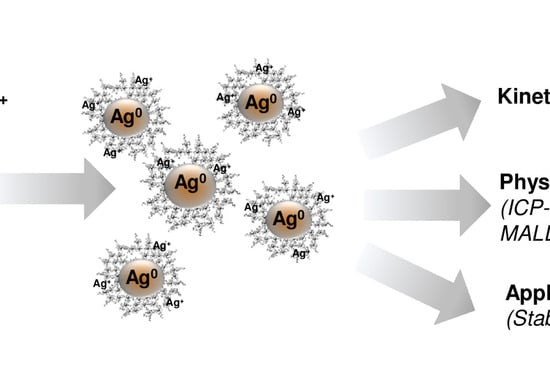
A New Approach for Spontaneous Silver Ions Immobilization onto Casein
Abstract
1. Introduction
2. Results and Discussion
2.1. Kinetics and Isotherm Study
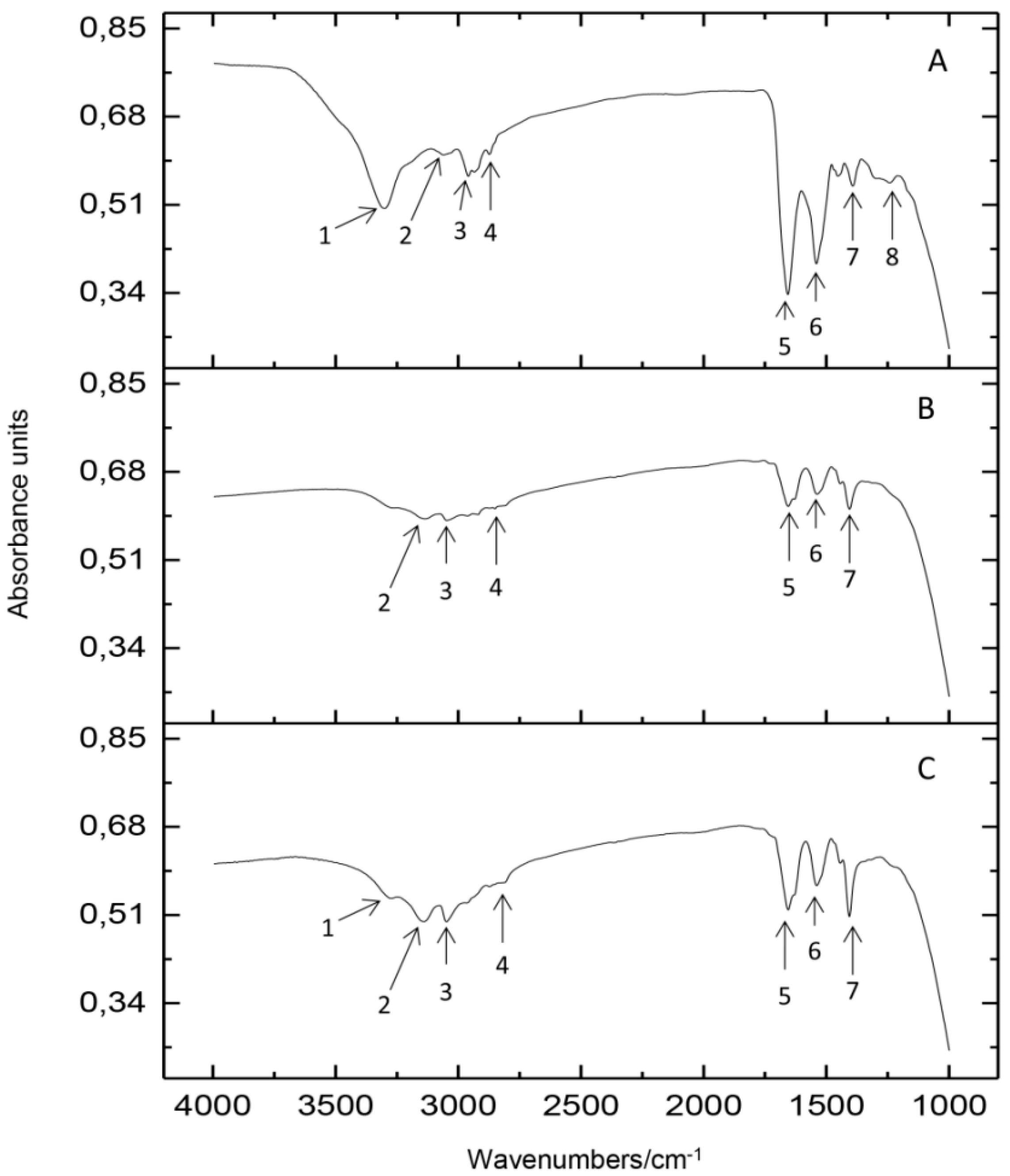
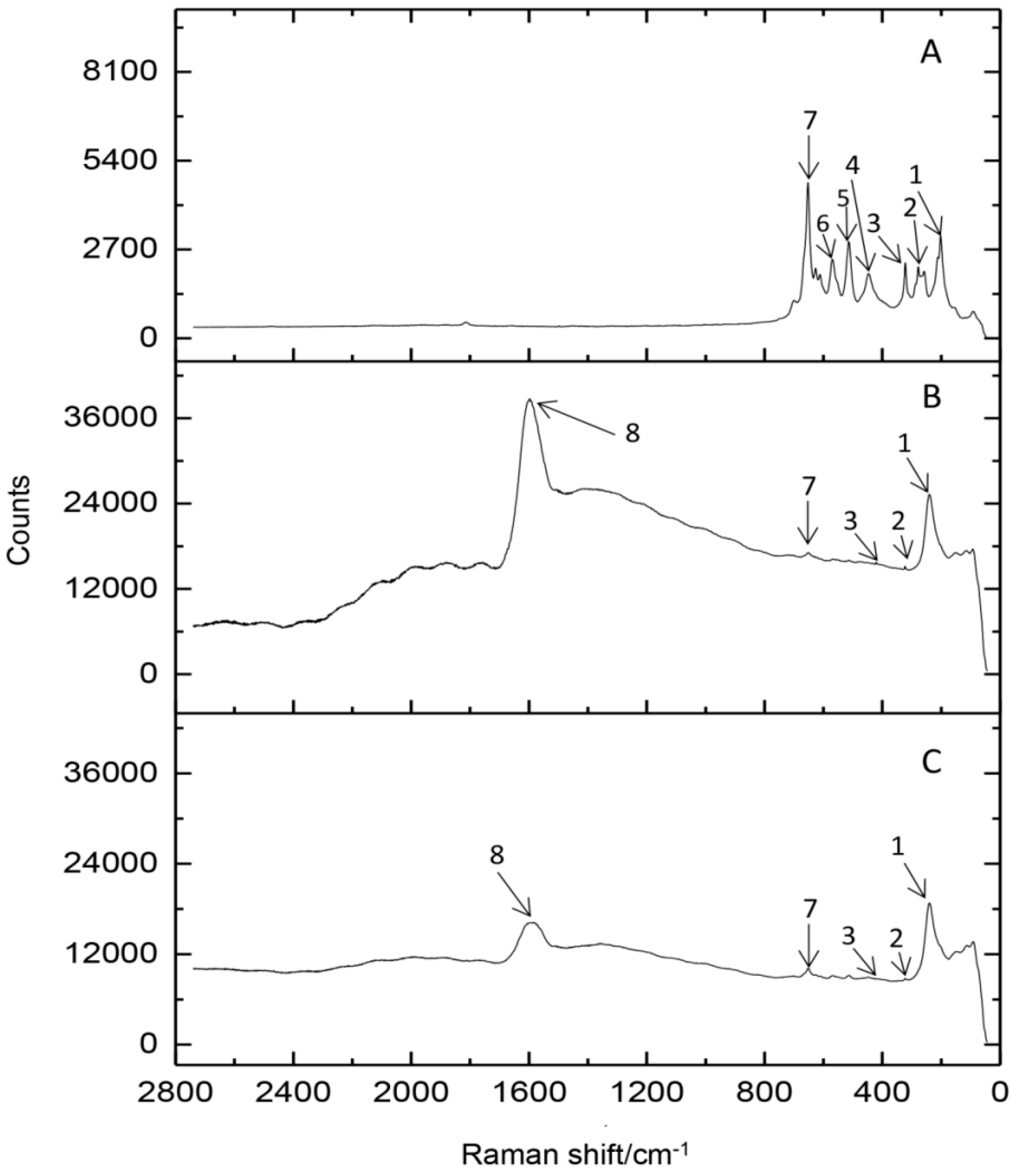
2.2. Spectroscopic Study of the Silver Binding Process
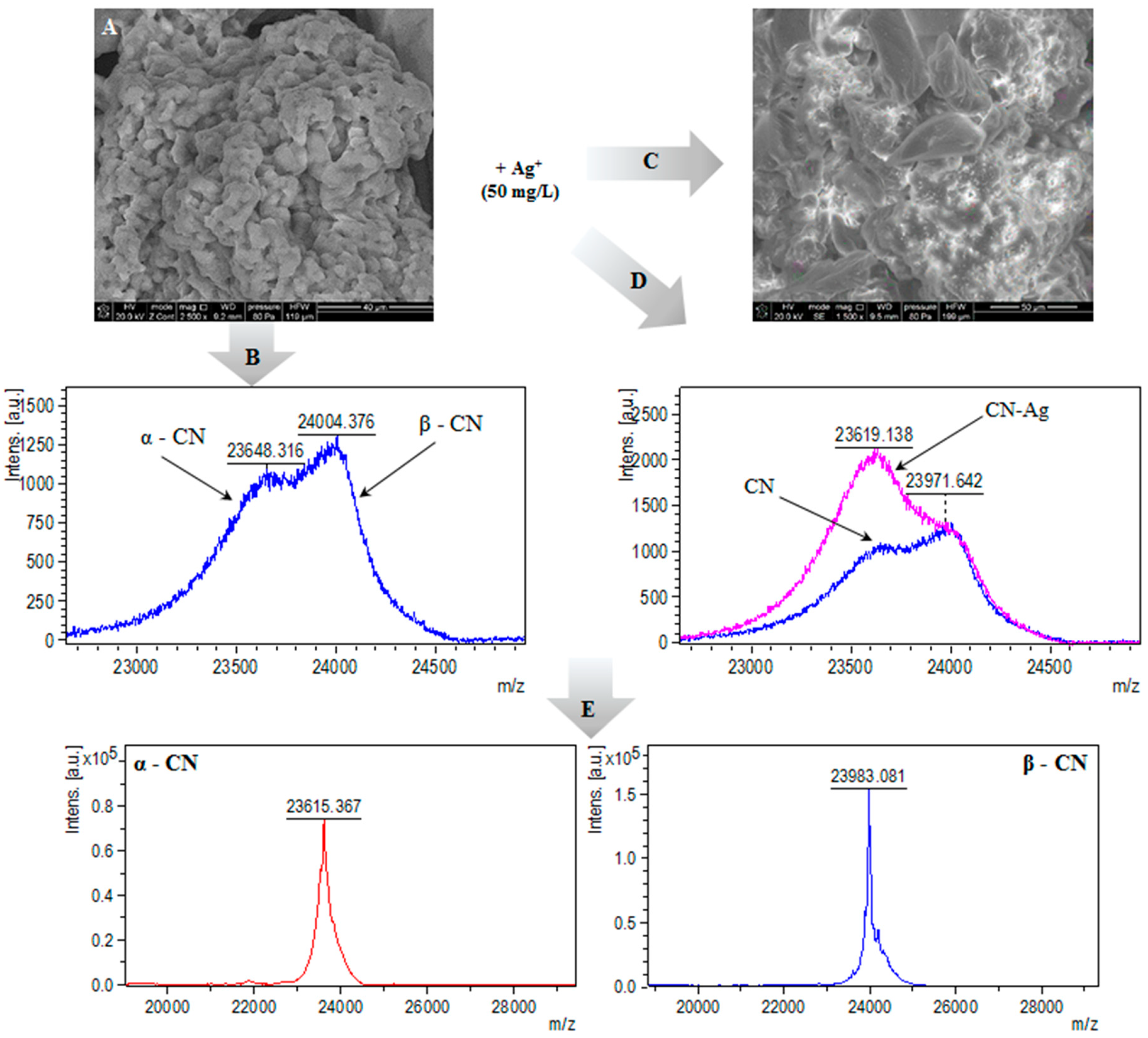
2.3. MALDI-TOF-MS Analysis
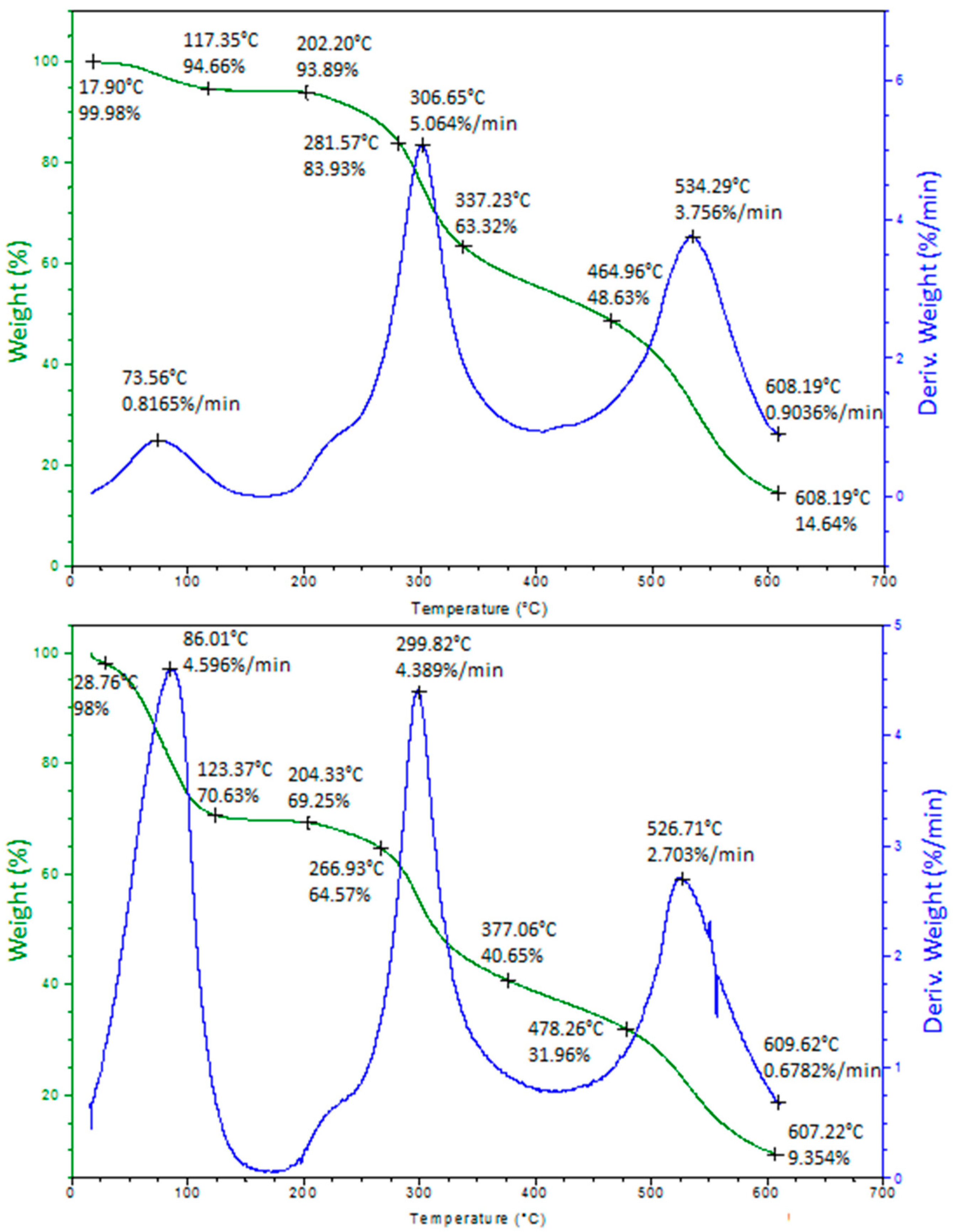
2.4. Thermogravimetric Analysis
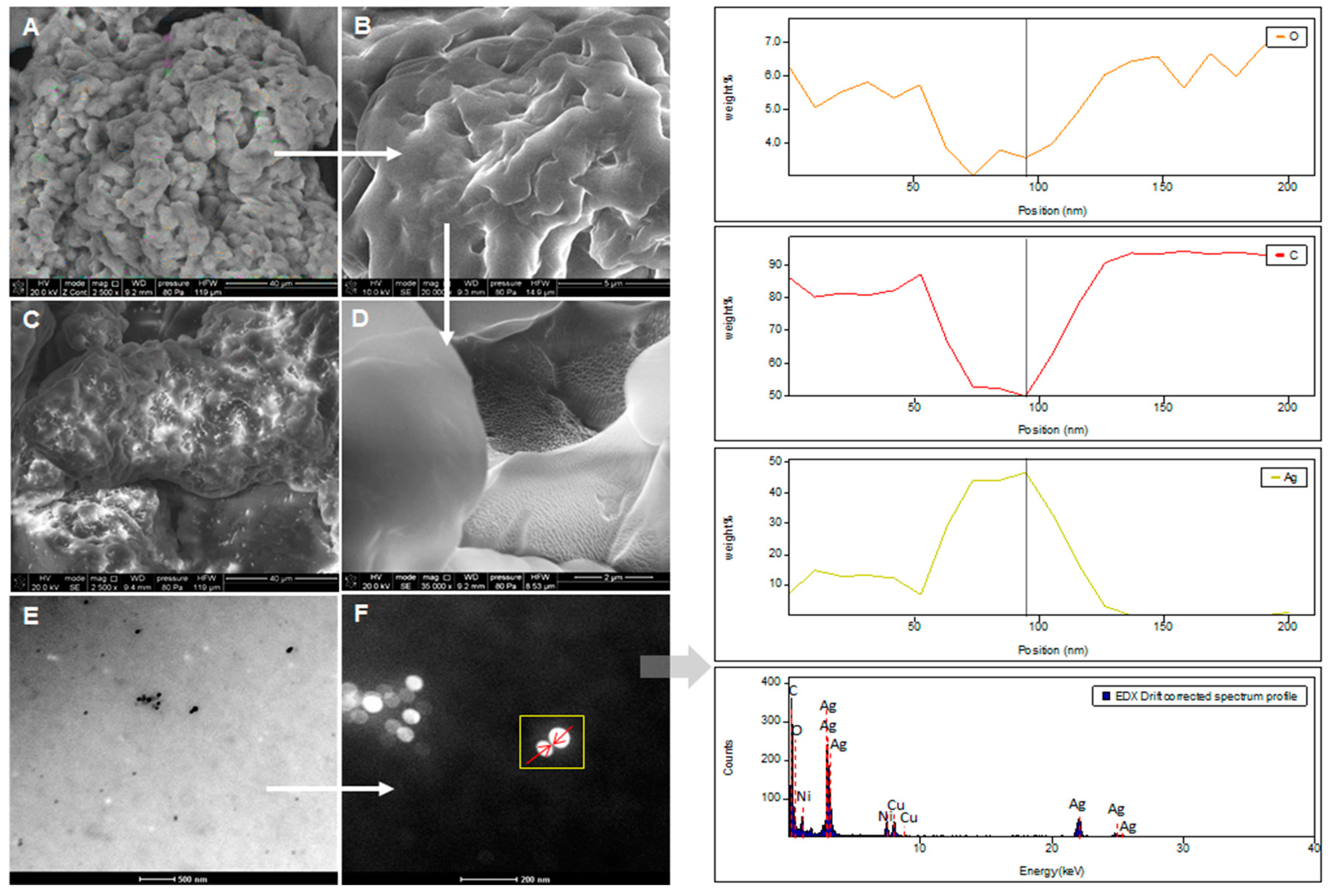
2.5. SEM and TEM/EDX
2.6. Stability of Casein-silver Complex in Synthetic Physiological Fluids
3. Materials and Methods
3.1. Kinetic Study of Silver Binding to Casein
3.1.1. Modeling of Sorption Kinetic Data
3.1.2. Determination of Thermodynamic Parameters
3.2. Isotherm Experiments
Modeling of Sorption Isotherm Data
3.3. Physicochemical Characterization of Silver-Casein Nanocomplexes
3.3.1. Fourier Transform Infrared Spectroscopy
3.3.2. MALDI-TOF MS Analysis
3.3.3. Thermogravimetric Analysis
3.3.4. Raman Spectroscopy
3.3.5. Electron Microscopy (SEM, TEM) and Energy Dispersive X-Ray (EDX) Analysis
3.4. Application of Silver-Casein Complexes
Stability in Synthetics Physiological Fluids
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| Ag-CN | Silver-casein |
| DTG | Derivative Thermogravimetry |
| EDS | Energy Dispersive Spectroscopy |
| EDX | Energy Dispersive X-ray detector |
| FT-IR | Fourier Transform Infrared Spectroscopy |
| HPLC | High Performance Liquid Chromatography |
| HSAB | Hard Soft Acid Base principle |
