Revisiting the Role of LXRs in PUFA Metabolism and Phospholipid Homeostasis
Abstract
:1. Introduction
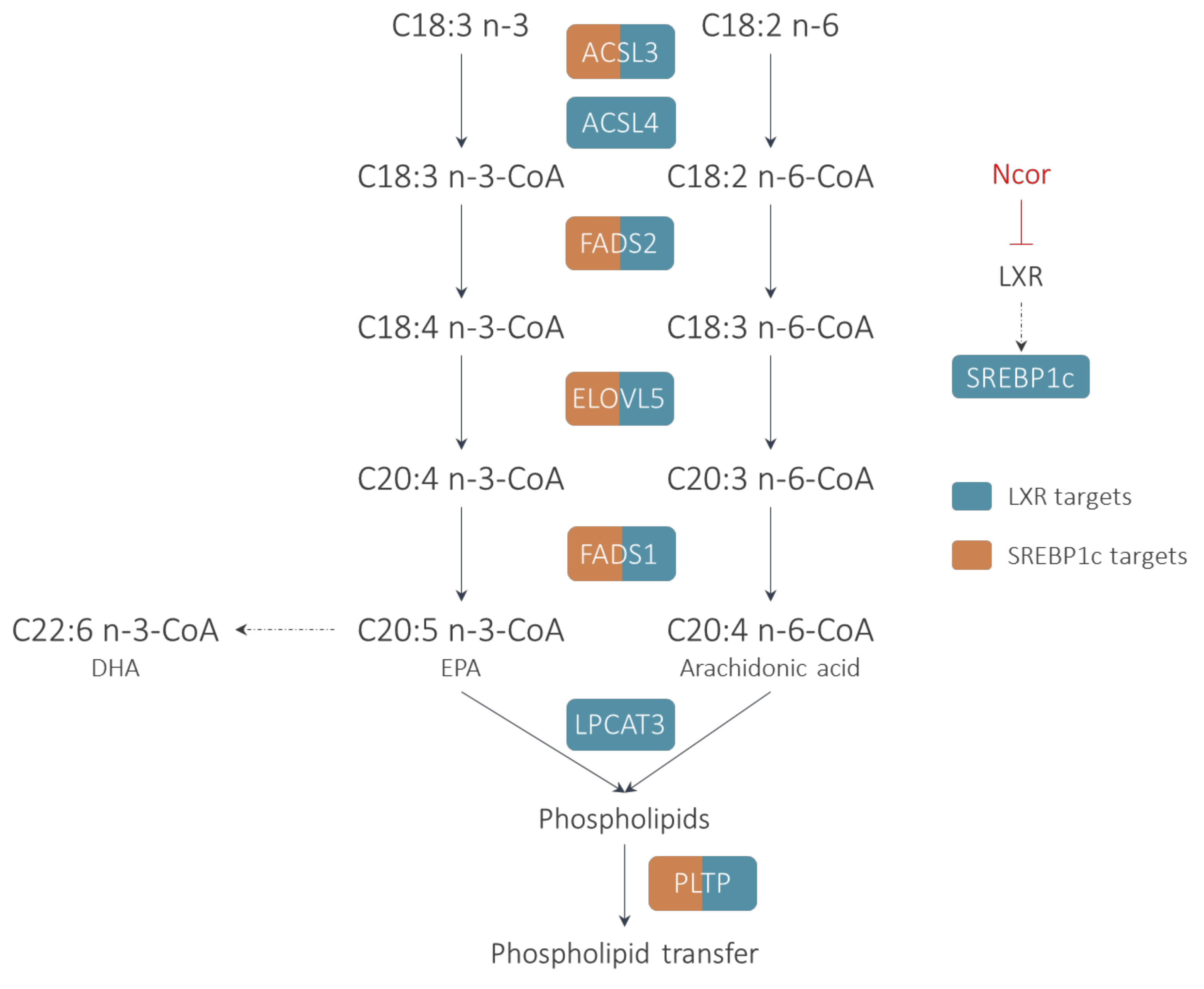
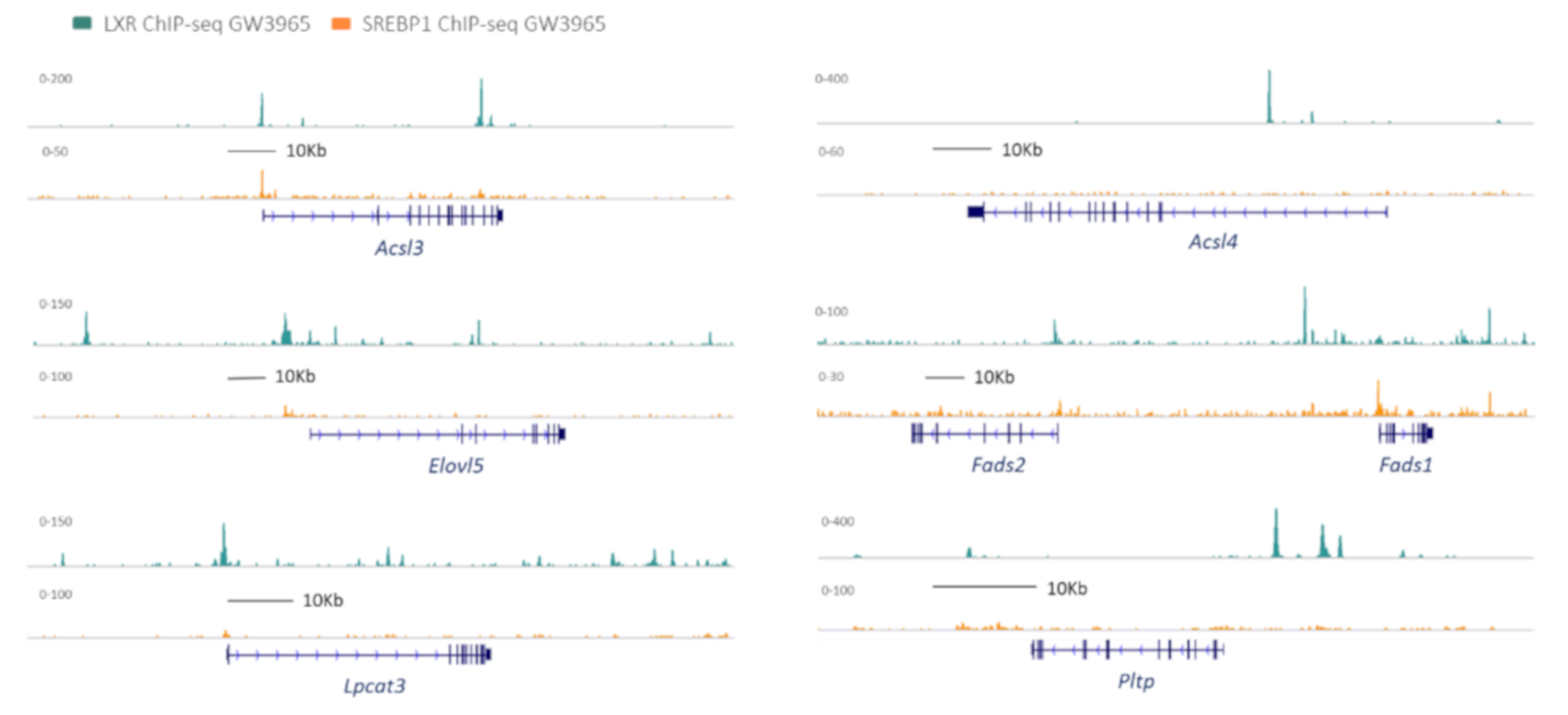
2. LXR and PUFA Synthesis
2.1. Fatty Acid Activation
2.2. Elongation
2.3. Desaturation
3. LXRs in Phospholipid Transfer and Remodeling
3.1. LPCAT3
3.2. PLTP
4. Control of Phospholipid Metabolism by LXRs in the Liver: Impact on Lipogenesis and VLDL Secretion
5. LXRs, PUFA Metabolism and Inflammation
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| FA | Fatty acid |
| AA | Arachidonic Acid |
| EPA | Eicosapentaenoic Acid |
| DHA | Docosahexaenoic Acid |
| LXR | Liver X receptor |
| PUFA | Polyunsaturated fatty acid |
| PL | Phospholipid |
| ER | Endoplasmic reticulum |
References
- Schultz, J.R.; Tu, H.; Luk, A.; Repa, J.J.; Medina, J.C.; Li, L.; Schwendner, S.; Wang, S.; Thoolen, M.; Mangelsdorf, D.J.; et al. Role of LXRs in control of lipogenesis. Genes Dev. 2000, 14, 2831–2838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Repa, J.J.; Liang, G.; Ou, J.; Bashmakov, Y.; Lobaccaro, J.M.; Shimomura, I.; Shan, B.; Brown, M.S.; Goldstein, J.L.; Mangelsdorf, D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev. 2000, 14, 2819–2830. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Tontonoz, P. Liver X receptors in lipid signalling and membrane homeostasis. Nat. Rev. Endocrinol. 2018, 14, 452–463. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hao, M.; Luo, Y.; Liang, C.; Silver, D.L.; Cheng, C.; Maxfield, F.R.; Tall, A.R. Stearoyl-CoA desaturase inhibits ATP-binding cassette transporter A1-mediated cholesterol efflux and modulates membrane domain structure. J. Biol. Chem. 2003, 278, 5813–5820. [Google Scholar] [CrossRef] [PubMed]
- Joseph, S.B.; Laffitte, B.A.; Patel, P.H.; Watson, M.A.; Matsukuma, K.E.; Walczak, R.; Collins, J.L.; Osborne, T.F.; Tontonoz, P. Direct and indirect mechanisms for regulation of fatty acid synthase gene expression by liver X receptors. J. Biol. Chem. 2002, 277, 11019–11025. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Yang, J.; Horton, J.D.; Hammer, R.E.; Goldstein, J.L.; Brown, M.S. Diminished hepatic response to fasting/refeeding and liver X receptor agonists in mice with selective deficiency of sterol regulatory element-binding protein-1c. J. Biol. Chem. 2002, 277, 9520–9528. [Google Scholar] [CrossRef] [PubMed]
