Deficiency of GD3 Synthase in Mice Resulting in the Attenuation of Bone Loss with Aging
Abstract
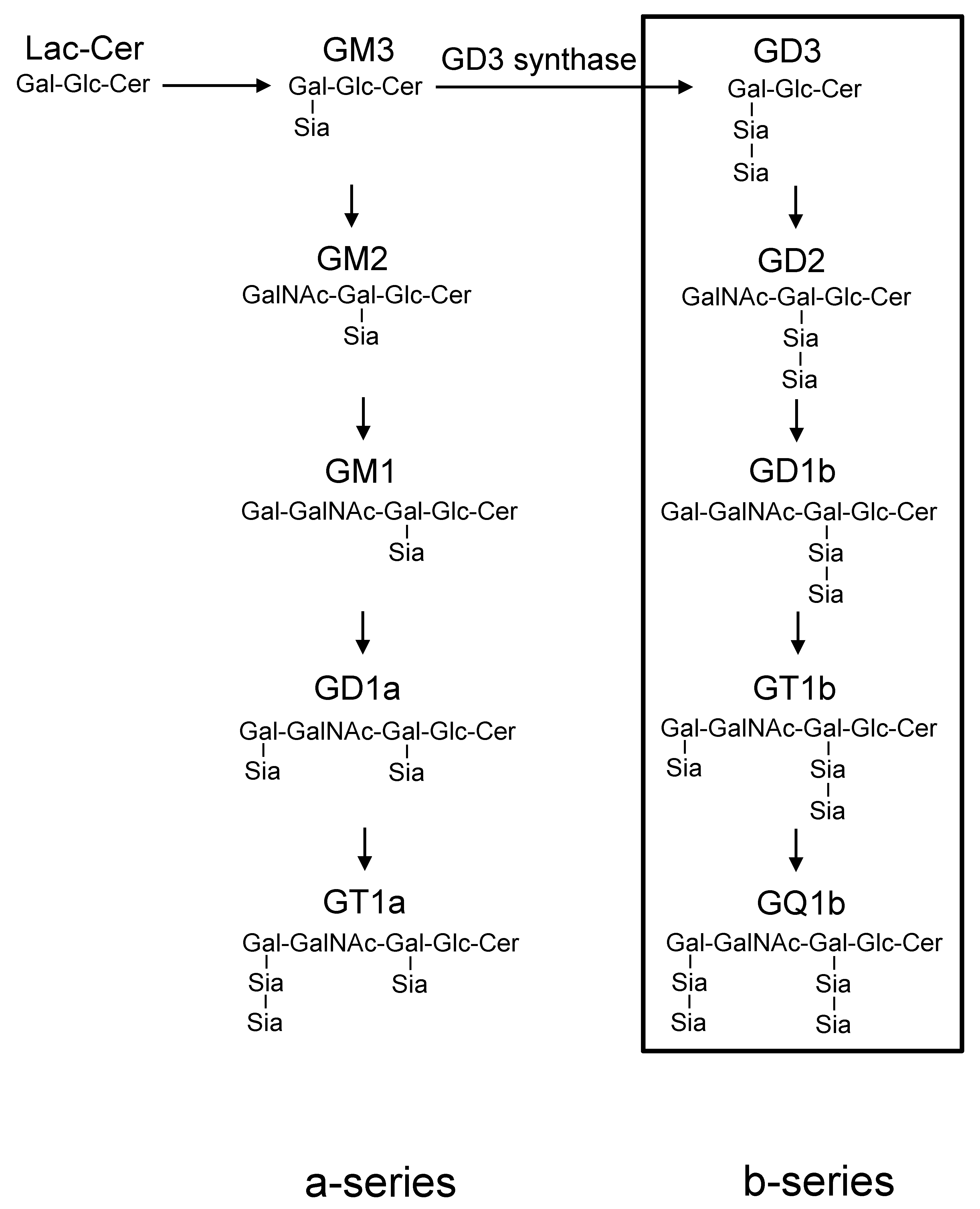
1. Introduction
2. Results
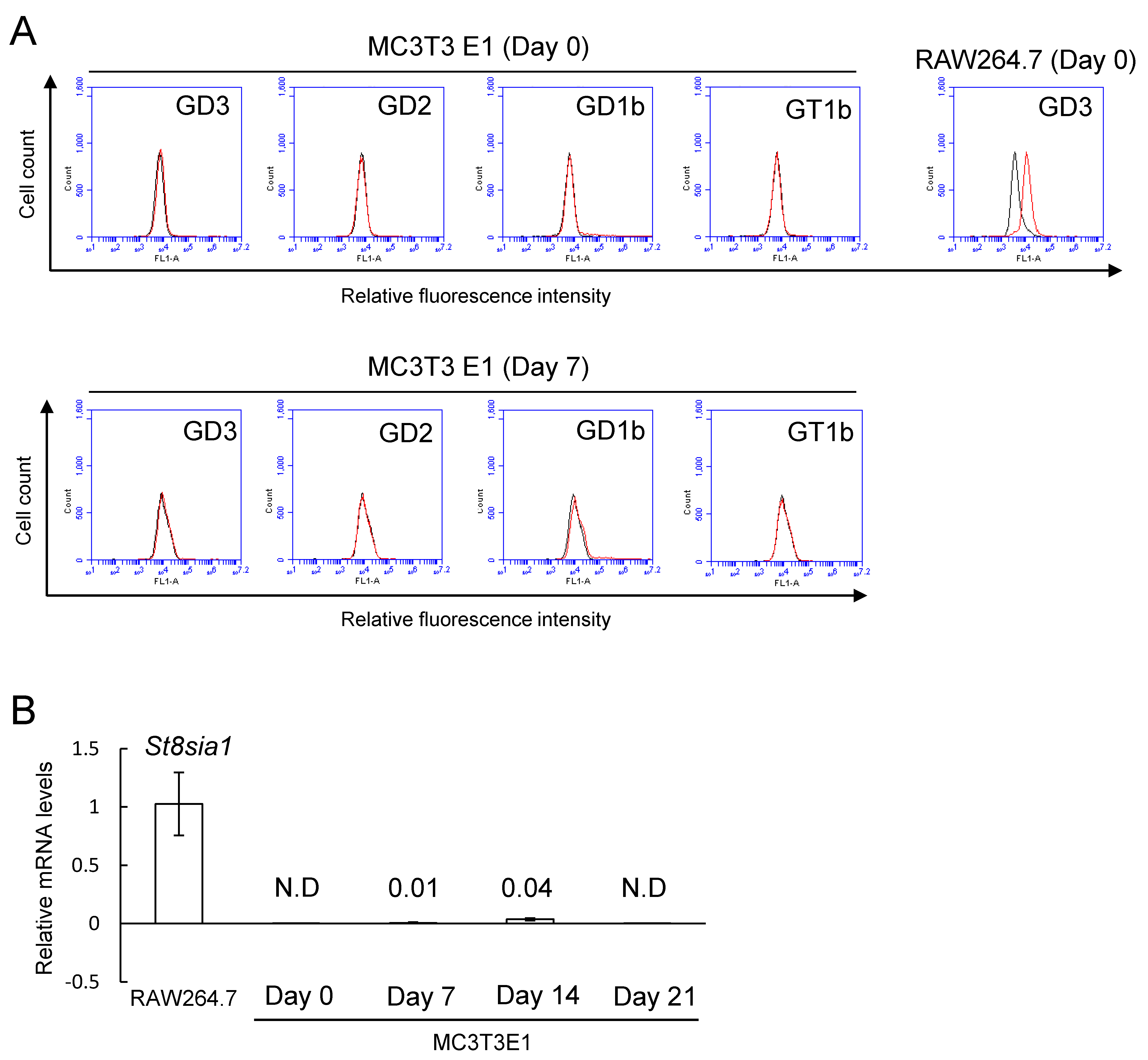
2.1. No Expression of b-Series Gangliosides and GD3 Synthase Gene in Osteoblasts
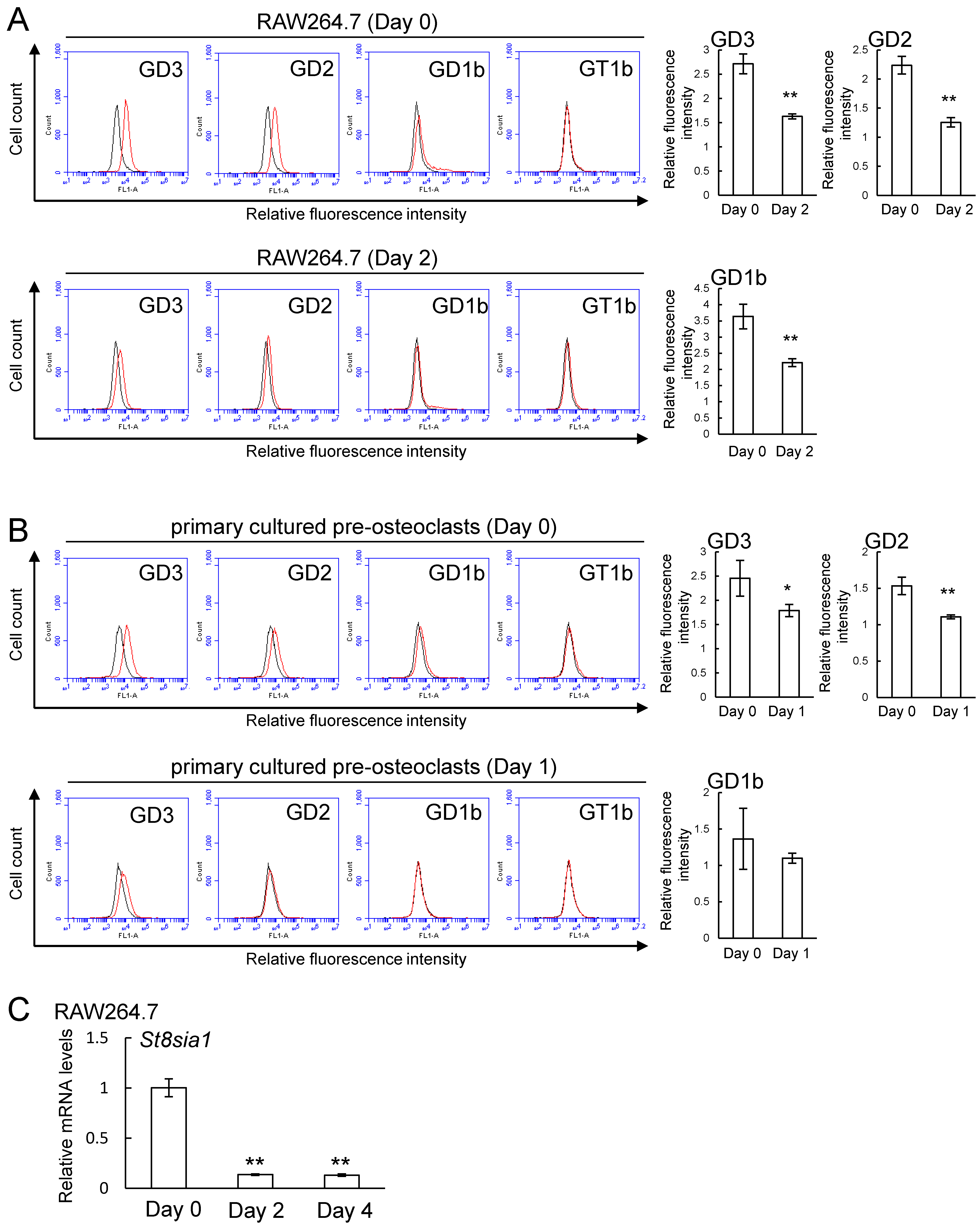
2.2. Expression of b-series Gangliosides and GD3 Synthase Gene in RAW264.7 Cells and Primary Cultured Pre-Osteoclasts Derived from Mouse Bone Marrow Cells in the Presence or Absence of Receptor Activator of Nuclear Factor Kappa-B Ligand (RANKL)
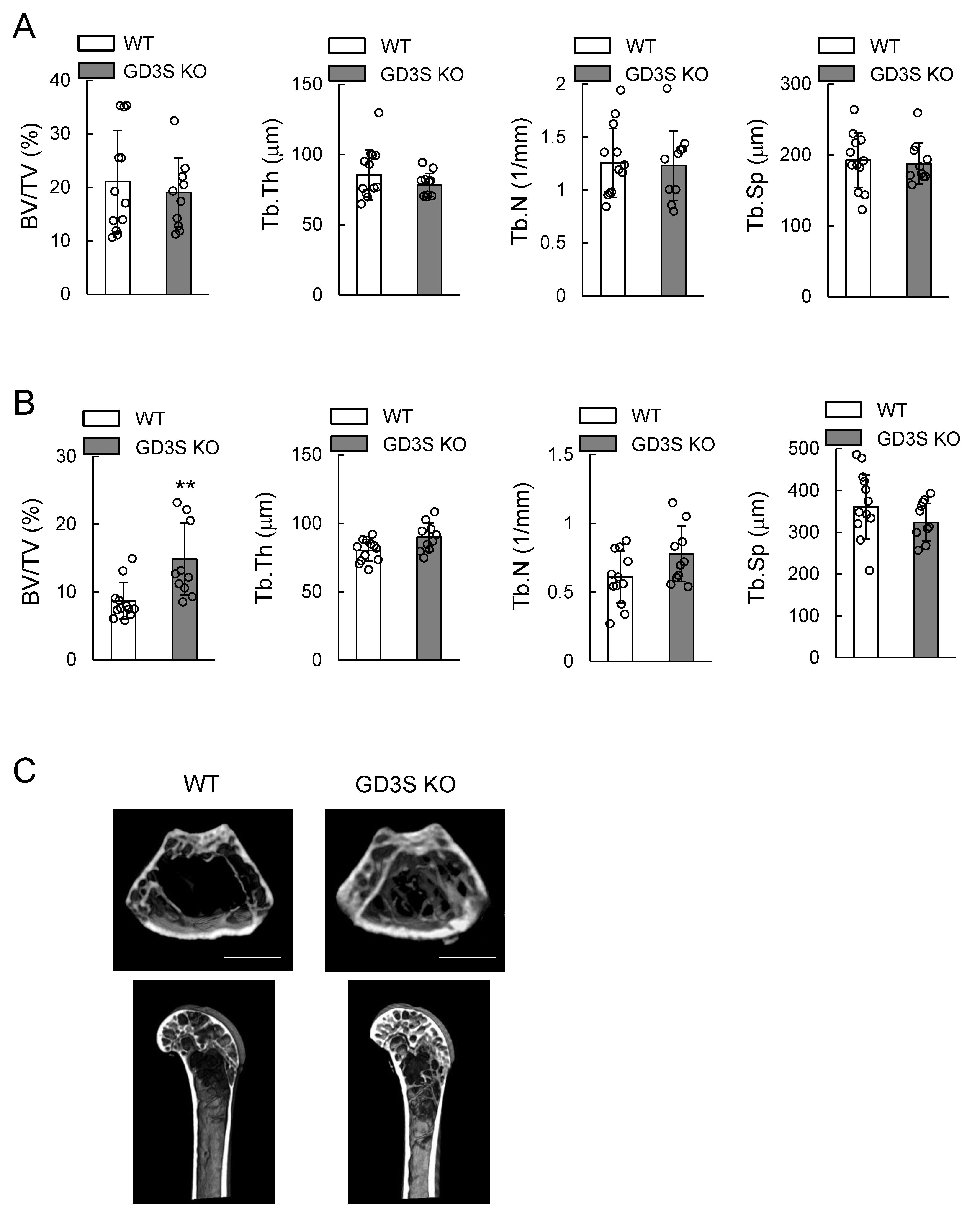
2.3. Attenuation of Loss of Femoral Cancellous Bone Mass with Aging in GD3 Synthase-Knockout (GD3S KO) Mice
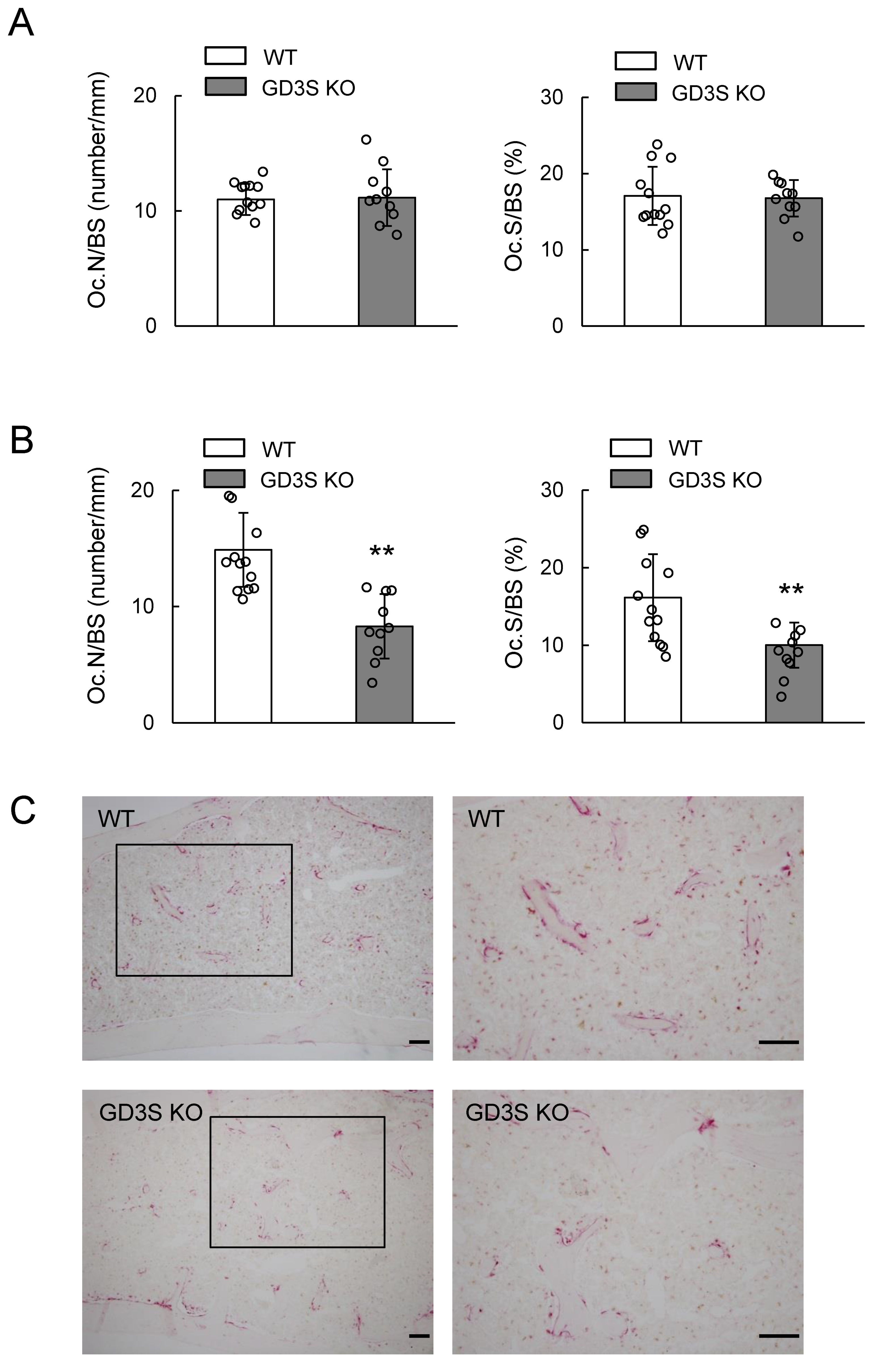
2.4. No Differences in Osteoblast Number in Femoral Cancellous Bone Between WT and GD3S KO Mice
2.5. Decrease of Bone Resorption Parameters by GD3 Synthase Deficiency
3. Discussion
4. Materials and Methods
4.1. Mice
4.2. Cell Culture
4.3. Antibodies
4.4. Induction to Mature Osteoblasts
4.5. In Vitro Osteoclast Induction
4.6. Flow Cytometry
4.7. qPCR
4.8. Three-Dimensional Micro-Computed Tomography (3D-μCT Analysis)
4.9. TRAP Staining
4.10. Bone Histomorphometric Analyses
