Effects of Spaceflight and Simulated Microgravity on YAP1 Expression in Cardiovascular Progenitors: Implications for Cell-Based Repair
Abstract
1. Introduction
2. Results
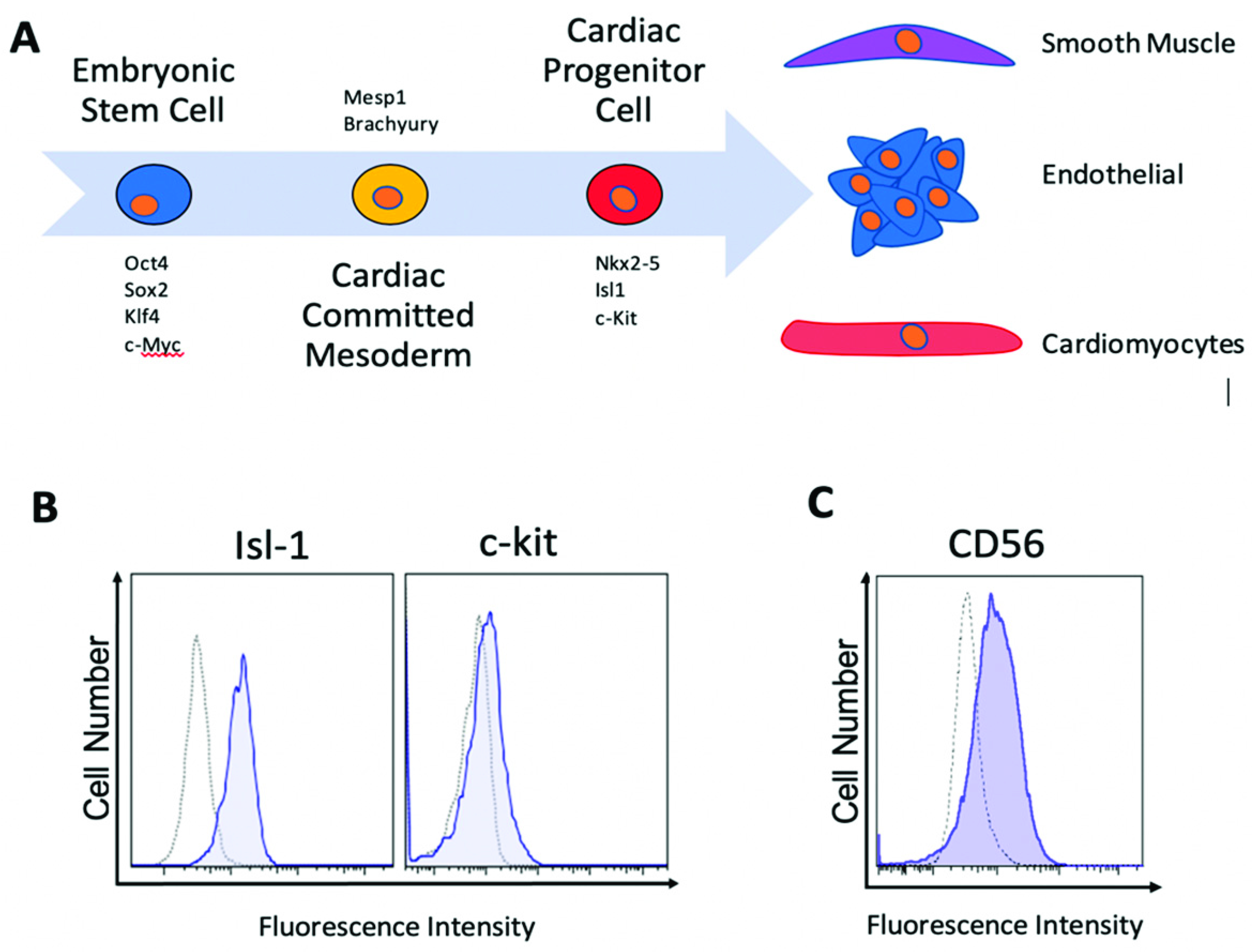
2.1. Human Islet-1+ Cardiac Progenitor Cells co-Express Several Markers of Early Cardiovascular Differentiation
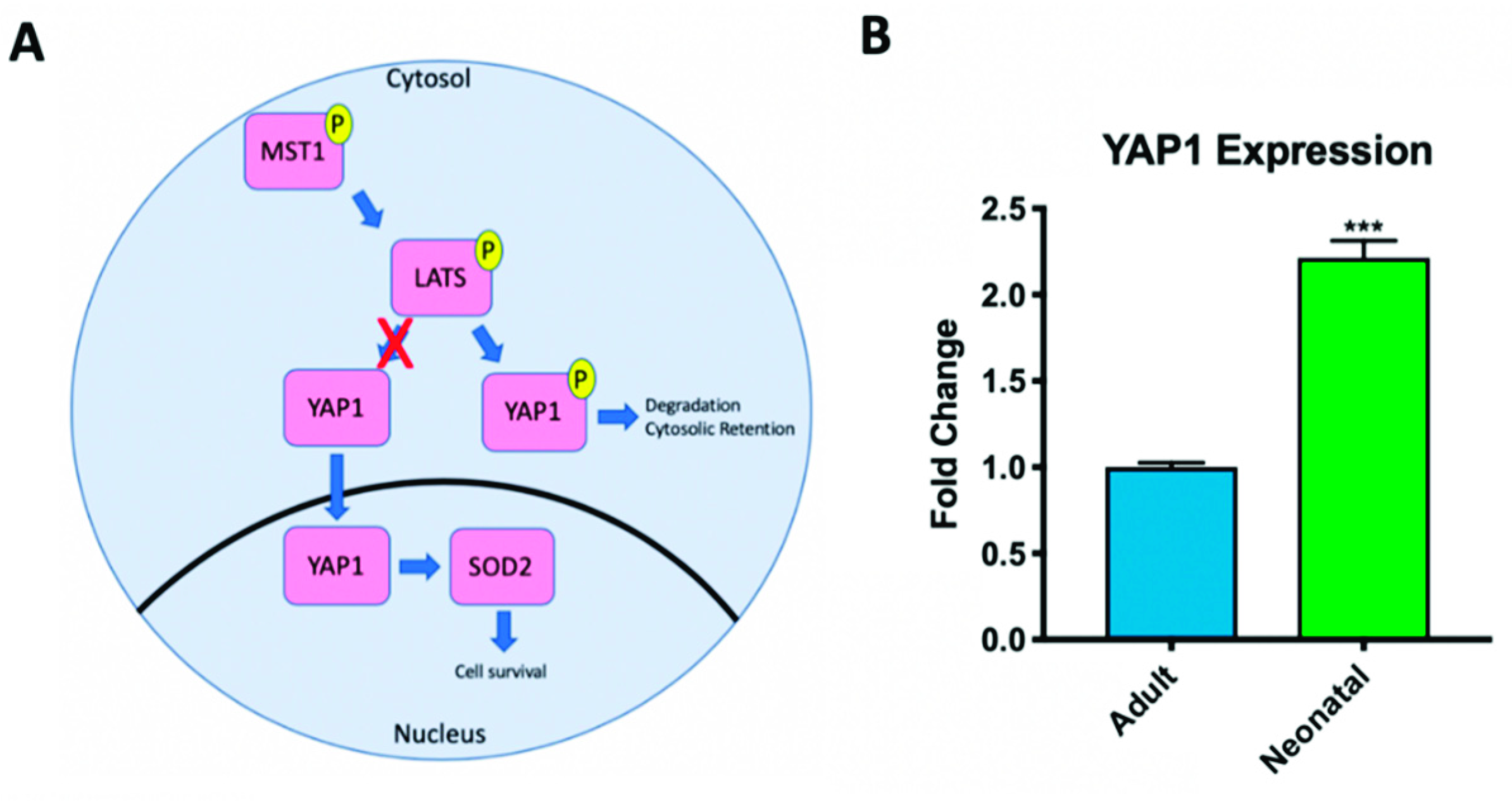
2.2. Hippo Signaling Pathway Activity Differs in Adult and Neonatal Cardiac Progenitor Cells
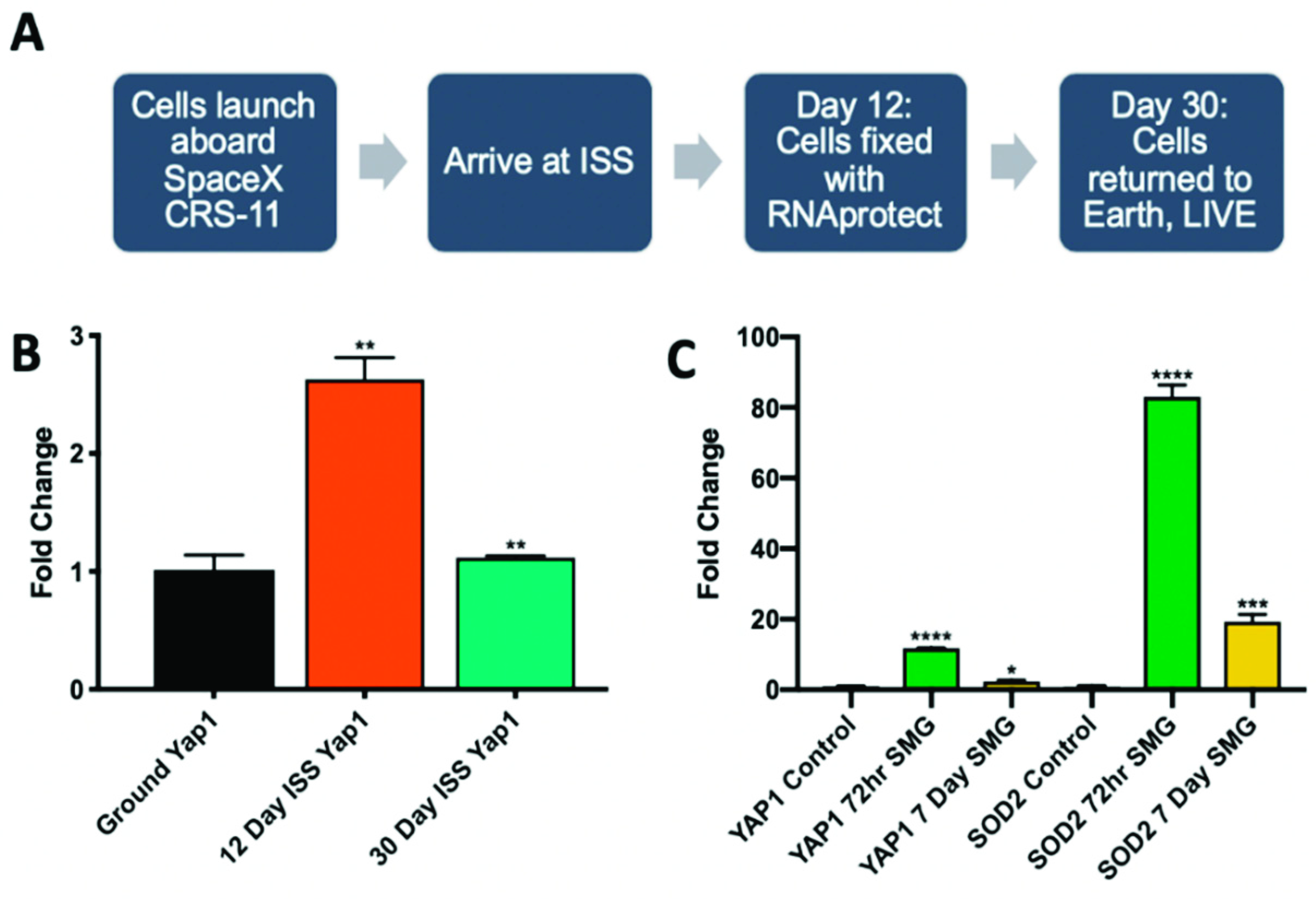
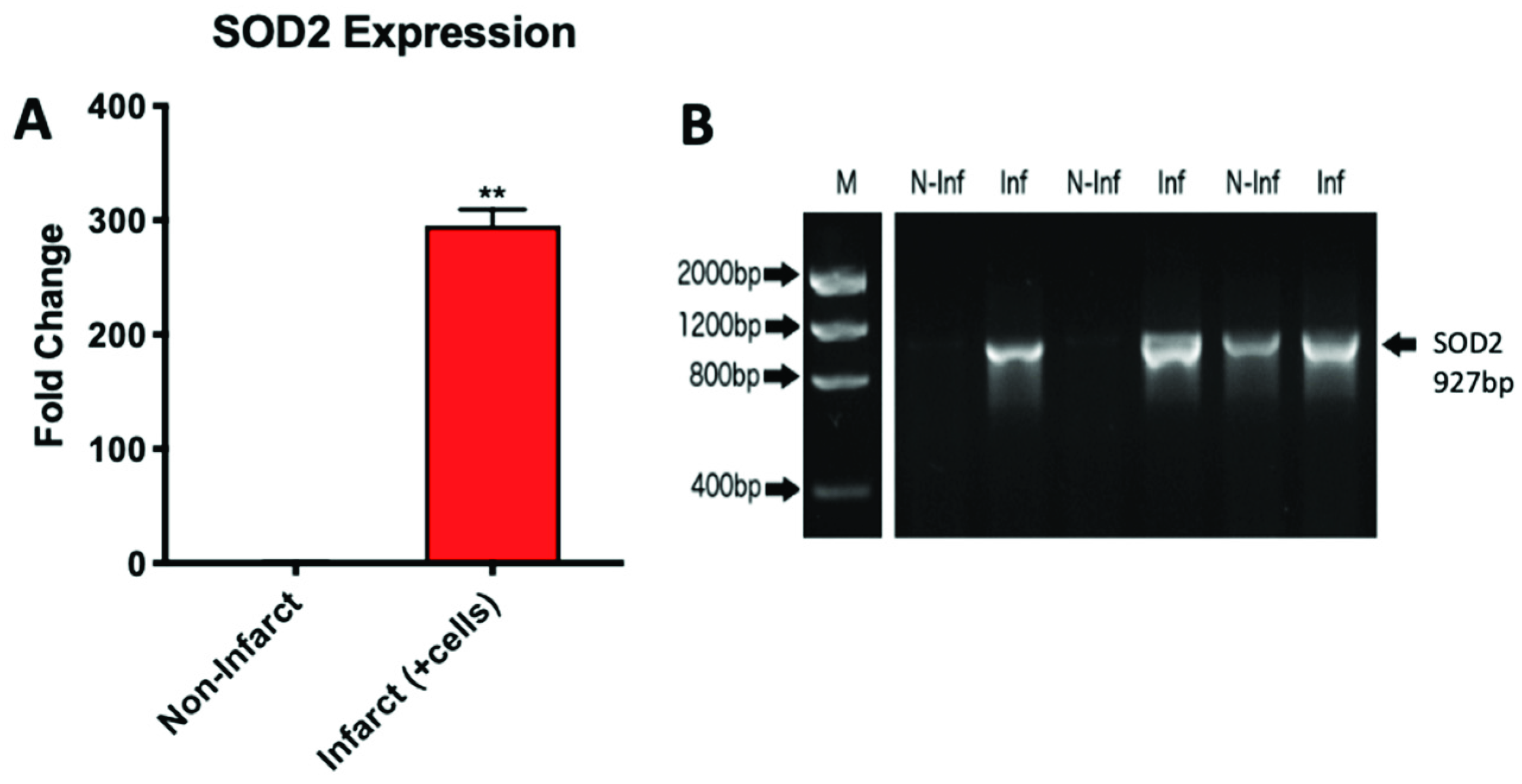
2.3. Microgravity Conditions Increase YAP1 and SOD2 Expression in Adult CPCs
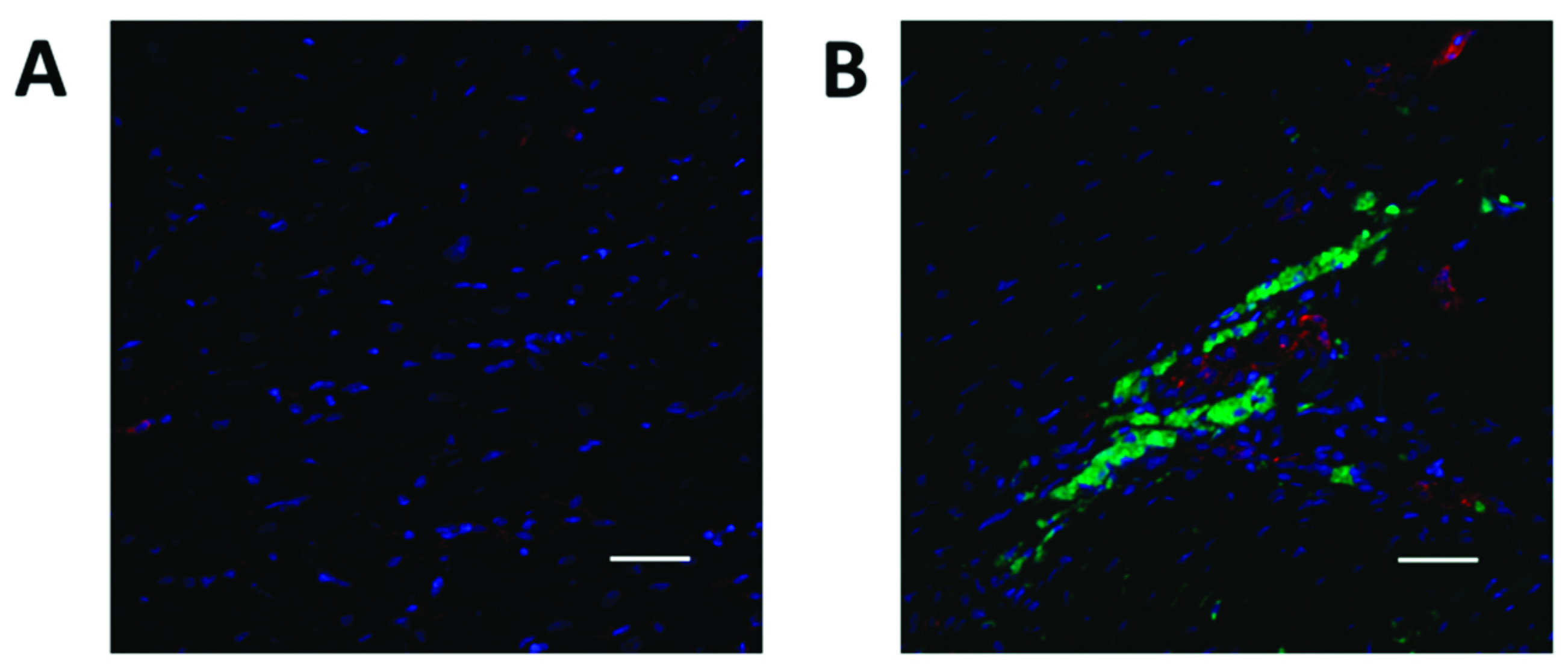
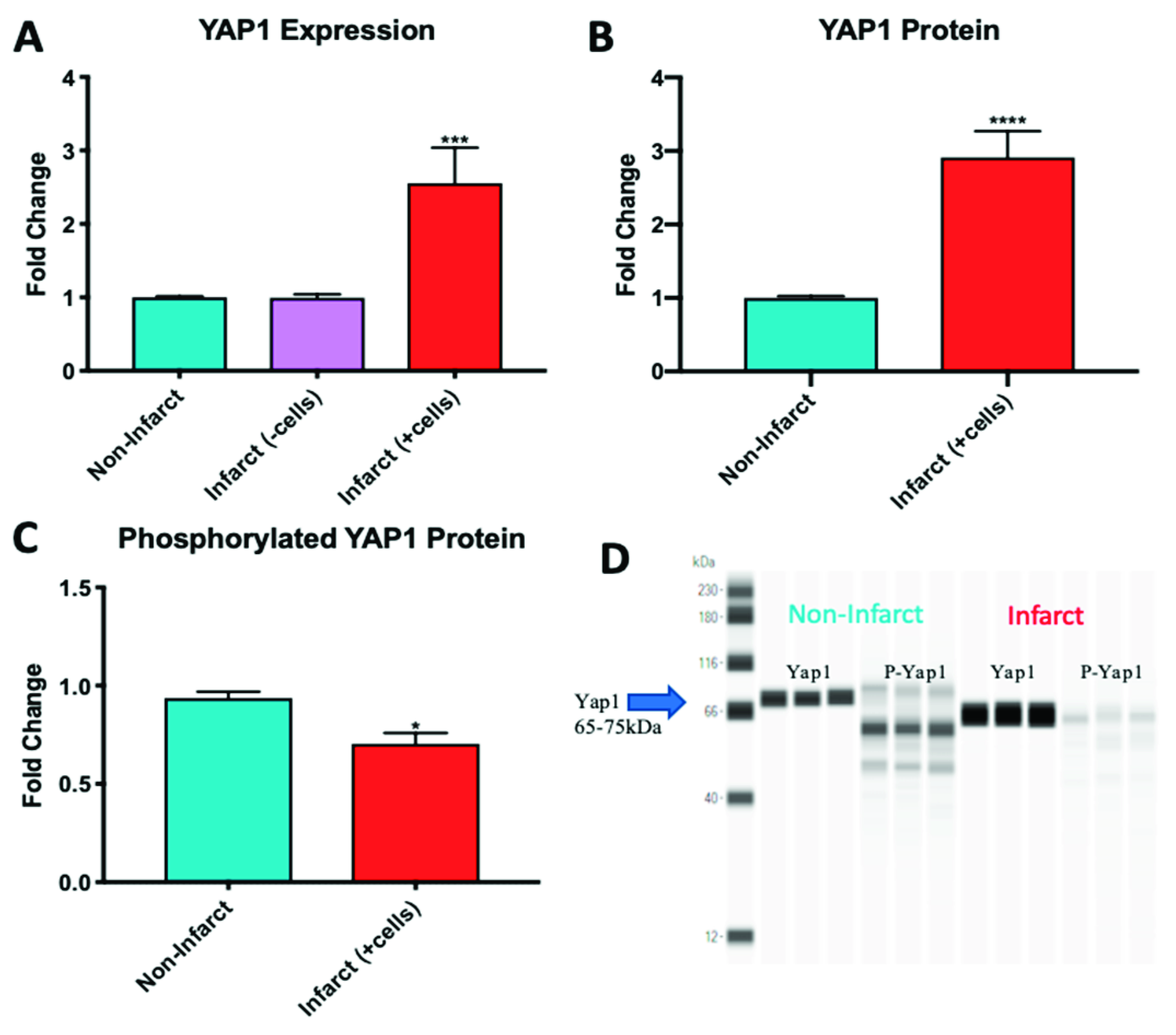
2.4. YAP1 Expression is Elevated in the Cardiovascular Repair Zone When YAP1 Expressing Neonatal CPC Are Introduced Following Myocardial Infarction
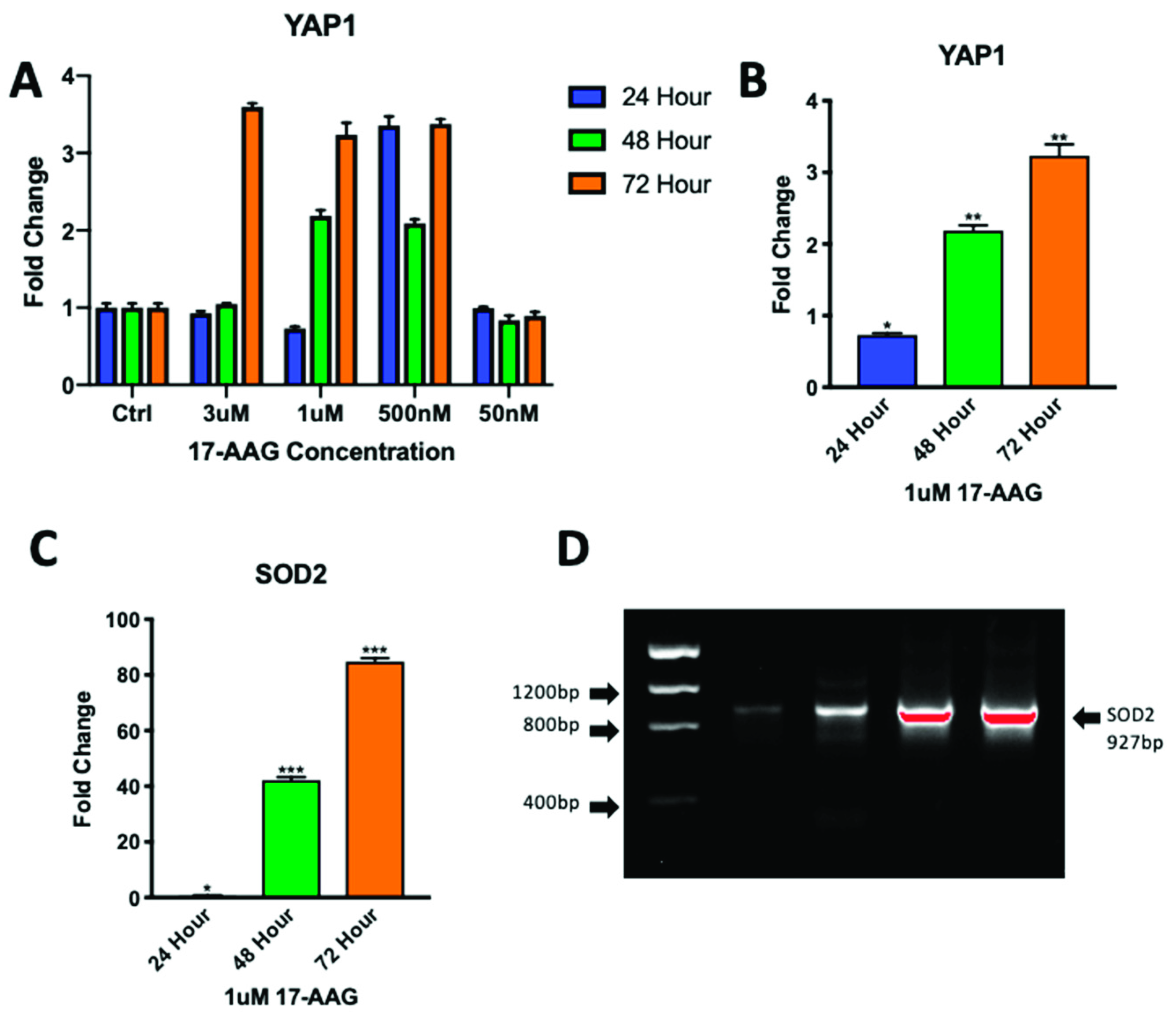
2.5. Adult CPCs Treated with 1uM of 17-AAG Demonstrated Increased Levels of YAP1 and SOD2
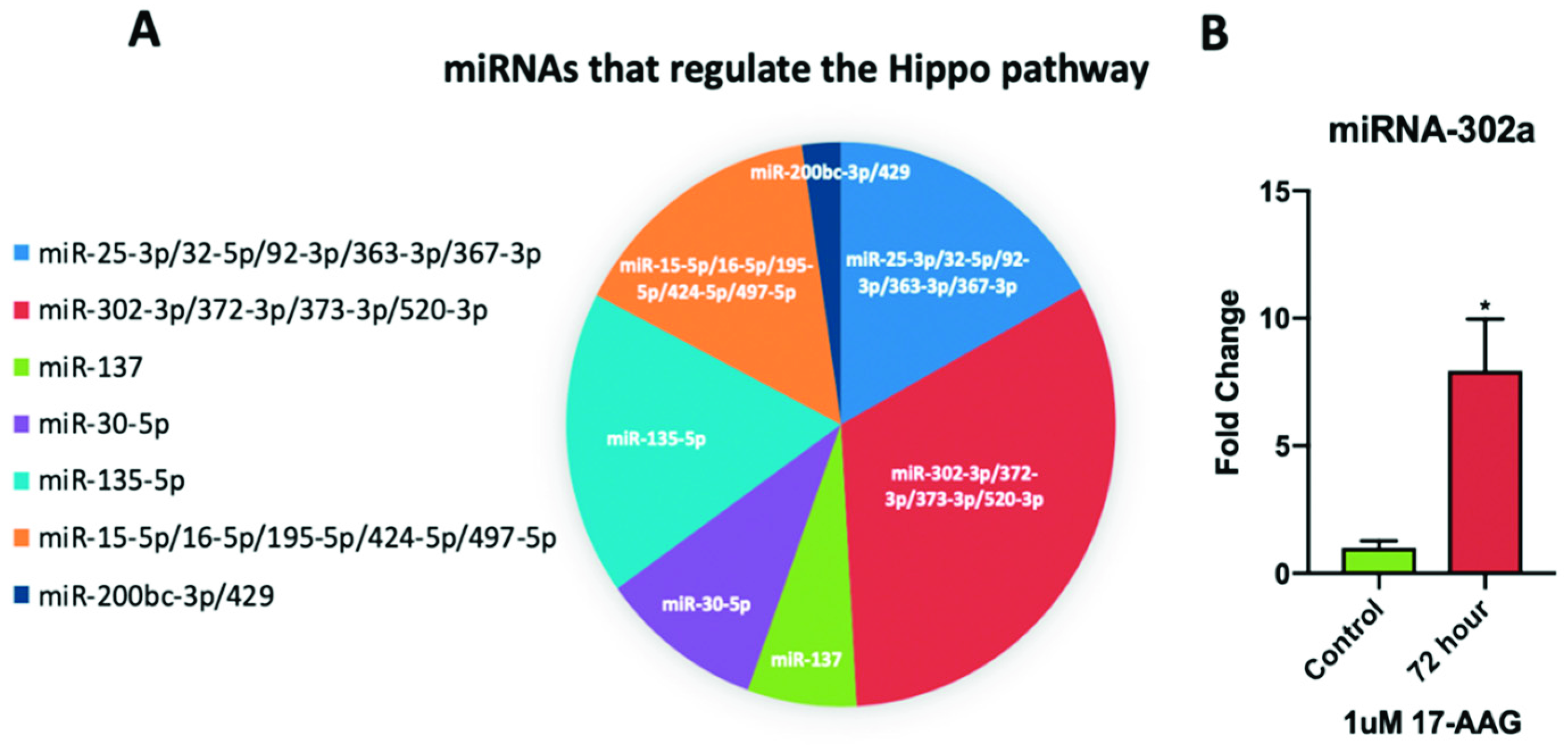
2.6. miRNA-302a is Elevated in Adult CPCs Treated with 17-AAG
3. Discussion
4. Materials and Methods
4.1. Isolation of Cardiac Progenitor Cells
4.2. Simulated Microgravity
4.3. Culture of CPCs Aboard the ISS
4.4. Sheep Model of Myocardial Infarction
4.5. Flow Cytometry
4.6. Treatment of Adult Cardiac Progenitor Cells with 17-AAG
4.7. Western Blot and Protein Purification
4.8. RNA Purification and RT-PCR
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ISS | International Space Station |
