Molecular Characterization of Ca2+/Calmodulin-Dependent Protein Kinase II Isoforms in Three Rice Planthoppers—Nilaparvata lugens, Laodelphax striatellus, and Sogatella furcifera
Abstract
1. Introduction
2. Results
2.1. Identification of CaMKII Isoforms and Sequence Analysis
2.2. Variable Insert Region of CaMKII in Rice Planthoppers
2.3. NlCaMKII Gene Structure
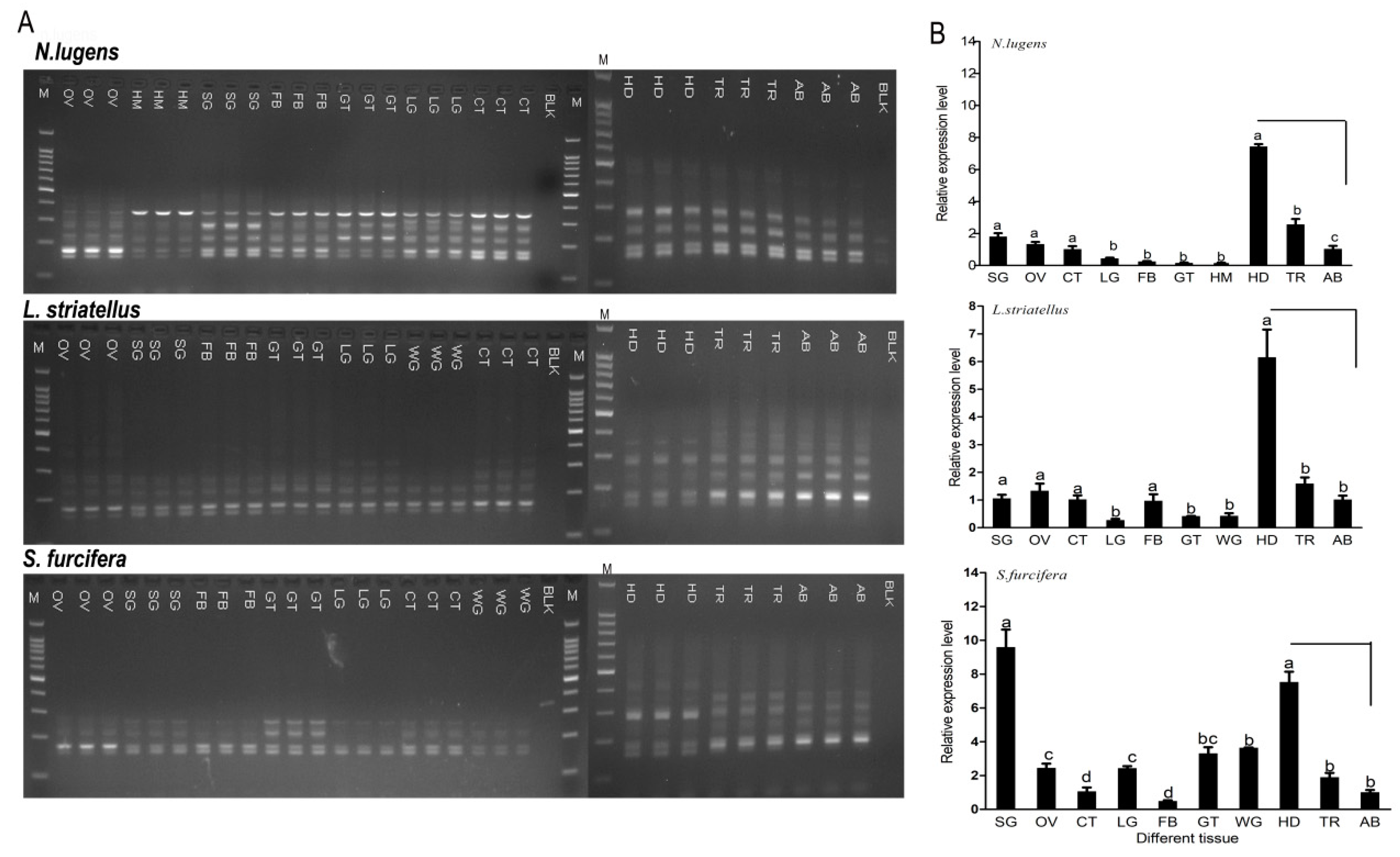
2.4. Expression Pattern of CaMKII in Diverse Tissues and Stages
2.5. Expression Profiling of NlCaMKII in Different Virulent Populations and Response to Resistant Rice
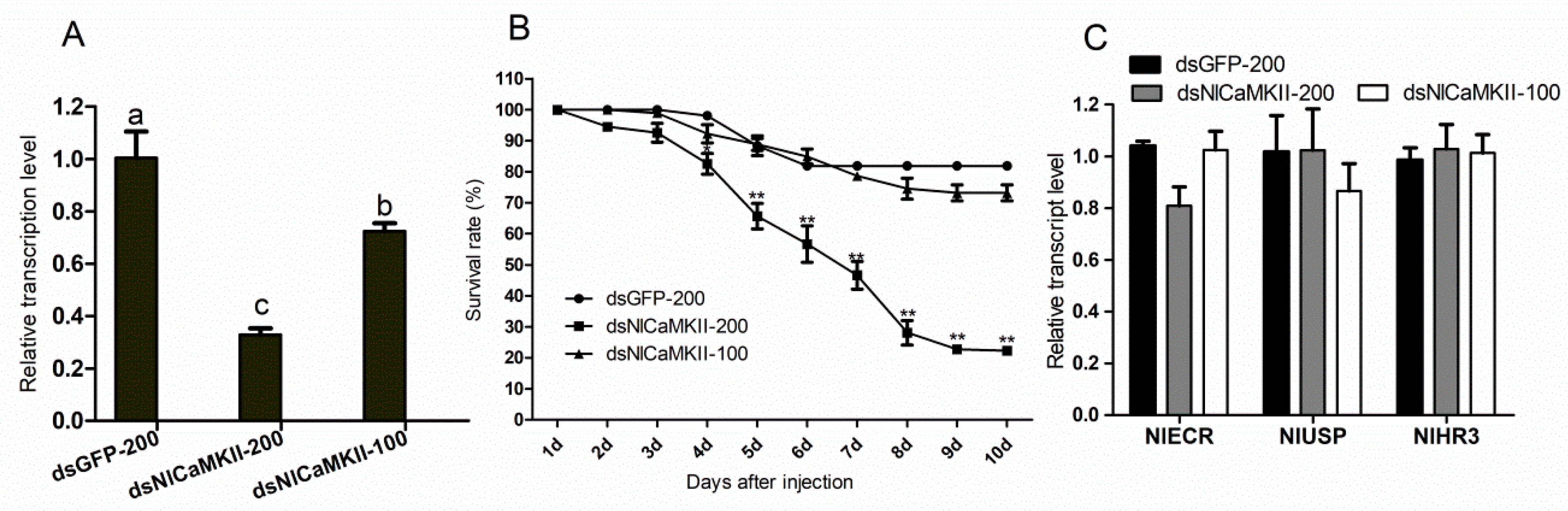
2.6. Gene-Silencing Effects of NlCaMKII on Development of N. lugens
3. Discussion
4. Materials and Methods
4.1. Insects and Rice
4.2. Total RNA Extraction and Reverse Transcription
4.3. Cloning of Full-Length cDNA Sequence and Sequence Analysis
4.4. Expression Analysis by RT-PCR and RT-qPCR
4.5. RNAi Experiment and Bioassay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| dsRNA | Double-strand RNA |
| CaMKII | Calcium/calmodulin-dependent protein kinase II |
| ORF | Open reading frame |
| GFP | Green fluorescent protein |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| RT-PCR | Reverse transcription polymerase chain reaction |
| RNAi | RNA interference |
| 20E | 20-Hydroxyecdysone |
| siRNA | Small interfering RNA |
References
- Barrion, A.T.; Litsinger, J.A. Taxonomy of rice insect pests and their arthropod parasites and predators. In Biology and Management of Rice Insects; Heinrichs, E.A., Ed.; Wiley Eastern Ltd.: New Delhi, India; IRRI: Manila, Philippines, 1994; pp. 13–362. [Google Scholar]
- Cheng, J.A. Rice Planthoppers in the past half century in China. In Rice Planthoppersecology, Management, Socio Economics and Policy; Heong, K.L., Cheng, J.A., Escalada, M.M., Eds.; Zhejiang University: Hangzhou, China, 2014; pp. 1–32. [Google Scholar]