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wiegandt, H. Gangliosides. In Glycolipid; Wiegandt, H., Ed.; Elsevier: New York, NY, USA, 1985; pp. 199–260. [Google Scholar]
- Yu, R.K.; Tsai, Y.T.; Ariga, T. Functional roles of gangliosides in neurodevelopment: An overview of recent advances. Neurochem. Res. 2012, 37, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Ohmi, Y.; Ohkawa, Y.; Tajima, O.; Furukawa, K. Glycosphingolipids in the regulation of the nerve system. Adv. Neurobiol. 2014, 9, 307–320. [Google Scholar] [PubMed]
- Schengrund, C.L. Gangliosides: Glycosphingolipids essential for normal neural development and function. Trends Biochem. Sci. 2015, 40, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Schnaar, R.L. Gangliosides of the vertebrate nervous system. J. Mol. Biol. 2016, 428, 3325–3336. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, K.; Yamamoto, A.; Furukawa, K.; Zhao, J.; Fukumoto, S.; Yamashiro, S.; Okada, M.; Haraguchi, M.; Shin, M.; Kishikawa, M.; et al. Complex gangliosides are essential in spermatogenesis of mice: Possible roles in the transport of testosterone. Proc. Natl. Acad. Sci. USA 1998, 95, 12147–12152. [Google Scholar] [CrossRef]
