Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity
Abstract
1. Introduction
2. Clinical Problem—HCMV Pneumonitis
2.1. High Risk Groups
2.2. Clinical Symptoms and Diagnosis
2.3. Transmission and Ports of Entry
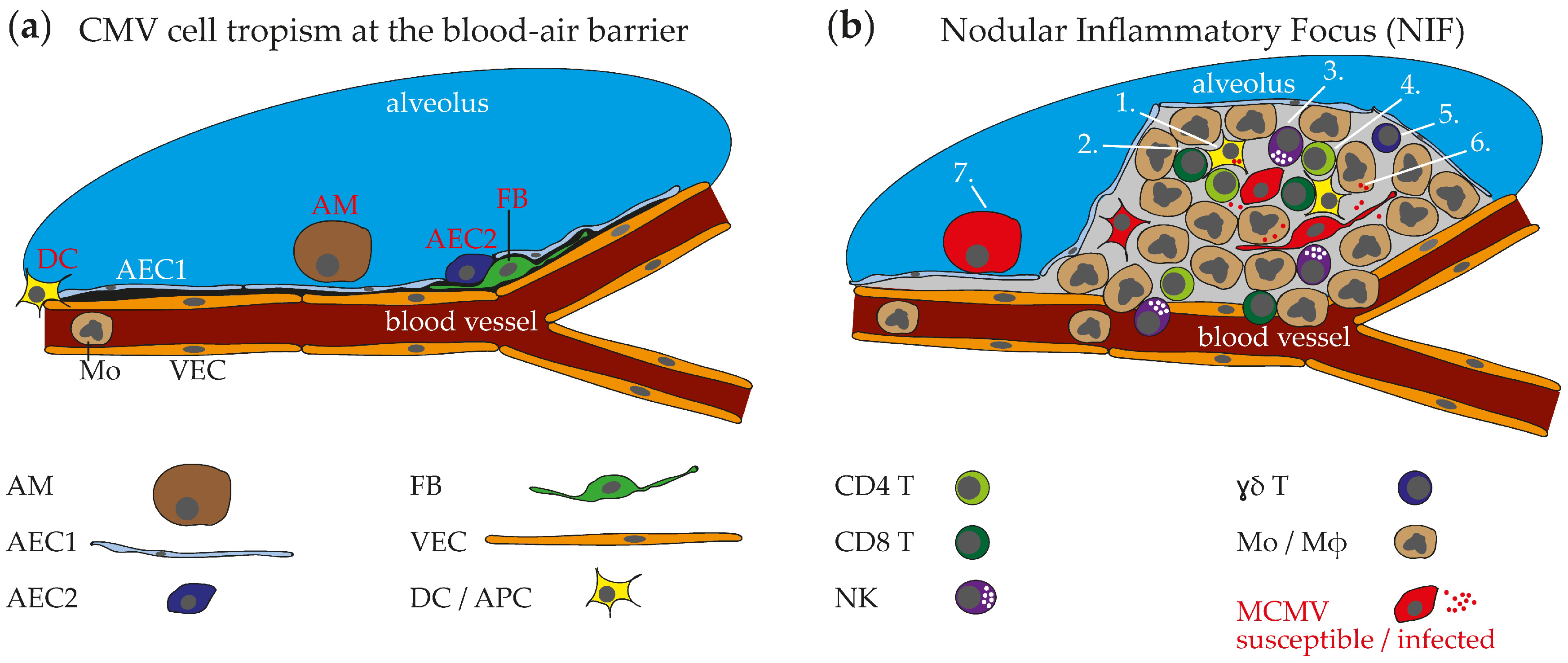
2.4. HCMV Cell Tropism in the Respiraory Tract and Histopathological Findings
2.5. Summary
3. Insights Obtained from the Animal Model—MCMV Pneumonitis
3.1. MCMV Cell Tropism in the Respiratory Tract and Ports of Entry
3.2. Nodular Inflammatory Focus as Site of Immune Control in Lungs
3.3. Immune Cells in MCMV Pneumonitis
3.3.1. αβ T Cells
3.3.2. NK Cells
3.3.3. Myeloid Cells
3.3.4. Antibodies, ɣδ T Cells, and More
3.3.5. Integral Model of Anti-MCMV Immune Response in NIFs
3.4. Summary
4. Perspective
4.1. Age-Related Differences in CMV Pneumonitis
4.2. CMV Transmission via Airway Exposure
4.3. CMV Determinants of Pathogenicity
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Arvin, A.; Campadelli-Fiume, G.; Mocarski, E.; Moore, P.S.; Roizman, B.; Whitley, R.; Yamanishi, K. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis, 2011/02/25 ed.; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutre, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Griffiths, P.D. Burden of disease associated with human cytomegalovirus and prospects for elimination by universal immunisation. Lancet Infect. Dis. 2012, 12, 790–798. [Google Scholar] [CrossRef]
- Reddehase, M.J.; Lemmermann, N.A.W. Mouse Model of Cytomegalovirus Disease and Immunotherapy in the Immunocompromised Host: Predictions for Medical Translation that Survived the “Test of Time”. Viruses 2018, 10, 693. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, G.M.; Horak, D.A.; Niland, J.C.; Duncan, S.R.; Forman, S.J.; Zaia, J.A. A randomized, controlled trial of prophylactic ganciclovir for cytomegalovirus pulmonary infection in recipients of allogeneic bone marrow transplants; The City of Hope-Stanford-Syntex CMV Study Group. N. Engl. J. Med. 1991, 324, 1005–1011. [Google Scholar] [CrossRef] [PubMed]
- Boeckh, M.; Ljungman, P. How we treat cytomegalovirus in hematopoietic cell transplant recipients. Blood 2009, 113, 5711–5719. [Google Scholar] [CrossRef] [PubMed]
- Fishman, J.A. Infection in solid-organ transplant recipients. N. Engl. J. Med. 2007, 357, 2601–2614. [Google Scholar] [CrossRef]
- Hill, R.B., Jr.; Rowlands, D.T., Jr.; Rifkind, D. Infectious Pulmonary Disease in Patients Receiving Immunosuppressive Therapy for Organ Transplantation. N. Engl. J. Med. 1964, 271, 1021–1027. [Google Scholar] [CrossRef]
- Snyder, L.D.; Finlen-Copeland, C.A.; Turbyfill, W.J.; Howell, D.; Willner, D.A.; Palmer, S.M. Cytomegalovirus pneumonitis is a risk for bronchiolitis obliterans syndrome in lung transplantation. Am. J. Respir. Crit. Care Med. 2010, 181, 1391–1396. [Google Scholar] [CrossRef]
- Wallace, J.M.; Hannah, J. Cytomegalovirus pneumonitis in patients with AIDS. Findings in an autopsy series. Chest 1987, 92, 198–203. [Google Scholar] [CrossRef]