| CPC | Cardiac progenitor cell |
| YAP1 | Yes-associated protein 1 |
| LATS1/2 | Large tumor suppressor 1/2 |
| SOD2 | Superoxide dismutase 2 |
| 17-AAG | 17-allylamino-17-demethoxygeldanamycin |
| DMSO | Dimethylsulfoxide |
| CFSE | Carboxyfluorescein succinimidyl ester |
References
- Kovo, Y. Bioculture System. Available online: http://www.nasa.gov/ames/research/space-biosciences/bioculture-system (accessed on 29 January 2019).
- Davis, T.A.; Wiesmann, W.; Kidwell, W.; Cannon, T.; Kerns, L.; Serke, C.; Delaplaine, T.; Pranger, A.; Lee, K.P. Effect of spaceflight on human stem cell hematopoiesis: Suppression of erythropoiesis and myelopoiesis. J. Leukoc. Biol. 1996, 60, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, J.; Gass, S.; Nebuloni, S.; Echegoyen, D.; Riwaldt, S.; Baake, C.; Bauer, J.; Corydon, T.J.; Egli, M.; Infanger, M.; et al. Three-dimensional growth of human endothelial cells in an automated cell culture experiment container during the SpaceX CRS-8 ISS space mission—The SPHEROIDS project. Biomaterials 2017, 124, 126–156. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.; Martinez, A.F.; Silva, I.; Hoehn, C.V.; Countryman, S.; Bailey, L.; Hasaniya, N.; Pecaut, M.J.; Kearns-Jonker, M. Cardiovascular progenitor cells cultured aboard the International Space Station exhibit altered developmental and functional properties. Npj Microgravity 