Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia
Abstract
1. Introduction
2. HHcy Affects Gene Expression
3. HHcy and DNA Methylation
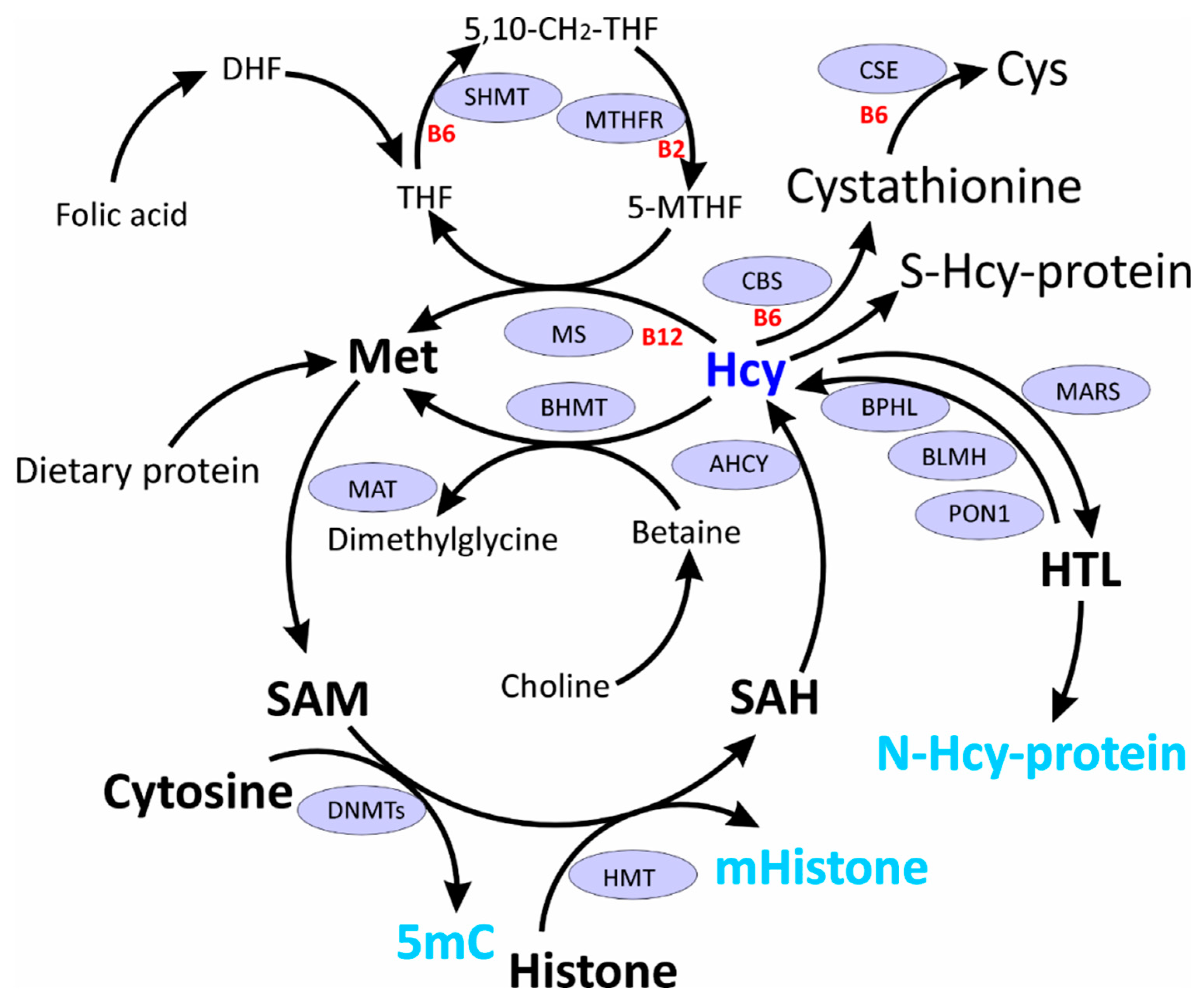
3.1. SAM/SAH Homeostasis and DNA Methylation
3.2. HHcy & SAH/SAM Levels
3.3. HHcy and DNA Methyltransferases
3.4. HHcy and DNA Methylation in Disease
3.4.1. Atherosclerosis
3.4.2. Uraemia
3.4.3. Cognition and Alzheimer’s Disease
3.5. HHcy and DNA Methylation in Humans
4. Histone Modifications in HHcy
4.1. Histone Modification
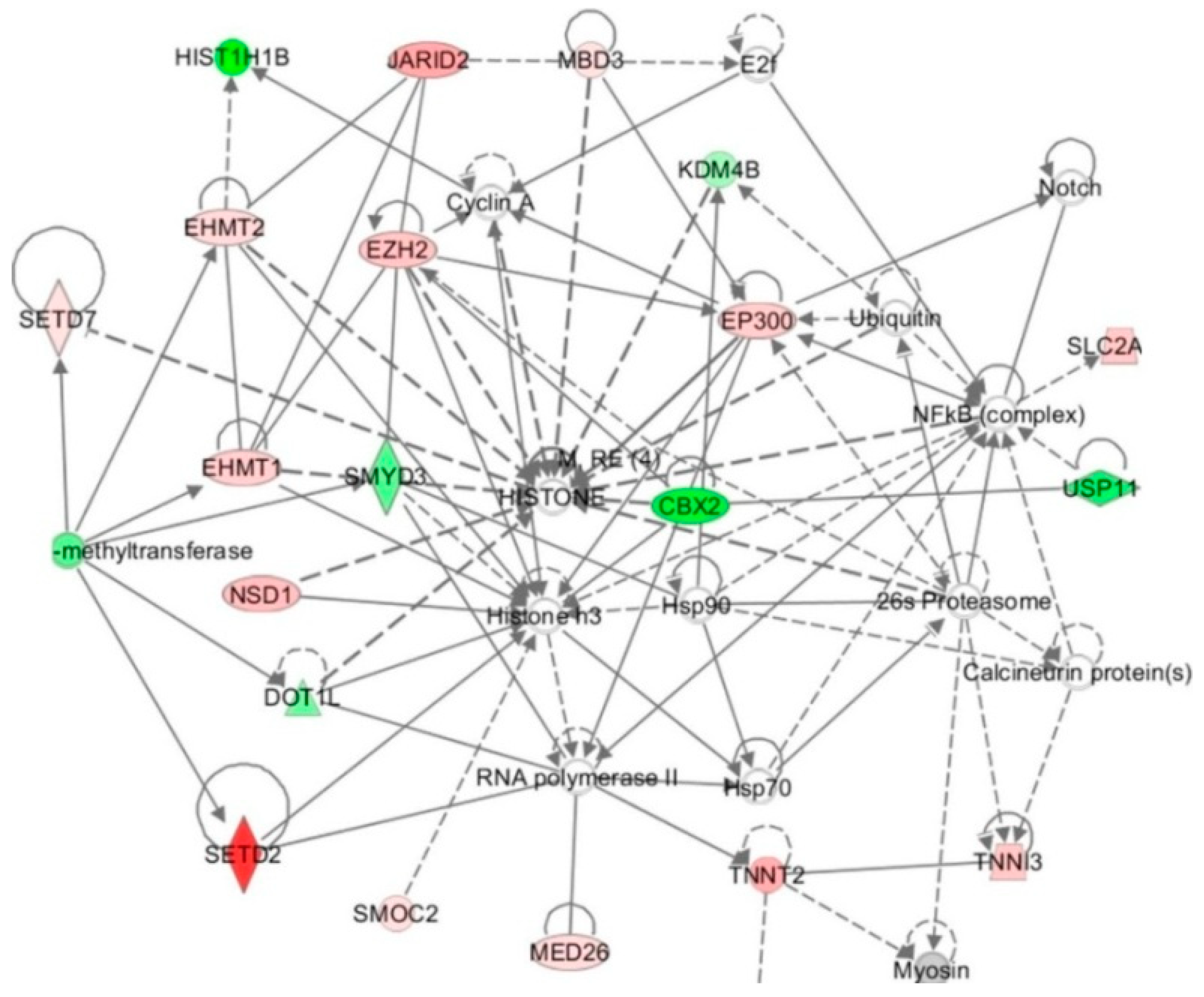
4.2. Histone-Modifying Enzymes in HHcy
4.3. Histone Modifications and HHcy
4.4. Crosstalk between DNA Methylation and Histone Modification
5. HHcy and Noncoding RNA Regulation
5.1. MiRNAs
5.2. LncRNAs
5.3. CircRNAs
6. Summary/Prospects
Supplementary Materials
Funding
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| DOAJ | Directory of open access journals |
| TLA | Three letter acronym |
| LD | linear dichroism |
| AD | Alzheimer’s disease |
| ADMA | Asymmetric dimethylarginine |
| AHCY | S-adenosylhomocysteine hydrolase |
| APP | Amyloid-beta precursor protein |
| BACE | β-secretase |
| BBB | Blood-brain barrier |
| BHMT | Betaine:Hcy methyltransferase |
| BLMH | Bleomycin hydrolase |
| BPHL | Biphenyl hydrolase like |
| CBS | Cystathionine β-synthase |
| CFTR | Cystic fibrosis transmembrane conductance regulator |
| CRC | Colorectal cancer |
| CSE | Cystathionine γ-lyase |
| DNMT | DNA methyltransferase |
| ECs | Endothelial cells |
| EPCs | Endothelial progenitor cells |
| EPRS | Glutamyl-prolyl-tRNA synthetase |
| FABP4 | Fatty acid-binding protein 4 |
| FATP1 | Long-chain fatty acid transport protein 1 |
| HAT | Histone acetyltransferase |
| HDAC | Histone deacetylase |
| HMT | Histone methyltransferase |
| HTL | Homocysteine thiolactone |
| HUVECs | Human umbilical vein endothelial cells |
| 5LO | 5-Lipoxygenase |
| MAPK | Mitogen-activated protein kinase |
| MARS | Met-tRNA synthetase |
| MBP | Methylcytosine-binding proteins |
| MFN2 | Mitofusin-2 |
| MMP | Matrix metalloproteinase |
| MTHFR | 5,10-methylenetetrahydrofolate reductase |
| MS | Met synthase |
| NASH | Nonalcoholic fatty liver disease |
| NTD | Neural tube defect |
| PDGF | Platelet-derived growth factor |
| PON1 | Paraoxonase 1 |
| PPAR | Peroxisome proliferator-activated receptor |
| PS1 | Presenilin-1 |
| ROS | Reactive oxygen species |
| SAH | S-adenosylhomocysteine |
| SAM | S-adenosylmethionine |
| She | Soluble epoxide hydrolase |
| SHMT | Serine hydroxymethyltransferase |
| SOD-2 | Superoxide dismutase |
| SP1 | Specificity protein-1 |
| TIMP | Tissue inhibitors of metaloproteinases |
| TERT | Telomerase reverse transcriptase |
| TLR4 | Toll-like receptor 4 |
| VSMCs | Vascular smooth muscle cells |
| TLA | Three letter acronym |
| LD | linear dichroism |
References
- Perla-Kajan, J.; Twardowski, T.; Jakubowski, H. Mechanisms of homocysteine toxicity in humans. Amino Acids 2007, 32, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Maron, B.A.; Loscalzo, J. The treatment of hyperhomocysteinemia. Annu. Rev. Med. 2009, 60, 39–54. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine in Protein Structure/Function and Human Disease; Springer: Vienna, Austria, 2013. [Google Scholar]
- Wald, D.S.; Law, M.; Morris, J.K. Homocysteine and cardiovascular disease: Evidence on causality from a meta-analysis. BMJ 2002, 325, 1202. [Google Scholar] [CrossRef] [PubMed]
- Seshadri, S.; Beiser, A.; Selhub, J.; Jacques, P.F.; Rosenberg, I.H.; D’Agostino, R.B.; Wilson, P.W.; Wolf, P.A. Plasma homocysteine as a risk factor for dementia and alzheimer’s disease. N. Engl. J. Med. 2002, 346, 476–483. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.A.; Hewedi, D.H.; Eissa, A.M.; Frydecka, D.; Misiak, B. Homocysteine levels in schizophrenia and affective disorders-focus on cognition. Front. Behav. Neurosci. 2014, 8, 343. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Daly, S.; Cotter, A.; Molloy, A.E.; Scott, J. Homocysteine and folic acid: Implications for pregnancy. Semin. Vasc. Med. 2005, 5, 190–200. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhao, R.; Qu, Y.Y.; Mei, X.Y.; Zhang, X.; Zhou, Q.; Li, Y.; Yang, S.B.; Zuo, Z.G.; Chen, Y.M.; et al. Colonic lysine homocysteinylation induced by high-fat diet suppresses DNA damage repair. Cell Rep. 2018, 25, 398–412. [Google Scholar] [CrossRef]
- Jakubowski, H. Protein n-homocysteinylation and colorectal cancer. Trends Cancer 2019, 5, 7–10. [Google Scholar] [CrossRef]
- García-Tevijano, E.R.; Berasain, C.; Rodríguez, J.A.; Corrales, F.J.; Arias, R.; Martín-Duce, A.; Caballería, J.; Mato, J.M.; Avila, M.A. Hyperhomocysteinemia in liver cirrhosis: Mechanisms and role in vascular and hepatic fibrosis. Hypertension 2001, 38, 1217–1221. [Google Scholar] [CrossRef]
- Gjesdal, C.G.; Vollset, S.E.; Ueland, P.M.; Refsum, H.; Drevon, C.A.; Gjessing, H.K.; Tell, G.S. Plasma total homocysteine level and bone mineral density: The hordaland homocysteine study. Arch. Intern. Med. 2006, 166, 88–94. [Google Scholar] [CrossRef]
- Robinson, K.; Gupta, A.; Dennis, V.; Arheart, K.; Chaudhary, D.; Green, R.; Vigo, P.; Mayer, E.L.; Selhub, J.; Kutner, M.; et al. Hyperhomocysteinemia confers an independent increased risk of atherosclerosis in end-stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation 1996, 94, 2743–2748. [Google Scholar] [CrossRef] [PubMed]
- Leach, N.V.; Dronca, E.; Vesa, S.C.; Sampelean, D.P.; Craciun, E.C.; Lupsor, M.; Crisan, D.; Tarau, R.; Rusu, R.; Para, I.; et al. Serum homocysteine levels, oxidative stress and cardiovascular risk in non-alcoholic steatohepatitis. Eur. J. Intern. Med. 2014, 25, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Dai, H.; Wang, W.; Tang, X.; Chen, R.; Chen, Z.; Lu, Y.; Yuan, H. Association between homocysteine and non-alcoholic fatty liver disease in chinese adults: A cross-sectional study. Nutr. J. 2016, 15, 102. [Google Scholar] [CrossRef] [PubMed]
- Mandaviya, P.R.; Stolk, L.; Heil, S.G. Homocysteine and DNA methylation: A review of animal and human literature. Mol. Genet. Metab. 2014, 113, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Harker, L.A.; Slichter, S.J.; Scott, C.R.; Ross, R. Homocystinemia. Vascular injury and arterial thrombosis. N. Engl. J. Med. 1974, 291, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Tinelli, C.; Di Pino, A.; Ficulle, E.; Marcelli, S.; Feligioni, M. Hyperhomocysteinemia as a risk factor and potential nutraceutical target for certain pathologies. Front. Nutr. 2019, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Esse, R.; Barroso, M.; Tavares de Almeida, I.; Castro, R. The contribution of homocysteine metabolism disruption to endothelial dysfunction: State-of-the-art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Homocysteine modification in protein structure/function and human disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef]
- Mudd, S.H.; Finkelstein, J.D.; Refsum, H.; Ueland, P.M.; Malinow, M.R.; Lentz, S.R.; Jacobsen, D.W.; Brattstrom, L.; Wilcken, B.; Wilcken, D.E.; et al. Homocysteine and its disulfide derivatives: A suggested consensus terminology. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1704–1706. [Google Scholar] [CrossRef]
- Glowacki, R.; Jakubowski, H. Cross-talk between cys34 and lysine residues in human serum albumin revealed by n-homocysteinylation. J. Biol. Chem. 2004, 279, 10864–10871. [Google Scholar] [CrossRef]
- Sikora, M.; Marczak, Ł.; Perła-Kajan, J.; Jakubowski, H. Sex affects n-homocysteinylation at lysine residue 212 of albumin in mice. Sci. Rep. 2019, 9, 2669. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. The molecular basis of homocysteine thiolactone-mediated vascular disease. Clin. Chem. Lab. Med. 2007, 45, 1704–1716. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Quality control in trna charging-editing of homocysteine. Acta Biochim. Pol. 2011, 58, 149–163. [Google Scholar] [CrossRef] [PubMed]