| ICP-MS | Inductevely Coupled Plasma Mass Spectrometry |
| MALDI-TOF MS | Matrix-Assisted Laser Desorption/Ionization Time Of Flight Mass Spectrometry |
| SEM | Scanning Electron Microscope |
| SGF | Synthetic Gastric Fluid |
| SIF | Synthetic Intestinal Fluid |
| TEM | Transmission Electron Microscopy |
| TGA | Thermogravimetric analysis |
References
- Mills, S.; Ross, R.P.; Hill, C.; Fitzgerald, G.F.; Stanton, C. Milk intelligence: Mining milk for bioactive substances associated with human health. Int. Dairy J. 2011, 21, 377–401. [Google Scholar] [CrossRef]
- Kumaresan, A.; Selma, C.; Reshma, N.; Jacinth, N.A. Quantitative analysis of casein precipitation from the various milk samples. J. Chem. Pharm. Res. 2017, 9, 113–115. [Google Scholar]
- Badem, A.; Uçar, G. Production of caseins and their usages. Int. J. Food Sci. Nutr. 2017, 2, 4–9. [Google Scholar]
- Müller-Buschbaum, P.; Gebhardt, R.; Roth, S.V.; Metwalli, E.; Doster, W. Effect of calcium concentration on the structure of casein micelles in thin films. Biophys. J. 2007, 93, 960–968. [Google Scholar] [CrossRef] [PubMed]
- Glantz, M.; Devold, T.G.; Vegarud, G.E.; Lindmark Månsson, H.; Stålhammar, H.; Paulsson, M. Importance of casein micelle size and milk composition for milk gelation. J. Dairy Sci. 2010, 93, 1444–1451. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.S. Casein micelle structure: Models and muddles. Curr. Opin. Colloid Interface Sci. 2006, 11, 148–153. [Google Scholar] [CrossRef]