2018, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Blaber, E.A.; Finkelstein, H.; Dvorochkin, N.; Sato, K.Y.; Yousuf, R.; Burns, B.P.; Globus, R.K.; Almeida, E.A.C. Microgravity reduces the differentiation and regenerative potential of embryonic stem cells. Stem Cells Dev. 2015, 24, 2605–2621. [Google Scholar] [CrossRef] [PubMed]
- My, K. Alteration of cell cytoskeleton and functions of cell recovery of normal human osteoblast cells caused by factors associated with real space flight. Malays. J. Pathol. 2013, 11, 153–163. [Google Scholar]
- Liu, Y.; Wang, E. Transcriptional analysis of normal human fibroblast responses to microgravity stress. Genom. Proteom. Bioinform. 2008, 6, 29–41. [Google Scholar] [CrossRef]
- Blaber, E.A.; Dvorochkin, N.; Torres, M.L.; Yousuf, R.; Burns, B.P.; Globus, R.K.; Almeida, E.A.C. Mechanical unloading of bone in microgravity reduces mesenchymal and hematopoietic stem cell-mediated tissue regeneration. Stem Cell Res. 2014, 13, 181–201. [Google Scholar] [CrossRef]
- Herranz, R.; Anken, R.; Boonstra, J.; Braun, M.; Christianen, P.C.M.; de Geest, M.; Hauslage, J.; Hilbig, R.; Hill, R.J.A.; Lebert, M.; et al. Ground-based facilities for simulation of microgravity: Organism-specific recommendations for their use, and recommended terminology. Astrobiology 2013, 13, 1–17. [Google Scholar] [CrossRef]
- Grigoryan, E.N.; Radugina, E.A. Behavior of stem-like cells, precursors for tissue regeneration in Urodela, under conditions of microgravity. Stem Cells Dev. 2019, 28, 423–437. [Google Scholar] [CrossRef]