- Borowczyk, K.; Piechocka, J.; Głowacki, R.; Dhar, I.; Midtun, Ø.; Tell, G.S.; Ueland, P.M.; Nygård, O.; Jakubowski, H. Urinary excretion of homocysteine thiolactone and the risk of acute myocardial infarction in coronary artery disease patients: The wenbit trial. J. Intern. Med. 2019, 285, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Handy, D.E.; Castro, R.; Loscalzo, J. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease. Circulation 2011, 123, 2145–2156. [Google Scholar] [CrossRef] [PubMed]
- Loscalzo, J.; Handy, D.E. Epigenetic modifications: Basic mechanisms and role in cardiovascular disease (2013 grover conference series). Pulm. Circ. 2014, 4, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Esse, R.; Imbard, A.; Florindo, C.; Gupta, S.; Quinlivan, E.P.; Davids, M.; Teerlink, T.; Tavares de Almeida, I.; Kruger, W.D.; Blom, H.J.; et al. Protein arginine hypomethylation in a mouse model of cystathionine beta-synthase deficiency. FASEB J. 2014, 28, 2686–2695. [Google Scholar] [CrossRef]
- Lee, H.O.; Wang, L.; Kuo, Y.M.; Gupta, S.; Slifker, M.J.; Li, Y.S.; Andrews, A.J.; Kruger, W.D. Lack of global epigenetic methylation defects in cbs deficient mice. J. Inherit. Metab. Dis. 2017, 40, 113–120. [Google Scholar] [CrossRef]
- Jakubowski, H. Quality control in trna charging. Wiley Interdiscip. Rev. RNA 2012, 3, 295–310. [Google Scholar] [CrossRef]
- Jakubowski, H. Protein homocysteinylation: Possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J. 1999, 13, 2277–2283. [Google Scholar] [CrossRef]
- Jakubowski, H. Metabolism of homocysteine thiolactone in human cell cultures—Possible mechanism for pathological consequences of elevated homocysteine levels. J. Biol. Chem. 1997, 272, 1935–1942. [Google Scholar] [PubMed]
- Jackson, A.O.; Regine, M.A.; Subrata, C.; Long, S. Molecular mechanisms and genetic regulation in atherosclerosis. Int. J. Cardiol. Heart Vasc. 2018, 21, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Kamat, P.K.; Tyagi, S.C.; Tyagi, N. Synergy of homocysteine, microrna, and epigenetics: A novel therapeutic approach for stroke. Mol. Neurobiol. 2013, 48, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yu, J.T.; Tan, M.S.; Jiang, T.; Tan, L. Epigenetic mechanisms in alzheimer’s disease: Implications for pathogenesis and therapy. Ageing Res. Rev. 2013, 12, 1024–1041. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Bai, B.; Mei, X.; Wan, C.; Cao, H.; Li, D.; Wang, S.; Zhang, M.; Wang, Z.; Wu, J.; et al. Elevated h3k79 homocysteinylation causes abnormal gene expression during neural development and subsequent neural tube defects. Nat. Commun. 2018, 9, 3436. [Google Scholar] [CrossRef] [PubMed]
- Belužić, L.; Grbeša, I.; Belužić, R.; Park, J.H.; Kong, H.K.; Kopjar, N.; Espadas, G.; Sabidó, E.; Lepur, A.; Rokić, F.; et al. Knock-down of ahcy and depletion of adenosine induces DNA damage and cell cycle arrest. Sci. Rep. 2018, 8, 14012. [Google Scholar] [CrossRef] [PubMed]
- Gurda, D.; Handschuh, L.; Kotkowiak, W.; Jakubowski, H. Homocysteine thiolactone and n-homocysteinylated protein induce pro-atherogenic changes in gene expression in human vascular endothelial cells. Amino Acids 2015, 47, 1319–1339. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Wang, J.; Yan, H.; Zhao, Y.X.; Liu, X.Y. A proteomics study of hyperhomocysteinemia injury of the hippocampal neurons using itraq. Mol. Med. Rep. 2014, 10, 2511–2516. [Google Scholar] [CrossRef]
- Suszynska-Zajczyk, J.; Jakubowski, H. Paraoxonase 1 and dietary hyperhomocysteinemia modulate the expression of mouse proteins involved in liver homeostasis. Acta Biochim. Pol. 2014, 61, 815–823. [Google Scholar] [CrossRef]
- Suszynska-Zajczyk, J.; Wroblewski, J.; Utyro, O.; Luczak, M.; Marczak, L.; Jakubowski, H. Bleomycin hydrolase and hyperhomocysteinemia modulate the expression of mouse proteins involved in liver homeostasis. Amino Acids 2014, 46, 1471–1480. [Google Scholar] [CrossRef]
- Suszynska-Zajczyk, J.; Sikora, M.; Jakubowski, H. Paraoxonase 1 deficiency and hyperhomocysteinemia alter the expression of mouse kidney proteins involved in renal disease. Mol. Genet. Metab. 2014, 113, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Suszynska-Zajczyk, J.; Utyro, O.; Jakubowski, H. Methionine-induced hyperhomocysteinemia and bleomycin hydrolase deficiency alter the expression of mouse kidney proteins involved in renal disease. Mol. Genet. Metab. 2014, 112, 339–346. [Google Scholar] [CrossRef] [PubMed]
- Suszynska-Zajczyk, J.; Luczak, M.; Marczak, L.; Jakubowski, H. Hyperhomocysteinemia and bleomycin hydrolase modulate the expression of mouse brain proteins involved in neurodegeneration. J. Alzheimers Dis. JAD 2014, 40, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Suszynska-Zajczyk, J.; Luczak, M.; Marczak, L.; Jakubowski, H. Inactivation of the paraoxonase 1 gene affects the expression of mouse brain proteins involved in neurodegeneration. J. Alzheimers Dis. JAD 2014, 42, 247–260. [Google Scholar] [CrossRef] [PubMed]
- DiBello, P.M.; Dayal, S.; Kaveti, S.; Zhang, D.; Kinter, M.; Lentz, S.R.; Jacobsen, D.W. The nutrigenetics of hyperhomocysteinemia: Quantitative proteomics reveals differences in the methionine cycle enzymes of gene-induced versus diet-induced hyperhomocysteinemia. Mol. Cell. Proteom. 2010, 9, 471–485. [Google Scholar] [CrossRef]
- Feng, P.N.; Liang, Y.R.; Lin, W.B.; Yao, Z.R.; Chen, D.B.; Chen, P.S.; Ouyang, J. Homocysteine induced oxidative stress in human umbilical vein endothelial cells via regulating methylation of sorbs1. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 6948–6958. [Google Scholar] [PubMed]
- Zhang, J.G.; Liu, J.X.; Li, Z.H.; Wang, L.Z.; Jiang, Y.D.; Wang, S.R. Dysfunction of endothelial no system originated from homocysteine-induced aberrant methylation pattern in promoter region of ddah2 gene. Chin. Med. J. (Engl.) 2007, 120, 2132–2137. [Google Scholar] [CrossRef]
- Jia, S.J.; Lai, Y.Q.; Zhao, M.; Gong, T.; Zhang, B.K. Homocysteine-induced hypermethylation of ddah2 promoter contributes to apoptosis of endothelial cells. Pharmazie 2013, 68, 282–286. [Google Scholar]
- Ma, S.C.; Hao, Y.J.; Jiao, Y.; Wang, Y.H.; Xu, L.B.; Mao, C.Y.; Yang, X.L.; Yang, A.N.; Tian, J.; Zhang, M.H.; et al. Homocysteine-induced oxidative stress through tlr4/nf-κb/dnmt1-mediated lox-1 DNA methylation in endothelial cells. Mol. Med. Rep. 2017, 16, 9181–9188. [Google Scholar] [CrossRef]
- Zhang, D.; Chen, Y.; Xie, X.; Liu, J.; Wang, Q.; Kong, W.; Zhu, Y. Homocysteine activates vascular smooth muscle cells by DNA demethylation of platelet-derived growth factor in endothelial cells. J. Mol. Cell. Cardiol. 2012, 53, 487–496. [Google Scholar] [CrossRef]
- Kim, C.S.; Kim, Y.R.; Naqvi, A.; Kumar, S.; Hoffman, T.A.; Jung, S.B.; Kumar, A.; Jeon, B.H.; McNamara, D.M.; Irani, K. Homocysteine promotes human endothelial cell dysfunction via site-specific epigenetic regulation of p66shc. Cardiovasc. Res. 2011, 92, 466–475. [Google Scholar] [CrossRef] [PubMed]
- Jamaluddin, M.D.; Chen, I.; Yang, F.; Jiang, X.; Jan, M.; Liu, X.; Schafer, A.I.; Durante, W.; Yang, X.; Wang, H. Homocysteine inhibits endothelial cell growth via DNA hypomethylation of the cyclin a gene. Blood 2007, 110, 3648–3655. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Xie, X.; Chen, Y.; Hammock, B.D.; Kong, W.; Zhu, Y. Homocysteine upregulates soluble epoxide hydrolase in vascular endothelium in vitro and in vivo. Circ. Res. 2012, 110, 808–817. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Sun, X.; Liu, J.; Xie, X.; Cui, W.; Zhu, Y. Homocysteine accelerates senescence of endothelial cells via DNA hypomethylation of human telomerase reverse transcriptase. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Sun, Y.; Gao, Y.; Yang, S.; Mao, C.; Ding, N.; Deng, M.; Wang, Y.; Yang, X.; Jia, Y.; et al. Reciprocal regulation between mir-148a/152 and DNA methyltransferase 1 is associated with hyperhomocysteinemia-accelerated atherosclerosis. DNA Cell Biol. 2017, 36, 462–474. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.N.; Zhang, H.P.; Sun, Y.; Yang, X.L.; Wang, N.; Zhu, G.; Zhang, H.; Xu, H.; Ma, S.C.; Zhang, Y.; et al. High-methionine diets accelerate atherosclerosis by hhcy-mediated fabp4 gene demethylation pathway via dnmt1 in apoe–/– mice. FEBS Lett. 2015, 589, 3998–4009. [Google Scholar] [CrossRef] [PubMed]
