Vascular Adhesion Protein-1 (VAP-1) as Predictor of Radiographic Severity in Symptomatic Knee Osteoarthritis in the New York University Cohort
Abstract
1. Introduction
2. Results
2.1. Baseline Characteristics of the Subjects in the New York University (NYU) Cohort
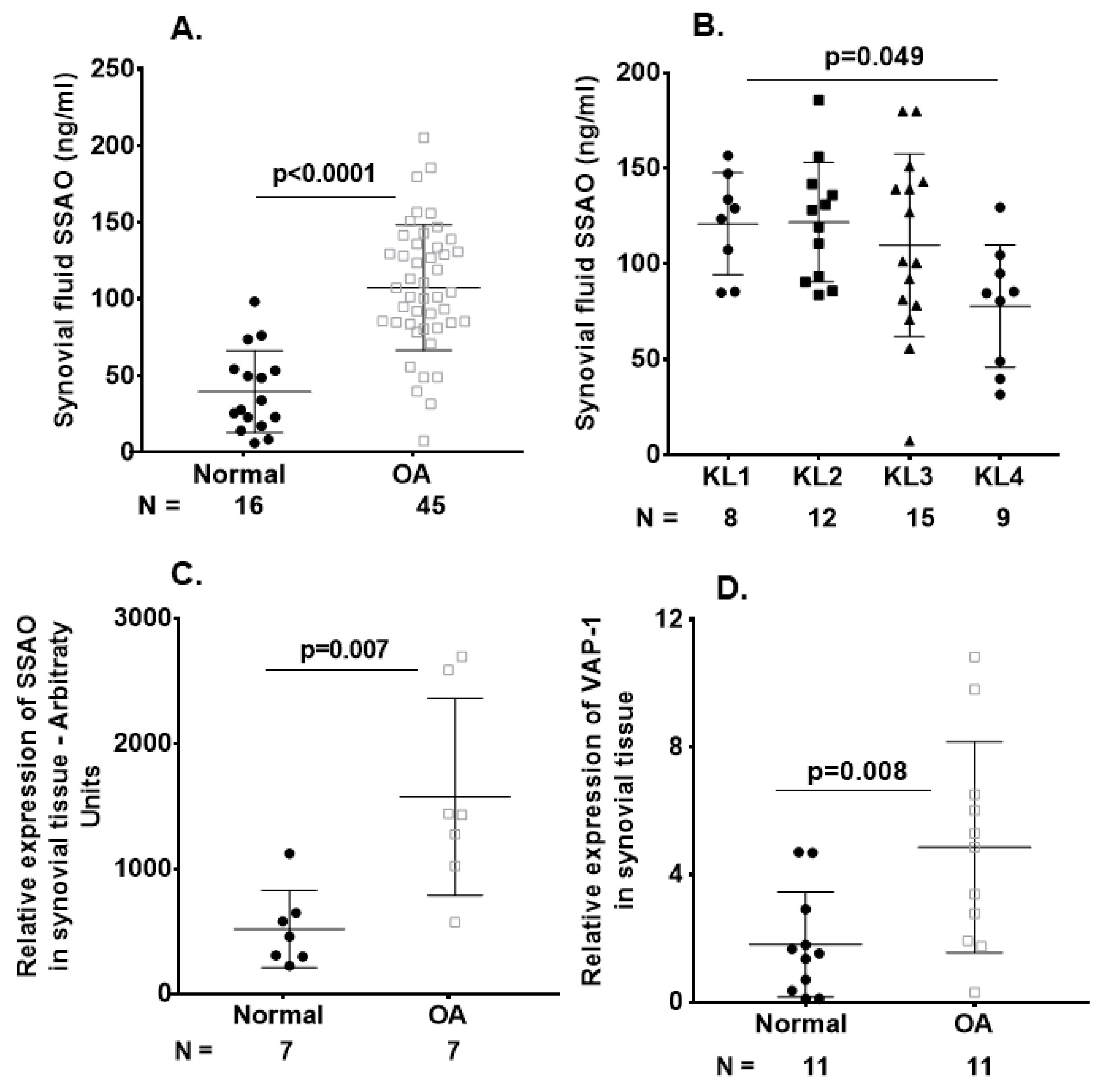
2.2. Elevated SSAO/sVAP-1 Levels in Synovial Fluid of OA Patients
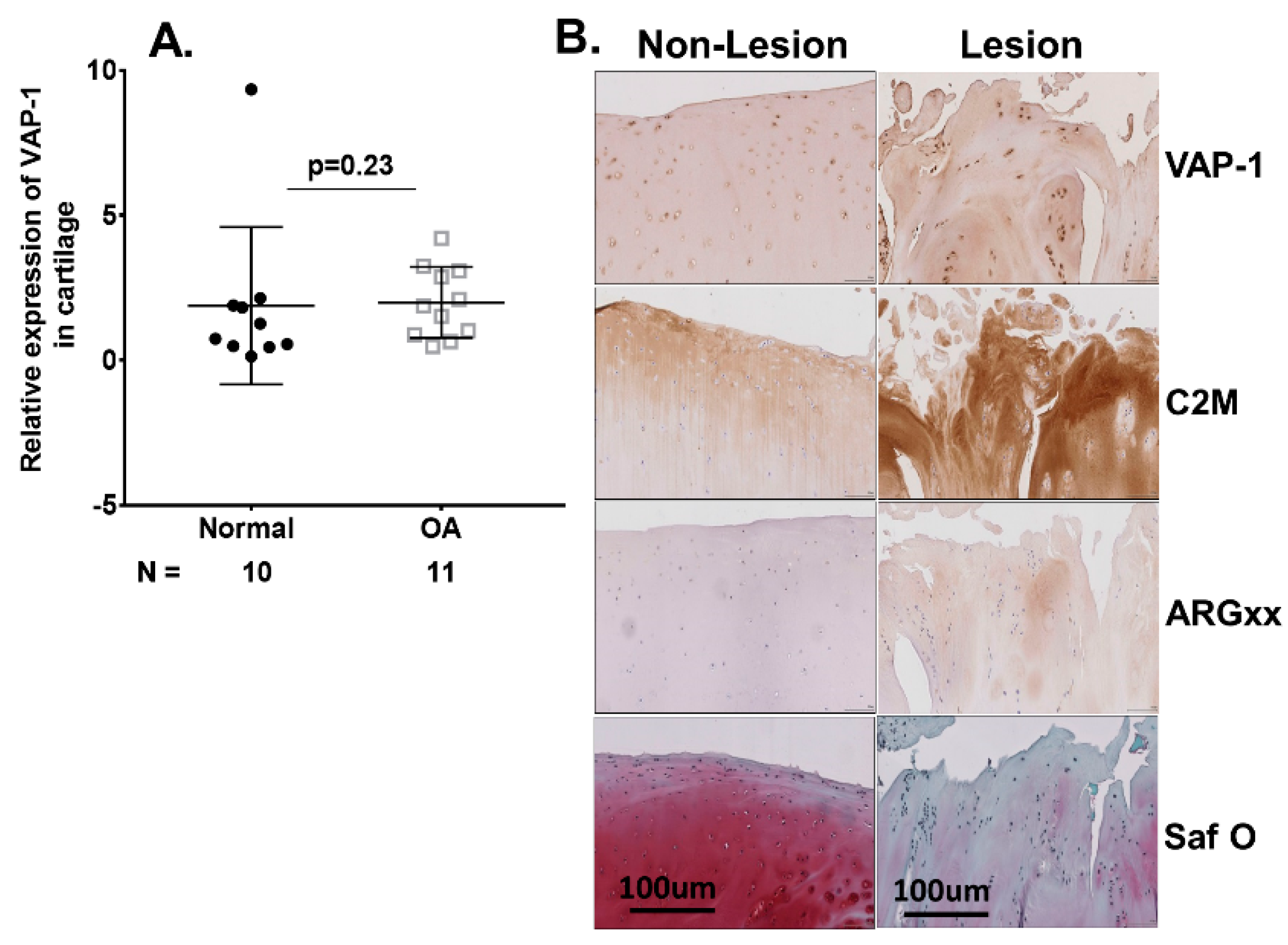
2.3. VAP-1 Locally Overexpressed in the Synovium and Not Cartilage of End-Stage Knee OA Patients
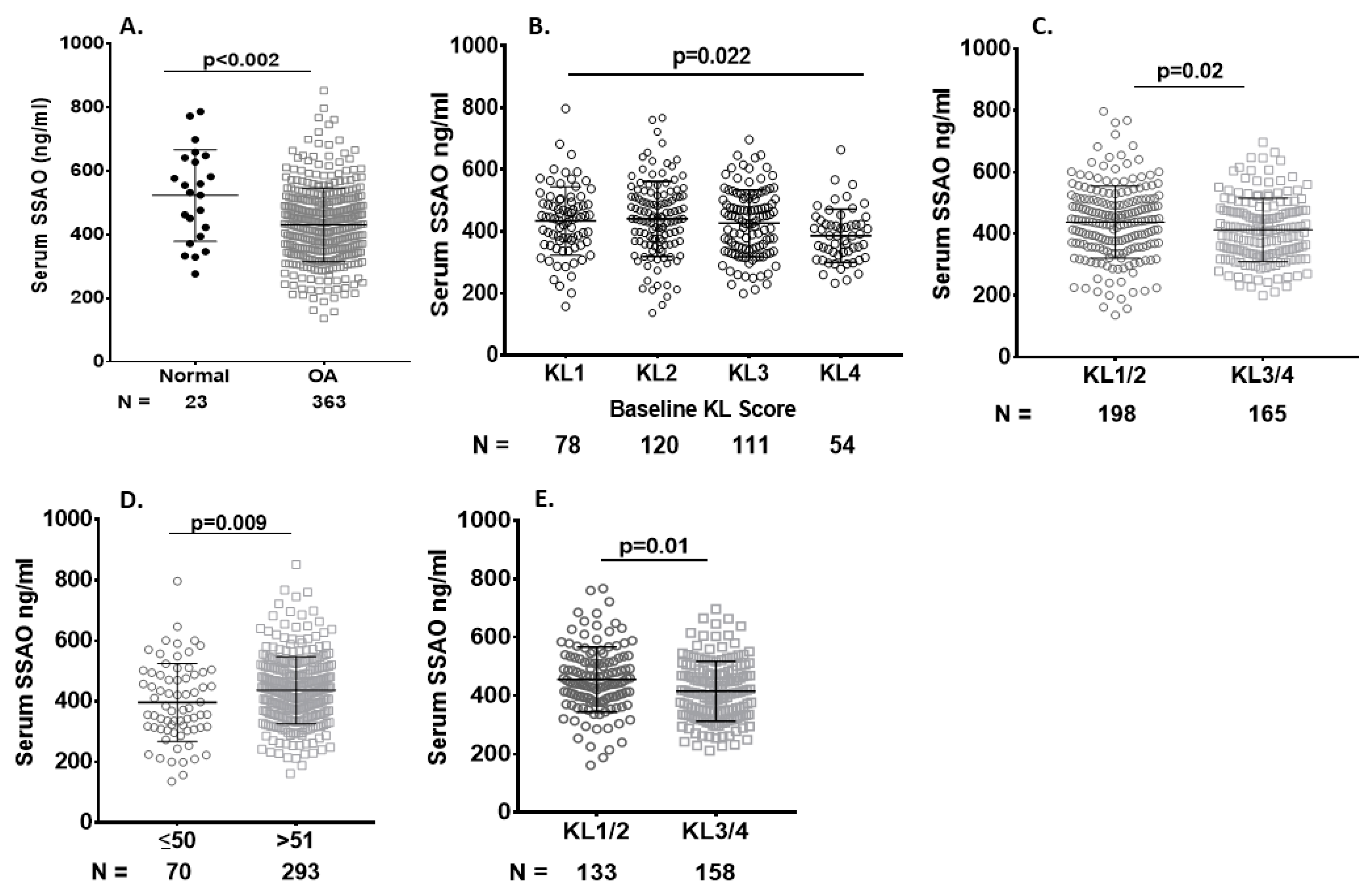
2.4. Serum sVAP-1 Levels Correlated with Pain
2.5. Correlation of Baseline Serum sVAP-1 Levels with Other Biomarkers
3. Discussion
4. Materials and Methods
4.1. Symptomatic Knee OA (SKOA), New York University (NYU) Patient Cohort
4.2. Synovial Fluid, Synovium, and Cartilage from Non-OA Subjects and OA Patients
4.3. Microarray Gene Expression Analysis
4.4. Cytokine and sVAP-1 Measurement
4.5. Real-Time Quantitative Polymerase Chain Reaction (qRT-PCR)
4.6. Histological and Immunohistochemical Analysis of Human Synovial Tissue and Cartilage
4.7. Statistical Analysis
4.8. Ethics Approval
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vina, E.R.; Kwoh, C.K. Epidemiology of osteoarthritis: literature update. Curr. Opin. Rheumatol. 2018, 30, 160–167. [Google Scholar] [CrossRef]
- Abramson, S.B.; Attur, M.; Yazici, Y. Prospects for disease modification in osteoarthritis. Nat. Clin. Pract. Rheumatol. 2006, 2, 304–312. [Google Scholar]
- Berenbaum, F. Osteoarthritis as an inflammatory disease (osteoarthritis is not osteoarthrosis!). Osteoarthr. Cartil. 2013, 21, 16–21. [Google Scholar] [CrossRef]