- Grimm, D.; Wehland, M.; Pietsch, J.; Aleshcheva, G.; Wise, P.; van Loon, J.; Ulbrich, C.; Magnusson, N.E.; Infanger, M.; Bauer, J. Growing tissues in real and simulated microgravity: New methods for tissue engineering. Tissue Eng. Part B Rev. 2014, 20, 555–566. [Google Scholar] [CrossRef]
- Hemmersbach, R.; Strauch, S.M.; Seibt, D.; Schuber, M. Comparative studies on gravisensitive protists on ground (2D and 3D clinostats) and in microgravity. Microgravity Sci. Technol. 2006, 18, 257–259. [Google Scholar] [CrossRef]
- Fuentes, T.I.; Appleby, N.; Raya, M.; Bailey, L.; Hasaniya, N.; Stodieck, L.; Kearns-Jonker, M. Simulated microgravity exerts an age-dependent effect on the differentiation of cardiovascular progenitors isolated from the human heart. PLoS ONE 2015, 10, e0132378. [Google Scholar] [CrossRef] [PubMed]
- Yuge, L.; Sasaki, A.; Kawahara, Y.; Wu, S.; Matsumoto, M.; Manabe, T.; Kajiume, T.; Takeda, M.; Magaki, T.; Takahashi, T.; et al. Simulated microgravity maintains the undifferentiated state and enhances the neural repair potential of bone marrow stromal cells. Stem Cells Dev. 2011, 20, 893–900. [Google Scholar] [CrossRef] [PubMed]
- Mitsuhara, T.; Takeda, M.; Yamaguchi, S.; Manabe, T.; Matsumoto, M.; Kawahara, Y.; Yuge, L.; Kurisu, K. Simulated microgravity facilitates cell migration and neuroprotection after bone marrow stromal cell transplantation in spinal cord injury. Stem Cell Res. Ther. 2013, 4, 35. [Google Scholar] [CrossRef] [PubMed]