- Szczawinska-Poplonyk, A.; Jonczyk-Potoczna, K.; Ossowska, L.; Breborowicz, A.; Bartkowska-Sniatkowska, A.; Wachowiak, J. Cytomegalovirus pneumonia as the first manifestation of severe combined immunodeficiency. Cent. Eur. J. Immunol. 2014, 39, 392–395. [Google Scholar] [CrossRef]
- Coclite, E.; di Natale, C.; Nigro, G. Congenital and perinatal cytomegalovirus lung infection. J. Matern Fetal. Neonatal Med. 2013, 26, 1671–1675. [Google Scholar] [CrossRef] [PubMed]
- Cunha, B.A.; Pherez, F.; Walls, N. Severe cytomegalovirus (CMV) community-acquired pneumonia (CAP) in a nonimmunocompromised host. Heart Lung 2009, 38, 243–248. [Google Scholar] [CrossRef] [PubMed]
- Grilli, E.; Galati, V.; Bordi, L.; Taglietti, F.; Petrosillo, N. Cytomegalovirus pneumonia in immunocompetent host: Case report and literature review. J. Clin. Virol. 2012, 55, 356–359. [Google Scholar] [CrossRef] [PubMed]
- Goncalves, C.; Cipriano, A.; Videira Santos, F.; Abreu, M.; Mendez, J.; Sarmento, E.C.R. Cytomegalovirus acute infection with pulmonary involvement in an immunocompetent patient. IDCases 2018, 14, e00445. [Google Scholar] [CrossRef] [PubMed]
- Grundy, J.E.; Shanley, J.D.; Griffiths, P.D. Is Cytomegalovirus Interstitial Pneumonitis in Transplant Recipients an Immunopathological Condition. Lancet 1987, 2, 996–999. [Google Scholar] [CrossRef]
- Barry, S.M.; Johnson, M.A.; Janossy, G. Cytopathology or immunopathology? The puzzle of cytomegalovirus pneumonitis revisited. Bone Marrow Transplant 2000, 26, 591–597. [Google Scholar] [CrossRef] [PubMed]
- Rafailidis, P.I.; Mourtzoukou, E.G.; Varbobitis, I.C.; Falagas, M.E. Severe cytomegalovirus infection in apparently immunocompetent patients: A systematic review. Virol. J. 2008, 5, 47. [Google Scholar] [CrossRef]
- Moon, J.H.; Kim, E.A.; Lee, K.S.; Kim, T.S.; Jung, K.J.; Song, J.H. Cytomegalovirus pneumonia: High-resolution CT findings in ten non-AIDS immunocompromised patients. Korean J. Radiol. 2000, 1, 73–78. [Google Scholar] [CrossRef]
- Restrepo-Gualteros, S.M.; Gutierrez, M.J.; Villamil-Osorio, M.; Arroyo, M.A.; Nino, G. Challenges and Clinical Implications of the Diagnosis of Cytomegalovirus Lung Infection in Children. Curr. Infect. Dis. Rep. 2019, 21, 24. [Google Scholar] [CrossRef]
- Pinana, J.L.; Gimenez, E.; Gomez, M.D.; Perez, A.; Gonzalez, E.M.; Vinuesa, V.; Hernandez-Boluda, J.C.; Montoro, J.; Salavert, M.; Tormo, M.; et al. Pulmonary cytomegalovirus (CMV) DNA shedding in allogeneic hematopoietic stem cell transplant recipients: Implications for the diagnosis of CMV pneumonia. J. Infect. 2019, 78, 393–401. [Google Scholar] [CrossRef]
- Lippold, S.; Braun, B.; Kruger, F.; Harms, M.; Muller, J.A.; Gross, R.; Munch, J.; von Einem, J. Natural Inhibitor of Human Cytomegalovirus in Human Seminal Plasma. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [PubMed]