- Yideng, J.; Zhihong, L.; Jiantuan, X.; Jun, C.; Guizhong, L.; Shuren, W. Homocysteine-mediated pparalpha, gamma DNA methylation and its potential pathogenic mechanism in monocytes. DNA Cell Biol. 2008, 27, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.C.; Zhang, H.P.; Jiao, Y.; Wang, Y.H.; Zhang, H.; Yang, X.L.; Yang, A.N.; Jiang, Y.D. Homocysteine-induced proliferation of vascular smooth muscle cells occurs via pten hypermethylation and is mitigated by resveratrol. Mol. Med. Rep. 2018, 17, 5312–5319. [Google Scholar] [CrossRef]
- Cao, C.; Zhang, H.; Zhao, L.; Zhou, L.; Zhang, M.; Xu, H.; Han, X.; Li, G.; Yang, X.; Jiang, Y. Mir-125b targets dnmt3b and mediates p53 DNA methylation involving in the vascular smooth muscle cells proliferation induced by homocysteine. Exp. Cell Res. 2016, 347, 95–104. [Google Scholar] [CrossRef]
- Zhang, H.P.; Wang, Y.H.; Cao, C.J.; Yang, X.M.; Ma, S.C.; Han, X.B.; Yang, X.L.; Yang, A.N.; Tian, J.; Xu, H.; et al. A regulatory circuit involving mir-143 and dnmt3a mediates vascular smooth muscle cell proliferation induced by homocysteine. Mol. Med. Rep. 2016, 13, 483–490. [Google Scholar] [CrossRef]
- Xu, L.; Hao, H.; Hao, Y.; Wei, G.; Li, G.; Ma, P.; Ding, N.; Ma, S.; Chen, A.F.; Jiang, Y. Aberrant mfn2 transcription facilitates homocysteine-induced vsmcs proliferation via the increased binding of c-myc to dnmt1 in atherosclerosis. J. Cell Mol. Med. 2019, 23, 4611–4626. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, P.; Kalani, A.; Givvimani, S.; Kamat, P.K.; Familtseva, A.; Tyagi, S.C. Differential regulation of DNA methylation versus histone acetylation in cardiomyocytes during hhcy in vitro and in vivo: An epigenetic mechanism. Physiol. Genom. 2014, 46, 245–255. [Google Scholar] [CrossRef]
- Yang, A.; Jiao, Y.; Yang, S.; Deng, M.; Yang, X.; Mao, C.; Sun, Y.; Ding, N.; Li, N.; Zhang, M.; et al. Homocysteine activates autophagy by inhibition of cftr expression via interaction between DNA methylation and h3k27me3 in mouse liver. Cell Death Dis. 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Elmasry, K.; Mohamed, R.; Sharma, I.; Elsherbiny, N.M.; Liu, Y.; Al-Shabrawey, M.; Tawfik, A. Epigenetic modifications in hyperhomocysteinemia: Potential role in diabetic retinopathy and age-related macular degeneration. Oncotarget 2018, 9, 12562–12590. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Tyagi, S.C.; Tyagi, N. Hyperhomocysteinemia induced endothelial progenitor cells dysfunction through hyper-methylation of cbs promoter. Biochem. Biophys. Res. Commun. 2019, 510, 135–141. [Google Scholar] [CrossRef]
- Kamat, P.K.; Kalani, A.; Tyagi, S.C.; Tyagi, N. Hydrogen sulfide epigenetically attenuates homocysteine-induced mitochondrial toxicity mediated through nmda receptor in mouse brain endothelial (bend3) cells. J. Cell. Physiol. 2015, 230, 378–394. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Kamat, P.K.; Familtseva, A.; Chaturvedi, P.; Muradashvili, N.; Narayanan, N.; Tyagi, S.C.; Tyagi, N. Role of microrna29b in blood-brain barrier dysfunction during hyperhomocysteinemia: An epigenetic mechanism. J. Cereb. Blood Flow Metab. 2014, 34, 1212–1222. [Google Scholar] [CrossRef]
- Veeranki, S.; Winchester, L.J.; Tyagi, S.C. Hyperhomocysteinemia associated skeletal muscle weakness involves mitochondrial dysfunction and epigenetic modifications. Biochim. Biophys. Acta 2015, 1852, 732–741. [Google Scholar] [CrossRef]
- Li, J.G.; Barrero, C.; Gupta, S.; Kruger, W.D.; Merali, S.; Pratico, D. Homocysteine modulates 5-lipoxygenase expression level via DNA methylation. Aging Cell 2017, 16, 273–280. [Google Scholar] [CrossRef]
- Gupta, S.; Kuhnisch, J.; Mustafa, A.; Lhotak, S.; Schlachterman, A.; Slifker, M.J.; Klein-Szanto, A.; High, K.A.; Austin, R.C.; Kruger, W.D. Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 2009, 23, 883–893. [Google Scholar] [CrossRef]
- Zhang, D.; Wen, X.; Wu, W.; Xu, E.; Zhang, Y.; Cui, W. Homocysteine-related htert DNA demethylation contributes to shortened leukocyte telomere length in atherosclerosis. Atherosclerosis 2013, 231, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.H.; Wen, X.M.; Zhang, L.; Cui, W. DNA methylation of human telomerase reverse transcriptase associated with leukocyte telomere length shortening in hyperhomocysteinemia-type hypertension in humans and in a rat model. Circ. J. 2014, 78, 1915–1923. [Google Scholar] [CrossRef] [PubMed]
- Kalani, A.; Kamat, P.K.; Givvimani, S.; Brown, K.; Metreveli, N.; Tyagi, S.C.; Tyagi, N. Nutri-epigenetics ameliorates blood-brain barrier damage and neurodegeneration in hyperhomocysteinemia: Role of folic acid. J. Mol. Neurosci. 2014, 52, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Pogribny, I.P.; Dreval, K.; Kindrat, I.; Melnyk, S.; Jimenez, L.; de Conti, A.; Tryndyak, V.; Pogribna, M.; Ortega, J.F.; James, S.J.; et al. Epigenetically mediated inhibition of s-adenosylhomocysteine hydrolase and the associated dysregulation of 1-carbon metabolism in nonalcoholic steatohepatitis and hepatocellular carcinoma. FASEB J. 2018, 32, 1591–1601. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Kutanzi, K.; Melnyk, S.; de Conti, A.; Tryndyak, V.; Montgomery, B.; Pogribna, M.; Muskhelishvili, L.; Latendresse, J.R.; James, S.J.; et al. Strain-dependent dysregulation of one-carbon metabolism in male mice is associated with choline- and folate-deficient diet-induced liver injury. FASEB J. 2013, 27, 2233–2243. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Ross, S.A.; Tryndyak, V.P.; Pogribna, M.; Poirier, L.A.; Karpinets, T.V. Histone h3 lysine 9 and h4 lysine 20 trimethylation and the expression of suv4-20h2 and suv-39h1 histone methyltransferases in hepatocarcinogenesis induced by methyl deficiency in rats. Carcinogenesis 2006, 27, 1180–1186. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Ross, S.A.; Wise, C.; Pogribna, M.; Jones, E.A.; Tryndyak, V.P.; James, S.J.; Dragan, Y.P.; Poirier, L.A. Irreversible global DNA hypomethylation as a key step in hepatocarcinogenesis induced by dietary methyl deficiency. Mutat. Res. 2006, 593, 80–87. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Karpf, A.R.; James, S.R.; Melnyk, S.; Han, T.; Tryndyak, V.P. Epigenetic alterations in the brains of fisher 344 rats induced by long-term administration of folate/methyl-deficient diet. Brain Res. 2008, 1237, 25–34. [Google Scholar] [CrossRef]
- Jiang, Y.; Sun, T.; Xiong, J.; Cao, J.; Li, G.; Wang, S. Hyperhomocysteinemia-mediated DNA hypomethylation and its potential epigenetic role in rats. Acta Biochim. Biophys. Sin. (Shanghai) 2007, 39, 657–667. [Google Scholar] [CrossRef]
- Bauer, A.J.; Martin, K.A. Coordinating regulation of gene expression in cardiovascular disease: Interactions between chromatin modifiers and transcription factors. Front. Cardiovasc. Med. 2017, 4, 19. [Google Scholar] [CrossRef]
- Fuks, F.; Burgers, W.A.; Godin, N.; Kasai, M.; Kouzarides, T. Dnmt3a binds deacetylases and is recruited by a sequence-specific repressor to silence transcription. EMBO J. 2001, 20, 2536–2544. [Google Scholar] [CrossRef]
- Ma, P.; de Waal, E.; Weaver, J.R.; Bartolomei, M.S.; Schultz, R.M. A dnmt3a2-hdac2 complex is essential for genomic imprinting and genome integrity in mouse oocytes. Cell Rep. 2015, 13, 1552–1560. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, A.; Jurkowska, R.Z. Allosteric control of mammalian DNA methyltransferases—A new regulatory paradigm. Nucleic Acids Res. 2016, 44, 8556–8575. [Google Scholar] [CrossRef] [PubMed]
- Otani, J.; Nankumo, T.; Arita, K.; Inamoto, S.; Ariyoshi, M.; Shirakawa, M. Structural basis for recognition of h3k4 methylation status by the DNA methyltransferase 3a atrx-dnmt3-dnmt3l domain. EMBO Rep. 2009, 10, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Dhayalan, A.; Rajavelu, A.; Rathert, P.; Tamas, R.; Jurkowska, R.Z.; Ragozin, S.; Jeltsch, A. The dnmt3a pwwp domain reads histone 3 lysine 36 trimethylation and guides DNA methylation. J. Biol. Chem. 2010, 285, 26114–26120. [Google Scholar] [CrossRef] [PubMed]