- Cha, J.-Y.; Repa, J.J. The liver X receptor (LXR) and hepatic lipogenesis. The carbohydrate-response element-binding protein is a target gene of LXR. J. Biol. Chem. 2007, 282, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.B.; Moon, H.M.; Kim, W.S.; Lee, Y.S.; Jeong, H.W.; Yoo, E.J.; Ham, J.; Kang, H.; Park, M.-G.; Steffensen, K.R.; et al. Activated liver X receptors stimulate adipocyte differentiation through induction of peroxisome proliferator-activated receptor gamma expression. Mol. Cell. Biol. 2004, 24, 3430–3444. [Google Scholar] [CrossRef]
- Chisholm, J.W.; Hong, J.; Mills, S.A.; Lawn, R.M. The LXR ligand T0901317 induces severe lipogenesis in the db/db diabetic mouse. J. Lipid Res. 2003, 44, 2039–2048. [Google Scholar] [CrossRef] [Green Version]
- Masson, D.; Staels, B.; Gautier, T.; Desrumaux, C.; Athias, A.; Le Guern, N.; Schneider, M.; Zak, Z.; Dumont, L.; Deckert, V.; et al. Cholesteryl ester transfer protein modulates the effect of liver X receptor agonists on cholesterol transport and excretion in the mouse. J. Lipid Res. 2004, 45, 543–550. [Google Scholar] [CrossRef] [Green Version]
- Grefhorst, A.; Elzinga, B.M.; Voshol, P.J.; Plösch, T.; Kok, T.; Bloks, V.W.; van der Sluijs, F.H.; Havekes, L.M.; Romijn, J.A.; Verkade, H.J.; et al. Stimulation of lipogenesis by pharmacological activation of the liver X receptor leads to production of large, triglyceride-rich very low density lipoprotein particles. J. Biol. Chem. 2002, 277, 34182–34190. [Google Scholar] [CrossRef] [PubMed]
- Kirchgessner, T.G.; Sleph, P.; Ostrowski, J.; Lupisella, J.; Ryan, C.S.; Liu, X.; Fernando, G.; Grimm, D.; Shipkova, P.; Zhang, R.; et al. Beneficial and Adverse Effects of an LXR Agonist on Human Lipid and Lipoprotein Metabolism and Circulating Neutrophils. Cell Metab. 2016, 24, 223–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choe, S.S.; Choi, A.H.; Lee, J.-W.; Kim, K.H.; Chung, J.-J.; Park, J.; Lee, K.-M.; Park, K.-G.; Lee, I.-K.; Kim, J.B. Chronic activation of liver X receptor induces beta-cell apoptosis through hyperactivation of lipogenesis: liver X receptor-mediated lipotoxicity in pancreatic beta-cells. Diabetes 2007, 56, 1534–1543. [Google Scholar] [CrossRef] [PubMed]