- Ji, S.; Ohkawa, Y.; Tokizane, K.; Ohmi, Y.; Banno, R.; Furukawa, K.; Kiyama, H.; Furukawa, K. b-series gangliosides crucially regulate leptin secretion in adipose tissues. Biochem. Biophys. Res. Commun. 2015, 459, 189–195. [Google Scholar] [CrossRef]
- Furukawa, K.; Ohmi, Y.; Ji, S.; Zhang, P.; Bhuiyan, R.H.; Ohkawa, Y.; Tajima, O.; Hashimoto, N.; Furukawa, K. Glycolipids: Essential regulator of neuro-inflammation, metabolism and gliomagenesis. Biochim. Biophys. Acta Gen. Subj. 2017, 1861, 2479–2484. [Google Scholar] [CrossRef]
- Ohmi, Y.; Tajima, O.; Ohkawa, Y.; Mori, A.; Sugiura, Y.; Furukawa, K.; Furukawa, K. Gangliosides play pivotal roles in the regulation of complement systems and in the maintenance of integrity in nerve tissues. Proc. Natl. Acad. Sci. USA 2009, 106, 22405–22410. [Google Scholar] [CrossRef]
- Ohmi, Y.; Ohkawa, Y.; Tajima, O.; Sugiura, Y.; Furukawa, K.; Furukawa, K. Ganglioside deficiency causes inflammation and neurodegeneration via the activation of complement system in the spinal cord. J. Neuroinflammation 2014, 11, 61. [Google Scholar] [CrossRef]
- Okada, M.; Itoh, M.; Haraguchi, M.; Okajima, T.; Inoue, M.; Oishi, H.; Matsuda, Y.; Iwamoto, T.; Kawano, T.; Fukumoto, S.; et al. b-series ganglioside deficiency exhibits no definite changes in the neurogenesis and the sensitivity to Fas-mediated apoptosis but impairs regeneration of the lesioned hypoglossal nerve. J. Biol. Chem. 2002, 277, 1633–1636. [Google Scholar] [CrossRef]
