HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk
Abstract
1. Introduction
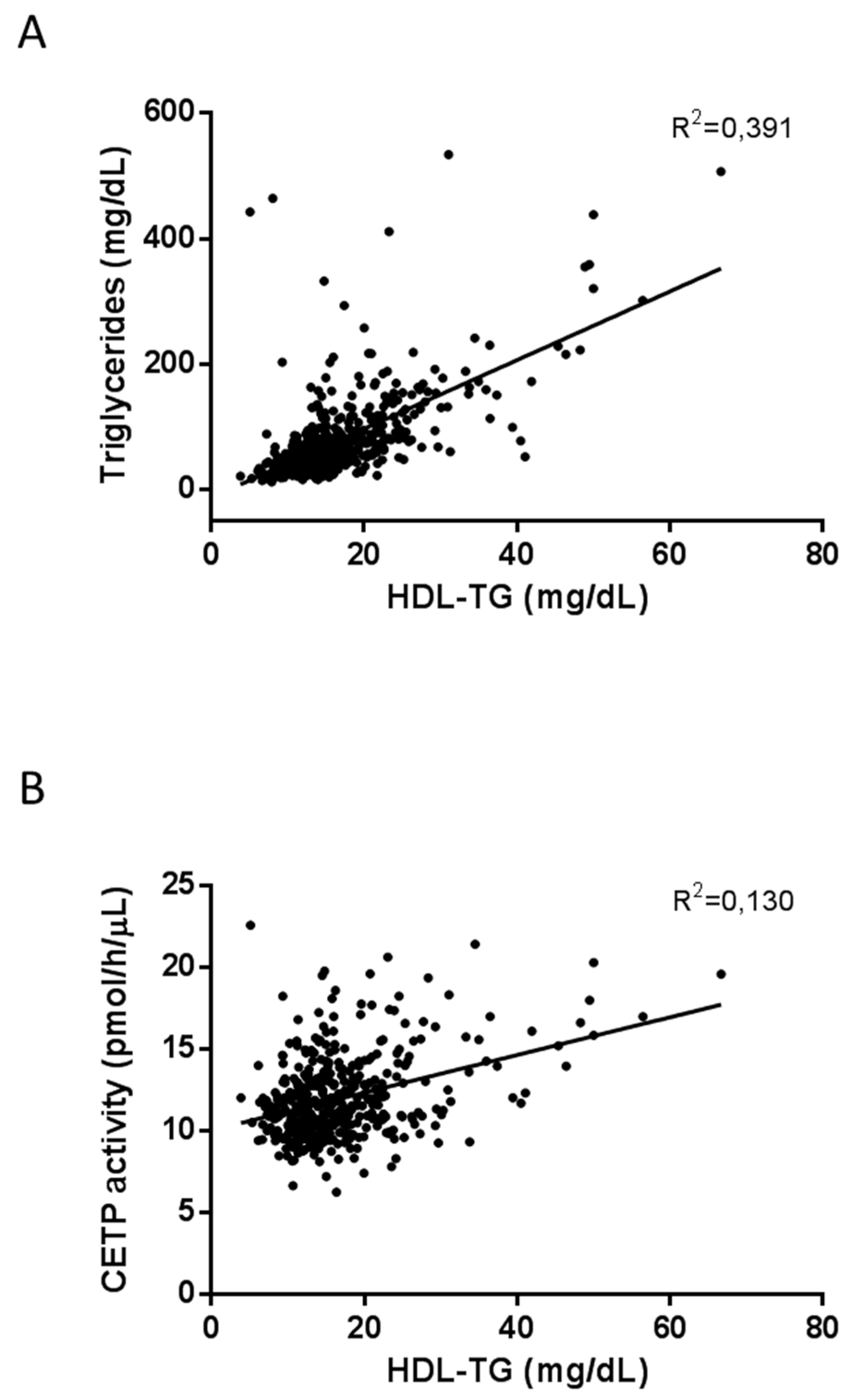
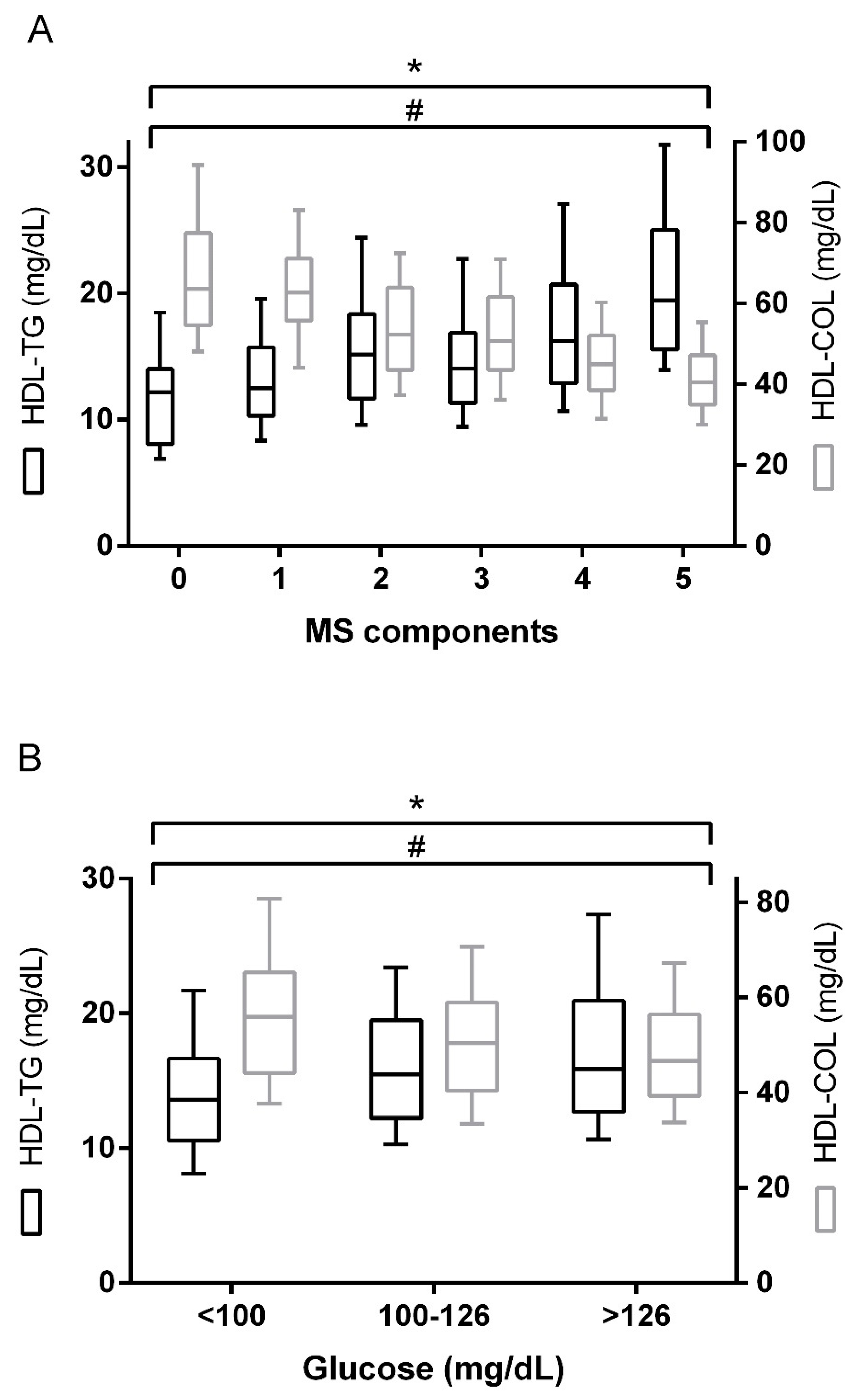
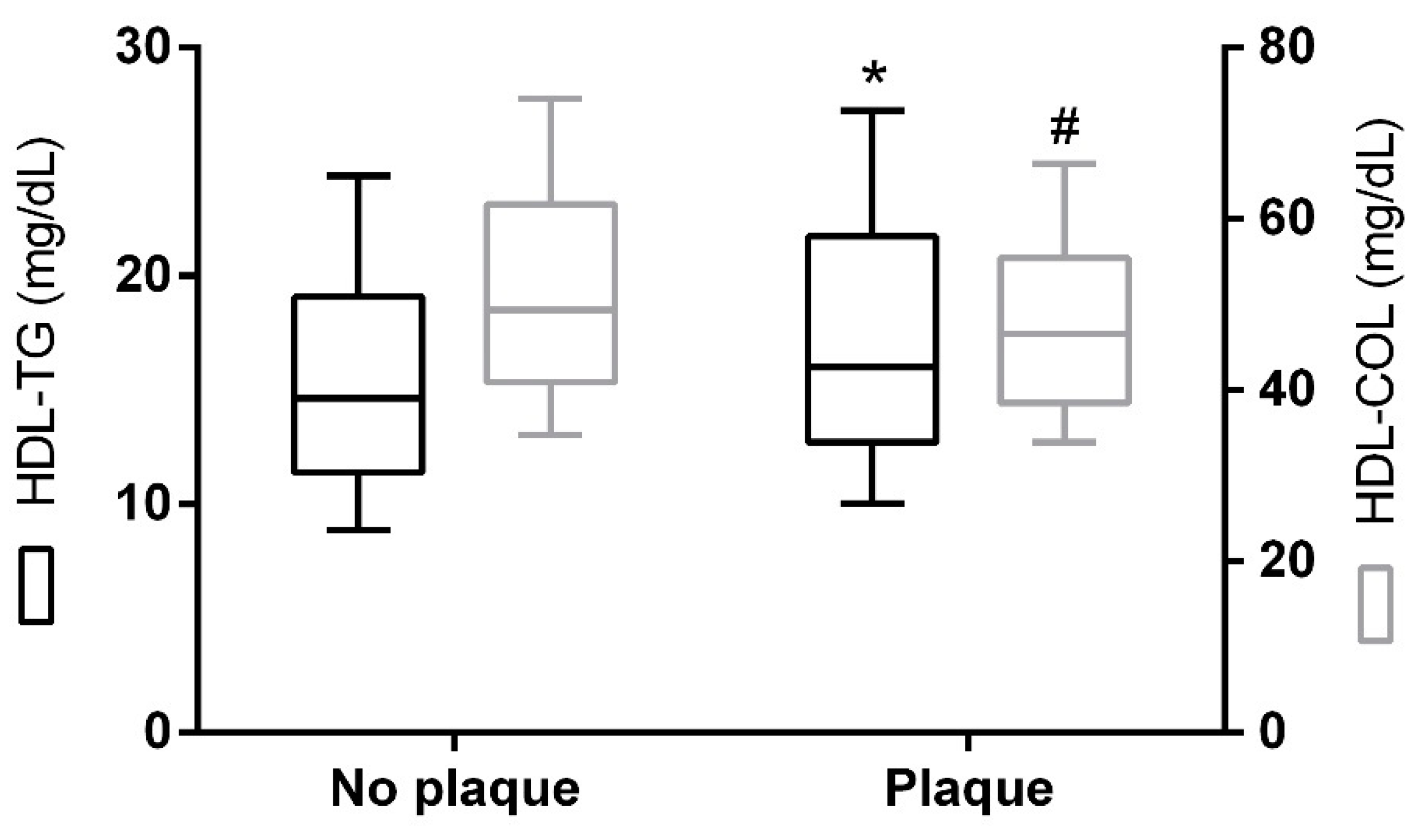
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Blood Analyses
4.3. Carotid Examination
4.4. Statistical Analyses
Author Contributions
Funding
Conflicts of Interest
References
- Rye, K.-A.; Bursill, C.A.; Lambert, G.; Tabet, F.; Barter, P.J. The metabolism and anti-atherogenic properties of HDL. J. Lipid Res. 2009, 50, S195–S200. [Google Scholar] [CrossRef] [PubMed]
- Spady, D.K. Reverse Cholesterol Transport and Atherosclerosis Regression. Circulation 1999, 100, 576–578. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J.; Hovingh, G.K. HDL and cardiovascular disease. Lancet 2014, 384, 618–625. [Google Scholar] [CrossRef]
- The Emerging Risk Factors Collaboration; Di Angelantonio, E.; Sarwar, N.; Perry, P.; Kaptoge, S.; Ray, K.K.; Thompson, A.; Wood, A.M.; Lewington, S.; Sattar, N.; et al. Major Lipids, Apolipoproteins, and Risk of Vascular Disease. JAMA 2009, 302, 1993. [Google Scholar] [PubMed]
- Ramasamy, I. Update on the molecular biology of dyslipidemias. Clin. Chim. Acta 2016, 454, 143–185. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.G.; Olsson, A.G.; Abt, M.; Ballantyne, C.M.; Barter, P.J.; Brumm, J.; Chaitman, B.R.; Holme, I.M.; Kallend, D.; Leiter, L.A.; et al. Effects of Dalcetrapib in Patients with a Recent Acute Coronary Syndrome. N. Engl. J. Med. 2012, 367, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Haynes, R.; Jiang, L.; Hopewell, J.C.; Li, J.; Chen, F.; Parish, S.; Landray, M.J.; Collins, R.; Armitage, J.; Collins, R.; et al. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: Trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 2013, 34, 1279–1291. [Google Scholar]