- Cheng, J.A. Rice planthopper problems and relevant causes in China. In Planthoppers: New Threats to the Sustainability of Intensive Rice Production Systems in Asia; Heong, K.L., Hardy, B., Eds.; International Rice Research Institute: Los Banos, CA, USA, 2009; pp. 157–178. [Google Scholar]
- Bass, C.; Carvalho, R.A.; Oliphant, L.; Puinean, A.M.; Field, L.M.; Nauen, R.; Williamson, M.S.; Moores, G.; Gorman, K. Overexpression of a cytochrome P450 monooxygenase, CYP6ER1, is associated with resistance to imidacloprid in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2011, 20, 763–773. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Williamson, M.S.; Lansdell, S.J.; Denholm, I.; Han, Z.; Millar, N.S. A nicotinic acetylcholine receptor mutation conferring target-site resistance to imidacloprid in Nilaparvata lugens (brown planthopper). Proc. Natl. Acad. Sci. USA 2005, 102, 8420–8425. [Google Scholar] [CrossRef] [PubMed]
- Small, G.J.; Hemingway, J. Molecular characterization of the amplified carboxylesterase gene associated with organophosphorus insecticide resistance in the brown planthopper, Nilaparvata lugens. Insect Mol. Biol. 2000, 9, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Hudmon, A.; Schulman, H. Neuronal Ca2+/calmodulin-cependentproteinkinase II: The role of structure and autoregulation in cellular function. Annu. Rev. Biochem. 2002, 71, 473–510. [Google Scholar] [CrossRef]
- Lu, C.; Hodge, J.; Mehren, J.; Sun, X.; Griffith, L. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron 2003, 40, 1185–1197. [Google Scholar] [CrossRef]
- Inagaki, S.; Kaku, K.; Dunlap, D.Y.; Matsumura, F. Sequences of cDNAs encoding calmodulin, and partial structures of calmodulin kinase, and a calcium channel of kdr-resistant and -susceptible German cockroaches, Blattella germanica. Comp. Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1998, 120, 225–233. [Google Scholar] [CrossRef]
- Mehren, J.E.; Griffith, L.C. Calcium-independent calcium/calmodulin-dependent protein kinase II in the adult Drosophila CNS enhances the training of pheromonal cues. J. Neurosci. 2004, 24, 10584–10593. [Google Scholar] [CrossRef]