- Cozzone, D.; Debard, C.; Dif, N.; Ricard, N.; Disse, E.; Vouillarmet, J.; Rabasa-Lhoret, R.; Laville, M.; Pruneau, D.; Rieusset, J.; et al. Activation of liver X receptors promotes lipid accumulation but does not alter insulin action in human skeletal muscle cells. Diabetologia 2006, 49, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Archer, A.; Stolarczyk, E.; Doria, M.L.; Helguero, L.; Domingues, R.; Howard, J.K.; Mode, A.; Korach-André, M.; Gustafsson, J.-Å. LXR activation by GW3965 alters fat tissue distribution and adipose tissue inflammation in ob/ob female mice. J. Lipid Res. 2013, 54, 1300–1311. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Kurdi-Haidar, B.; Oram, J.F. LXR-mediated activation of macrophage stearoyl-CoA desaturase generates unsaturated fatty acids that destabilize ABCA1. J. Lipid Res. 2004, 45, 972–980. [Google Scholar] [CrossRef] [Green Version]
- Varin, A.; Thomas, C.; Ishibashi, M.; Ménégaut, L.; Gautier, T.; Trousson, A.; Bergas, V.; de Barros, J.P.P.; Narce, M.; Lobaccaro, J.M.A.; et al. Liver X receptor activation promotes polyunsaturated fatty acid synthesis in macrophages: relevance in the context of atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1357–1365. [Google Scholar] [CrossRef]
- Wagner, B.L.; Valledor, A.F.; Shao, G.; Daige, C.L.; Bischoff, E.D.; Petrowski, M.; Jepsen, K.; Baek, S.H.; Heyman, R.A.; Rosenfeld, M.G.; et al. Promoter-specific roles for liver X receptor/corepressor complexes in the regulation of ABCA1 and SREBP1 gene expression. Mol. Cell. Biol. 2003, 23, 5780–5789. [Google Scholar] [CrossRef]
- Demeure, O.; Lecerf, F.; Duby, C.; Desert, C.; Ducheix, S.; Guillou, H.; Lagarrigue, S. Regulation of LPCAT3 by LXR. Gene 2011, 470, 7–11. [Google Scholar] [CrossRef]
- Laffitte, B.A.; Joseph, S.B.; Chen, M.; Castrillo, A.; Repa, J.; Wilpitz, D.; Mangelsdorf, D.; Tontonoz, P. The phospholipid transfer protein gene is a liver X receptor target expressed by macrophages in atherosclerotic lesions. Mol. Cell. Biol. 2003, 23, 2182–2191. [Google Scholar] [CrossRef]
- Cao, G.; Beyer, T.P.; Yang, X.P.; Schmidt, R.J.; Zhang, Y.; Bensch, W.R.; Kauffman, R.F.; Gao, H.; Ryan, T.P.; Liang, Y.; et al. Phospholipid transfer protein is regulated by liver X receptors in vivo. J. Biol. Chem. 2002, 277, 39561–39565. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, M.; Varin, A.; Filomenko, R.; Lopez, T.; Athias, A.; Gambert, P.; Blache, D.; Thomas, C.; Gautier, T.; Lagrost, L.; et al. Liver x receptor regulates arachidonic acid distribution and eicosanoid release in human macrophages: a key role for lysophosphatidylcholine acyltransferase 3. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Spann, N.J.; Garmire, L.X.; McDonald, J.G.; Myers, D.S.; Milne, S.B.; Shibata, N.; Reichart, D.; Fox, J.N.; Shaked, I.; Heudobler, D.; et al. Regulated accumulation of desmosterol integrates macrophage lipid metabolism and inflammatory responses. Cell 2012, 151, 138–152. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Spann, N.J.; Link, V.M.; Muse, E.D.; Strid, T.; Edillor, C.; Kolar, M.J.; Matsuzaka, T.; Hayakawa, S.; Tao, J.; et al. SREBP1 Contributes to Resolution of Pro-inflammatory TLR4 Signaling by Reprogramming Fatty Acid Metabolism. Cell Metab. 2017, 25, 412–427. [Google Scholar] [CrossRef] [PubMed]
- Fujino, T.; Kang, M.J.; Suzuki, H.; Iijima, H.; Yamamoto, T. Molecular characterization and expression of rat acyl-CoA synthetase 3. J. Biol. Chem. 1996, 271, 16748–16752. [Google Scholar] [CrossRef] [PubMed]
- Dong, B.; Kan, C.F.K.; Singh, A.B.; Liu, J. High-fructose diet downregulates long-chain acyl-CoA synthetase 3 expression in liver of hamsters via impairing LXR/RXR signaling pathway. J. Lipid Res. 2013, 54, 1241–1254. [Google Scholar] [CrossRef] [Green Version]
- Weedon-Fekjaer, M.S.; Dalen, K.T.; Solaas, K.; Staff, A.C.; Duttaroy, A.K.; Nebb, H.I. Activation of LXR increases acyl-CoA synthetase activity through direct regulation of ACSL3 in human placental trophoblast cells. J. Lipid Res. 2010, 51, 1886–1896. [Google Scholar] [CrossRef] [Green Version]
- Kang, M.J.; Fujino, T.; Sasano, H.; Minekura, H.; Yabuki, N.; Nagura, H.; Iijima, H.; Yamamoto, T.T. A novel arachidonate-preferring acyl-CoA synthetase is present in steroidogenic cells of the rat adrenal, ovary, and testis. Proc. Natl. Acad. Sci. USA 1997, 94, 2880–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Dalen, K.T.; Gustafsson, J.-A.; Nebb, H.I. Regulation of hepatic fatty acid elongase 5 by LXRalpha-SREBP-1c. Biochim. Biophys. Acta 2009, 1791, 140–147. [Google Scholar] [CrossRef]
- Li, P.; Spann, N.J.; Kaikkonen, M.U.; Lu, M.; Oh, D.Y.; Fox, J.N.; Bandyopadhyay, G.; Talukdar, S.; Xu, J.; Lagakos, W.S.; et al. NCoR repression of LXRs restricts macrophage biosynthesis of insulin-sensitizing omega 3 fatty acids. Cell 2013, 155, 200–214. [Google Scholar] [CrossRef]
- Nara, T.Y.; He, W.S.; Tang, C.; Clarke, S.D.; Nakamura, M.T. The E-box like sterol regulatory element mediates the suppression of human Delta-6 desaturase gene by highly unsaturated fatty acids. Biochem. Biophys. Res. Commun. 2002, 296, 111–117. [Google Scholar] [CrossRef]