- Handa, Y.; Ozaki, N.; Honda, T.; Furukawa, K.; Tomita, Y.; Inoue, M.; Furukawa, K.; Okada, M.; Sugiura, Y. GD3 synthase gene knockout mice exhibit thermal hyperalgesia and mechanical allodynia but decreased response to formalin-induced prolonged noxious stimulation. Pain 2005, 117, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Hamamura, K.; Furukawa, K.; Hayashi, T.; Nakano, J.; Nakashima, H.; Okuda, T.; Mizutani, H.; Hattori, H.; Ueda, M.; Urano, T.; et al. Ganglioside GD3 promotes cell growth and invasion through p130Cas and paxillin in malignant melanoma cells. Proc. Natl. Acad. Sci. USA 2005, 102, 11041–11046. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, H.; Hamamura, K.; Hotta, H.; Matsumoto, Y.; Nishida, Y.; Hattori, H.; Furukawa, K.; Ueda, M.; Furukawa, K. Enhancement of malignant properties of human osteosarcoma cells with disialyl gangliosides GD2/GD3. Cancer Sci. 2012, 103, 1656–1664. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, K.; Hamamura, K.; Ohkawa, Y.; Ohmi, Y.; Furukawa, K. Disialyl gangliosides enhance tumor phenotypes with differential modalities. Glycoconj. J. 2012, 29, 579–584. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.M.; Jung, J.U.; Ryu, J.S.; Jin, J.W.; Yang, H.J.; Ko, K.; You, H.K.; Jung, K.Y.; Choo, Y.K. Effects of gangliosides on the differentiation of human mesenchymal stem cells into osteoblasts by modulating epidermal growth factor receptors. Biochem. Biophys. Res. Commun. 2008, 371, 866–871. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.J.; Jung, K.Y.; Kwak, D.H.; Lee, S.H.; Ryu, J.S.; Kim, J.S.; Chang, K.T.; Lee, J.W.; Choo, Y.K. Inhibition of ganglioside GD1a synthesis suppresses the differentiation of human mesenchymal stem cells into osteoblasts. Develop. Growth Differ. 2011, 53, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Bergante, S.; Torretta, E.; Creo, P.; Sessarego, N.; Papini, N.; Piccoli, M.; Fania, C.; Cirillo, F.; Conforti, E.; Ghiroldi, A.; et al. Gangliosides as a potential new class of stem cell markers: The case of GD1a in human bone marrow mesenchymal stem cells. J. Lipid Res. 2014, 55, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.K.; Lee, J.W.; Kim, K.S.; Mun, S.W.; Kim, D.H.; Kim, H.J.; Kim, C.H.; Lee, Y.C. Serum deprivation-induced human GM3 synthase (hST3Gal V) gene expression is mediated by Runx2 in human osteoblastic MG-63 cells. Int. J. Mol. Sci. 2016, 17, 35. [Google Scholar] [CrossRef] [PubMed]