- Ziemann, M.; Thiele, T. Transfusion-transmitted CMV infection—Current knowledge and future perspectives. Transfus. Med. 2017, 27, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Azenkot, T.; Zaniello, B.; Green, M.L.; Selke, S.; Huang, M.L.; Magaret, A.; Wald, A.; Johnston, C. Cytomegalovirus shedding from breastmilk and mucosal sites in healthy postpartum women: A pilot study. J. Med. Virol. 2018, 91, 894–898. [Google Scholar] [CrossRef] [PubMed]
- Cannon, M.J.; Stowell, J.D.; Clark, R.; Dollard, P.R.; Johnson, D.; Mask, K.; Stover, C.; Wu, K.; Amin, M.; Hendley, W.; et al. Repeated measures study of weekly and daily cytomegalovirus shedding patterns in saliva and urine of healthy cytomegalovirus-seropositive children. BMC Infect. Dis. 2014, 14, 569. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.P. Molecular epidemiology of cytomegalovirus: Viral transmission among children attending a day care center, their parents, and caretakers. J. Pediatr. 1988, 112, 366–372. [Google Scholar] [CrossRef]
- Jackson, J.W.; Sparer, T. There Is Always Another Way! Cytomegalovirus′ Multifaceted Dissemination Schemes. Viruses 2018, 10, 383. [Google Scholar] [CrossRef] [PubMed]
- Herriot, R.; Gray, E.S. Images in clinical medicine. Owl’s-eye cells. N. Engl. J. Med. 1994, 331, 649. [Google Scholar] [CrossRef]
- Sinzger, C.; Grefte, A.; Plachter, B.; Gouw, A.S.; The, T.H.; Jahn, G. Fibroblasts, epithelial cells, endothelial cells and smooth muscle cells are major targets of human cytomegalovirus infection in lung and gastrointestinal tissues. J. Gen. Virol. 1995, 76, 741–750. [Google Scholar] [CrossRef]
- Andrade, Z.R.; Garippo, A.L.; Saldiva, P.H.; Capelozzi, V.L. Immunohistochemical and in situ detection of cytomegalovirus in lung autopsies of children immunocompromised by secondary interstitial pneumonia. Pathol. Res. Pract. 2004, 200, 25–32. [Google Scholar] [CrossRef]
- Restrepo-Gualteros, S.M.; Jaramillo-Barberi, L.E.; Gonzalez-Santos, M.; Rodriguez-Martinez, C.E.; Perez, G.F.; Gutierrez, M.J.; Nino, G. Characterization of cytomegalovirus lung infection in non-HIV infected children. Viruses 2014, 6, 2038–2051. [Google Scholar] [CrossRef]
- Mui, T.S.; Kapp, M.; Einsele, H.; Grigoleit, G.U. T-cell therapy for cytomegalovirus infection. Curr. Opin. Organ Transpl. 2010, 15, 744–750. [Google Scholar] [CrossRef] [PubMed]
- Jordan, M.C. Interstitial pneumonia and subclinical infection after intranasal inoculation of murine cytomegalovirus. Infect. Immun. 1978, 21, 275–280. [Google Scholar] [PubMed]
- Balthesen, M.; Messerle, M.; Reddehase, M.J. Lungs are a major organ site of cytomegalovirus latency and recurrence. J. Virol. 1993, 67, 5360–5366. [Google Scholar] [PubMed]
- Reddehase, M.J.; Balthesen, M.; Rapp, M.; Jonjic, S.; Pavic, I.; Koszinowski, U.H. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 1994, 179, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Steffens, H.P.; Kurz, S.; Holtappels, R.; Reddehase, M.J. Preemptive CD8 T-cell immunotherapy of acute cytomegalovirus infection prevents lethal disease, limits the burden of latent viral genomes, and reduces the risk of virus recurrence. J. Virol. 1998, 72, 1797–1804. [Google Scholar] [PubMed]