- Matsuzaka, T.; Shimano, H.; Yahagi, N.; Amemiya-Kudo, M.; Yoshikawa, T.; Hasty, A.H.; Tamura, Y.; Osuga, J.; Okazaki, H.; Iizuka, Y.; et al. Dual regulation of mouse Delta(5)- and Delta(6)-desaturase gene expression by SREBP-1 and PPARalpha. J. Lipid Res. 2002, 43, 107–114. [Google Scholar]
- Shindou, H.; Hishikawa, D.; Harayama, T.; Yuki, K.; Shimizu, T. Recent progress on acyl CoA: lysophospholipid acyltransferase research. J. Lipid Res. 2009, 50, S46–S51. [Google Scholar] [CrossRef] [Green Version]
- Shindou, H.; Hishikawa, D.; Harayama, T.; Eto, M.; Shimizu, T. Generation of membrane diversity by lysophospholipid acyltransferases. J. Biochem. 2013, 154, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Rong, X.; Wang, B.; Dunham, M.M.; Hedde, P.N.; Wong, J.S.; Gratton, E.; Young, S.G.; Ford, D.A.; Tontonoz, P. Lpcat3-dependent production of arachidonoyl phospholipids is a key determinant of triglyceride secretion. Elife 2015, 4. [Google Scholar] [CrossRef]
- Hashidate-Yoshida, T.; Harayama, T.; Hishikawa, D.; Morimoto, R.; Hamano, F.; Tokuoka, S.M.; Eto, M.; Tamura-Nakano, M.; Yanobu-Takanashi, R.; Mukumoto, Y.; et al. Fatty acid remodeling by LPCAT3 enriches arachidonate in phospholipid membranes and regulates triglyceride transport. Elife 2015, 4. [Google Scholar] [CrossRef]
- Rong, X.; Albert, C.J.; Hong, C.; Duerr, M.A.; Chamberlain, B.T.; Tarling, E.J.; Ito, A.; Gao, J.; Wang, B.; Edwards, P.A.; et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013, 18, 685–697. [Google Scholar] [CrossRef]
- Masson, D.; Jiang, X.-C.; Lagrost, L.; Tall, A.R. The role of plasma lipid transfer proteins in lipoprotein metabolism and atherogenesis. J. Lipid Res. 2009, 50, S201–S206. [Google Scholar] [CrossRef] [Green Version]
- Okazaki, H.; Goldstein, J.L.; Brown, M.S.; Liang, G. LXR-SREBP-1c-phospholipid transfer protein axis controls very low density lipoprotein (VLDL) particle size. J. Biol. Chem. 2010, 285, 6801–6810. [Google Scholar] [CrossRef]
- Jiang, X.-C.; Qin, S.; Qiao, C.; Kawano, K.; Lin, M.; Skold, A.; Xiao, X.; Tall, A.R. Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency. Nat. Med. 2001, 7, 847. [Google Scholar] [CrossRef]
- Manchekar, M.; Liu, Y.; Sun, Z.; Richardson, P.E.; Dashti, N. Phospholipid transfer protein plays a major role in the initiation of apolipoprotein B-containing lipoprotein assembly in mouse primary hepatocytes. J. Biol. Chem. 2015, 290, 8196–8205. [Google Scholar] [CrossRef]