- Wisniewski, H.G.; Colón, E.; Liublinska, V.; Karia, R.J.; Stabler, T.V.; Attur, M.; Abramson, S.B.; Band, P.A.; Kraus, V.B. TSG-6 activity as a novel biomarker of progression in knee osteoarthritis. Osteoarthr. Cartil. 2014, 22, 235–241. [Google Scholar] [CrossRef]
- Pelletier, J.P.; Martel-Pelletier, J.; Abramson, S.B. Osteoarthritis, an inflammatory disease: Potential implication for the selection of new therapeutic targets. Arthritis Rheumatol. 2001, 44, 1237–1247. [Google Scholar] [CrossRef]
- Sohn, D.H.; Sokolove, J.; Sharpe, O.; Erhart, J.C.; Chandra, P.E.; Lahey, L.J.; Lindstrom, T.M.; Hwang, I.; Boyer, K.A.; Andriacchi, T.P.; et al. Plasma proteins present in osteoarthritic synovial fluid can stimulate cytokine production via Toll-like receptor 4. Arthritis Res. Ther. 2012, 14, R7. [Google Scholar] [CrossRef]
- Attur, M.; Belitskaya-Lévy, I.; Oh, C.; Krasnokutsky, S.; Greenberg, J.; Samuels, J.; Smiles, S.; Lee, S.; Patel, J.; Al-Mussawir, H.; et al. Increased interleukin-1β gene expression in peripheral blood leukocytes is associated with increased pain and predicts risk for progression of symptomatic knee osteoarthritis. Arthritis Rheumatol. 2011, 63, 1908–1917. [Google Scholar] [CrossRef]
- Attur, M.; Statnikov, A.; Samuels, J.; Li, Z.; Alekseyenko, A.V.; Greenberg, J.D.; Krasnokutsky, S.; Rybak, L.; Lu, Q.A.; Todd, J.; et al. Plasma levels of interleukin-1 receptor antagonist (IL1Ra) predict radiographic progression of symptomatic knee osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1915–1924. [Google Scholar] [CrossRef]
- Salmi, M.; Jalkanen, S. A 90-kilodalton endothelial cell molecule mediating lymphocyte binding in humans. Science 1992, 257, 1407–1409. [Google Scholar] [CrossRef]