- Denis, H.; Ndlovu, M.N.; Fuks, F. Regulation of mammalian DNA methyltransferases: A route to new mechanisms. EMBO Rep. 2011, 12, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Gabory, A.; Attig, L.; Junien, C. Epigenetic mechanisms involved in developmental nutritional programming. World J. Diabetes 2011, 2, 164–175. [Google Scholar] [CrossRef] [PubMed]
- Wyatt, G.R.; Cohen, S.S. The bases of the nucleic acids of some bacterial and animal viruses: The occurrence of 5-hydroxymethylcytosine. Biochem. J. 1953, 55, 774–782. [Google Scholar] [CrossRef]
- Penn, N.W.; Suwalski, R.; O’Riley, C.; Bojanowski, K.; Yura, R. The presence of 5-hydroxymethylcytosine in animal deoxyribonucleic acid. Biochem. J. 1972, 126, 781–790. [Google Scholar] [CrossRef]
- Richa, R.; Sinha, R.P. Hydroxymethylation of DNA: An epigenetic marker. EXCLI J. 2014, 13, 592–610. [Google Scholar]
- Kinney, S.M.; Chin, H.G.; Vaisvila, R.; Bitinaite, J.; Zheng, Y.; Estève, P.O.; Feng, S.; Stroud, H.; Jacobsen, S.E.; Pradhan, S. Tissue-specific distribution and dynamic changes of 5-hydroxymethylcytosine in mammalian genomes. J. Biol. Chem. 2011, 286, 24685–24693. [Google Scholar] [CrossRef] [PubMed]
- Kriaucionis, S.; Heintz, N. The nuclear DNA base 5-hydroxymethylcytosine is present in purkinje neurons and the brain. Science 2009, 324, 929–930. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.U.; Su, Y.; Zhong, C.; Ming, G.L.; Song, H. Hydroxylation of 5-methylcytosine by tet1 promotes active DNA demethylation in the adult brain. Cell 2011, 145, 423–434. [Google Scholar] [CrossRef] [PubMed]
- Ehrlich, M.; Ehrlich, K.C. DNA cytosine methylation and hydroxymethylation at the borders. Epigenomics 2014, 6, 563–566. [Google Scholar] [CrossRef] [PubMed]
- McKee, S.E.; Zhang, S.; Chen, L.; Rabinowitz, J.D.; Reyes, T.M. Perinatal high fat diet and early life methyl donor supplementation alter one carbon metabolism and DNA methylation in the brain. J. Neurochem. 2018, 145, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Glier, M.B.; Ngai, Y.F.; Sulistyoningrum, D.C.; Aleliunas, R.E.; Bottiglieri, T.; Devlin, A.M. Tissue-specific relationship of s-adenosylhomocysteine with allele-specific h19/igf2 methylation and imprinting in mice with hyperhomocysteinemia. Epigenetics 2013, 8, 44–53. [Google Scholar] [CrossRef]
- Lu, S.C. S-adenosylmethionine. Int. J. Biochem. Cell Biol. 2000, 32, 391–395. [Google Scholar] [CrossRef]
- Saavedra, O.M.; Isakovic, L.; Llewellyn, D.B.; Zhan, L.; Bernstein, N.; Claridge, S.; Raeppel, F.; Vaisburg, A.; Elowe, N.; Petschner, A.J.; et al. Sar around (l)-s-adenosyl-l-homocysteine, an inhibitor of human DNA methyltransferase (dnmt) enzymes. Bioorg. Med. Chem. Lett. 2009, 19, 2747–2751. [Google Scholar] [CrossRef]
- Zappia, V.; Zydek-Cwick, R.; Schlenk, F. The specificity of s-adenosylmethionine derivatives in methyl transfer reactions. J. Biol. Chem. 1969, 244, 4499–4509. [Google Scholar]
- Ponnaluri, V.K.C.; Estève, P.O.; Ruse, C.I.; Pradhan, S. S-adenosylhomocysteine hydrolase participates in DNA methylation inheritance. J. Mol. Biol. 2018, 430, 2051–2065. [Google Scholar] [CrossRef]
- Lee, H.O.; Wang, L.; Kuo, Y.M.; Andrews, A.J.; Gupta, S.; Kruger, W.D. S-adenosylhomocysteine hydrolase over-expression does not alter s-adenosylmethionine or s-adenosylhomocysteine levels in cbs deficient mice. Mol. Genet. Metab. Rep. 2018, 15, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Paudel, K.R.; Panth, N.; Kim, D.W. Circulating endothelial microparticles: A key hallmark of atherosclerosis progression. Scientifica (Cairo) 2016, 2016, 8514056. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Ogura, S.; Chen, J.; Little, P.J.; Moss, J.; Liu, P. Lox-1 in atherosclerosis: Biological functions and pharmacological modifiers. Cell. Mol. Life Sci. 2013, 70, 2859–2872. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.J.; Jia, S.J.; Dai, Z.; Li, Y.J. Asymmetric dimethylarginine induces apoptosis via p38 mapk/caspase-3-dependent signaling pathway in endothelial cells. J. Mol. Cell. Cardiol. 2006, 40, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Palm, F.; Onozato, M.L.; Luo, Z.; Wilcox, C.S. Dimethylarginine dimethylaminohydrolase (ddah): Expression, regulation, and function in the cardiovascular and renal systems. Am. J. Physiol. Heart Circ. Physiol. 2007, 293, H3227–H3245. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.; Jiang, D.J.; Huang, H.; Jia, S.J.; Jiang, J.L.; Hu, C.P.; Li, Y.J. Taurine protects against low-density lipoprotein-induced endothelial dysfunction by the ddah/adma pathway. Vasc. Pharmacol. 2007, 46, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xia, J.; Cheng, J.; Huang, H.; Zhou, Y.; Yang, X.; Su, X.; Ke, Y.; Ling, W. Inhibition of s-adenosylhomocysteine hydrolase induces endothelial dysfunction via epigenetic regulation of p66shc-mediated oxidative stress pathway. Circulation 2019, 139, 2260–2277. [Google Scholar] [CrossRef]
- Liao, J.K. Linking endothelial dysfunction with endothelial cell activation. J. Clin. Investig. 2013, 123, 540–541. [Google Scholar] [CrossRef]
- Sher, T.; Yi, H.F.; McBride, O.W.; Gonzalez, F.J. Cdna cloning, chromosomal mapping, and functional characterization of the human peroxisome proliferator activated receptor. Biochemistry 1993, 32, 5598–5604. [Google Scholar] [CrossRef]
- Khateeb, J.; Gantman, A.; Kreitenberg, A.J.; Aviram, M.; Fuhrman, B. Paraoxonase 1 (pon1) expression in hepatocytes is upregulated by pomegranate polyphenols: A role for ppar-gamma pathway. Atherosclerosis 2010, 208, 119–125. [Google Scholar] [CrossRef]
- Panayiotou, A.G.; Nicolaides, A.N.; Griffin, M.; Tyllis, T.; Georgiou, N.; Bond, D.; Martin, R.M.; Hoppensteadt, D.; Fareed, J.; Humphries, S.E. Leukocyte telomere length is associated with measures of subclinical atherosclerosis. Atherosclerosis 2010, 211, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Spence, J.D. Nutrition and risk of stroke. Nutrients 2019, 11, 647. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xu, G.; Wen, Q.; Peng, X.; Chen, H.; Zhang, J.; Xu, S.; Zhang, C.; Zhang, M.; Ma, J.; et al. Cbs promoter hypermethylation increases the risk of hypertension and stroke. Clinics (Sao Paulo) 2019, 74, e630. [Google Scholar] [CrossRef] [PubMed]
- Ingrosso, D.; Cimmino, A.; Perna, A.F.; Masella, L.; De Santo, N.G.; De Bonis, M.L.; Vacca, M.; D’Esposito, M.; D’Urso, M.; Galletti, P.; et al. Folate treatment and unbalanced methylation and changes of allelic expression induced by hyperhomocysteinaemia in patients with uraemia. Lancet 2003, 361, 1693–1699. [Google Scholar] [CrossRef]
- Hainsworth, A.H.; Yeo, N.E.; Weekman, E.M.; Wilcock, D.M. Homocysteine, hyperhomocysteinemia and vascular contributions to cognitive impairment and dementia (vcid). Biochim. Biophys. Acta 2016, 1862, 1008–1017. [Google Scholar] [CrossRef]
- Scarpa, S.; Fuso, A.; D’Anselmi, F.; Cavallaro, R.A. Presenilin 1 gene silencing by s-adenosylmethionine: A treatment for alzheimer disease? FEBS Lett. 2003, 541, 145–148. [Google Scholar] [CrossRef]
- Fuso, A.; Seminara, L.; Cavallaro, R.A.; D’Anselmi, F.; Scarpa, S. S-adenosylmethionine/homocysteine cycle alterations modify DNA methylation status with consequent deregulation of ps1 and bace and beta-amyloid production. Mol. Cell. Neurosci. 2005, 28, 195–204. [Google Scholar] [CrossRef]
- Gilbert, N.C.; Bartlett, S.G.; Waight, M.T.; Neau, D.B.; Boeglin, W.E.; Brash, A.R.; Newcomer, M.E. The structure of human 5-lipoxygenase. Science 2011, 331, 217–219. [Google Scholar] [CrossRef]
- Chinnici, C.M.; Yao, Y.; Praticò, D. The 5-lipoxygenase enzymatic pathway in the mouse brain: Young versus old. Neurobiol. Aging 2007, 28, 1457–1462. [Google Scholar] [CrossRef]
- Ikonomovic, M.D.; Abrahamson, E.E.; Uz, T.; Manev, H.; Dekosky, S.T. Increased 5-lipoxygenase immunoreactivity in the hippocampus of patients with alzheimer’s disease. J. Histochem. Cytochem. 2008, 56, 1065–1073. [Google Scholar] [CrossRef]