- Rong, X.; Wang, B.; Palladino, E.N.; de Aguiar Vallim, T.Q.; Ford, D.A.; Tontonoz, P. ER phospholipid composition modulates lipogenesis during feeding and in obesity. J. Clin. Invest. 2017, 127, 3640–3651. [Google Scholar] [CrossRef] [Green Version]
- Ou, J.; Tu, H.; Shan, B.; Luk, A.; DeBose-Boyd, R.A.; Bashmakov, Y.; Goldstein, J.L.; Brown, M.S. Unsaturated fatty acids inhibit transcription of the sterol regulatory element-binding protein-1c (SREBP-1c) gene by antagonizing ligand-dependent activation of the LXR. Proc. Natl. Acad. Sci. USA 2001, 98, 6027–6032. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.; Shimano, H.; Yahagi, N.; Ide, T.; Amemiya-Kudo, M.; Matsuzaka, T.; Nakakuki, M.; Tomita, S.; Okazaki, H.; Tamura, Y.; et al. Polyunsaturated fatty acids suppress sterol regulatory element-binding protein 1c promoter activity by inhibition of liver X receptor (LXR) binding to LXR response elements. J. Biol. Chem. 2002, 277, 1705–1711. [Google Scholar] [CrossRef]
- Jung, U.J.; Millman, P.N.; Tall, A.R.; Deckelbaum, R.J. n-3 fatty acids ameliorate hepatic steatosis and dysfunction after LXR agonist ingestion in mice. Biochim. Biophys. Acta 2011, 1811, 491–497. [Google Scholar] [CrossRef]
- Ghisletti, S.; Huang, W.; Ogawa, S.; Pascual, G.; Lin, M.-E.; Willson, T.M.; Rosenfeld, M.G.; Glass, C.K. Parallel SUMOylation-dependent pathways mediate gene- and signal-specific transrepression by LXRs and PPARgamma. Mol. Cell 2007, 25, 57–70. [Google Scholar] [CrossRef]
- Ito, A.; Hong, C.; Rong, X.; Zhu, X.; Tarling, E.J.; Hedde, P.N.; Gratton, E.; Parks, J.; Tontonoz, P. LXRs link metabolism to inflammation through Abca1-dependent regulation of membrane composition and TLR signaling. Elife 2015, 4, e08009. [Google Scholar] [CrossRef]
- Thomas, D.G.; Doran, A.C.; Fotakis, P.; Westerterp, M.; Antonson, P.; Jiang, H.; Jiang, X.-C.; Gustafsson, J.-Å.; Tabas, I.; Tall, A.R. LXR Suppresses Inflammatory Gene Expression and Neutrophil Migration through cis-Repression and Cholesterol Efflux. Cell Reports 2018, 25, 3774–3785. [Google Scholar] [CrossRef]
- Kappus, M.S.; Murphy, A.J.; Abramowicz, S.; Ntonga, V.; Welch, C.L.; Tall, A.R.; Westerterp, M. Activation of liver X receptor decreases atherosclerosis in Ldlr−/− mice in the absence of ATP-binding cassette transporters A1 and G1 in myeloid cells. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 279–284. [Google Scholar] [CrossRef]