- Yuge, L.; Kajiume, T.; Tahara, H.; Kawahara, Y.; Umeda, C.; Yoshimoto, R.; Wu, S.-L.; Yamaoka, K.; Asashima, M.; Kataoka, K.; et al. Microgravity potentiates stem cell proliferation while sustaining the capability of differentiation. Stem Cells Dev. 2006, 15, 921–929. [Google Scholar] [CrossRef]
- Baio, J.; Martinez, A.F.; Bailey, L.; Hasaniya, N.; Pecaut, M.J.; Kearns-Jonker, M. Spaceflight activates protein kinase C alpha signaling and modifies the developmental stage of human neonatal cardiovascular progenitor cells. Stem Cells Dev. 2018, 27, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Di Cara, F.; Maile, T.M.; Parsons, B.D.; Magico, A.; Basu, S.; Tapon, N.; King-Jones, K. The Hippo pathway promotes cell survival in response to chemical stress. Cell Death Differ. 2015, 22, 1526–1539. [Google Scholar] [CrossRef]
- Mach, J.; Atkins, M.; Gajewski, K.M.; Mottier-Pavie, V.; Sansores-Garcia, L.; Xie, J.; Mills, R.A.; Kowalczyk, W.; Huffel, L.V.; Mills, G.B.; et al. Modulation of the Hippo pathway and organ growth by RNA processing proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 10684–10689. [Google Scholar] [CrossRef]
- Pan, D. Hippo signaling in organ size control. Genes Dev. 2007, 21, 886–897. [Google Scholar] [CrossRef]
- Yu, F.-X.; Zhao, B.; Guan, K.-L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Song, Y.; Fu, J.; Zhou, M.; Xiao, L.; Feng, X.; Chen, H.; Huang, W. Activated Hippo/Yes-associated protein pathway promotes cell proliferation and anti-apoptosis in endometrial stromal cells of endometriosis. J. Clin. Endocrinol. Metab. 2016, 101, 1552–1561. [Google Scholar] [CrossRef] [PubMed]