- Mattoli, S. Involvement of fibrocytes in asthma and clinical implications. Clin. Exp. Allergy 2015, 45, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Stone, K.C.; Mercer, R.R.; Gehr, P.; Stockstill, B.; Crapo, J.D. Allometric relationships of cell numbers and size in the mammalian lung. Am. J. Respir.Cell Mol. Biol. 1992, 6, 235–243. [Google Scholar] [CrossRef]
- Bohmwald, K.; Espinoza, J.A.; Pulgar, R.A.; Jara, E.L.; Kalergis, A.M. Functional Impairment of Mononuclear Phagocyte System by the Human Respiratory Syncytial Virus. Front. Immunol. 2017, 8, 1643. [Google Scholar] [CrossRef]
- Reddehase, M.J.; Weiland, F.; Munch, K.; Jonjic, S.; Luske, A.; Koszinowski, U.H. Interstitial murine cytomegalovirus pneumonia after irradiation: Characterization of cells that limit viral replication during established infection of the lungs. J. Virol. 1985, 55, 264–273. [Google Scholar]
- Podlech, J.; Holtappels, R.; Pahl-Seibert, M.F.; Steffens, H.P.; Reddehase, M.J. Murine model of interstitial cytomegalovirus pneumonia in syngeneic bone marrow transplantation: Persistence of protective pulmonary CD8-T-cell infiltrates after clearance of acute infection. J. Virol. 2000, 74, 7496–7507. [Google Scholar] [CrossRef]
- Stahl, F.R.; Heller, K.; Halle, S.; Keyser, K.A.; Busche, A.; Marquardt, A.; Wagner, K.; Boelter, J.; Bischoff, Y.; Kremmer, E.; et al. Nodular inflammatory foci are sites of T cell priming and control of murine cytomegalovirus infection in the neonatal lung. PLoS Pathog. 2013, 9, e1003828. [Google Scholar] [CrossRef] [PubMed]
- Stahl, F.R.; Keyser, K.A.; Heller, K.; Bischoff, Y.; Halle, S.; Wagner, K.; Messerle, M.; Forster, R. Mck2-dependent infection of alveolar macrophages promotes replication of MCMV in nodular inflammatory foci of the neonatal lung. Mucosal. Immunol. 2015, 8, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.E.; Lawler, C.; Oliveira, M.T.; Davis-Poynter, N.; Stevenson, P.G. Alveolar Macrophages Are a Prominent but Nonessential Target for Murine Cytomegalovirus Infecting the Lungs. J. Virol. 2015, 90, 2756–2766. [Google Scholar] [CrossRef] [PubMed]
- Brody, A.R.; Craighead, J.E. Pathogenesis of pulmonary cytomegalovirus infection in immunosuppressed mice. J. Infect. Dis. 1974, 129, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Wagner, F.M.; Brizic, I.; Prager, A.; Trsan, T.; Arapovic, M.; Lemmermann, N.A.; Podlech, J.; Reddehase, M.J.; Lemnitzer, F.; Bosse, J.B.; et al. The viral chemokine MCK-2 of murine cytomegalovirus promotes infection as part of a gH/gL/MCK-2 complex. PLoS Pathog. 2013, 9, e1003493. [Google Scholar] [CrossRef] [PubMed]
- Lueder, Y.; Heller, K.; Ritter, C.; Keyser, K.A.; Wagner, K.; Liu, X.; Messerle, M.; Stahl, F.R.; Halle, S.; Forster, R. Control of primary mouse cytomegalovirus infection in lung nodular inflammatory foci by cooperation of interferon-gamma expressing CD4 and CD8 T cells. PLoS Pathog. 2018, 14, e1007252. [Google Scholar] [CrossRef]