- Mehren, J.E.; Griffith, L.C. Cholinergic neurons mediate CaMKII-dependent enhancement of courtship suppression. Learn. Mem. 2006, 13, 686–689. [Google Scholar] [CrossRef][Green Version]
- Ohnishi, A.; Hull, J.J.; Kaji, M.; Hashimoto, K.; Lee, J.M.; Tsuneizumi, K.; Suzuki, T.; Dohmae, N.; Matsumoto, S. Hormone signaling linked to silkmoth sex pheromone biosynthesis involves Ca2+/Calmodulin-dependent proteinkinase II-mediated phosphorylation of the insect PAT family protein Bombyx mori lipid storage droplet protein-1(BmLsd1). J. Biol. Chem. 2011, 286, 24101–24112. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.P.; Liu, W.; Wang, J.X.; Zhao, X.F. The Steroid hormone 20-hydroxyecdysone via nongenomic pathway activates Ca2+/Calmodulin-dependent protein kinase II to regulate gene expression. J. Biol. Chem. 2015, 290, 8469–8481. [Google Scholar] [CrossRef]
- Burkert, P.; Duch, C. Developmental changes of CaMKII localization, activity and function during postembryonic CNS remodelling in Manduca sexta. Eur. J. Neurosci. 2006, 23, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Kamikouchi, A.; Takeuchi, H.; Sawata, M.; Natori, S.; Kubo, T. Concentrated expression of Ca2+/calmodulin-dependent protein kinase II and protein kinase C in the mushroom bodies of the brain of the honeybee Apis mellifera L. J. Comp. Neurol. 2000, 417, 501–510. [Google Scholar] [CrossRef]
- Malik, B.R.; Gillespie, J.M.; Hodge, J.J. CASK and CaMKII function in the mushroom body α’/β’ neurons during Drosophila memory formation. Front. Neural Circuits 2013, 7, 52. [Google Scholar] [CrossRef] [PubMed]
- Miller, S.G.; Kennedy, M.B. Distinct forebrain and cerebellar isozymes of type II calcium⁄calmodulin dependent protein kinase associated differently with postsynaptic density fraction. J. Biol. Chem. 1985, 260, 9039–9046. [Google Scholar] [PubMed]
- Griffith, L.C.; Greenspan, R.J. The diversity of calcium dependent protein kinase II isoforms in Drosophila is generated by alternative splicing of a single gene. J. Neurochem. 1993, 61, 1534–1537. [Google Scholar] [CrossRef] [PubMed]