- Chu, J.; Giannopoulos, P.F.; Ceballos-Diaz, C.; Golde, T.E.; Praticò, D. 5-lipoxygenase gene transfer worsens memory, amyloid, and tau brain pathologies in a mouse model of alzheimer disease. Ann. Neurol. 2012, 72, 442–454. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, A.; Arosio, B.; Falconi, A.; Micioni Di Bonaventura, M.V.; Karimi, M.; Mari, D.; Casati, M.; Maccarrone, M.; D’Addario, C. Global changes in DNA methylation in alzheimer’s disease peripheral blood mononuclear cells. Brain Behav. Immun. 2015, 45, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Bednarska-Makaruk, M.; Graban, A.; Sobczyńska-Malefora, A.; Harrington, D.J.; Mitchell, M.; Voong, K.; Dai, L.; Łojkowska, W.; Bochyńska, A.; Ryglewicz, D.; et al. Homocysteine metabolism and the associations of global DNA methylation with selected gene polymorphisms and nutritional factors in patients with dementia. Exp. Gerontol. 2016, 81, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Chouliaras, L.; Mastroeni, D.; Delvaux, E.; Grover, A.; Kenis, G.; Hof, P.R.; Steinbusch, H.W.; Coleman, P.D.; Rutten, B.P.; van den Hove, D.L. Consistent decrease in global DNA methylation and hydroxymethylation in the hippocampus of alzheimer’s disease patients. Neurobiol. Aging 2013, 34, 2091–2099. [Google Scholar] [CrossRef] [PubMed]
- Condliffe, D.; Wong, A.; Troakes, C.; Proitsi, P.; Patel, Y.; Chouliaras, L.; Fernandes, C.; Cooper, J.; Lovestone, S.; Schalkwyk, L.; et al. Cross-region reduction in 5-hydroxymethylcytosine in alzheimer’s disease brain. Neurobiol. Aging 2014, 35, 1850–1854. [Google Scholar] [CrossRef] [PubMed]
- Mastroeni, D.; Grover, A.; Delvaux, E.; Whiteside, C.; Coleman, P.D.; Rogers, J. Epigenetic changes in alzheimer’s disease: Decrements in DNA methylation. Neurobiol. Aging 2010, 31, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Coppieters, N.; Dieriks, B.V.; Lill, C.; Faull, R.L.; Curtis, M.A.; Dragunow, M. Global changes in DNA methylation and hydroxymethylation in alzheimer’s disease human brain. Neurobiol. Aging 2014, 35, 1334–1344. [Google Scholar] [CrossRef] [PubMed]
- Cacabelos, R.; Torrellas, C. Epigenetics of aging and alzheimer’s disease: Implications for pharmacogenomics and drug response. Int. J. Mol. Sci. 2015, 16, 30483–30543. [Google Scholar] [CrossRef]
- Smith, A.D.; Refsum, H. Homocysteine, b vitamins, and cognitive impairment. Annu. Rev. Nutr. 2016, 36, 211–239. [Google Scholar] [CrossRef]
- Flitton, M.; Rielly, N.; Warman, R.; Warden, D.; Smith, A.D.; Macdonald, I.A.; Knight, H.M. Interaction of nutrition and genetics via dnmt3l-mediated DNA methylation determines cognitive decline. Neurobiol. Aging 2019, 78, 64–73. [Google Scholar] [CrossRef]
- Mandaviya, P.R.; Aïssi, D.; Dekkers, K.F.; Joehanes, R.; Kasela, S.; Truong, V.; Stolk, L.; Heemst, D.V.; Ikram, M.A.; Lindemans, J.; et al. Homocysteine levels associate with subtle changes in leukocyte DNA methylation: An epigenome-wide analysis. Epigenomics 2017, 9, 1403–1422. [Google Scholar] [CrossRef] [PubMed]
- Egging, D.; van den Berkmortel, F.; Taylor, G.; Bristow, J.; Schalkwijk, J. Interactions of human tenascin-x domains with dermal extracellular matrix molecules. Arch. Dermatol. Res. 2007, 298, 389–396. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arif, A.; Terenzi, F.; Potdar, A.A.; Jia, J.; Sacks, J.; China, A.; Halawani, D.; Vasu, K.; Li, X.; Brown, J.M.; et al. Eprs is a critical mtorc1-s6k1 effector that influences adiposity in mice. Nature 2017, 542, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Friso, S.; Choi, S.W.; Girelli, D.; Mason, J.B.; Dolnikowski, G.G.; Bagley, P.J.; Olivieri, O.; Jacques, P.F.; Rosenberg, I.H.; Corrocher, R.; et al. A common mutation in the 5,10-methylenetetrahydrofolate reductase gene affects genomic DNA methylation through an interaction with folate status. Proc. Natl. Acad. Sci. USA 2002, 99, 5606–5611. [Google Scholar] [CrossRef] [PubMed]
- Nash, A.J.; Mandaviya, P.R.; Dib, M.J.; Uitterlinden, A.G.; van Meurs, J.; Heil, S.G.; Andrew, T.; Ahmadi, K.R. Interaction between plasma homocysteine and the mthfr c.677c > t polymorphism is associated with site-specific changes in DNA methylation in humans. FASEB J. 2018, 33, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Mandaviya, P.R.; Joehanes, R.; Aïssi, D.; Kühnel, B.; Marioni, R.E.; Truong, V.; Stolk, L.; Beekman, M.; Bonder, M.J.; Franke, L.; et al. Genetically defined elevated homocysteine levels do not result in widespread changes of DNA methylation in leukocytes. PLoS ONE 2017, 12, e0182472. [Google Scholar] [CrossRef] [PubMed]
- Geisel, J.; Schorr, H.; Bodis, M.; Isber, S.; Hübner, U.; Knapp, J.P.; Obeid, R.; Herrmann, W. The vegetarian lifestyle and DNA methylation. Clin. Chem. Lab. Med. 2005, 43, 1164–1169. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Qu, Q.M. Hypermethylation of era-a gene and high serum homocysteine level are correlated with cognitive impairment in white matter hyperintensity patients. QJM Int. J. Med. 2019, 112, 351–354. [Google Scholar] [CrossRef]
- Tessarz, P.; Kouzarides, T. Histone core modifications regulating nucleosome structure and dynamics. Nat. Rev. Mol. Cell Biol. 2014, 15, 703–708. [Google Scholar] [CrossRef]
- Smolle, M.; Workman, J.L. Transcription-associated histone modifications and cryptic transcription. Biochim. Biophys. Acta 2013, 1829, 84–97. [Google Scholar] [CrossRef]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Eberharter, A.; Becker, P.B. Histone acetylation: A switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002, 3, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Chernaya, V.; Johnson, C.; Yang, W.Y.; Cueto, R.; Sha, X.; Zhang, Y.; Qin, X.; Sun, J.; Choi, E.T.; et al. Metabolic diseases downregulate the majority of histone modification enzymes, making a few upregulated enzymes novel therapeutic targets—“Sand out and gold stays”. J. Cardiovasc. Transl. Res. 2016, 9, 49–66. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Swanson, S.K.; Gogol, M.; Florens, L.; Washburn, M.P.; Workman, J.L.; Suganuma, T. Serine and sam responsive complex sesame regulates histone modification crosstalk by sensing cellular metabolism. Mol. Cell 2015, 60, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Wellen, K.E.; Rabinowitz, J.D. Metabolic control of methylation and acetylation. Curr. Opin. Chem. Biol. 2016, 30, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H.; Zhang, L.; Bardeguez, A.; Aviv, A. Homocysteine thiolactone and protein homocysteinylation in human endothelial cells—Implications for atherosclerosis. Circ. Res. 2000, 87, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Behera, J.; Kelly, K.E.; Voor, M.J.; Metreveli, N.; Tyagi, S.C.; Tyagi, N. Hydrogen sulfide promotes bone homeostasis by balancing inflammatory cytokine signaling in cbs-deficient mice through an epigenetic mechanism. Sci. Rep. 2018, 8, 15226. [Google Scholar] [CrossRef]
- Mentch, S.J.; Mehrmohamadi, M.; Huang, L.; Liu, X.; Gupta, D.; Mattocks, D.; Gómez Padilla, P.; Ables, G.; Bamman, M.M.; Thalacker-Mercer, A.E.; et al. Histone methylation dynamics and gene regulation occur through the sensing of one-carbon metabolism. Cell Metab. 2015, 22, 861–873. [Google Scholar] [CrossRef]
- Gurda, D.; Marczak, L.; Jakubowski, H. Histones are targeted for nhomocysteinylation in human endothelial cells. Acta Biochim. Pol. 2014, 61, 127. [Google Scholar]
- Tóthová, B.; Kovalská, M.; Kalenská, D.; Tomašcová, A.; Lehotský, J. Histone hyperacetylation as a response to global brain ischemia associated with hyperhomocysteinemia in rats. Int. J. Mol. Sci. 2018, 19, 3147. [Google Scholar] [CrossRef]