- Ziegler, H.; Thale, R.; Lucin, P.; Muranyi, W.; Flohr, T.; Hengel, H.; Farrell, H.; Rawlinson, W.; Koszinowski, U.H. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity 1997, 6, 57–66. [Google Scholar] [CrossRef]
- Kavanagh, D.G.; Koszinowski, U.H.; Hill, A.B. The murine cytomegalovirus immune evasion protein m4/gp34 forms biochemically distinct complexes with class I MHC at the cell surface and in a pre-Golgi compartment. J. Immunol. 2001, 167, 3894–3902. [Google Scholar] [CrossRef]
- Thiel, N.; Keyser, K.A.; Lemmermann, N.A.; Oduro, J.D.; Wagner, K.; Elsner, C.; Halenius, A.; Lenac Rovis, T.; Brinkmann, M.M.; Jonjic, S.; et al. The Mouse Cytomegalovirus Gene m42 Targets Surface Expression of the Protein Tyrosine Phosphatase CD45 in Infected Macrophages. PLoS Pathog. 2016, 12, e1006057. [Google Scholar] [CrossRef]
- Yunis, J.; Farrell, H.E.; Bruce, K.; Lawler, C.; Sidenius, S.; Wyer, O.; Davis-Poynter, N.; Stevenson, P.G. Murine cytomegalovirus degrades MHC class II to colonize the salivary glands. PLoS Pathog. 2018, 14, e1006905. [Google Scholar] [CrossRef]
- Farrell, H.E.; Bruce, K.; Lawler, C.; Oliveira, M.; Cardin, R.; Davis-Poynter, N.; Stevenson, P.G. Murine Cytomegalovirus Spreads by Dendritic Cell Recirculation. MBio 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Coles, M.C.; Veiga-Fernandes, H.; Foster, K.E.; Norton, T.; Pagakis, S.N.; Seddon, B.; Kioussis, D. Role of T and NK cells and IL7/IL7r interactions during neonatal maturation of lymph nodes. Proc. Natl. Acad. Sci. USA 2006, 103, 13457–13462. [Google Scholar] [CrossRef] [PubMed]
- Farrell, H.E.; Lawler, C.; Tan, C.S.; MacDonald, K.; Bruce, K.; Mach, M.; Davis-Poynter, N.; Stevenson, P.G. Murine Cytomegalovirus Exploits Olfaction To Enter New Hosts. MBio 2016, 7, e00251-16. [Google Scholar] [CrossRef] [PubMed]
- Sacher, T.; Podlech, J.; Mohr, C.A.; Jordan, S.; Ruzsics, Z.; Reddehase, M.J.; Koszinowski, U.H. The major virus-producing cell type during murine cytomegalovirus infection, the hepatocyte, is not the source of virus dissemination in the host. Cell Host Microbe 2008, 3, 263–272. [Google Scholar] [CrossRef] [PubMed]
- Teichert, M.; Milde, L.; Holm, A.; Stanicek, L.; Gengenbacher, N.; Savant, S.; Ruckdeschel, T.; Hasanov, Z.; Srivastava, K.; Hu, J.; et al. Pericyte-expressed Tie2 controls angiogenesis and vessel maturation. Nat. Commun. 2017, 8, 16106. [Google Scholar] [CrossRef] [PubMed]
- Holtappels, R.; Podlech, J.; Geginat, G.; Steffens, H.P.; Thomas, D.; Reddehase, M.J. Control of murine cytomegalovirus in the lungs: Relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J. Virol. 1998, 72, 7201–7212. [Google Scholar]
- Krmpotic, A.; Bubic, I.; Polic, B.; Lucin, P.; Jonjic, S. Pathogenesis of murine cytomegalovirus infection. Microbes Infect. 2003, 5, 1263–1277. [Google Scholar] [CrossRef]