- Taillebois, E.; Heuland, E.; Bourdin, C.M.; Griveau, A.; Quinchard, S.; Tricoire-Leignel, H.; Legros, C.; Thany, S.H. Ca2+/calmodulin-Dependent protein kinase II in the Cockroach Periplaneta americana: Identification of five isoforms and their tissues distribution. Arch. Insect Biochem. Physiol. 2013, 83, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Renger, J.J.; Griffith, C.C.; Greenspan, R.J.; Wu, C.F. Concomitant alterations of physiological and developmental plasticity in Drosophila CaM Kinase II-inhibited synapse. Neuron 1994, 13, 1373–1384. [Google Scholar] [CrossRef]
- Xue, J.; Zhou, X.; Zhang, C.X.; Yu, L.L.; Cheng, J.A. Genomes of the rice pest brown planthopper and its endosymbionts reveal complex complementary contributions for host adaptation. Genome Biol. 2014, 15, 521. [Google Scholar] [CrossRef] [PubMed]
- Tombes, R.M.; Faisona, M.O.; Turbeville, J.M. Organization and evolution of multifunctional Ca2+/CaM-dependent protein kinase genes. Gene 2003, 322, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Guptaroy, B.; Marwaha, N.; Pla, M. Alternative splicing of Drosophila calcium/calmodulin-dependent protein kinase II regulates substrate specificity and activation. Mol. Brain Res. 2000, 80, 26–34. [Google Scholar] [CrossRef]
- Schworer, C.M.; Colbran, R.J.; Keefer, J.R.; Soderling, T.R. Ca2+/calmodulin-dependent protein kinase II. Identification of a regulatory autophosphorylation site adjacent to the inhibitory and calmodulin-binding domains. J. Biol. Chem. 1988, 263, 13486–13489. [Google Scholar] [PubMed]
- Stratton, M.M.; Chao, L.H.; Schulman, H.; Kuriyan, J. Structural studies on the regulation of Ca2+/calmodulin dependent protein kinase II. Curr. Opin. Struct. Biol. 2013, 23, 292–301. [Google Scholar] [CrossRef] [PubMed]