- Campbell, K.N.; Wong, J.S.; Gupta, R.; Asanuma, K.; Sudol, M.; He, J.C.; Mundel, P. Yes-associated protein (YAP) promotes cell survival by inhibiting proapoptotic dendrin signaling. J. Biol. Chem. 2013, 288, 17057–17062. [Google Scholar] [CrossRef] [PubMed]
- Uygur, A.; Lee, R.T. Mechanisms of cardiac regeneration. Dev. Cell 2016, 36, 362–374. [Google Scholar] [CrossRef]
- Kikuchi, K.; Poss, K.D. Cardiac regenerative capacity and mechanisms. Annu. Rev. Cell Dev. Biol. 2012, 28, 719–741. [Google Scholar] [CrossRef]
- Xin, M.; Olson, E.N.; Bassel-Duby, R. Mending broken hearts: Cardiac development as a basis for adult heart regeneration and repair. Nat. Rev. Mol. Cell Biol. 2013, 14, 529–541. [Google Scholar] [CrossRef]
- Lin, Z.; von Gise, A.; Zhou, P.; Gu, F.; Ma, Q.; Jiang, J.; Yau, A.L.; Buck, J.N.; Gouin, K.A.; van Gorp, P.R.R.; et al. Cardiac-specific YAP activation improves cardiac function and survival in an experimental murine MI model. Circ. Res. 2014, 115, 354–363. [Google Scholar] [CrossRef]
- LeBlanc, L.; Lee, B.-K.; Yu, A.C.; Kim, M.; Kambhampati, A.V.; Dupont, S.M.; Seruggia, D.; Ryu, B.U.; Orkin, S.H.; Kim, J. Yap1 Safeguards Mouse Embryonic Stem Cells from Excessive Apoptosis during Differentiation. Available online: https://elifesciences.org/articles/40167 (accessed on 1 February 2019).
