfamilies: Towards improved functional annotations of α-amylase-related proteins. Protein Eng. Des. Sel. 2006, 19, 555–562. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, 490–495. [Google Scholar] [CrossRef]
- Verhaeghe, T.; Aerts, D.; Diricks, M.; Soetaert, W.; Desmet, T. The quest for a thermostable sucrose phosphorylase reveals sucrose 6′-phosphate phosphorylase as a novel specificity. Appl. Microbiol. Biotechnol. 2014, 98, 7027–7037. [Google Scholar] [CrossRef]
- Dirks-Hofmeister, M.E.; Verhaeghe, T.; De Winter, K.; Desmet, T. Creating space for large acceptors: Rational biocatalyst design for resveratrol glycosylation in an aqueous system. Angew. Chem. 2015, 127, 9421–9424. [Google Scholar] [CrossRef]
- Decuyper, L.; Franceus, J.; Dhaene, S.; Debruyne, M.; Vandoorne, K.; Piens, N.; Dewitte, G.; Desmet, T.; D’hooghe, M. Chemoenzymatic approach toward the Synthesis of 3-O-(α/β)-Glucosylated 3-Hydroxy-β-lactams. ACS Omega 2018, 3, 15235–15245. [Google Scholar] [CrossRef]
- Franceus, J.; Pinel, D.; Desmet, T. Glucosylglycerate phosphorylase, an enzyme with novel specificity involved in compatible solute metabolism. Appl. Environ. Microbiol. 2017, 83, e01434-17. [Google Scholar] [CrossRef]
- Franceus, J.; Decuyper, L.; D’hooghe, M.; Desmet, T. Exploring the sequence diversity in glycoside hydrolase family 13_18 reveals a novel glucosylglycerol phosphorylase. Appl. Microbiol. Biotechnol. 2018, 102, 3183–3191. [Google Scholar] [CrossRef]
- Tauzin, A.S.; Bruel, L.; Laville, E.; Nicoletti, C.; Navarro, D.; Henrissat, B.; Perrier, J.; Potocki-Veronese, G.; Giardina, T.; Lafond, M. Sucrose 6F-phosphate phosphorylase: A novel insight in the human gut microbiome. Microb. Genom. 2019, 1–14. [Google Scholar] [CrossRef]
- Fujinami, S.; Takarada, H.; Kasai, H.; Sekine, M.; Omata, S.; Fukai, R.; Hosoyama, A.; Horikawa, H.; Kato, Y.; Fujita, N.; et al. Complete genome sequence of Ilumatobacter coccineum YM16–304. Stand. Genom. Sci. 2013, 8, 430–440. [Google Scholar] [CrossRef]
- Sprogoe, D.; van den Broek, L.A.M.; Mirza, O.; Kastrup, J.S.; Voragen, A.G.J.; Gajhede, M.; Skov, L.K. Crystal structure of sucrose phosphorylase from Bifidobacterium adolescentis. Biochemistry 2004, 43, 1156–1162. [Google Scholar] [CrossRef]
- Mirza, O.; Skov, L.K.; Sprogøe, D.; van den Broek, L.M.; Beldman, G.; Kastrup, J.S.; Gajhede, M. Structural rearrangements of sucrose phosphorylase from Bifidobacterium adolescentis during sucrose conversion. J. Biol. Chem. 2006, 281, 35576–35584. [Google Scholar] [CrossRef]
- Schwarz, A.; Nidetzky, B. Asp-196—Ala mutant of Leuconostoc mesenteroides sucrose phosphorylase exhibits altered stereochemical course and kinetic mechanism of glucosyl transfer to and from phosphate. FEBS Lett. 2006, 580, 3905–3910. [Google Scholar] [CrossRef]
- Schwarz, A.; Brecker, L.; Nidetzky, B. Acid–base catalysis in Leuconostoc mesenteroides sucrose phosphorylase probed by site-directed mutagenesis and detailed kinetic comparison of wild-type and Glu237→Gln mutant enzymes. Biochem. J. 2007, 403, 441–449. [Google Scholar] [CrossRef]
- Verhaeghe, T.; Diricks, M.; Aerts, D.; Soetaert, W.; Desmet, T. Mapping the acceptor site of sucrose phosphorylase from Bifidobacterium adolescentis by alanine scanning. J. Mol. Catal. B Enzym. 2013, 96, 81–88. [Google Scholar] [CrossRef]
- Goedl, C.; Sawangwan, T.; Mueller, M.; Schwarz, A.; Nidetzky, B. A high-yielding biocatalytic process for the production of 2-O-(α-D-glucopyranosyl)-sn-glycerol, a natural osmolyte and useful moisturizing ingredient. Angew. Chem. Int. Ed. Engl. 2008, 47, 10086–10089. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Luley-Goedl, C.; Leitner, E.; Sawangwan, T.; Nidetzky, B. Production of glucosyl glycerol by immobilized sucrose phosphorylase: Options for enzyme fixation on a solid support and application in microscale flow format. J. Biotechnol. 2017, 257, 131–138. [Google Scholar] [CrossRef]