- Ohsako, S.; Nishida, Y.; Ryo, H.; Yamaguchi, T. Molecular characterization and expression of the Drosophila Ca2+/calmodulin-dependent kinase II gene. Identification of four forms of the enzyme generated from a single gene by alternative splicing. J. Biol. Chem. 1993, 268, 2052–2062. [Google Scholar] [PubMed]
- Cho, K.O.; Wall, J.B.; Pugh, P.C.; Ito, M.; Mueller, S.A.; Kennedy, M.B. The alpha subunit of type II Ca2+/calmodulin-dependent protein kinase is highly conserved in Drosophila. Neuron 1991, 7, 439–450. [Google Scholar] [CrossRef]
- Giese, K.P.; Mizuno, K. The roles of protein kinases in learning and memory. Learn. Mem. 2013, 20, 540–552. [Google Scholar] [CrossRef]
- Lohr, C.; Bergstein, S.; Hirnet, D. Developmental distribution of CaM kinase II in the antennal lobe of the sphinx moth Manduca sexta. Cell Tissue Res. 2007, 327, 189–197. [Google Scholar] [CrossRef]
- Liu, S.H.; Ding, Z.P.; Zhang, C.W.; Yang, B.J.; Liu, Z.W. Gene knockdown by intro-thoracic injection of double-stranded RNA in the brown planthopper. Insect. Biochem. Mol. 2010, 40, 666–671. [Google Scholar] [CrossRef]
- Consoulas, C.; Duch, C.; Bayline, R.J.; Levine, R.B. Behavioral transformations during metamorphosis: Remodeling of neural and motor systems. Brain Res. Bull. 2000, 53, 571–583. [Google Scholar] [CrossRef]
- Tissot, M.; Stocker, R.F. Metamorphosis in Drosophila and other insects: The fate of neurons throughout the stages. Prog. Neurobiol. 2000, 62, 89–101. [Google Scholar] [CrossRef]
- Wan, P.J.; Jia, S.; Li, N.; Fan, J.M.; Li, G.Q. RNA interference depletion of the halloween gene disembodied implies its potential application for management of planthopper Sogatella furcifera and Laodelphax striatellus. PLoS ONE 2014, 9, e86675. [Google Scholar] [CrossRef]
- Wang, W.X.; Li, K.L.; Chen, Y.; Lai, F.X.; Fu, Q. Identification and Function Analysis of enolase Gene NlEno1 from Nilaparvata lugens (Stål)(Hemiptera: Delphacidae). J. Insect Sci. 2015, 15, 66. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
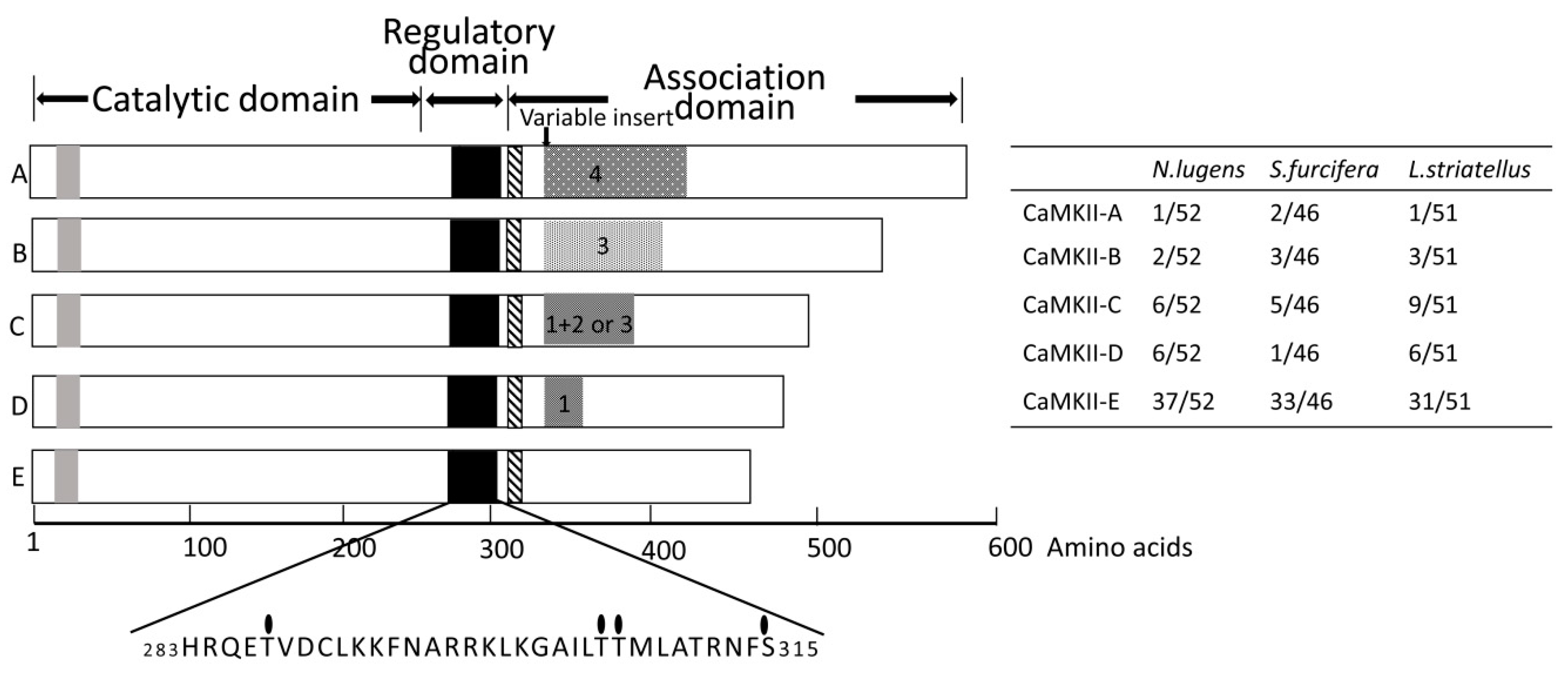
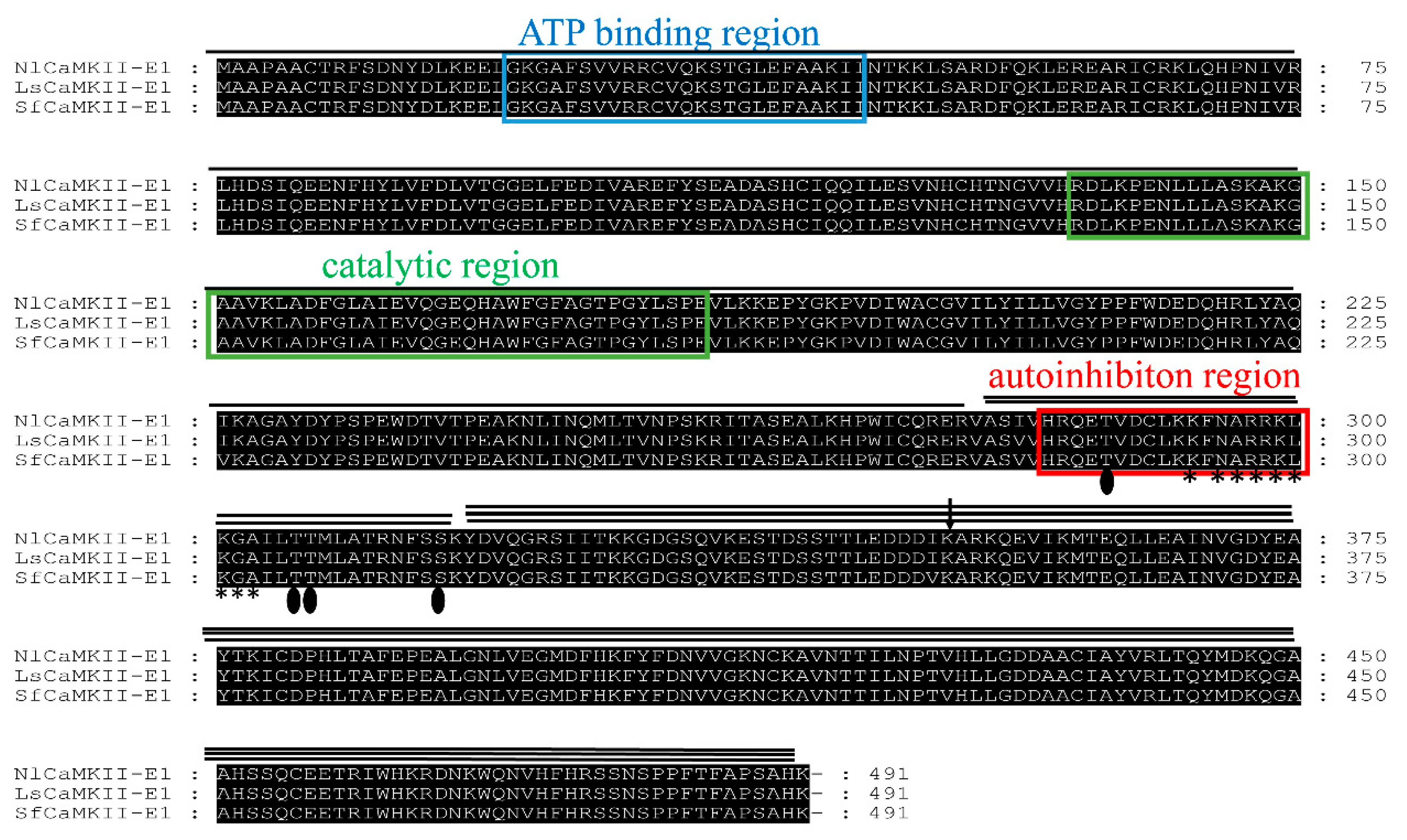
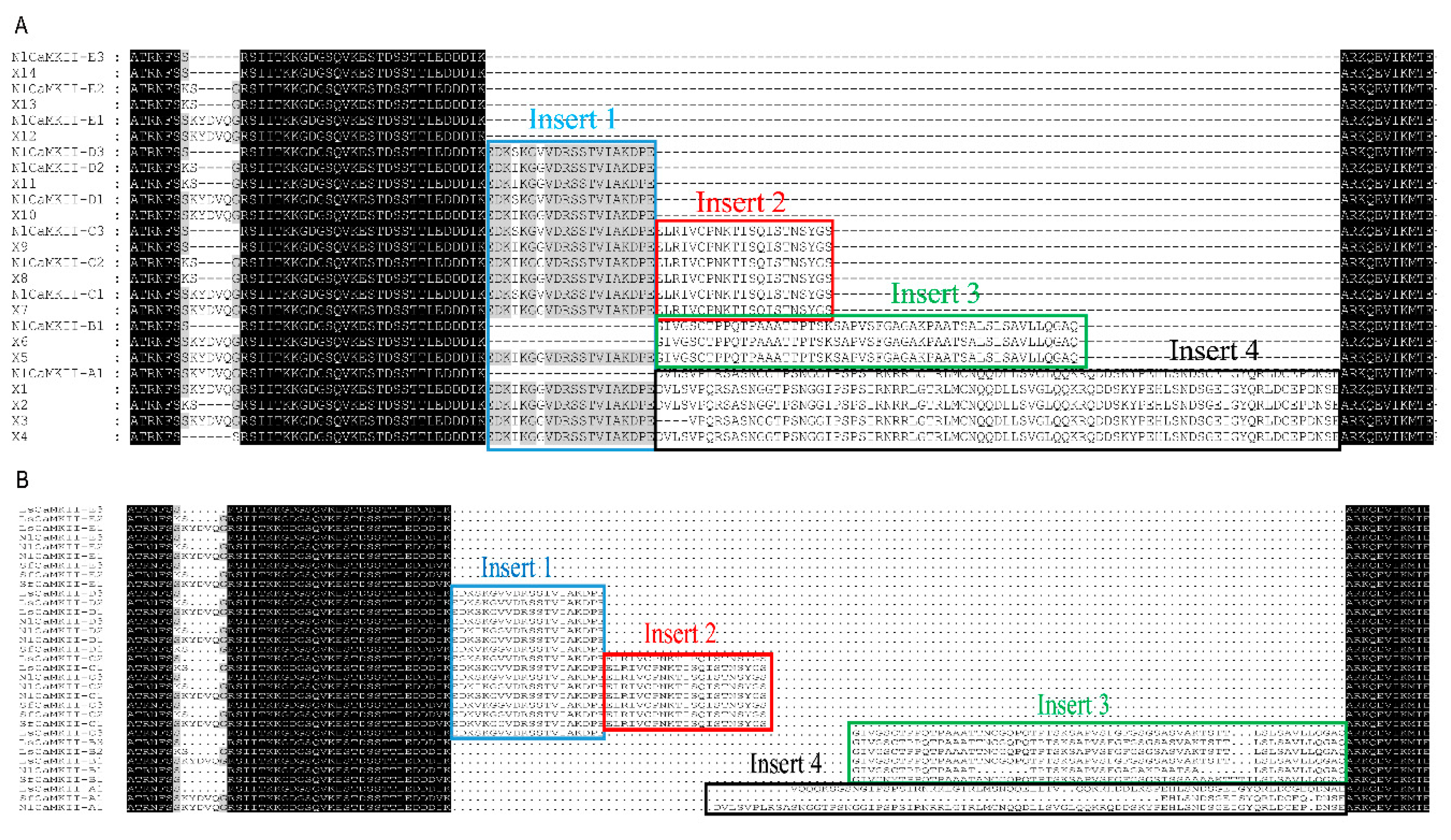