- Smith, D.J.; Salmi, M.; Bono, P.; Hellman, J.; Leu, T.; Jalkanen, S. Cloning of vascular adhesion protein 1 reveals a novel multifunctional adhesion molecule. J. Exp. Med. 1998, 188, 17–27. [Google Scholar] [CrossRef]
- Airas, L.; Salmi, M.; Jalkanen, S. Lymphocyte-vascular adhesion protein-2 is a novel 70-kDa molecule involved in lymphocyte adhesion to vascular endothelium. J. Immunol. 1993, 151, 4228–4238. [Google Scholar] [PubMed]
- Salmi, M.; Kalimo, K.; Jalkanen, S. Induction and function of vascular adhesion protein-1 at sites of inflammation. J. Exp. Med. 1993, 178, 2255–2260. [Google Scholar] [CrossRef]
- Jaakkola, K.; Nikula, T.; Holopainen, R.; Vähäsilta, T.; Matikainen, M.T.; Laukkanen, M.L.; Huupponen, R.; Halkola, L.; Nieminen, L.; Hiltunen, J.; et al. In vivo detection of vascular adhesion protein-1 in experimental inflammation. Am. J. Pathol. 2000, 157, 463–471. [Google Scholar] [CrossRef]
- Koskinen, K.; Nevalainen, S.; Karikoski, M.; Hänninen, A.; Jalkanen, S.; Salmi, M. VAP-1-deficient mice display defects in mucosal immunity and antimicrobial responses: Implications for antiadhesive applications. J. Immunol. 2007, 179, 6160–6168. [Google Scholar] [CrossRef] [PubMed]
- Salmi, M.; Jalkanen, S. Ectoenzymes in leukocyte migration and their therapeutic potential. Semin. Immunopathol. 2014, 36, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Marttila-Ichihara, F.; Smith, D.J.; Stolen, C.; Yegutkin, G.G.; Elima, K.; Mercier, N.; Kiviranta, R.; Pihlavisto, M.; Alaranta, S.; Pentikäinen, U.; et al. Vascular amine oxidases are needed for leukocyte extravasation into inflamed joints in vivo. Arthritis Rheumatol. 2006, 54, 2852–2862. [Google Scholar] [CrossRef]