- Esse, R.; Florindo, C.; Imbard, A.; Rocha, M.S.; de Vriese, A.S.; Smulders, Y.M.; Teerlink, T.; Tavares de Almeida, I.; Castro, R.; Blom, H.J. Global protein and histone arginine methylation are affected in a tissue-specific manner in a rat model of diet-induced hyperhomocysteinemia. Biochim. Biophys. Acta 2013, 1832, 1708–1714. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Mentch, S.J.; Gao, X.; Nichenametla, S.N.; Locasale, J.W. Methionine metabolism influences genomic architecture and gene expression through h3k4me3 peak width. Nat. Commun. 2018, 9, 1955. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Chen, J.; Gao, J.; Yu, H.; Yang, P. Crosstalk of homocysteinylation, methylation and acetylation on histone h3. Analyst 2015, 140, 3057–3063. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Liu, J.; Qiao, Z.; Liu, Y.; Yang, Y.; Jiang, C.; Wang, X.; Wang, C. Chemical proteomic profiling of protein. Chem. Sci. 2018, 9, 2826–2830. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, D.R.; Schreiber, S.L. Molecular association between atr and two components of the nucleosome remodeling and deacetylating complex, hdac2 and chd4. Biochemistry 1999, 38, 14711–14717. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H. Proofreading invivo—Editing of homocysteine by methionyl-transfer rna-synthetase in the yeast saccharomyces-cerevisiae. EMBO J. 1991, 10, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Jakubowski, H.; Goldman, E. Synthesis of homocysteine thiolactone by methionyl-transfer rna-synthetase in cultured-mammalian-cells. FEBS Lett. 1993, 317, 237–240. [Google Scholar] [CrossRef]
- Bossenmeyer-Pourie, C.; Smith, A.D.; Lehmann, S.; Deramecourt, V.; Sablonniere, B.; Camadro, J.M.; Pourie, G.; Kerek, R.; Helle, D.; Umoret, R.; et al. N-homocysteinylation of tau and map1 is increased in autopsy specimens of alzheimer’s disease and vascular dementia. J. Pathol. 2019, 248, 291–303. [Google Scholar] [CrossRef]
- Noh, K.M.; Allis, C.D.; Li, H. Reading between the lines: “Add”-ing histone and DNA methylation marks toward a new epigenetic “sum”. ACS Chem. Biol. 2016, 11, 554–563. [Google Scholar] [CrossRef]
- Zhang, Y.; Jurkowska, R.; Soeroes, S.; Rajavelu, A.; Dhayalan, A.; Bock, I.; Rathert, P.; Brandt, O.; Reinhardt, R.; Fischle, W.; et al. Chromatin methylation activity of dnmt3a and dnmt3a/3l is guided by interaction of the add domain with the histone h3 tail. Nucleic Acids Res. 2010, 38, 4246–4253. [Google Scholar] [CrossRef]
- Li, B.Z.; Huang, Z.; Cui, Q.Y.; Song, X.H.; Du, L.; Jeltsch, A.; Chen, P.; Li, G.; Li, E.; Xu, G.L. Histone tails regulate DNA methylation by allosterically activating de novo methyltransferase. Cell Res. 2011, 21, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Wang, L.; Li, J.; Ding, Z.; Xiao, J.; Yin, X.; He, S.; Shi, P.; Dong, L.; Li, G.; et al. Structural insight into autoinhibition and histone h3-induced activation of dnmt3a. Nature 2015, 517, 640–644. [Google Scholar] [CrossRef]
- Goll, M.G.; Bestor, T.H. Eukaryotic cytosine methyltransferases. Annu. Rev. Biochem. 2005, 74, 481–514. [Google Scholar] [CrossRef] [PubMed]
- Geisel, J.; Schorr, H.; Heine, G.H.; Bodis, M.; Hübner, U.; Knapp, J.P.; Herrmann, W. Decreased p66shc promoter methylation in patients with end-stage renal disease. Clin. Chem. Lab. Med. 2007, 45, 1764–1770. [Google Scholar] [CrossRef] [PubMed]
- Impagnatiello, F.; Guidotti, A.R.; Pesold, C.; Dwivedi, Y.; Caruncho, H.; Pisu, M.G.; Uzunov, D.P.; Smalheiser, N.R.; Davis, J.M.; Pandey, G.N.; et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc. Natl. Acad. Sci. USA 1998, 95, 15718–15723. [Google Scholar] [CrossRef] [PubMed]
- D’Arcangelo, G.; Miao, G.G.; Curran, T. Detection of the reelin breakpoint in reeler mice. Brain Res. Mol. Brain Res. 1996, 39, 234–236. [Google Scholar] [CrossRef]
- Tremolizzo, L.; Carboni, G.; Ruzicka, W.B.; Mitchell, C.P.; Sugaya, I.; Tueting, P.; Sharma, R.; Grayson, D.R.; Costa, E.; Guidotti, A. An epigenetic mouse model for molecular and behavioral neuropathologies related to schizophrenia vulnerability. Proc. Natl. Acad. Sci. USA 2002, 99, 17095–17100. [Google Scholar] [CrossRef]
- Tremolizzo, L.; Doueiri, M.S.; Dong, E.; Grayson, D.R.; Davis, J.; Pinna, G.; Tueting, P.; Rodriguez-Menendez, V.; Costa, E.; Guidotti, A. Valproate corrects the schizophrenia-like epigenetic behavioral modifications induced by methionine in mice. Biol. Psychiatry 2005, 57, 500–509. [Google Scholar] [CrossRef]
- Dong, E.; Nelson, M.; Grayson, D.R.; Costa, E.; Guidotti, A. Clozapine and sulpiride but not haloperidol or olanzapine activate brain DNA demethylation. Proc. Natl. Acad. Sci. USA 2008, 105, 13614–13619. [Google Scholar] [CrossRef]
- Dong, E.; Guidotti, A.; Grayson, D.R.; Costa, E. Histone hyperacetylation induces demethylation of reelin and 67-kda glutamic acid decarboxylase promoters. Proc. Natl. Acad. Sci. USA 2007, 104, 4676–4681. [Google Scholar] [CrossRef]
- Bartel, D.P. Micrornas: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Cheng, A.M.; Byrom, M.W.; Shelton, J.; Ford, L.P. Antisense inhibition of human mirnas and indications for an involvement of mirna in cell growth and apoptosis. Nucleic Acids Res. 2005, 33, 1290–1297. [Google Scholar] [CrossRef] [PubMed]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. Mir-155 gene: A typical multifunctional microrna. Biochim. Biophys. Acta 2009, 1792, 497–505. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C. Microrna and vascular smooth muscle cell phenotype: New therapy for atherosclerosis? Genome Med. 2009, 1, 85. [Google Scholar] [CrossRef] [PubMed]
- Hosin, A.A.; Prasad, A.; Viiri, L.E.; Davies, A.H.; Shalhoub, J. Micrornas in atherosclerosis. J. Vasc. Res. 2014, 51, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.J.; Zhou, Y.; Chen, L.; Wang, Y.Q.; Wang, X.; Pi, Y.; Gao, C.Y.; Li, J.C.; Zhang, L.L. An overview of potential molecular mechanisms involved in vsmc phenotypic modulation. Histochem. Cell Biol. 2016, 145, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, X.; Yang, J.; Lin, Y.; Xu, D.Z.; Lu, Q.; Deitch, E.A.; Huo, Y.; Delphin, E.S.; Zhang, C. Microrna-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 2009, 105, 158–166. [Google Scholar] [CrossRef]
- Hutcheson, R.; Terry, R.; Chaplin, J.; Smith, E.; Musiyenko, A.; Russell, J.C.; Lincoln, T.; Rocic, P. Microrna-145 restores contractile vascular smooth muscle phenotype and coronary collateral growth in the metabolic syndrome. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 727–736. [Google Scholar] [CrossRef]
- Antoniades, C.; Bakogiannis, C.; Tousoulis, D.; Antonopoulos, A.S.; Stefanadis, C. The cd40/cd40 ligand system: Linking inflammation with atherothrombosis. J. Am. Coll. Cardiol. 2009, 54, 669–677. [Google Scholar] [CrossRef]
- Dong, S.; Xiong, W.; Yuan, J.; Li, J.; Liu, J.; Xu, X. Mirna-146a regulates the maturation and differentiation of vascular smooth muscle cells by targeting nf-κb expression. Mol. Med. Rep. 2013, 8, 407–412. [Google Scholar] [CrossRef]
- Rangrez, A.Y.; Massy, Z.A.; Metzinger-Le Meuth, V.; Metzinger, L. Mir-143 and mir-145: Molecular keys to switch the phenotype of vascular smooth muscle cells. Circ. Cardiovasc. Genet. 2011, 4, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Moshal, K.S.; Kumar, M.; Tyagi, N.; Mishra, P.K.; Metreveli, N.; Rodriguez, W.E.; Tyagi, S.C. Restoration of contractility in hyperhomocysteinemia by cardiac-specific deletion of nmda-r1. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H887–H892. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, N.; Vacek, J.C.; Givvimani, S.; Sen, U.; Tyagi, S.C. Cardiac specific deletion of n-methyl-d-aspartate receptor 1 ameliorates mtmmp-9 mediated autophagy/mitophagy in hyperhomocysteinemia. J. Recept. Signal Transduct. Res. 2010, 30, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.K.; Tyagi, N.; Kundu, S.; Tyagi, S.C. Micrornas are involved in homocysteine-induced cardiac remodeling. Cell Biochem. Biophys. 2009, 55, 153–162. [Google Scholar] [CrossRef] [PubMed]
- Nandi, S.S.; Mishra, P.K. H2S and homocysteine control a novel feedback regulation of cystathionine beta synthase and cystathionine gamma lyase in cardiomyocytes. Sci. Rep. 2017, 7, 3639. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, S.C.; Lominadze, D.; Roberts, A.M. Homocysteine in microvascular endothelial cell barrier permeability. Cell Biochem. Biophys. 2005, 43, 37–44. [Google Scholar] [CrossRef]