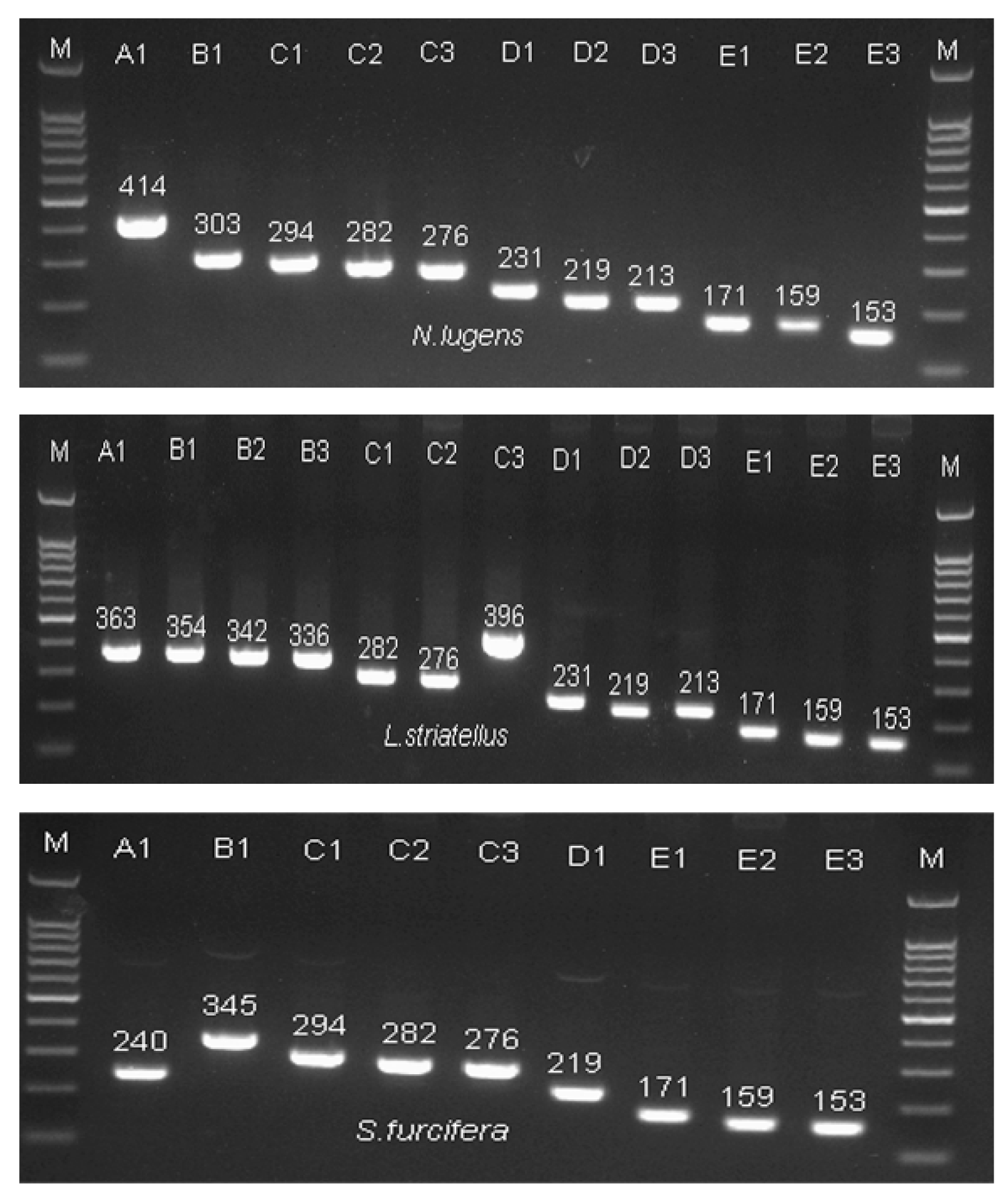

| CaMKII Isoform | Accession Number | Full Length | ORF 1 | CDS 2 Size | MW 3 | Beginning AD 4 | Insert | Clone No. |
|---|---|---|---|---|---|---|---|---|
| N. lugens | ||||||||
| NlCamMKII-A1 | MH807664 | 2028 | 1719 | 572 | 64.3 | SKYDVQG | 4 | 1/52 |
| NlCamMKII-B1 | MH807665 | 1917 | 1608 | 535 | 59.4 | S | 3 | 2/52 |
| NlCamMKII-C1 | MH807666 | 1908 | 1599 | 533 | 59.9 | SKYDVQG | 1 + 2 | 3/52 |
| NlCamMKII-C2 | MH807667 | 1897 | 1587 | 529 | 59.4 | KSG | 1 + 2 | 1/52 |
| NlCamMKII-C3 | MH807668 | 1891 | 1581 | 527 | 59.2 | S | 1 + 2 | 2/52 |
| NlCamMKII-D1 | MH807669 | 1846 | 1536 | 512 | 57.5 | SKYDVQG | 1 | 1/52 |
| NlCamMKII-D2 | MH807670 | 1834 | 1524 | 508 | 57.1 | KSG | 1 | 2/52 |
| NlCamMKII-D3 | MH807671 | 1828 | 1518 | 506 | 56.9 | S | 1 | 3/52 |
| NlCamMKII-E1 | MH807672 | 1786 | 1476 | 492 | 55.5 | SKYDVQG | NONE | 14/52 |
| NlCamMKII-E2 | MH807673 | 1773 | 1464 | 488 | 54.9 | KSG | NONE | 8/52 |
| NlCamMKII-E3 | MH807674 | 1767 | 1458 | 486 | 54.8 | S | NONE | 15/52 |
| L. striatellus | ||||||||
| LsCamMKII-A1 | MH807675 | 1815 | 1668 | 556 | 62.6 | S | 4 | 1/51 |
| LsCamMKII-B1 | MH807676 | 1806 | 1659 | 553 | 61.3 | SKYDVQG | 3 | 1/51 |
| LsCamMKII-B2 | MH807677 | 1794 | 1647 | 549 | 60.8 | KSG | 3 | 1/51 |
| LsCamMKII-B3 | MH807678 | 1788 | 1641 | 547 | 60.6 | S | 3 | 1/51 |
| LsCamMKII-C1 | MH807679 | 1734 | 1587 | 529 | 59.4 | KSG | 1 + 2 | 4/51 |
| LsCamMKII-C2 | MH807680 | 1728 | 1581 | 527 | 59.2 | S | 1 + 2 | 4/51 |
| LsCamMKII-C3 | MH807681 | 1848 | 1701 | 567 | 62.8 | S | 1 + 3 | 1/51 |
| LsCamMKII-D1 | MH807682 | 1683 | 1536 | 512 | 57.6 | SKYDVQG | 1 | 2/51 |
| LsCamMKII-D2 | MH807683 | 1671 | 1524 | 508 | 57.1 | KSG | 1 | 2/51 |
| LsCamMKII-D3 | MH807684 | 1665 | 1518 | 506 | 56.9 | S | 1 | 3/51 |
| LsCamMKII-E1 | MH807685 | 1623 | 1476 | 492 | 55.5 | SKYDVQG | NONE | 4/51 |
| LsCamMKII-E2 | MH807686 | 1611 | 1464 | 488 | 59.7 | KSG | NONE | 10/51 |