- Travis, W.D.; Colby, T.V.; Koss, M.N.; Rosado-de-Christenson, M.L.; Müller, N.L.; King, T.E., Jr. Lung Infections. In Non-Neoplastic Disorders of the Lower Respiratory Tract (AFIP Atlas of Nontumor Pathology Series Vol. 2), 1st ed.; Amer Registry of Pathology Press: Washington, DC, USA, 2002; pp. 639–641. [Google Scholar]
- Khairallah, C.; Netzer, S.; Villacreces, A.; Juzan, M.; Rousseau, B.; Dulanto, S.; Giese, A.; Costet, P.; Praloran, V.; Moreau, J.F.; et al. Gammadelta T cells confer protection against murine cytomegalovirus (MCMV). PLoS Pathog. 2015, 11, e1004702. [Google Scholar] [CrossRef]
- Seckert, C.K.; Grieβl, M.; Büttner, J.K.; Freitag, K.; Lemmermann, N.A.W.; Hummel, M.A.; Liu, X.-F.; Abecassis, M.I.; Angulo, A.; Messerle, M.; et al. Immune Surveillance of Cytomegalovirus Latency and Reactivation in Murine Models: Link to ‘Memory Inflation’. In Cytomegaloviruses: From Molecular Pathogenesis to Intervention; Reddehase, M.J., Ed.; Caister Academic Press: London, UK, 2013; Volume 1. [Google Scholar]
- Wong, M.T.; Ong, D.E.; Lim, F.S.; Teng, K.W.; McGovern, N.; Narayanan, S.; Ho, W.Q.; Cerny, D.; Tan, H.K.; Anicete, R.; et al. A High-Dimensional Atlas of Human T Cell Diversity Reveals Tissue-Specific Trafficking and Cytokine Signatures. Immunity 2016, 45, 442–456. [Google Scholar] [CrossRef]
- Reddehase, M.J.; Mutter, W.; Munch, K.; Buhring, H.J.; Koszinowski, U.H. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J. Virol. 1987, 61, 3102–3108. [Google Scholar]
- Podlech, J.; Holtappels, R.; Wirtz, N.; Steffens, H.P.; Reddehase, M.J. Reconstitution of CD8 T cells is essential for the prevention of multiple-organ cytomegalovirus histopathology after bone marrow transplantation. J. Gen. Virol. 1998, 79, 2099–2104. [Google Scholar] [CrossRef] [PubMed]
- Holtappels, R.; Lemmermann, N.A.; Podlech, J.; Ebert, S.; Reddehase, M.J. Reconstitution of CD8 T Cells Protective against Cytomegalovirus in a Mouse Model of Hematopoietic Cell Transplantation: Dynamics and Inessentiality of Epitope Immunodominance. Front. Immunol. 2016, 7, 232. [Google Scholar] [CrossRef] [PubMed]
- Jonjic, S.; Mutter, W.; Weiland, F.; Reddehase, M.J.; Koszinowski, U.H. Site-restricted persistent cytomegalovirus infection after selective long-term depletion of CD4+ T lymphocytes. J. Exp. Med. 1989, 169, 1199–1212. [Google Scholar] [CrossRef] [PubMed]
- Jonjic, S.; Pavic, I.; Lucin, P.; Rukavina, D.; Koszinowski, U.H. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 1990, 64, 5457–5464. [Google Scholar] [PubMed]
- Walton, S.M.; Wyrsch, P.; Munks, M.W.; Zimmermann, A.; Hengel, H.; Hill, A.B.; Oxenius, A. The dynamics of mouse cytomegalovirus-specific CD4 T cell responses during acute and latent infection. J. Immunol. 2008, 181, 1128–1134. [Google Scholar] [CrossRef] [PubMed]
- Mandaric, S.; Walton, S.M.; Rulicke, T.; Richter, K.; Girard-Madoux, M.J.; Clausen, B.E.; Zurunic, A.; Kamanaka, M.; Flavell, R.A.; Jonjic, S.; et al. IL-10 suppression of NK/DC crosstalk leads to poor priming of MCMV-specific CD4 T cells and prolonged MCMV persistence. PLoS Pathog. 2012, 8, e1002846. [Google Scholar] [CrossRef] [PubMed]