- AIM-HIGH Investigators; Boden, W.E.; Probstfield, J.L.; Anderson, T.; Chaitman, B.R.; Desvignes-Nickens, P.; Koprowicz, K.; McBride, R.; Teo, K.; Weintraub, W. Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. N. Engl. J. Med. 2011, 365, 2255–2267. [Google Scholar]
- Keech, A.; Simes, R.J.; Barter, P.; Best, J.; Scott, R.; Taskinen, M.R.; Forder, P.; Pillai, A.; Davis, T.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R.; Leiter, L.A.; Linz, P.; Friedewald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar]
- Barter, P.J.; Caulfield, M.; Eriksson, M.; Grundy, S.M.; Kastelein, J.J.P.; Komajda, M.; Lopez-Sendon, J.; Mosca, L.; Tardif, J.-C.; Waters, D.D.; et al. Effects of Torcetrapib in Patients at High Risk for Coronary Events. N. Engl. J. Med. 2007, 357, 2109–2122. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Nicholls, S.J.; Riesmeyer, J.S.; Barter, P.J.; Brewer, H.B.; Fox, K.A.A.; Gibson, C.M.; Granger, C.; Menon, V.; Montalescot, G.; et al. Evacetrapib and Cardiovascular Outcomes in High-Risk Vascular Disease. N. Engl. J. Med. 2017, 376, 1933–1942. [Google Scholar] [CrossRef] [PubMed]
- Elam, M.; Lovato, L.; Ginsberg, H. The ACCORD-Lipid study: Implications for treatment of dyslipidemia in Type 2 diabetes mellitus. Clin. Lipidol. 2011, 6, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Masana, L.; Cabré, A.; Heras, M.; Amigó, N.; Correig, X.; Martínez-Hervás, S.; Real, J.T.; Ascaso, J.F.; Quesada, H.; Julve, J.; et al. Remarkable quantitative and qualitative differences in HDL after niacin or fenofibrate therapy in type 2 diabetic patients. Atherosclerosis 2015, 238, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.A.K. Dysfunctional HDL in diabetes mellitus and its role in the pathogenesis of cardiovascular disease. Mol. Cell Biochem. 2018, 440, 167–187. [Google Scholar] [CrossRef]
- Vergès, B. Lipid modification in type 2 diabetes: The role of LDL and HDL. Fundam. Clin. Pharmacol. 2009, 23, 681–685. [Google Scholar] [CrossRef]