- de Kruif, C.G.; Huppertz, T.; Urban, V.S.; Petukhov, A.V. Casein micelles and their internal structure. Adv. Colloid Interface Sci. 2012, 171–172, 36–52. [Google Scholar] [CrossRef] [PubMed]
- Horne, D.S. Casein Interactions: Casting light on the black boxes, the structure in dairy products. Int. Dairy J. 1998, 8, 171–177. [Google Scholar] [CrossRef]
- Lucey, J.A.; Horne, D.S. Perspectives on casein interactions. Int. Dairy J. 2018, 85, 56–65. [Google Scholar] [CrossRef]
- Sarkar, B. Metal protein interactions. Prog. Food Nutr. Sci. 1987, 11, 363–400. [Google Scholar]
- Hall Sedlak, R.; Hnilova, M.; Grosh, C.; Fong, H.; Baneyx, F.; Schwartz, D.; Sarikaya, M.; Tamerler, C.; Traxler, B. Engineered escherichia coli silver-binding periplasmic protein that promotes silver tolerance. Appl. Environ. Microbiol. 2012, 78, 2289–2296. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Michael Siu, K.W.; Guevremont, R.; Yves Le Blanc, J.C. Complexes of silver(I) with peptides and proteins as produced in electrospray mass spectrometry. J. Am. Soc. Mass Spectrom. 1997, 8, 781–792. [Google Scholar] [CrossRef]
- Pomastowski, P.; Sprynsky, M.; Žuvela, P.; Rafińska, K.; Milanowski, M.; Liu, J.J.; Yi, M.; Buszewski, B. Silver-Lactoferrin nanocomplexes as a potent antimicrobial agent. J. Am. Chem. Soc. 2016, 138, 7899–7909. [Google Scholar] [CrossRef] [PubMed]
- Railean-Plugaru, V.; Pomastowski, P.; Meller, K.; Złoch, M.; Rafinska, K.; Buszewski, B. Lactococcus lactis as a safe and inexpensive source of bioactive silver composites. Appl. Microbiol. Biotechnol. 2017, 101, 7141–7153. [Google Scholar]