- Lin, Z.; Pu, W.T. Harnessing Hippo in the heart: Hippo/Yap signaling and applications to heart regeneration and rejuvenation. Stem Cell Res. 2014, 13, 571–581. [Google Scholar] [CrossRef]
- Fuentes, T.I.; Appleby, N.; Tsay, E.; Martinez, J.J.; Bailey, L.; Hasaniya, N.; Kearns-Jonker, M. Human neonatal cardiovascular progenitors: Unlocking the secret to regenerative ability. PLoS ONE 2013, 8, e77464. [Google Scholar] [CrossRef]
- Di Felice, V.; Zummo, G. Stem cell populations in the heart and the role of Isl1 positive cells. Eur. J. Histochem. 2013, 57, 83–85. [Google Scholar] [CrossRef]
- Domian, I.J.; Chiravuri, M.; van der Meer, P.; Feinberg, A.W.; Shi, X.; Shao, Y.; Wu, S.M.; Parker, K.K.; Chien, K.R. Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science 2009, 326, 426–429. [Google Scholar] [CrossRef]
- Bartulos, O.; Zhuang, Z.W.; Huang, Y.; Mikush, N.; Suh, C.; Bregasi, A.; Wang, L.; Chang, W.; Krause, D.S.; Young, L.H.; et al. ISL1 cardiovascular progenitor cells for cardiac repair after myocardial infarction. JCI Insight 2016, 1, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Evseenko, D.; Zhu, Y.; Schenke-Layland, K.; Kuo, J.; Latour, B.; Ge, S.; Scholes, J.; Dravid, G.; Li, X.; MacLellan, W.R.; et al. Mapping the first stages of mesoderm commitment during differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. USA 2010, 107, 13742–13747. [Google Scholar] [CrossRef] [PubMed]
- Baio, J.M.; Walden, R.C.; Fuentes, T.I.; Lee, C.C.; Hasaniya, N.W.; Bailey, L.L.; Kearns-Jonker, M.K. A hyper-crosslinked carbohydrate polymer scaffold facilitates lineage commitment and maintains a reserve pool of proliferating cardiovascular progenitors. Transpl. Direct 2017, 3, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Irvine, K.D.; Harvey, K.F. Control of organ growth by patterning and Hippo signaling in drosophila. Cold Spring Harb Perspect Biol. 2015, 7, 1–15. [Google Scholar] [CrossRef]
- Von Gise, A.; Lin, Z.; Schlegelmilch, K.; Honor, L.B.; Pan, G.M.; Buck, J.N.; Ma, Q.; Ishiwata, T.; Zhou, B.; Camargo, F.D.; et al. YAP1, the nuclear target of Hippo signaling, stimulates heart growth through cardiomyocyte proliferation but not hypertrophy. Proc. Natl. Acad. Sci. USA 2012, 109, 2394–2399. [Google Scholar] [CrossRef]
- Lin, P.; Chen, Y.-R. Overexpression of SOD2 in myocytes protects mitochondrial function from myocardial ischemia and reperfusion injury. Faseb J. 2017, 31, 845.4. [Google Scholar]
- Fukai, T.; Ushio-Fukai, M. Superoxide dismutases: Role in redox signaling, vascular function, and diseases. Antioxid Redox Signal 2011, 15, 1583–1606. [Google Scholar] [CrossRef]
- Del Re, D.P.; Yang, Y.; Nakano, N.; Cho, J.; Zhai, P.; Yamamoto, T.; Zhang, N.; Yabuta, N.; Nojima, H.; Pan, D.; et al. Yes-associated protein isoform 1 (Yap1) promotes cardiomyocyte survival and growth to protect against myocardial ischemic injury. J. Biol. Chem. 2013, 288, 3977–3988. [Google Scholar] [CrossRef]
- Odashima, M.; Usui, S.; Takagi, H.; Hong, C.; Liu, J.; Yokota, M.; Sadoshima, J. Inhibition of endogenous Mst1 prevents apoptosis and cardiac dysfunction without affecting cardiac hypertrophy after myocardial infarction. Circ. Res. 2007, 100, 1344–1352. [Google Scholar] [CrossRef]
- Matsuda, T.; Zhai, P.; Sciarretta, S.; Zhang, Y.; Jeong, J.I.; Ikeda, S.; Park, J.; Hsu, C.-P.; Tian, B.; Pan, D.; et al. NF2 activates Hippo signaling and promotes ischemia/reperfusion injury in heart. Circ. Res. 2016, 119, 596–606. [Google Scholar] [CrossRef]
- Hou, X.; Appleby, N.; Fuentes, T.; Longo, L.D.; Bailey, L.L.; Hasaniya, N.; Kearns-Jonker, M. Isolation, characterization, and spatial distribution of cardiac progenitor cells in the sheep heart. J. Clin. Exp. Cardiol. 2012, 6, 1–16. [Google Scholar]
- Fukui, M.; Zhu, B.T. Mitochondrial superoxide dismutase SOD2, but not cytosolic SOD1, plays a critical role in protection against glutamate-induced oxidative stress and cell death in HT22 neuronal cells. Free Radic. Biol. Med. 2010, 48, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Zhang, L.; Shi, X.; Wu, M.; Zhou, X.; Liu, X.; Huo, T. SOD2 mediates amifostine-induced protection against glutamate in PC12 cells. Oxid. Med. Cell Longev. 2016, 2016, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Xin, M.; Kim, Y.; Sutherland, L.B.; Murakami, M.; Qi, X.; McAnally, J.; Porrello, E.R.; Mahmoud, A.I.; Tan, W.; Shelton, J.M.; et al. Hippo pathway effector Yap promotes cardiac regeneration. Proc. Natl. Acad. Sci. USA 2013, 110, 13839–13844. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Liu, Y.; Wang, T.; Zhou, N.; Kong, J.; Chen, L.; Snitow, M.; Morley, M.; Li, D.; Petrenko, N.; et al. A microRNA-Hippo pathway that promotes cardiomyocyte proliferation and cardiac regeneration in mice. Sci. Transl. Med. 2015, 7, ra38–ra279. [Google Scholar] [CrossRef] [PubMed]
- Yimlamai, D.; Christodoulou, C.; Galli, G.G.; Yanger, K.; Pepe-Mooney, B.; Gurung, B.; Shrestha, K.; Cahan, P.; Stanger, B.Z.; Camargo, F.D. Hippo pathway activity influences liver cell fate. Cell 2014, 157, 1324–1338. [Google Scholar] [CrossRef]
- Zhao, B.; Lei, Q.-Y.; Guan, K.-L. The Hippo-YAP pathway: New connections between regulation of organ size and cancer. Curr. Opin. Cell Biol. 2008, 20, 638–646. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Li, L.; Lei, Q.; Guan, K.-L. The Hippo–YAP pathway in organ size control and tumorigenesis: An updated version. Genes Dev. 2010, 24, 862–874. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Li, X.; He, Y.; Li, W.; Wang, Y.; Wang, H.; Jiang, S.; Xin, Y. YAP1 enhances cell proliferation, migration, and invasion of gastric cancer in vitro and in vivo. Oncotarget 2016, 7, 81062–81076. [Google Scholar] [CrossRef]
- Study Finds Astronauts’ Hearts Become More Spherical in Space. Available online: https://www.acc.org/about-acc/press-releases/2014/03/29/09/09/may-hearts-in-space (accessed on 14 March 2019).