- Horváth, Á.; Tékus, V.; Bencze, N.; Szentes, N.; Scheich, B.; Bölcskei, K.; Szőke, É.; Mócsai, A.; Tóth-Sarudy, É.; Mátyus, P.; et al. Analgesic effects of the novel semicarbazide-sensitive amine oxidase inhibitor SZV 1287 in mouse pain models with neuropathic mechanisms: Involvement of transient receptor potential vanilloid 1 and ankyrin 1 receptors. Pharmacol. Res. 2018, 131, 231–243. [Google Scholar] [CrossRef]
- Akin, E.; Aversa, J.; Steere, A.C. Expression of adhesion molecules in synovia of patients with treatment-resistant lyme arthritis. Infect. Immun. 2001, 69, 1774–1780. [Google Scholar] [CrossRef]
- Filip, A.; Pinzano, A.; Bianchi, A.; Fève, B.; Jalkanen, S.; Gillet, P.; Mainard, D.; Lacolley, P.; Magdalou, J.; Mercier, N. Expression of the semicarbazide-sensitive amine oxidase in articular cartilage: Its role in terminal differentiation of chondrocytes in rat and human. Osteoarthr. Cartil. 2016, 24, 1223–1234. [Google Scholar] [CrossRef]
- Ling, S.M.; Patel, D.D.; Garnero, P.; Zhan, M.; Vaduganathan, M.; Muller, D.; Taub, D.; Bathon, J.M.; Hochberg, M.; Abernethy, D.R.; et al. Serum protein signatures detect early radiographic osteoarthritis. Osteoarthr. Cartil. 2009, 17, 43–48. [Google Scholar] [CrossRef]
- Bellamy, N.; Buchanan, W.W.; Goldsmith, C.H.; Campbell, J.; Stitt, L.W. Validation study of WOMAC: A health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J. Rheumatol. 1988, 15, 1833–1840. [Google Scholar]
- Srikanth, V.K.; Fryer, J.L.; Zhai, G.; Winzenberg, T.M.; Hosmer, D.; Jones, G. A meta-analysis of sex differences prevalence, incidence and severity of osteoarthritis. Osteoarthr. Cartil. 2005, 13, 769–781. [Google Scholar] [CrossRef]
- Shane Anderson, A.; Loeser, R.F. Why is osteoarthritis an age-related disease? Best Pract Res. Clin. Rheumatol. 2010, 24, 15–26. [Google Scholar] [CrossRef]