- Buszewski, B.; Railean-Plugaru, V.; Pomastowski, P.; Rafinska, K.; Szultka-Mlynska, M.; Kowalkowski, T. Antimicrobial effectiveness of bioactive silver nanoparticles synthesized by actinomycetes HGG16n strain. Curr. Pharm. Biotechnol. 2017, 18, 168–176. [Google Scholar] [CrossRef]
- Singh, R.; Shedbalkar, U.U.; Wadhwani, S.A.; Chopade, B.A. Bacteriagenic silver nanoparticles: Synthesis, mechanism, and applications. Appl. Microbiol. Biotechnol. 2015, 99, 4579–4593. [Google Scholar] [CrossRef]
- Bahrudin, N.N.; Nawi, M.A. Lelifajri Kinetics and isotherm modeling of phenol adsorption by immobilizable activated carbon. React. Kinet. Mech. Catal. 2019, 126, 61–82. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Kowalkowski, T.; Tutu, H.; Cukrowska, E.M.; Buszewski, B. Ionic liquid modified diatomite as a new effective adsorbent for uranium ions removal from aqueous solution. Colloids Surfaces A Physicochem. Eng. Asp. 2015, 465, 159–167. [Google Scholar] [CrossRef]
- Pabón, M.L.; Lönnerdal, B. Bioavailability of zinc and its binding to casein in milks and formulas. J. Trace Elem. Med. Biol. 2000, 14, 146–153. [Google Scholar] [CrossRef]
- Pomastowski, P.; Sprynskyy, M.; Buszewski, B. The study of zinc ions binding to casein. Colloids Surf. B Biointerfaces 2014, 120, 21–27. [Google Scholar] [CrossRef]
- Kalsi, P.S. Spectroscopy of Organic Compounds; New Age International: New Delhi, India, 2006; ISBN 8122415431. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta 2007, 1767, 1073–1101. [Google Scholar] [CrossRef] [PubMed]