- Arena, C.; De Micco, V.; Macaeva, E.; Quintens, R. Space radiation effects on plant and mammalian cells. Acta Astronaut. 2014, 104, 419–431. [Google Scholar] [CrossRef]
- Boerma, M.; Nelson, G.A.; Sridharan, V.; Mao, X.-W.; Koturbash, I.; Hauer-Jensen, M. Space radiation and cardiovascular disease risk. World J. Cardiol. 2015, 7, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Little, M.P. A review of non-cancer effects, especially circulatory and ocular diseases. Radiat. Env. Biophys. 2013, 52, 435–449. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, Y.; Takemoto, T.; Itoh, K.; Ishida, A.; Yamazaki, T. Dual role of superoxide dismutase 2 induced in activated microglia oxidative stress tolerance and convergence of inflammatory responses. J. Biol. Chem. 2015, 290, 22805–22817. [Google Scholar] [CrossRef] [PubMed]
- Diez-Cuñado, M.; Wei, K.; Bushway, P.J.; Maurya, M.R.; Perera, R.; Subramaniam, S.; Ruiz-Lozano, P.; Mercola, M. miRNAs that induce human cardiomyocyte proliferation converge on the Hippo pathway. Cell Rep. 2018, 23, 2168–2174. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Li, Y.; Li, Z.; Liu, Z.; Zhang, Z.; Chang, S.; Wu, J. Mechanism of folate deficiency-induced apoptosis in mouse embryonic stem cells: Cell cycle arrest/apoptosis in G1/G0 mediated by microRNA-302a and tumor suppressor gene Lats2. Int. J. Biochem. Cell Biol. 2012, 44, 1750–1760. [Google Scholar] [CrossRef] [PubMed]
- Card, D.A.G.; Hebbar, P.B.; Li, L.; Trotter, K.W.; Komatsu, Y.; Mishina, Y.; Archer, T.K. Oct4/sox2-regulated miR-302 targets cyclin D1 in human embryonic stem cells. Mol. Cell. Biol. 2008, 28, 6426–6438. [Google Scholar] [CrossRef] [PubMed]
- Sattler, S.; Rosenthal, N. The neonate versus adult mammalian immune system in cardiac repair and regeneration. Biochim. Et Biophys. Acta (Bba) Mol. Cell Res. 2016, 1863, 1813–1821. [Google Scholar] [CrossRef] [PubMed]
- Lam, N.T.; Sadek, H.A. Neonatal heart regeneration. Circulation 2018, 138, 412–423. [Google Scholar] [CrossRef]
- Zhou, W.; Zhao, M. How Hippo Signaling Pathway Modulates Cardiovascular Development And Diseases. Available online: https://www.hindawi.com/journals/jir/2018/3696914/ (accessed on 22 March 2019).
- Zhou, Q.; Li, L.; Zhao, B.; Guan, K.-L. The hippo pathway in heart development, regeneration, and diseases. Circ. Res. 2015, 116, 1431–1447. [Google Scholar] [CrossRef]
- Heallen, T.; Morikawa, Y.; Leach, J.; Tao, G.; Willerson, J.T.; Johnson, R.L.; Martin, J.F. Hippo signaling impedes adult heart regeneration. Development 2013, 140, 4683–4690. [Google Scholar] [CrossRef]
- Ziegler, A.; Gonzalez, L.; Blikslager, A. Large animal models: The key to translational discovery in digestive disease research. Cell Mol. Gastroenterol. Hepatol. 2016, 2, 716–724. [Google Scholar] [CrossRef] [PubMed]
- Zwetsloot, P.P.; Kouwenberg, L.H.J.A.; Sena, E.S.; Eding, J.E.; den Ruijter, H.M.; Sluijter, J.P.G.; Pasterkamp, G.; Doevendans, P.A.; Hoefer, I.E.; Chamuleau, S.a.J.; et al. Optimization of large animal MI models; a systematic analysis of control groups from preclinical studies. Sci. Rep. 2017, 7, 14218. [Google Scholar] [CrossRef] [PubMed]
- Camacho, P.; Fan, H.; Liu, Z.; He, J.-Q. Large mammalian animal models of heart disease. J. Cardiovasc. Dev. Dis. 2016, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Ajorlou, E.; Khosroushahi, A.Y. Trends on polymer- and lipid-based nanostructures for parenteral drug delivery to tumors. Cancer Chemother. Pharm. 2017, 79, 251–265. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Zhang, Y.; Kidane, Y.; Feiveson, A.; Stodieck, L.; Karouia, F.; Ramesh, G.; Rohde, L.; Wu, H. Cellular responses and gene expression profile changes due to bleomycin-induced DNA damage in human fibroblasts in space. PLoS ONE 2017, 12, e0170358. [Google Scholar] [CrossRef] [PubMed]
| Antibody | Manufacturer | Isotype | Species | Clone | Catalog No. | Lot No. |
|---|---|---|---|---|---|---|
| Islet-1 | Abcam | IgG1 | Mouse | 1H9 | ab86472 | GR273015-3 |
| cKit-Dylight650 | Novus Biologicals | IgG2B Kappa | Mouse | 2B8 | NB100-77477c | B147020-A |
| CD56 | Biolegend | IgG1 | Mouse | 5.1H11 | 362545 | B245476 |
| Antibody | Manufacturer | Sample Used (mg/mL) | Antibody Dilution | Species | Size (kDa) | Catalog No. | Lot No. |
|---|---|---|---|---|---|---|---|
| GAPDH | Cell Signaling | 0.4 | 1:50 | Mouse | 37 | 97166S | 4 |
| YAP1 | Cell Signaling | 0.4 | 1:200 | Rabbit | 65–75 | 14074S | 2 |
| Phosphorylated YAP1 | Cell Signaling | 0.4 | 1:10 | Rabbit | 65–75 | 13008S | 5 |
| Gene | Forward Sequence | Reverse Sequence |
|---|---|---|
| GAPDH | TGCACCACCAACTGCTTAGC | GGCATGGACTGTGGTCATGAG |
| YAP1 | TCCCAGATGAACGTCACAGC | TCATGGCAAAACGAGGGTCA |
| SOD2 | ACCACGCGGCCTACGTGAAC | AGAAAGCCGAGTGTTTCCCTT |
| Sheep GAPDH | CCAGCCGCATCCCTGAGACAA | GACCCTCTTGGCGCCACCCT |
| Sheep YAP1 | GCACCTTCGACAGTCTTCCT | TTCTCTGGTTCATGGCAAAACG |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Camberos, V.; Baio, J.; Bailey, L.; Hasaniya, N.; Lopez, L.V.; Kearns-Jonker, M. Effects of Spaceflight and Simulated Microgravity on YAP1 Expression in Cardiovascular Progenitors: Implications for Cell-Based Repair. Int. J. Mol. Sci. 2019, 20, 2742. https://doi.org/10.3390/ijms20112742
Camberos V, Baio J, Bailey L, Hasaniya N, Lopez LV, Kearns-Jonker M. Effects of Spaceflight and Simulated Microgravity on YAP1 Expression in Cardiovascular Progenitors: Implications for Cell-Based Repair. International Journal of Molecular Sciences. 2019; 20(11):2742. https://doi.org/10.3390/ijms20112742
Chicago/Turabian StyleCamberos, Victor, Jonathan Baio, Leonard Bailey, Nahidh Hasaniya, Larry V. Lopez, and Mary Kearns-Jonker. 2019. "Effects of Spaceflight and Simulated Microgravity on YAP1 Expression in Cardiovascular Progenitors: Implications for Cell-Based Repair" International Journal of Molecular Sciences 20, no. 11: 2742. https://doi.org/10.3390/ijms20112742
APA StyleCamberos, V., Baio, J., Bailey, L., Hasaniya, N., Lopez, L. V., & Kearns-Jonker, M. (2019). Effects of Spaceflight and Simulated Microgravity on YAP1 Expression in Cardiovascular Progenitors: Implications for Cell-Based Repair. International Journal of Molecular Sciences, 20(11), 2742. https://doi.org/10.3390/ijms20112742