- Amigó, N.; Mallol, R.; Heras, M.; Martínez-Hervás, S.; Blanco-Vaca, F.; Escolà-Gil, J.C.; Plana, N.; Yanes, Ó.; Masana, L.; Correig, X. Lipoprotein hydrophobic core lipids are partially extruded to surface in smaller HDL: “Herniated” HDL, a common feature in diabetes. Sci. Rep. 2016, 6, 19249. [Google Scholar] [CrossRef]
- Holmes, M.V.; Millwood, I.Y.; Kartsonaki, C.; Hill, M.R.; Bennett, D.A.; Boxall, R.; Guo, Y.; Xu, X.; Bian, Z.; Hu, R.; et al. Lipids, Lipoproteins, and Metabolites and Risk of Myocardial Infarction and Stroke. J. Am. Coll. Cardiol. 2018, 71, 620–632. [Google Scholar] [CrossRef]
- Madsen, C.M.; Varbo, A.; Nordestgaard, B.G. Extreme high high-density lipoprotein cholesterol is paradoxically associated with high mortality in men and women: Two prospective cohort studies. Eur. Heart J. 2017, 38, 2478–2486. [Google Scholar] [CrossRef]
- Shah, A.S.; Tan, L.; Long, J.L.; Davidson, W.S. Proteomic diversity of high density lipoproteins: Our emerging understanding of its importance in lipid transport and beyond. J. Lipid Res. 2013, 54, 2575–2585. [Google Scholar] [CrossRef]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 13, 423–433. [Google Scholar] [CrossRef] [PubMed]
- Rohatgi, A.; Khera, A.; Berry, J.D.; Givens, E.G.; Ayers, C.R.; Wedin, K.E.; Neeland, I.J.; Yuhanna, I.S.; Rader, D.R.; de Lemos, J.A.; et al. HDL Cholesterol Efflux Capacity and Incident Cardiovascular Events. N. Engl. J. Med. 2014, 371, 2383–2393. [Google Scholar] [CrossRef] [PubMed]
- Giannini, C.; Santoro, N.; Caprio, S.; Kim, G.; Lartaud, D.; Shaw, M.; Pierpont, B.; Weiss, R. The triglyceride-to-HDL cholesterol ratio: Association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care 2011, 34, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Zhu, L.; Cui, X.; Feng, L.; Zhao, X.; He, S.; Ping, F.; Li, W.; Li, Y. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of β cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis. 2016, 15, 104. [Google Scholar] [CrossRef] [PubMed]