- Bergante, S.; Creo, P.; Piccoli, M.; Ghiroldi, A.; Menon, A.; Cirillo, F.; Rota, P.; Monasky, M.M.; Ciconte, G.; Pappone, C.; et al. GM1 ganglioside promotes osteogenic differentiation of human tendon stem cells. Stem Cells Int. 2018, 4706943. [Google Scholar] [CrossRef]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Friedman, J.M.; Halaas, J.L. Leptin and the regulation of body weight in mammals. Nature 1998, 395, 763–770. [Google Scholar] [CrossRef] [PubMed]
- Paracchini, V.; Pedotti, P.; Taioli, E. Genetics of leptin and obesity: A HuGE review. Am. J. Epidemiol. 2005, 162, 101–114. [Google Scholar] [CrossRef]
- Elefteriou, F.; Ahn, J.D.; Takeda, S.; Starbuck, M.; Yang, X.; Liu, X.; Kondo, H.; Richards, W.G.; Bannon, T.W.; Noda, M.; et al. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 2005, 434, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Ji, S.; Tokizane, K.; Ohkawa, Y.; Ohmi, Y.; Banno, R.; Okajima, T.; Kiyama, H.; Furukawa, K.; Furukawa, K. Increased a-series gangliosides positively regulate leptin/Ob receptor-mediated signals in hypothalamus of GD3 synthase-deficient mice. Biochem. Biophys. Res. Commun. 2016, 479, 453–460. [Google Scholar] [CrossRef]