- Bedson, J.; Croft, P.R. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet. Disord. 2008, 9, 116. [Google Scholar] [CrossRef]
- Hannan, M.T.; Felson, D.T.; Pincus, T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J. Rheumatol. 2000, 27, 1513–1517. [Google Scholar]
- Deveza, L.A.; Loeser, R.F. Is osteoarthritis one disease or a collection of many? Rheumatology 2018, 57 (Suppl. 4), iv34–iv42. [Google Scholar] [CrossRef]
- Liu-Bryan, R.; Terkeltaub, R. Emerging regulators of the inflammatory process in osteoarthritis. Nat. Rev. Rheumatol. 2015, 11, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Robinson, W.H.; Lepus, C.M.; Wang, Q.; Raghu, H.; Mao, R.; Lindstrom, T.M.; Sokolove, J. Low-grade inflammation as a key mediator of the pathogenesis of osteoarthritis. Nat. Rev. Rheumatol. 2016, 12, 580–592. [Google Scholar] [CrossRef]
- Attur, M.; Krasnokutsky-Samuels, S.; Samuels, J.; Abramson, S.B. Prognostic biomarkers in osteoarthritis. Curr. Opin. Rheumatol. 2013, 25, 136–144. [Google Scholar] [CrossRef]
- Attur, M.; Krasnokutsky, S.; Statnikov, A.; Samuels, J.; Li, Z.; Friese, O.; Hellio Le Graverand-Gastineau, M.P.; Rybak, L.; Kraus, V.B.; Jordan, J.M.; et al. Low-grade inflammation in symptomatic knee osteoarthritis: Prognostic value of inflammatory plasma lipids and peripheral blood leukocyte biomarkers. Arthritis Rheumatol. 2015, 67, 2905–2915. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.H.; Wei, J.N.; Yang, C.Y.; Ou, H.Y.; Wu, H.T.; Fan, K.C.; Wang, S.H.; Hua, C.H.; Hsiao, C.H.; Lee, M.K.; et al. Serum vascular adhesion protein-1 is up-regulated in hyperglycemia and is associated with incident diabetes negatively. Int. J. Obes. 2019, 43, 512–522. [Google Scholar] [CrossRef] [PubMed]