- Huppertz, T.; Fox, P.F.; Kelly, A.L. The caseins: Structure, stability, and functionality. Proteins Food Process. 2018, 49–92. [Google Scholar]
- Dumas, B.R.; Brignon, G.; Grosclaude, F.; Mercier, J.-C. Structure primaire de la caseine beta bovine. Sequence complete. Eur. J. Biochem. 1972, 25, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Zhu, X.; Fan, Q.; Wan, X. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta Part. A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195. [Google Scholar] [CrossRef] [PubMed]
- Li-Chan, E.C.Y.; Li-Chan, E.C.Y. Vibrational spectroscopy applied to the study of milk proteins. Editions 2007, 87, 443–458. [Google Scholar] [CrossRef]
- Li-Chan, E.; Nakai, S.; Hirotsuka, M. Raman spectroscopy as a probe of protein structure in food systems. In Protein Structure-Function Relationships in Foods; Springer US: Boston, MA, USA, 1994; pp. 163–197. [Google Scholar]
- Pomastowski, P.; Walczak, J.; Gawin, M.; Bocian, S.; Piekoszewski, W.; Buszewski, B. HPLC separation of casein components on a diol-bonded silica column with MALDI TOF/TOF MS identification. Anal. Methods 2014, 6, 5236–5244. [Google Scholar] [CrossRef]
- Meisel, H. Overview on Milk Protein-derived Peptides. Int. Dairy J. 1998, 8, 363–373. [Google Scholar] [CrossRef]
- Lien, Y.C.; Nawar, W.W. Thermal decomposition of some amino acids. valine, leucine and lsoleucine. J. Food Sci. 1974, 39, 911–913. [Google Scholar] [CrossRef]