- Khanal, A.; McLoughlin, S.Y.; Kershner, J.P.; Copley, S.D. Differential effects of a mutation on the normal and promiscuous activities of orthologs: Implications for natural and directed evolution. Mol. Biol. Evol. 2015, 32, 100–108. [Google Scholar] [CrossRef]
- Newton, M.S.; Arcus, V.L.; Gerth, M.L.; Patrick, W.M. Enzyme evolution: Innovation is easy, optimization is complicated. Curr. Opin. Struct. Biol. 2018, 48, 110–116. [Google Scholar] [CrossRef]
- De Winter, K.; Cerdobbel, A.; Soetaert, W.; Desmet, T. Operational stability of immobilized sucrose phosphorylase: Continuous production of α-glucose-1-phosphate at elevated temperatures. Process Biochem. 2011, 46, 1074–1078. [Google Scholar] [CrossRef]
- Madeira, F.; Park, Y.M.; Lee, J.; Buso, N.; Gur, T.; Madhusoodanan, N.; Basutkar, P.; Tivey, A.R.N.; Potter, S.C.; Finn, R.D.; et al. The EMBL-EBI search and sequence analysis tools APIs in 2019. Nucleic Acids Res. 2019, 47, W636–W641. [Google Scholar] [CrossRef]
- Guindon, S.; Dufayard, J.-F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 2–5. [Google Scholar] [CrossRef]
- Crooks, G.; Hon, G.; Chandonia, J.; Brenner, S. WebLogo: A sequence logo generator. Genome Res. 2004, 14, 1188–1190. [Google Scholar] [CrossRef]
- Robert, X.; Gouet, P. Deciphering key features in protein structures with the new ENDscript server. Nucleic Acids Res. 2014, 42, 320–324. [Google Scholar] [CrossRef]
- Reetz, M.; Kahakeaw, D.; Sanchis, J. Shedding light on the efficacy of laboratory evolution based on iterative saturation mutagenesis. Mol. Biosyst. 2009, 5, 115–122. [Google Scholar] [CrossRef]
- Silverstein, R.; Voet, J.; Reed, D.; Abeles, R. Purification and mechanism of action of sucrose phosphorylase. J. Biol. Chem. 1967, 242, 1338–1346. [Google Scholar]
- Gawronski, J.D.; Benson, D.R. Microtiter assay for glutamine synthetase biosynthetic activity using inorganic phosphate detection. Anal. Biochem. 2004, 327, 114–118. [Google Scholar] [CrossRef]
- Blecher, M.; Glassman, A.B. Determination of glucose in the presence of sucrose using glucose oxidase; effect of pH on absorption spectrum of oxidized o-dianisidine. Anal. Biochem. 1962, 3, 343–352. [Google Scholar] [CrossRef]
- Kabsch, W. Integration, scaling, space-group assignment and post-refinement. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 133–144. [Google Scholar] [CrossRef]
- CCP4 The CCP4 suite: Programs for protein crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 1994, 50, 760–763. [CrossRef]
- McCoy, A.J. Solving structures of protein complexes by molecular replacement with Phaser. Acta Crystallogr. Sect. D Biol. Crystallogr. 2006, 63, 32–41. [Google Scholar] [CrossRef]
- Langer, G.; Cohen, S.X.; Lamzin, V.S.; Perrakis, A. Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat. Protoc. 2008, 3, 1171–1179. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef]
- Adams, P.D.; Afonine, P.V.; Bunkóczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef]
- Chen, V.B.; Arendall, W.B.; Headd, J.J.; Keedy, D.A.; Immormino, R.M.; Kapral, G.J.; Murray, L.W.; Richardson, J.S.; Richardson, D.C. MolProbity: All-atom structure validation for macromolecular crystallography. Acta Crystallogr. Sect. D Biol. Crystallogr. 2010, 66, 12–21. [Google Scholar] [CrossRef]
- Krieger, E.; Vriend, G. YASARA View—Molecular graphics for all devices—From smartphones to workstations. Bioinformatics 2014, 30, 2981–2982. [Google Scholar] [CrossRef]
- Schrödinger LLC. The PyMOL Molecular Graphics System, v 2.0. Available online: https://sourceforge.net/p/pymol/mailman/message/36047137/ (accessed on 20 September 2017).