- Pantoja-Torres, B.; Toro-Huamanchumo, C.J.; Urrunaga-Pastor, D.; Guarnizo-Poma, M.; Lazaro-Alcantara, H.; Paico-Palacios, S.; del Carmen Ranilla-Seguin, V.; Benites-Zapata, V.A. Insulin Resistance and Metabolic Syndrome Research Group High triglycerides to HDL-cholesterol ratio is associated with insulin resistance in normal-weight healthy adults. Diabetes Metab Syndr. Clin. Res. Rev. 2019, 13, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Fu, J.; Jiang, Y.; Dong, G.; Wang, X.; Wu, W. TriGlycerides and high-density lipoprotein cholesterol ratio compared with homeostasis model assessment insulin resistance indexes in screening for metabolic syndrome in the chinese obese children: A cross section study. BMC Pediatr. 2015, 15, 138. [Google Scholar] [CrossRef] [PubMed]
- Jepsen, A.-M.K.; Langsted, A.; Varbo, A.; Bang, L.E.; Kamstrup, P.R.; Nordestgaard, B.G. Increased Remnant Cholesterol Explains Part of Residual Risk of All-Cause Mortality in 5414 Patients with Ischemic Heart Disease. Clin. Chem. 2016, 62, 593–604. [Google Scholar] [CrossRef]
- Varbo, A.; Freiberg, J.J.; Nordestgaard, B.G. Remnant Cholesterol and Myocardial Infarction in Normal Weight, Overweight, and Obese Individuals from the Copenhagen General Population Study. Clin. Chem. 2018, 64, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Bedogni, G.; Bellentani, S.; Miglioli, L.; Masutti, F.; Passalacqua, M.; Castiglione, A.; Tiribelli, C. The Fatty Liver Index: A simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006, 6, 33. [Google Scholar] [CrossRef]
- Mallol, R.; Amigó, N.; Rodríguez, M.A.; Heras, M.; Vinaixa, M.; Plana, N.; Rock, E.; Ribalta, J.; Yanes, O.; Masana, L.; et al. Liposcale: A novel advanced lipoprotein test based on 2D diffusion-ordered 1H NMR spectroscopy. J. Lipid Res. 2015, 56, 737–746. [Google Scholar] [CrossRef]