- Attur, M.; Yang, Q.; Shimada, K.; Tachida, Y.; Nagase, H.; Mignatti, P.; Statman, L.; Palmer, G.; Kirsch, T.; Beier, F.; et al. Elevated expression of periostin in human osteoarthritic cartilage and its potential role in matrix degradation via matrix metalloproteinase-13. FASEB J. 2015, 29, 4107–4121. [Google Scholar] [CrossRef]
- Mix, K.S.; McMahon, K.; McMorrow, J.P.; Walkenhorst, D.E.; Smyth, A.M.; Petrella, B.L.; Gogarty, M.; Fearon, U.; Veale, D.; Attur, M.G.; et al. Orphan nuclear receptor NR4A2 induces synoviocyte proliferation, invasion, and matrix metalloproteinase 13 transcription. Arthritis Rheumatol. 2012, 64, 2126–2136. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−∆∆Ct Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

| Variable | Normal (N = 24) | SKOA (N = 372) | p Values | Adjusted for AGB p Values |
|---|---|---|---|---|
| Age (years) | 54.63 (9.37), [40.00–75.00] | 61.21 (10.51), [37.00–88.00] | 0.003 | |
| Gender (%) | ||||
| Male | 50% | 36.93% | ||
| Female | 50% | 63.07% | ||
| BMI (kg/m2) | 26.30 (3.92), [20.00–32.40] | 26.35 (3.50), [15.40–32.60] | 0.943 | 0.943 |
| VAS (0–100) | 0 | 46.97 (26.47), [0.00–100.00] | NA | NA |
| WOMAC (0–100) | 0 | 30.97 (24.73), [0.00–98.60] | NA | NA |
| JSW (mm) | NA | 3.39 (1.34), [0.00–7.30] | NA | NA |
| KL Grades | ||||
| KL0 | 63.64% | 5.41% | ||
| KL1 | 36.36% | 15.68% | ||
| KL2 | 0.00% | 32.70% | ||
| KL3 | 0.00% | 31.35% | ||
| KL4 | 0.00% | 14.86% | ||
| IL-1Ra (pg/mL) | 354.53 (172.28), [91.40–726.43] | 350.04 (156.37), [97.97–999.98] | 0.901 | 0.943 |
| MMP-1 (pg/mL) | 2388.24 (1140.35), [1137.52–4679.16] | 3151.94 (2383.33), [401.28–17,537.19] | 0.146 | 0.351 |