- Jeitziner, S.M.; Walton, S.M.; Torti, N.; Oxenius, A. Adoptive transfer of cytomegalovirus-specific effector CD4+ T cells provides antiviral protection from murine CMV infection. Eur. J. Immunol. 2013, 43, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, J.F.; Woda, B.A.; Welsh, R.M. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol. 1984, 52, 119–128. [Google Scholar] [PubMed]
- Sumaria, N.; van Dommelen, S.L.; Andoniou, C.E.; Smyth, M.J.; Scalzo, A.A.; Degli-Esposti, M.A. The roles of interferon-gamma and perforin in antiviral immunity in mice that differ in genetically determined NK-cell-mediated antiviral activity. Immunol. Cell Biol. 2009, 87, 559–566. [Google Scholar] [CrossRef]
- Sell, S.; Dietz, M.; Schneider, A.; Holtappels, R.; Mach, M.; Winkler, T.H. Control of murine cytomegalovirus infection by gammadelta T cells. PLoS Pathog. 2015, 11, e1004481. [Google Scholar] [CrossRef]
- Stacey, M.A.; Marsden, M.; Pham, N.T.; Clare, S.; Dolton, G.; Stack, G.; Jones, E.; Klenerman, P.; Gallimore, A.M.; Taylor, P.R.; et al. Neutrophils recruited by IL-22 in peripheral tissues function as TRAIL-dependent antiviral effectors against MCMV. Cell Host Microbe 2014, 15, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Klenovsek, K.; Weisel, F.; Schneider, A.; Appelt, U.; Jonjic, S.; Messerle, M.; Bradel-Tretheway, B.; Winkler, T.H.; Mach, M. Protection from CMV infection in immunodeficient hosts by adoptive transfer of memory B cells. Blood 2007, 110, 3472–3479. [Google Scholar] [CrossRef] [PubMed]
- Ebert, S.; Becker, M.; Lemmermann, N.A.; Buttner, J.K.; Michel, A.; Taube, C.; Podlech, J.; Bohm, V.; Freitag, K.; Thomas, D.; et al. Mast cells expedite control of pulmonary murine cytomegalovirus infection by enhancing the recruitment of protective CD8 T cells to the lungs. PLoS Pathog. 2014, 10, e1004100. [Google Scholar] [CrossRef] [PubMed]
- Shanley, J.D.; Pesanti, E.L.; Nugent, K.M. The pathogenesis of pneumonitis due to murine cytomegalovirus. J. Infect. Dis. 1982, 146, 388–396. [Google Scholar] [CrossRef] [PubMed]
- Oduro, J.D.; Redeker, A.; Lemmermann, N.A.; Ebermann, L.; Marandu, T.F.; Dekhtiarenko, I.; Holzki, J.K.; Busch, D.H.; Arens, R.; Cicin-Sain, L. Murine cytomegalovirus (CMV) infection via the intranasal route offers a robust model of immunity upon mucosal CMV infection. J. Gen. Virol. 2016, 97, 185–195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Caldeira-Dantas, S.; Smith, C.J.; Snyder, C.M. Persistent viral replication and the development of T-cell responses after intranasal infection by MCMV. Med. Microbiol. Immunol. 2019, 208, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Shanley, J.D.; Thrall, R.S.; Forman, S.J. Murine cytomegalovirus replication in the lungs of athymic BALB/c nude mice. J. Infect. Dis. 1997, 175, 309–315. [Google Scholar] [CrossRef][Green Version]