| Reaction | Substrate | KM (mM) | kcat (s−1) | kcat/KM (mM−1s−1) |
|---|---|---|---|---|
| Phosphorolysis | sucrose 6F-phosphate | 11.3 ± 1.8 | 126 ± 15 | 11.2 |
| phosphate | 7.8 ± 1.2 | 110 ± 9 | 14.1 | |
| Synthesis | α-d-glucose 1-phosphate | 18.8 ± 3.6 | 45 ± 3 | 2.4 |
| fructose 6-phosphate | 2.0 ± 0.3 | 40 ± 4 | 20 |
| Mutant | Kinetics Fru6P | vacceptor/vwater | ||||
|---|---|---|---|---|---|---|
| KM (mM) | kcat (s−1) | Fru6P | Fructose | Glycerol | d-Glycerate | |
| Wild-type | 2.0 ± 0.3 | 40.0 ± 3.8 | 1360 ± 120 | - | - | - |
| R152A | 3.3 ± 0.7 | 1.42 ± 0.11 | 98 ± 5 | 6.9 ± 0.3 | 2.4 ± 0.3 | 2.5 ± 0.2 |
| K364S | 6.3 ± 1.1 | 0.61 ± 0.04 | 18 ± 2 | 1.3 ± 0.1 | 1.3 ± 0.1 | 1.3 ± 0.1 |
| Y377H | 2.8 ± 0.3 | 0.80 ± 0.08 | 21 ± 2 | - | - | - |
| K434R | 10.6 ± 1.9 | 6.84 ± 0.15 | 680 ± 45 | 8.1 ± 0.4 | - | 4.9 ± 0.4 |
| K434A | 33.2 ± 2.9 | 0.10 ± 0.01 | 3.5 ± 0.5 | 2.8 ± 0.4 | 1.4 ± 0.2 | - |
| Primer | DNA sequence (5′-3′) |
|---|---|
| IcSPP_Fw_R152A | CGTCTGTTTATGGCTAAACCGGGTCTGC |
| IcSPP_Fw_K364S | GGTGGTCGTGTGAGTAATCTGTATGGTG |
| IcSPP_Fw_Y377H | GGTACAAAAGTGAGCTATCATCAGGTTAACGCC |
| IcSPP_Fw_K434R | GGTGCGGATGGTCATCGTGAAATCAATCG |
| IcSPP_Fw_K434A | GGTGCGGATGGTCATGCAGAAATCAATCG |
| pET21a_Rv_seq1 | TCCGCGCACATTTCC |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franceus, J.; Capra, N.; Desmet, T.; Thunnissen, A.-M.W.H. Structural Comparison of a Promiscuous and a Highly Specific Sucrose 6F-Phosphate Phosphorylase. Int. J. Mol. Sci. 2019, 20, 3906. https://doi.org/10.3390/ijms20163906
Franceus J, Capra N, Desmet T, Thunnissen A-MWH. Structural Comparison of a Promiscuous and a Highly Specific Sucrose 6F-Phosphate Phosphorylase. International Journal of Molecular Sciences. 2019; 20(16):3906. https://doi.org/10.3390/ijms20163906
Chicago/Turabian StyleFranceus, Jorick, Nikolas Capra, Tom Desmet, and Andy-Mark W.H. Thunnissen. 2019. "Structural Comparison of a Promiscuous and a Highly Specific Sucrose 6F-Phosphate Phosphorylase" International Journal of Molecular Sciences 20, no. 16: 3906. https://doi.org/10.3390/ijms20163906
APA StyleFranceus, J., Capra, N., Desmet, T., & Thunnissen, A.-M. W. H. (2019). Structural Comparison of a Promiscuous and a Highly Specific Sucrose 6F-Phosphate Phosphorylase. International Journal of Molecular Sciences, 20(16), 3906. https://doi.org/10.3390/ijms20163906