- Fontaine, C.; Rigamonti, E.; Nohara, A.; Gervois, P.; Teissier, E.; Fruchart, J.-C.; Staels, B.; Chinetti-Gbaguidi, G. Liver X receptor activation potentiates the lipopolysaccharide response in human macrophages. Circ. Res. 2007, 101, 40–49. [Google Scholar] [CrossRef]
- Thomas, C.; Jalil, A.; Magnani, C.; Ishibashi, M.; Queré, R.; Bourgeois, T.; Bergas, V.; Ménégaut, L.; Patoli, D.; Le Guern, N.; et al. LPCAT3 deficiency in hematopoietic cells alters cholesterol and phospholipid homeostasis and promotes atherosclerosis. Atherosclerosis 2018, 275, 409–418. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Z.; Huan, C.; Jiang, X.-C. Macrophage Lysophosphatidylcholine Acyltransferase 3 Deficiency-Mediated Inflammation Is Not Sufficient to Induce Atherosclerosis in a Mouse Model. Front. Cardiovasc. Med. 2018, 5, 192. [Google Scholar] [CrossRef]
- Bäck, M.; Hansson, G.K. Omega-3 fatty acids, cardiovascular risk, and the resolution of inflammation. FASEB J. 2019, 33, 1536–1539. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Steg, P.G.; Miller, M.; Brinton, E.A.; Jacobson, T.A.; Ketchum, S.B.; Doyle, R.T.; Juliano, R.A.; Jiao, L.; Granowitz, C.; et al. Cardiovascular Risk Reduction with Icosapent Ethyl for Hypertriglyceridemia. N. Engl. J. Med. 2019, 380, 11–22. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jalil, A.; Bourgeois, T.; Ménégaut, L.; Lagrost, L.; Thomas, C.; Masson, D. Revisiting the Role of LXRs in PUFA Metabolism and Phospholipid Homeostasis. Int. J. Mol. Sci. 2019, 20, 3787. https://doi.org/10.3390/ijms20153787
Jalil A, Bourgeois T, Ménégaut L, Lagrost L, Thomas C, Masson D. Revisiting the Role of LXRs in PUFA Metabolism and Phospholipid Homeostasis. International Journal of Molecular Sciences. 2019; 20(15):3787. https://doi.org/10.3390/ijms20153787
Chicago/Turabian StyleJalil, Antoine, Thibaut Bourgeois, Louise Ménégaut, Laurent Lagrost, Charles Thomas, and David Masson. 2019. "Revisiting the Role of LXRs in PUFA Metabolism and Phospholipid Homeostasis" International Journal of Molecular Sciences 20, no. 15: 3787. https://doi.org/10.3390/ijms20153787
APA StyleJalil, A., Bourgeois, T., Ménégaut, L., Lagrost, L., Thomas, C., & Masson, D. (2019). Revisiting the Role of LXRs in PUFA Metabolism and Phospholipid Homeostasis. International Journal of Molecular Sciences, 20(15), 3787. https://doi.org/10.3390/ijms20153787