- Weiss, I.M.; Muth, C.; Drumm, R.; Kirchner, H.O.K. Thermal decomposition of the amino acids glycine, cysteine, aspartic acid, asparagine, glutamic acid, glutamine, arginine and histidine. BMC Biophys. 2018, 11, 2–17. [Google Scholar] [CrossRef]
- Haitinger, L. Vorlaufige mittheilung uber glutaminsaure und pyrrol. Monatshefte fur Chemie 1882, 3, 228–229. [Google Scholar] [CrossRef]
- Dunn, M.S.; Brophy, T.W. Decomposition points of the amino acids. J. Biol. Chem. 1932, 99, 221–229. [Google Scholar]
- Purevsuren, B.; Davaajav, Y. Thermal analysis of casein. J. Therm. Anal. Calorim. 2001, 65, 147–152. [Google Scholar] [CrossRef]
- Moldoveanu, C.; Odochian, L.; Paius, C.M.; Lorela, I.; Baiceanu, A. Study on the thermal behavior of casein in air. ACTA Chem. IASI 2013, 21, 31–46. [Google Scholar] [CrossRef]
- Elavazhagan, T.; Elavazhagan, T. Memecylon edule leaf extract mediated green synthesis of silver and gold nanoparticles. Int. J. Nanomedicine 2011, 6, 1265–1278. [Google Scholar] [CrossRef] [PubMed]
- Buszewski, B.; Railean-Plugaru, V.; Pomastowski, P.; Rafińska, K.; Szultka-Mlynska, M.; Golinska, P.; Wypij, M.; Laskowski, D.; Dahm, H. Antimicrobial activity of biosilver nanoparticles produced by a novel Streptacidiphilus durhamensis strain. J. Microbiol. Immunol. Infect. 2018, 51, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Hadrup, N.; Lam, H.R. Oral toxicity of silver ions, silver nanoparticles and colloidal silver—A review. Regul. Toxicol. Pharmacol. 2014, 68, 1–7. [Google Scholar] [CrossRef]