- Hamamura, K.; Chen, A.; Nishimura, A.; Tanjung, N.; Sudo, A.; Yokota, H. Predicting and validating the pathway of Wnt3a-driven suppression of osteoclastogenesis. Cell. Signal. 2014, 26, 2358–2369. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, H.; Fukumoto, S.; Okada, M.; Hasegawa, T.; Furukawa, K. Expression cloning of rat cDNA encoding UDP-galactose: GD2 β1,3-galactosyltransferase that determines the expression of GD1b/GM1/GA1. J. Biol. Chem. 1997, 272, 24794–24799. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Hamamura, K.; Yo, S.; Hamajima, K.; Ootani, K.; Honda, M.; Ishizuka, K.; Kondo, H.; Tanaka, K.; Kodama, D.; et al. Conditioned medium from rat dental pulp reduces the number of osteoclasts via attenuation of adhesiveness in osteoclast precursors. J. Oral Sci. 2018, 60, 352–359. [Google Scholar] [CrossRef]
- Tanaka, K.; Hirai, T.; Kodama, D.; Kondo, H.; Hamamura, K.; Togari, A. α1B-Adrenoceptor signaling regulates bone formation through the up-regulation of CCAAT/enhancer-binding protein δ expression in osteoblasts. Br. J. Pharmacol. 2016, 173, 1058–1069. [Google Scholar] [CrossRef] [PubMed]


| Target | Forward Primer | Backward Primer |
|---|---|---|
| St8sia1 | 5′-TGCTTTTTGCTAACCCCAAC-3′ | 5′-AAGGGCCAGAAGCCATAGAT-3′ |