- Brannan, C.I.; Dees, E.C.; Ingram, R.S.; Tilghman, S.M. The product of the h19 gene may function as an rna. Mol. Cell. Biol. 1990, 10, 28–36. [Google Scholar] [CrossRef]
- Gabory, A.; Jammes, H.; Dandolo, L. The h19 locus: Role of an imprinted non-coding rna in growth and development. Bioessays 2010, 32, 473–480. [Google Scholar] [CrossRef]
- Leighton, P.A.; Saam, J.R.; Ingram, R.S.; Stewart, C.L.; Tilghman, S.M. An enhancer deletion affects both h19 and igf2 expression. Genes Dev. 1995, 9, 2079–2089. [Google Scholar] [CrossRef]
- Gao, Z.H.; Suppola, S.; Liu, J.; Heikkilä, P.; Jänne, J.; Voutilainen, R. Association of h19 promoter methylation with the expression of h19 and igf-ii genes in adrenocortical tumors. J. Clin. Endocrinol. Metab. 2002, 87, 1170–1176. [Google Scholar] [CrossRef]
- Devlin, A.M.; Bottiglieri, T.; Domann, F.E.; Lentz, S.R. Tissue-specific changes in h19 methylation and expression in mice with hyperhomocysteinemia. J. Biol. Chem. 2005, 280, 25506–25511. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Yang, L.; Zhong, T.; Mueller, M.; Men, Y.; Zhang, N.; Xie, J.; Giang, K.; Chung, H.; Sun, X.; et al. H19 lncrna alters DNA methylation genome wide by regulating s-adenosylhomocysteine hydrolase. Nat. Commun. 2015, 6, 10221. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Zhu, Y.; Liang, Y.; Huang, H.; Xu, Y.; Zhong, C. Circular rnas in vascular functions and diseases. Adv. Exp. Med. Biol. 2018, 1087, 287–297. [Google Scholar] [PubMed]
- Bayoumi, A.S.; Aonuma, T.; Teoh, J.P.; Tang, Y.L.; Kim, I.M. Circular noncoding rnas as potential therapies and circulating biomarkers for cardiovascular diseases. Acta Pharmacol. Sin. 2018, 39, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Sandhu, H.S.; Tyagi, S.C. Circular rnas profiling in the cystathionine-β-synthase mutant mouse reveals novel gene targets for hyperhomocysteinemia induced ocular disorders. Exp. Eye Res. 2018, 174, 80–92. [Google Scholar] [CrossRef]
- Singh, M.; George, A.K.; Homme, R.P.; Majumder, A.; Laha, A.; Sandhu, H.S.; Tyagi, S.C. Expression analysis of the circular rna molecules in the human retinal cells treated with homocysteine. Curr. Eye Res. 2019, 44, 287–293. [Google Scholar] [CrossRef]
- Pizzolo, F.; Blom, H.J.; Choi, S.W.; Girelli, D.; Guarini, P.; Martinelli, N.; Stanzial, A.M.; Corrocher, R.; Olivieri, O.; Friso, S. Folic acid effects on s-adenosylmethionine, s-adenosylhomocysteine, and DNA methylation in patients with intermediate hyperhomocysteinemia. J. Am. Coll. Nutr. 2011, 30, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, Z.; Xu, G. Notable epigenetic role of hyperhomocysteinemia in atherogenesis. Lipids Health Dis. 2014, 13, 134. [Google Scholar] [CrossRef]
- Capelli, I.; Cianciolo, G.; Gasperoni, L.; Zappulo, F.; Tondolo, F.; Cappuccilli, M.; La Manna, G. Folic acid and vitamin B12 administration in CKD, Why Not? Nutrients. 2019, 11, 383. [Google Scholar] [CrossRef]
- Undas, A.; Stepien, E.; Glowacki, R.; Tisonczyk, J.; Tracz, W.; Jakubowski, H. Folic acid administration and antibodies against homocysteinylated proteins in subjects with hyperhomocysteinemia. Thromb. Haemost. 2006, 96, 342–347. [Google Scholar] [CrossRef]
- Jakubowski, H. Protective mechanisms against protein damage in hyperhomocysteinemia: Systemic and renal detoxification of homocysteine-thiolactone. Biomed. Genet. Genom. 2016, 1, 40–43. [Google Scholar] [CrossRef]
- Perla-Kajan, J.; Borowczyk, K.; Glowacki, R.; Nygard, O.; Jakubowski, H. Paraoxonase 1 q192r genotype and activity affect homocysteine thiolactone levels in humans. FASEB J. 2018, 32, 6019–6024. [Google Scholar] [CrossRef] [PubMed]
| Cell Line/Model Organism | Treatment | Effect on | Reference | ||
|---|---|---|---|---|---|
| SAM, SAH, SAM/SAH | DNMT | Promoter DNA Methylation/Gene Expression mRNA/Protein | |||
| HUVEC | Hcy 1 mM, 5 days | NA | NA | ↓genomic DNA methylation ↑SORBS1/↓/↓ | [47] |
| HUVEC | Hcy 10, 30 μM, 72 h Hcy 100, 300 μM, 72 h | NA NA | NA NA | ↓DDAH2/↑/NA ↑DDAH2/↓/NA | [48] |
| HUVEC | Hcy 1 mM, 48 h | NA | ↑DNMT1 protein | ↑DDAH2/↓/NA | [49] |
| HUVEC | Hcy 100, 200, 500 μM, 72 h | NA | ↓DNMT1 mRNA, protein | ↓LOX-1/↑/↑ | [50] |
| HUVEC | Hcy 50, 100, 200 μM, 24 h | NA | ↓DNMT1 mRNA, protein ↓DNMT activity | ↓PDGF-A, -C, -D/↑/NA | [51] |
| HUVEC | Hcy 200 μM, 8 h | NA | ↑DNMT3B ↓DNMT activity | ↓p66shc/↑/↑ | [52] |
| HUVEC | D,L-Hcy 50 μM, Ado 40 μM, 10 μM Ado-deaminase inhibitor, 48 h | NA | ↓DNMT1 activity =DNMT3 activity | ↓Cyclin A/↓/NA | [53] |
| HUVEC | Hcy 25, 50, 100, 200 μM, 24, 48, 72 h | NA | NA | ↓sEH/↑/↑ mRNA | [54] |
| HUVEC | Hcy 25, 50, 100, 200 μM, 72 h | NA | NA | ↓hTERT/↓/↓ | [55] |
| Human foam cells | Hcy 50, 100, 200, 500 μM, 48 h | NA | ↓DNMT1 mRNA, protein | NA/↓miR-148a/152/NA | [56] |
| Human foam cells | Hcy 100 μM, 72 h | NA | NA | ↓FABP4/↑/↑ | [57] |
| Human monocytes | Hcy 50, 100, 200, 500 μM, 48 h | ↓SAM, ↑SAH ↓SAM/SAH | ↑DNMT activity | ↑PPARγ/↓/↓ | [58] |
| T/G HA-VSM | Hcy 50, 100, 200 and 500 μM, 72 h | NA | ↑DNMT1 mRNA, protein | ↑PTEN/↓/↓ | [59] |
| VSMC | D,L-Hcy 50, 100, 200, 500 μM, 72 h | NA | ↑DNMT3B protein | ↑p53/↓/↓ | [60] |
| VSMC | Hcy 50, 100, 200, 500 μM, 72 h | NA | ↑DNMT3A mRNA, protein | ↑/↓miR-143/NA | [61] |
| VSMCs | Hcy 50, 100, 200 and 500 μM, 72 h | NA | ↑DNMT1 mRNA, protein | ↑MFN2/↓/↓ | [62] |
| HL-1 cardiomyocytes | Hcy 5, 100 μM, 72 h | NA | ↑DNMT1 mRNA, protein | NA | [63] |
| Human hepatocytes (HL-7702) | Transfection with Ad-CFTR L-Hcy 100 μM, 24 h | NA | NA | ↑CRFT/↓/↓ | [64] |
| Human primary retinal endothelial cells (HRECs) | Hcy 20, 50, 100 μM | NA | ↑DNMT activity | NA | [65] |
| Human retinal pigments epithelial cells (ARPE-19) | Hcy 20, 50, 100 μM | NA | ↑DNMT activity | NA | [65] |
| Mouse Cbs−/−, Cbs+/− retinas | None | NA | ↑DNMT activity | NA | [65] |
| Mouse endothelial progenitor cells differentiated from primary bone marrow mononuclear cells isolated from femur and tibia of female C57BL/6J mice | High-Met, low-folate, low-vitamin B6 & B12 diet 8 weeks | NA | ↑DNMT1 mRNA, protein =DNMT3A mRNA, protein ↑DNMT activity | ↑global DNA methylation ↑Cbs/↓/↓ | [66] |
| Mouse brain endothelial cells (bEnd.3) | Hcy 100 μM, 24 h | NA | ↑ DNMT1 mRNA, protein ↑DNMT3A mRNA, protein ↓ DNMT3B mRNA, protein | NA | [67] |
| Mouse brain endothelial cell line (bEnd.3) | Hcy 50, 100, 200 μM, 24 h | NA | ↑DNMT3A protein ↓DNMT3B protein | NA | [68] |
| Mouse myoblast C2C12 cells | Hcy 500 μM, 3 days | NA | ↑DNMT3A protein ↑DNMT3B protein | NA | [69] |
| Mouse neuro 2A cells, neuroblastoma cell line stably expressing human APP carrying the K670N, M671L Swedish mutation (N2A-APPswe) | DL-Hcy 50 μM, adenosine 40 μM, erythro-9-(2-hydroxy-3-nonyl)-adenine hydrochloride 10 μM, 24 h | ↓SAM ↑SAH | ↓DNMT1 protein ↓DNMT3A protein ↓DNMT3B protein | ↓5LO/↑/↑ | [70] |
| Model Animal, Tissue | Treatment | Plasma/Tissue tHcy, μM | Effect on | Reference | ||
|---|---|---|---|---|---|---|
| SAM SAH SAM/SAH | DNMT | Promoter DNA Methylation/Gene Expression mRNA/Protein | ||||
| Male ApoE−/− mice, Aorta | High-Met diet 20 weeks | Plasma tHcy 2.67 ± 0.79 (control diet) vs. 13.79 ± 0.54 (ApoE-/-) and 6.40 ± 0.28 (ApoE+/+) (high-Met diet) | ↑SAM ↑SAH ↑SAM/SAH | ↓DNMT1 mRNA, protein =DNMT3A mRNA, protein =DNMT3B mRNA, protein | ↓FABP4/↑/↑ | [57] |
| Male ApoE−/− mice | High-Met diet 15 weeks | NA | NA | ↑DNMT3B protein | NA/↓miR-125b/NA | [60] |
| Male Tg-127T Cbs−/− mice 3-month-old Brain cortices | None | Plasma tHcy 296 vs. 5.5 (controls) [71] | NA | ↓DNMT1 protein ↓DNMT3A protein ↓DNMT3B protein | ↓5LO /↑/↑ | [70] |
| Male Cbs-deficient mice, 8–12-week-old, Heart | High-Met diet | NA | NA | ↑DNMT1 protein ↑DNMT activity | ↑Genomic DNA methylation | [63] |
| Male C57BL/6J mice, 8-week-old, Aortic intima | High-Met diet 4 or 8 weeks | Plasma tHcy 27.6 ± 4.5 or 61.5 ± 31.4 vs. 5.2 ± 1.3 in the control group after 4 or 8 weeks, respectively | NA | NA | ↓PDGF-A, -C and -D/↑/↑ | [51] |
| Male C57BL/6J mice, 6–8-week-old Blood | High-Met diet 8 weeks | Plasma tHcy 61.5 ± 31.4 vs. 5.2 ± 1.3 in the control group after 4 or 8 weeks, respectively [51] | NA | NA | ↓mTERT/↓/NA | [72] |