- Greulich, C.; Braun, D.; Peetsch, A.; Diendorf, J.; Siebers, B.; Epple, M.; Köller, M. The toxic effect of silver ions and silver nanoparticles towards bacteria and human cells occurs in the same concentration range. RSC Adv. 2012, 2, 6981–6987. [Google Scholar] [CrossRef]
- Liu, Y.; Guo, R. pH-dependent structures and properties of casein micelles. Biophys. Chem. 2008, 136, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Yuan, M.; Guo, R. Preparation and characterization of casein-stabilized gold nanoparticles for catalytic applications. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 18–25. [Google Scholar] [CrossRef]
- Pindáková, L.; Kašpárková, V.; Kejlová, K.; Dvořáková, M.; Krsek, D.; Jírová, D.; Kašparová, L. Behaviour of silver nanoparticles in simulated saliva and gastrointestinal fluids. Int. J. Pharm. 2017, 527, 12–20. [Google Scholar] [CrossRef]
- Rabbani, G.; Ahmad, E.; Zaidi, N.; Fatima, S.; Khan, R.H. pH-induced molten globule state of rhizopus niveus lipase is more resistant against thermal and chemical denaturation than its native state. Cell Biochem. Biophys. 2012, 62, 487–499. [Google Scholar] [CrossRef] [PubMed]
- Reed, B.E.; Matsumoto, M.R. Modeling cadmium adsorption by activated carbon using the langmuir and freundlich isotherm expressions. Sep. Sci. Technol. 1993, 28, 2179–2195. [Google Scholar] [CrossRef]
- The International Pharmacopoeia. Vol. 5, Tests and General Requirements for Dosage Forms: Quality Specifications for Pharmaceutical Substances and Dosages, 3rd ed.; World Health Organization: Geneva, Switzerland, 2003; ISBN 9241545364.