| Gapdh | 5′-TGCACCACCAACTGCTTAG-3′ | 5′-GGATGCAGGGATGATGTTC-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yo, S.; Hamamura, K.; Mishima, Y.; Hamajima, K.; Mori, H.; Furukawa, K.; Kondo, H.; Tanaka, K.; Sato, T.; Miyazawa, K.; et al. Deficiency of GD3 Synthase in Mice Resulting in the Attenuation of Bone Loss with Aging. Int. J. Mol. Sci. 2019, 20, 2825. https://doi.org/10.3390/ijms20112825
Yo S, Hamamura K, Mishima Y, Hamajima K, Mori H, Furukawa K, Kondo H, Tanaka K, Sato T, Miyazawa K, et al. Deficiency of GD3 Synthase in Mice Resulting in the Attenuation of Bone Loss with Aging. International Journal of Molecular Sciences. 2019; 20(11):2825. https://doi.org/10.3390/ijms20112825
Chicago/Turabian StyleYo, Shoyoku, Kazunori Hamamura, Yoshitaka Mishima, Kosuke Hamajima, Hironori Mori, Koichi Furukawa, Hisataka Kondo, Kenjiro Tanaka, Takuma Sato, Ken Miyazawa, and et al. 2019. "Deficiency of GD3 Synthase in Mice Resulting in the Attenuation of Bone Loss with Aging" International Journal of Molecular Sciences 20, no. 11: 2825. https://doi.org/10.3390/ijms20112825
APA StyleYo, S., Hamamura, K., Mishima, Y., Hamajima, K., Mori, H., Furukawa, K., Kondo, H., Tanaka, K., Sato, T., Miyazawa, K., Goto, S., & Togari, A. (2019). Deficiency of GD3 Synthase in Mice Resulting in the Attenuation of Bone Loss with Aging. International Journal of Molecular Sciences, 20(11), 2825. https://doi.org/10.3390/ijms20112825