| Clinical and Biochemical Variables | n = 502 |
|---|---|
| Age (y) | 61.0 (52.0–67.0) |
| Sex (%, women) | 50.9 |
| BMI (kg/m2) | 30.0 (27.0–34.8) |
| SBP (mm Hg) | 133.0 (124.0–146.0) |
| DBP (mm Hg) | 80.0 (71.0–85.0) |
| Total cholesterol (mg/dL) | 196.4 (172.8–231.2) |
| Total triglycerides (mg/dL) | 135.9 (91.2–210.8) |
| ApoB100 (mg/dL) | 97 (81–118) |
| ApoA1 (mg/dL) | 138.4 ± 14.94 |
| Disease | |
| Type 2 diabetes (%, yes) | 78.1 |
| Obesity (%, yes) | 51.2 |
| Metabolic syndrome (%, yes) | 80.1 |
| Atherogenic dyslipidemia (%, yes) | 30.6 |
| Subclinical atherosclerosis | |
| cIMT (mm) * | 0.68 (0.62–0.77) |
| Carotid atherosclerotic plaque (%, yes) ** | 32.8 |
| Lipidomics | |
| Cholesterol | |
| VLDL-C (mg/dL) | 19.4 (10.1–34.8) |
| IDL-C (mg/dL) | 11.2 (7.6–15.2) |
| LDL-C (mg/dL) | 110.1 (89.6–133.7) |
| HDL-C (mg/dL) | 49.1 (40.9–60.3) |
| Triglycerides | |
| VLDL-TG (mg/dL) | 73.0 (44.5–130.8) |
| IDL-TG (mg/dL) | 13.0 (9.7–16.2) |
| LDL-TG (mg/dL) | 18.8 (14.5–24.0) |
| HDL-TG (mg/dL) | 15.3 (12.2–19.5) |
| Particle number | |
| VLDL-P (nmol/L) | 58.2 (33.9–102.9) |
| Large VLDL-P (nmol/L) | 1.28 (0.82–2.10) |
| Medium VLDL-P (nmol/L) | 4.9 (2.9–8.9) |
| Small VLDL-P (nmol/L) | 51.7 (30.1–89.7) |
| LDL-P (nmol/L) | 831.8 (684.8–1020.6) |
| Large LDL-P (nmol/L) | 108.1 (89.4–132.4) |
| Medium LDL-P (nmol/L) | 269.2 (206.4–355.3) |
| Small LDL-P (nmol/L) | 448.9 (370.7–543.9) |
| HDL-P (µmol/L) | 27.9 (23.8–32.2) |
| Large HDL-P (µmol/L) | 0.28 (0.24–0.32) |
| Medium HDL-P (µmol/L) | 8.1 (6.7–9.6) |
| Small HDL-P (µmol/L) | 19.6 (16.3–22.7) |
| Particle size | |
| VLDL-Z (nm) | 41.9 (41.8–42.1) |
| LDL-Z (nm) | 20.9 (20.8–21.1) |
| HDL-Z (nm) | 8.2 (8.2–8.3) |
| Variables | HDL-TG | HDL-C | HDL-P | |||
|---|---|---|---|---|---|---|
| ρ (rho) | p | ρ (rho) | p | ρ (rho) | p | |
| Age | 0.062 | 0.167 | 0.097 | 0.031 | 0.087 | 0.052 |
| SBP | 0.217 | <0.001 | –0.201 | <0.001 | –0.101 | 0.044 |