| MMP-3 (pg/mL) | 16,525.13 (8777.40), [6684.15–33,139.95] | 17,517.27 (16,424.86), [3768.51–244,674.57] | 0.784 | 0.943 |
| MMP-9 (ng/mL) | 49,197.66 (56,813.60), [9256.92–257,706.04] | 38,409.48 (29,838.25), [9426.51–197,065.54] | 0.135 | 0.351 |
| IL-15 (pg/mL) | 1.18 (0.30), [0.70–1.90] | 1.15 (0.32), [0.6–3.29] | 0.714 | 0.943 |
| hsCRP (mg/L) | 4.06 (4.85), [0.14–19.85] | 2.73 (3.68), [0.00–37.00] | 0.117 | 0.351 |
| CD163 (ng/mL) | 679.84 (317.42), [291.74–1852.40] | 656.09 (236.68), [164.69–1501.75] | 0.692 | 0.943 |
| HA (ng/mL) | 29.59 (18.07), [1.91–72.72] | 30.20 (29.75), [0.76–193.22] | 0.929 | 0.943 |
| sRAGE (ng/mL) | 1303.70 (542.76), [435.92–2463.60] | 1106.13 (571.74), [239.11–4657.23] | 0.182 | 0.363 |
| sVAP-1 (ng/mL) | 569.90 (178.01), [276.32–1105.39] | 430.00 (115.50), [136.16–851.32] | <0.002 | <0.0001 |
| Variable | Normal (N = 14) | SKOA (N = 301) ≥51 Years | p Values | Adjusted for AGB p Values |
|---|---|---|---|---|
| Age (years) | 60.79 (6.94), [51.00–75.00] | 64.69 (8.34), [51.00–88.00] | 0.086 | 0.305 |
| Gender (%) | ||||
| Male | 64.29% | 35.55% | ||
| Female | 35.71% | 64.45% | ||
| BMI (kg/m2) | 26.62 (4.08), [20.00–31.90] | 26.35 (3.52), [15.40–32.60] | 0.779 | 0.934 |
| VAS (0–100) | 0 | 46.12 (25.83), [0.00–100.00] | NA | NA |
| WOMAC (0–100) | 0 | 30.66 (23.25), [0.00–91.60] | NA | NA |
| JSW (mm) | NA | 3.28 (1.37), [0.00–7.30] | NA | NA |
| KL Grades | ||||
| KL0 | 46.2% | 4.3% | ||
| KL1 | 53.8% | 12.6% | ||
| KL2 | 0.0% | 30.6% | ||
| Kl3 | 0.0% | 34.6% | ||
| KL4 | 0.0% | 17.9% | ||
| IL-1Ra (pg/mL) | 362.21 (163.82), [91.40–702.70] | 363.47 (154.69), [97.97–999.98] | 0.977 | 0.977 |