- Reusch, U.; Muranyi, W.; Lucin, P.; Burgert, H.G.; Hengel, H.; Koszinowski, U.H. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 1999, 18, 1081–1091. [Google Scholar] [CrossRef]
- Halle, S.; Keyser, K.A.; Stahl, F.R.; Busche, A.; Marquardt, A.; Zheng, X.; Galla, M.; Heissmeyer, V.; Heller, K.; Boelter, J.; et al. In Vivo Killing Capacity of Cytotoxic T Cells Is Limited and Involves Dynamic Interactions and T Cell Cooperativity. Immunity 2016, 44, 233–245. [Google Scholar] [CrossRef]
- Lisnic, B.; Lisnic, V.J.; Jonjic, S. NK cell interplay with cytomegaloviruses. Curr. Opin. Virol. 2015, 15, 9–18. [Google Scholar] [CrossRef]
- Del Rio, M.L.; Bernhardt, G.; Rodriguez-Barbosa, J.I.; Forster, R. Development and functional specialization of CD103+ dendritic cells. Immunol. Rev. 2010, 234, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Lemmermann, N.A.W.; Maxeiner, J.; Podlech, J.; Beckert, H.; Freitag, K.; Teschner, D.; Ries, F.; Taube, C.; Buhl, R.; et al. Coincident airway exposure to low-potency allergen and cytomegalovirus sensitizes for allergic airway disease by viral activation of migratory dendritic cells. PLoS Pathog. 2019, 15, e1007595. [Google Scholar] [CrossRef] [PubMed]
- Prendergast, A.J.; Klenerman, P.; Goulder, P.J. The impact of differential antiviral immunity in children and adults. Nat. Rev. Immunol. 2012, 12, 636–648. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, N.A.; Papadimitriou, J.M.; Shellam, G.R. Cytomegalovirus-induced pneumonitis and myocarditis in newborn mice. A model for perinatal human cytomegalovirus infection. Arch. Virol. 1990, 115, 75–88. [Google Scholar] [CrossRef] [PubMed]
| Cell Type | Cell Present in NIF | Function | References |
|---|---|---|---|
| CD8 T cell | yes | IFN-ɣ | [40,41,57,63,64,65] |
| CD4 T cell | yes | IFN-ɣ, TNF-α | [41,47,64,66,67,68,69,70] |
| NK cell | yes | Perforin, IFN-ɣ | [47,69,71,72] |
| ɣδ T cell | ? | IFN-ɣ? | [60,73] |
| Mɸ | yes | Phagocytosis | [42,47] |
| DC | yes | Antigen presentation | [42] |
| Neutrophil | ? | TRAIL | [74] |
| B cell / plasma cell | yes / ? | Antibodies | [75] |
| Mast cell | ? | Indirect via CD8 T cell | [76] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca Brito, L.; Brune, W.; Stahl, F.R. Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity. Int. J. Mol. Sci. 2019, 20, 3865. https://doi.org/10.3390/ijms20163865
Fonseca Brito L, Brune W, Stahl FR. Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity. International Journal of Molecular Sciences. 2019; 20(16):3865. https://doi.org/10.3390/ijms20163865
Chicago/Turabian StyleFonseca Brito, Luís, Wolfram Brune, and Felix R. Stahl. 2019. "Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity" International Journal of Molecular Sciences 20, no. 16: 3865. https://doi.org/10.3390/ijms20163865
APA StyleFonseca Brito, L., Brune, W., & Stahl, F. R. (2019). Cytomegalovirus (CMV) Pneumonitis: Cell Tropism, Inflammation, and Immunity. International Journal of Molecular Sciences, 20(16), 3865. https://doi.org/10.3390/ijms20163865