| DBP | 0.094 | 0.062 | –0.223 | <0.001 | –0.154 | 0.002 |
| Waist circumference | 0.145 | 0.002 | –0.352 | <0.001 | –0.283 | <0.001 |
| BMI | 0.157 | <0.001 | –0.250 | <0.001 | –0.183 | <0.001 |
| Cholesterol | 0.233 | <0.001 | 0.033 | 0.468 | 0.135 | 0.002 |
| Triglycerides | 0.652 | <0.001 | –0.536 | <0.001 | –0.197 | <0.001 |
| Apo B100 | 0.199 | <0.001 | –0.237 | <0.001 | –0.153 | 0.001 |
| Apo A1 | 0.003 | 0.938 | 0.525 | <0.001 | 0.528 | <0.001 |
| Glucose | 0.183 | <0.001 | –0.200 | <0.001 | –0.102 | 0.022 |
| Insulin * | 0.284 | <0.001 | –0.367 | <0.001 | –0.169 | 0.029 |
| HbA1c ** | 0.047 | 0.380 | –0.130 | 0.015 | –0.133 | 0.014 |
| Adiponectin | –0.020 | 0.674 | 0.421 | <0.001 | 0.362 | <0.001 |
| Glycerol | 0.410 | <0.001 | –0.355 | <0.001 | –0.113 | 0.012 |
| NEFA | 0.212 | <0.001 | –0.031 | 0.492 | 0.062 | 0.168 |
| CETP activity | 0.264 | <0.001 | –0.110 | 0.022 | 0.071 | 0.139 |
| LCAT activity | –0.006 | 0.901 | 0.282 | <0.001 | 0.211 | <0.001 |
| FLI | 0.363 | <0.001 | –0.460 | <0.001 | –0.268 | <0.001 |
| usCRP | 0.101 | 0.025 | –0.154 | 0.001 | –0.112 | 0.013 |
| cIMT *** | –0.023 | 0.682 | –0.081 | 0.152 | –0.126 | 0.027 |
| VLDL-C | 0.682 | <0.001 | –0.597 | <0.001 | –0.235 | <0.001 |
| IDL-C | 0.534 | <0.001 | –0.312 | <0.001 | –0.136 | 0.002 |
| LDL-C | –0.050 | 0.263 | 0.014 | 0.760 | –0.072 | 0.105 |
| HDL-C | –0.135 | 0.002 | - | 0.857 | <0.001 | |
| VLDL-TG | 0.631 | <0.001 | –0.607 | <0.001 | –0.251 | <0.001 |
| IDL-TG | 0.695 | <0.001 | –0.337 | <0.001 | –0.045 | 0.319 |
| LDL-TG | 0.427 | <0.001 | –0.099 | 0.029 | –0.008 | 0.851 |
| HDL-TG | - | –0.135 | 0.002 | 0.275 | <0.001 | |
| VLDL-P | 0.647 | <0.001 | –0.608 | <0.001 | –0.249 | <0.001 |
| Large VLDL-P | 0.622 | <0.001 | –0.624 | <0.001 | –0.266 | <0.001 |
| Medium VLDL-P | 0.600 | <0.001 | –0.540 | <0.001 | –0.205 | <0.001 |
| Small VLDL-P | 0.646 | <0.001 | –0.609 | <0.001 | –0.251 | <0.001 |