| LsCamMKII-E3 | MH807687 | 1605 | 1458 | 486 | 54.8 | S | NONE | 17/51 |
| S. furcifera | ||||||||
| SfCamMKII-A1 | MH807688 | 1665 | 1545 | 515 | 58.1 | SKYDVQG | 4 | 2/46 |
| SfCamMKII-B1 | MH807689 | 1772 | 1650 | 550 | 60.8 | S | 3 | 3/46 |
| SfCamMKII-C1 | MH807690 | 1715 | 1599 | 533 | 59.9 | SKYDVQG | 1 + 2 | 2/46 |
| SfCamMKII-C2 | MH807691 | 1707 | 1587 | 529 | 59.3 | KSG | 1 + 2 | 2/46 |
| SfCamMKII-C3 | MH807692 | 1701 | 1581 | 527 | 29.1 | S | 1 + 2 | 3/46 |
| SfCamMKII-D1 | MH807693 | 1645 | 1524 | 508 | 57.1 | KSG | 1 | 3/46 |
| SfCamMKII-E1 | MH807694 | 1597 | 1476 | 492 | 55.5 | SKYDVQG | NONE | 10/46 |
| SfCamMKII-E2 | MH807695 | 1585 | 1464 | 488 | 54.9 | KSG | NONE | 9/46 |
| SfCamMKII-E3 | MH807696 | 1573 | 1458 | 486 | 54.8 | S | NONE | 14/46 |
| Insert name | Sequence ID: Sequence |
|---|---|
| N. lugens | |
| Insert 1 | NlI1a: EDKSKGVVDRSSTVIAKDPE |
| Insert 1 | NlI1b: EDKIKGGVDRSSTVIAKDPE |
| Insert 2 | NlI2: ELRIVCPNKTISQISTNSYGS |
| Insert 3 | NlI3: GIVGSCTPPQTPAAATTPTSKSAPVSFGAGAKPAATSALSLSAVLLQGAQ |
| Insert 4 | NlI4:DVLSVPLRSASNGGTPSNGGIPSPSIRNRRLGTRLMCNQQDLLSVGLQQKRQDDSKYPEHLSNDSGEIGYQRLDCEPDNSE |
| L. striatellus | |
| Insert 1 | LsI1:EDKSKGVVDRSSTVIAKDPE |
| Insert 2 | LsI2:ELRIVCPNKTISQISTNSYGS |
| Insert 3 | LsI3:GIVGSCTPPQTPAAATTNCGQPQTPTSKSAPVSFGFGSGSASVAKTSTTLSLSAVLLQGAQ |
| Insert 4 | LsI4:VQQQKSGSNGIPSPSIRNRRLGTRLMSNQQELLTVQQKRHDDLKSPEHLSNDSGEIGYQRLDCGEQDNAE |
| S. furcifera | |
| Insert 1 | SfI1:EDKVKGGVDRSSTVIAKDPE |
| Insert 2 | SfI2:ELRIVCPNKTISQISTNSYGS |
| Insert 3 | SfI3:GIVGNYTPPQTPAAATANCGQPQTPTSKSAPVSFGFGSGSTSSAAAAKTTTTLSLSAVLLQGAQ |
| Insert 4 | SfI4:EHLSNDSGEISYQRLDCEQDNSE |
| Primer Name | Forward Sequence (5′–3′) | Reverse Sequence (5′–3′) | Amplicon Size (bp) |
|---|---|---|---|
| Primers for CaMKII Clone | |||
| NlCaMKII | GGGTAGTTGCTGAGCGAAGAG | TACCAGAACGCACCGACAGA | 1767–2028 |
| LsCaMKII | TAGTTGTTGAGCGAGTGGATGG | ATCAACAGAGTGAGTGGGGAA | 1605–1848 |
| SfCaMKII | GTTGGTGAGCGAGTGGATGG | GTCTTGTCAGCGGGCAGTAG | 1573–1772 |
| Primers for RT-qPCR | |||