| Male C57BL/6J mice, 6–8-week-old, Aorta | High-Met diet 4 or 8 weeks | Plasma tHcy 61.5 ± 31.4 vs. 5.2 ± 1.3 in the control group after 4 or 8 weeks, respectively [51] | NA | NA | ↓mTERT/↓/↓ | [55] |
| Male Sprague-Dawley rats, 4-week-old Blood | High-Met diet 8 weeks | Serum tHcy level 66.8±11.7 | NA | NA | ↓rTERT/↓/NA | [73] |
| Male Cbs-deficient mice 8–12-week-old Brain | High-Met diet | NA | NA | ↑DNMT1 mRNA, protein ↑DNMT3A mRNA, protein ↑DNMT activity | ↑Genomic DNA methylation | [74] |
| Male and female 3xTg-AD mice, Brain cortices | Folate, vitamin B6, B12-deficient diet 7 months | NA | ↓SAM ↑SAH | ↓DNMT1 protein ↓DNMT3A protein ↓DNMT3B protein | ↓5LO/↑/↑ | [70] |
| Cbs+/− mice, 8–10-week-old, Liver | None | 3.46 times higher than in Cbs+/+ mice | NA | ↑DNMT1 mRNA, protein =DNMT3A mRNA, protein =DNMT3B mRNA, protein | ↑CRFT/↓/↓ | [64] |
| Male C57BL/6J mice, Stelic animal model, Liver | Streptozotocin injection at 2nd day High-fat diet 6, 12, 20 weeks | =liver Hcy ↑plasma Hcy | ↑liver SAM ↑liver SAH ↓liver SAM/SAH ↑plasma SAH | ↑DNMT1 mRNA, protein ↑DNMT3A mRNA, protein ↑DNMT3B mRNA, protein | ↑Ahcy/↓/? | [75] |
| Male C57BL/6J mice, CFD model, Liver | Low-Met, w/o choline and folic acid (CFD) diet 12 weeks | ↑liver Hcy ↑plasma Hcy | ↓liver SAM =liver SAH | NA | NA Ahcy/↓/? | [76] |
| Male Fisher 344 rats, 4-week-old Liver, preneoplastic liver, and liver tumor | Low-Met, w/o choline and folic acid diet, 36, 54 weeks | NA | NA | NA | ↓LINE-1 | [77] |
| Male F344 rats 4-week-old Liver | Low-Met, w/o choline and folic acid diet, 9, 18, 24, and 36 weeks, followed by 18 weeks of feeding a methyl-adequate diet with sufficient content of Met, choline, and folic acid | NA | ↓ SAM ↓ SAM/SAH | NA | ↓global DNA methylation (reversible after 9 weeks and irreversible after 18-36 weeks of the methyl-deficient diet) | [78] |
| Male F344 rats 4-week-old Brain | Low-Met, w/o choline and folic acid diet, 18, 36 weeks | ↑Hcy After 36 weeks ~0.15 vs. 0.1 nmol/mg protein | =SAM =SAH =SAM/SAH | ↓DNMT1 protein ↑DNMT3A protein ↑DNMT3B protein | ↑DNA methylation within unmethylated GC-rich DNA domains =methylation within methylated GC-rich DNA regions | [79] |
| Male Sprague-Dawley rats, 8 weeks old | High-Met diet Low-Met diet 4 weeks | ↑plasma tHcy | ↓SAM ↑SAH ↓SAM/SAH | ↑DNMT3A mRNA, protein ↑DNMT3B mRNA, protein | ↓genome methylation in B1 repetitive elements | [80] |
| Male Cbs+/− and Cbs+/+ mice 8–10-week-old, Brain | 5′-aza (0.5 mg/kg body weight) intraperitoneally for 6 weeks, +/− Met, +/− folic acid (FA) | ↑Hcy | NA | ↓DNMT3B protein | ↑5-mC in brain DNA from CBS+/−, + Met mice; tended to decrease in FA-supplemented mice | [68] |
| Male ApoE−/− C57BL6J mice, 6-week-old, Aorta | High-Met, low folate/B12 diet 20 weeks | ↑Serum Hcy | NA | ↓DNMT1 mRNA, protein | NA | [56] |
| Male C57BL/6J sEH+/+ and sEH−/− mice, 8-week-old, Aortic intima | High-Met diet 4, 8 weeks | ↑Plasma tHcy | NA | NA | ↓sHE/↑/NA | [54] |
| Gene Name | Access. No. | Protein Name | Fold Change at HTL 10 μM 1000 μM | |
|---|---|---|---|---|
| SETD2 | 29072 | Histone-lysine N-methyltransferase SETD2 | 17.9 | 6.1 |
| SETD7 | 80854 | Lysine methyltransferase 7 | 1.1 | 3.9 |
| EZH2 | 2146 | Histone-lysine N-methyltransferase EZH2 | 2.3 | 5.4 |
| EHMT1 | 79813 | Euchromatic histone-lysine N-methyltransferase 1 | 2.0 | 3.7 |
| H2AFY | 9555 | H2A histone family, member Y | 1.7 | 2.5 |
| EHMT2 | 10919 | Euchromatic histone-lysine N-methyltransferase 2 | 1.7 | 2.5 |
| EPC1 | 80314 | Enhancer of polycomb homolog 1 (Histone acetylase) | 1.8 | 2.0 |
| EP300 | 2033 | E1A-binding protein (Histone acetylase) | 2.1 | 2.8 |
| HIST1H2BK | 85236 | Histone cluster 1, H2bk | 2.3 | 2.5 |
| JARID2 | 3720 | Jumonji, AT-rich interactive domin 2 | 3.6 | 2.7 |
| MBD3 | 53615 | Methyl-CpG binding domain protein 3 | 1.5 | 2.5 |
| SUV420H2 | 84787 | Histone-lysine N-methyltransferase | −1.9 | −3.3 |
| DOT1L | 84444 | Histone-Lys N-methyltransf, H3K79 specific | −2.5 | −2.3 |
| SMYD3 | 3009 | Histone-lysine N-methyltransferase | −2.8 | −11.3 |
| JMJD2B | 23030 | Lysine-specific demethylase 4B | −1.9 | −3.0 |
| BRD8 | 10902 | Bromodomain-containing protein 8 | −2.8 | −4.8 |
| CBX2 | 12416 | Chromobox prot homol 2 (H2AK119 ubiq.) | −6.0 | −7.1 |
| HIST1H2AJ | 8330 | Histone cluster 1, H2ak | −2.7 | −4.1 |
| HIST1H1B | 3009 | Histone cluster 1, H1b | −7.7 | −9.4 |
| Organism/Cell Line | Treatment | Effect | References |
|---|---|---|---|
| Acetylation | |||
| HUVEC | Hcy or HTL 1, 10, 100, 1000 μM | ↓H3K9ac | [19,150] |
| HUVEC | Hcy 50 μM 24 h | ↑H3K9ac ↑H3K14ac | [52] |
| HL-1 cardiomyocytes | Hcy 100 μM, 72 h | ↑H3K9ac | [63] |
| Human colorectal cancer cell line HCT116 | Met restriction 24 h | ↓H3K9ac | [149] |
| Male C57BL/6J mice Stelic animal model Liver | Streptozotocin injection at 2nd day High-fat diet from 4th week of age 6, 12, 20 weeks | ↓H4K16ac | [75] |
| Female Cbs+/− mice (129P2-Cbstm1Unc/J) 16-weeks old Femur bone | Met-rich, low folate vitamin B6, B12 diet 8 weeks | ↑H3K27ac | [148] |
| Male Wistar rats 4–6-months old Brain cortex | Hcy 1.2 μmol/g of body weight injected subcutaneously once a day 21 days | ↑H3K9ac ↑H4K12ac | [151] |
| Methylation | |||
| Male C57BL/6J mice Stelic model Liver | Streptozotocin injection at 2nd day High-fat diet from 4th week of age 6, 12, 20 weeks | ↑H3K27me3 in NASH-derived hepatocellular carcinoma | [75] |
| Female Wistar rats Brain Heart Liver | Met-rich, deficient in B vitamins (folic acid, B6 and B12) 8 weeks | ↓H3R8me2a (brain) = H3R8me2a (heart, liver) = H3R17me2a (brain, heart, liver) = H4R3me2a (brain, heart, liver) | [152] |
| Male Fisher 344 rats, 4-weeks old, Liver, Preneoplastic liver, Liver tumor | Low-Met and lacking in choline and folic acid diet 36, 54 weeks | ↓H4K20me3 ↑H3K9me3 (preneoplatic nodules and liver tumors) | [77] |
| Cbs+/− mice 8–10-weeks old, Liver | None | ↑H3K27me3 = H3K27me1 = H3K27me2 | [64] |
| Tg-I278T Cbs−/− mice, Brain, Liver, Heart, Kidney | None | ↓H4R3me2a (liver) = H4R3me2a (brain) | [28] |
| Human colon cancer cells HCT116 | Met restriction 24 h | ↓H3K4me3 ↓H3K9me3 ↓H3K27me3 | [149] |
| Human colon cancer cells SW620, SW480, HCT8, HT29, NCI-H5087 | Met restriction 24 h | ↓H3K4me3 | [149] |
| C57BL/6J mice 8-week-old, Liver | Low-Met diet 12 weeks | ↓H3K4me3 | [149] |
| Male C57BL/6J mice 7-week-old | Low-Met diet 12 weeks | ↓H3K4me3 | [153] |
| Human colon cancer cells HCT116 | Met restriction 24 h | ↓H3K4me3 | [153] |
| Human hepatocytes (HL-7702) | Transfection with Ad-CFTR L-Hcy 100 μM, 24 h | ↑H3K27me3 | [64] |
| N-homocysteinylation | |||
| HUVEC | HTL or Hcy 1, 10, 100, 1000 μM; 24 h | ↑N-Hcy-H1, -H2A, -H2B, -H3, -H4 | [19,150] |
| Human embryos | NA | 39 N-Hcy-sites: 8 in H2a, 16 in H2b, 8 in H3, 7 in H4 | [36] |
| Chicken embryos | 0.5 μL of 0.5 mM HTL injected into the neural groove | ↑N-Hcy-histone | [36] |
| Mouse neural stem cells (NE4C) | DL-Hcy or L-HTL 0.1, 0.5, 1 mM 8 h | ↑N-Hcy-histone | [36] |
| HEK293 cells | DL-Hcy or L-HTL 0.1, 0.5, 1 mM 8 h | ↑N-Hcy-histone | [36] |
| HEK293 cells | MARS knockdown | ↓ N-Hcy-histone | [36] |
| HEK293T, HCT116, HeLa cells | HTL 0.01, 0.1, 1 mM 24h | ↑ H3K23Hcy ↑ H3K79Hcy | [154] [155] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perła-Kaján, J.; Jakubowski, H. Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia. Int. J. Mol. Sci. 2019, 20, 3140. https://doi.org/10.3390/ijms20133140
Perła-Kaján J, Jakubowski H. Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia. International Journal of Molecular Sciences. 2019; 20(13):3140. https://doi.org/10.3390/ijms20133140
Chicago/Turabian StylePerła-Kaján, Joanna, and Hieronim Jakubowski. 2019. "Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia" International Journal of Molecular Sciences 20, no. 13: 3140. https://doi.org/10.3390/ijms20133140
APA StylePerła-Kaján, J., & Jakubowski, H. (2019). Dysregulation of Epigenetic Mechanisms of Gene Expression in the Pathologies of Hyperhomocysteinemia. International Journal of Molecular Sciences, 20(13), 3140. https://doi.org/10.3390/ijms20133140