| LDL-P | 0.047 | 0.291 | –0.063 | <0.001 | –0.094 | 0.035 |
| Large LDL-P | 0.032 | 0.471 | –0.041 | 0.354 | –0.111 | 0.012 |
| Medium LDL-P | –0.060 | 0.183 | 0.180 | <0.001 | 0.042 | 0.350 |
| Small LDL-P | 0.110 | 0.014 | –0.609 | <0.001 | –0.186 | <0.001 |
| HDL-P | 0.275 | <0.001 | 0.857 | <0.001 | ||
| Large HDL-P | 0.395 | <0.001 | 0.458 | <0.001 | 0.508 | <0.001 |
| Medium HDL-P | 0.281 | <0.001 | 0.745 | <0.001 | 0.734 | <0.001 |
| Small HDL-P | 0.234 | <0.001 | 0.771 | <0.001 | 0.955 | <0.001 |
| VLDL-Z | –0.271 | <0.001 | 0.282 | <0.001 | 0.169 | <0.001 |
| LDL-Z | –0.187 | <0.001 | 0.427 | <0.001 | 0.173 | <0.001 |
| HDL-Z | 0.061 | 0.170 | –0.009 | 0.834 | –0.186 | <0.001 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Girona, J.; Amigó, N.; Ibarretxe, D.; Plana, N.; Rodríguez-Borjabad, C.; Heras, M.; Ferré, R.; Gil, M.; Correig, X.; Masana, L. HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk. Int. J. Mol. Sci. 2019, 20, 3151. https://doi.org/10.3390/ijms20133151
Girona J, Amigó N, Ibarretxe D, Plana N, Rodríguez-Borjabad C, Heras M, Ferré R, Gil M, Correig X, Masana L. HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk. International Journal of Molecular Sciences. 2019; 20(13):3151. https://doi.org/10.3390/ijms20133151
Chicago/Turabian StyleGirona, Josefa, Núria Amigó, Daiana Ibarretxe, Núria Plana, Cèlia Rodríguez-Borjabad, Mercedes Heras, Raimon Ferré, Míriam Gil, Xavier Correig, and Lluís Masana. 2019. "HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk" International Journal of Molecular Sciences 20, no. 13: 3151. https://doi.org/10.3390/ijms20133151
APA StyleGirona, J., Amigó, N., Ibarretxe, D., Plana, N., Rodríguez-Borjabad, C., Heras, M., Ferré, R., Gil, M., Correig, X., & Masana, L. (2019). HDL Triglycerides: A New Marker of Metabolic and Cardiovascular Risk. International Journal of Molecular Sciences, 20(13), 3151. https://doi.org/10.3390/ijms20133151