| qCaMKII | AGTTATCGGTATGGCTGCTCC | TGGGGTGTTGAAGTTTCCGA | 224 |
| 18s-RNA | CAAGTATCAATTGGAGGGCAAGT | GCACACAGTATACAGGCGTGA | 342–368 |
| NlUSP | CAGATGTGCGAGACCTGAAG | CAGCGCTGTGTACACCTTCT | 58 |
| NlHr3 | AAGGAGACGTGACAGTGTGC | GGGAATTCGAGATGAGGTGT | 121 |
| NlECR | GCTGTGAAGCGAAAGGA | ATTCCACGCTGAAGTCG | 126 |
| Primers for dsRNA Synthesis | |||
| dsCaMKII | T7-AGTGTCAACCACTGCCACAC | T7-AGGGTGGTACTACTATCTGTAGACT | 668 |
| dsGFP | T7-CCTGAAGTTCATCTGCACCAC | T7-TGATGCCGTTCTTCTGCTTGT | 355 |
| Primers forRT-PCR | |||
| CaMKII-VR | GCC/AATTTTGACTACA/TATGCT | CTTTATAACTTCTTGCTTACGAG | 153–414 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.-X.; Lai, F.-X.; Wan, P.-J.; Fu, Q.; Zhu, T.-H. Molecular Characterization of Ca2+/Calmodulin-Dependent Protein Kinase II Isoforms in Three Rice Planthoppers—Nilaparvata lugens, Laodelphax striatellus, and Sogatella furcifera. Int. J. Mol. Sci. 2019, 20, 3014. https://doi.org/10.3390/ijms20123014
Wang W-X, Lai F-X, Wan P-J, Fu Q, Zhu T-H. Molecular Characterization of Ca2+/Calmodulin-Dependent Protein Kinase II Isoforms in Three Rice Planthoppers—Nilaparvata lugens, Laodelphax striatellus, and Sogatella furcifera. International Journal of Molecular Sciences. 2019; 20(12):3014. https://doi.org/10.3390/ijms20123014
Chicago/Turabian StyleWang, Wei-Xia, Feng-Xiang Lai, Pin-Jun Wan, Qiang Fu, and Ting-Heng Zhu. 2019. "Molecular Characterization of Ca2+/Calmodulin-Dependent Protein Kinase II Isoforms in Three Rice Planthoppers—Nilaparvata lugens, Laodelphax striatellus, and Sogatella furcifera" International Journal of Molecular Sciences 20, no. 12: 3014. https://doi.org/10.3390/ijms20123014
APA StyleWang, W.-X., Lai, F.-X., Wan, P.-J., Fu, Q., & Zhu, T.-H. (2019). Molecular Characterization of Ca2+/Calmodulin-Dependent Protein Kinase II Isoforms in Three Rice Planthoppers—Nilaparvata lugens, Laodelphax striatellus, and Sogatella furcifera. International Journal of Molecular Sciences, 20(12), 3014. https://doi.org/10.3390/ijms20123014