| Zero Order Kinetics model | Pseudofirst-Order Kineticsmodel | Intra-Particle Diffusion Model | |||
|---|---|---|---|---|---|
| In light | |||||
| First step k0 [mg L−1 min−1] | 7.842 | qe [mg g−1] k1 [min−1] Aapprox.[%] | 17.009 0.166 13.286 | A [mg g−1] Kip [mg g−1min−0.5] | 7.612 2.479 |
| Second step k0 [mg L−1 min−1] | 1.000 | ||||
| In dark | |||||
| First step k0 [mg L−1 min−1] | 7.040 | qe [mg g−1] k1 [min−1] Aapprox.[%] | 14.880 0.117 26.581 | A [mg g−1] Kip [mg g−1min−0.5] | 8.692 1.538 |
| Second step k0 [mg L−1 min−1] | 0.692 | ||||
| qe [mg/g] | Ce [mg/L] | Kd | T [K] | ΔG0 [kJmol−1] |
|---|---|---|---|---|
| In light | ||||
| 17.01 | 9.31 | 1830.00 | 295 | −18.42 |
| In dark | ||||
| 14.88 | 14.63 | 1016.86 | 295 | −16.98 |
| Freundlich Isotherm | Langmuir Isotherm | ||||||
|---|---|---|---|---|---|---|---|
| KF [L/g] | N | S | R2 | KL [L/mg] | qm [mg/g] | S | R2 |
| 8.62 | 0.37 | 18.57 | 0.96 | 0.02 | 77.54 | 21.50 | 0.97 |
| Type of Synthetic Fluid | [C] of Silver, ppb | ||
|---|---|---|---|
| 1 | 2 | 3 | |
| SGF | 1.526 | 0.781 | 1.358 |
| SGF with pepsin | 0.081 | 0.706 | 0.483 |
| SIF | 1.065 | 0.454 | 0.354 |
| SIF with pancreatine | 2.217 | 0.184 | 0.155 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pryshchepa, O.; Sagandykova, G.N.; Pomastowski, P.; Railean-Plugaru, V.; Król, A.; Rogowska, A.; Rodzik, A.; Sprynskyy, M.; Buszewski, B. A New Approach for Spontaneous Silver Ions Immobilization onto Casein. Int. J. Mol. Sci. 2019, 20, 3864. https://doi.org/10.3390/ijms20163864
Pryshchepa O, Sagandykova GN, Pomastowski P, Railean-Plugaru V, Król A, Rogowska A, Rodzik A, Sprynskyy M, Buszewski B. A New Approach for Spontaneous Silver Ions Immobilization onto Casein. International Journal of Molecular Sciences. 2019; 20(16):3864. https://doi.org/10.3390/ijms20163864
Chicago/Turabian StylePryshchepa, Oleksandra, Gulyaim N. Sagandykova, Paweł Pomastowski, Viorica Railean-Plugaru, Anna Król, Agnieszka Rogowska, Agnieszka Rodzik, Myroslav Sprynskyy, and Bogusław Buszewski. 2019. "A New Approach for Spontaneous Silver Ions Immobilization onto Casein" International Journal of Molecular Sciences 20, no. 16: 3864. https://doi.org/10.3390/ijms20163864
APA StylePryshchepa, O., Sagandykova, G. N., Pomastowski, P., Railean-Plugaru, V., Król, A., Rogowska, A., Rodzik, A., Sprynskyy, M., & Buszewski, B. (2019). A New Approach for Spontaneous Silver Ions Immobilization onto Casein. International Journal of Molecular Sciences, 20(16), 3864. https://doi.org/10.3390/ijms20163864