| MMP-1 (pg/mL) | 2511.82 (1290.26), [1137.52–4679.16] | 3230.39 (2430.58), [401.28–17,537.19] | 0.274 | 0.657 |
| MMP-3 (pg/mL) | 15,704.52 (7796.96), [6684.15–33,139.95] | 17,546.72 (17,564.45), [4685.58–244,674.57] | 0.697 | 0.934 |
| MMP-9 (ng/mL) | 41,278.95 (36,076.83), [9256.92–150,916.05] | 38,662.00 (28,912.33), [9426.51–184,835.39] | 0.745 | 0.934 |
| IL-15 (pg/mL) | 1.28 (0.28), [0.70–1.90] | 1.14 (0.30), [0.63–3.29] | 0.102 | 0.305 |
| hsCRP (mg/L) | 4.80 (5.59), [0.14–19.85] | 2.91 (3.96), [0.00–37.00] | 0.090 | 0.305 |
| CD163 (ng/mL) | 749.40 (348.24), [483.96–1852.40] | 687.27 (235.73), [223.48–1501.75] | 0.396 | 0.680 |
| HA (ng/mL) | 34.51 (17.67), [13.25–72.72] | 33.78 (31.59), [0.76–193.22] | 0.933 | 0.977 |
| sRAGE (pg/mL) | 1342.65 (492.87), [802.28–1927.11] | 1142.09 (615.22), [293.75–4657.23] | 0.396 | 0.680 |
| sVAP-1 (ng/mL) | 577.93 (192.95), [332.83–1105.39] | 437.86 (111.13), [161.94–851.32] | <0.0001 | <0.0001 |
| Variable | Normal (N = 20) | OA (N = 45) | p Values | Adjusted for AGB p Values |
|---|---|---|---|---|
| Age (years) | 75.00 (10.72), [51.00–90.00] | 62.15 (11.47), [43.00–87.00] | <0.00001 | |
| Gender (%) | ||||
| Male | 66.67% | 45.83% | ||
| Female | 33.33% | 54.17% | ||
| BMI | NA | 28.74 (5.08), [20.00–44.09] | NA | NA |
| KL Grades | ||||
| KL1 | NA | 17% | ||
| KL2 | NA | 26% | ||
| KL3 | NA | 33% | ||
| KL4 | NA | 20% | ||
| KL not known | 4% | |||
| IL-8 (pg/mL) | 22.38 (39.90), [3.21–134.79] | 24.68 (42.13), [1.04–249.18] | 0.839 | 0.979 |
| HSIL-6 (pg/mL) | 122.62 (193.02), [0.3–496.45] | 122.22 (140.02), [5.26–630.25] | 0.993 | 0.993 |
| CCL2 (pg/mL) | 682.53 (631.01), [0.10–2125.20] | 357.34 (179.17), [36.73–953.08] | 0.002 | 0.004 |
| CCL4 (pg/mL) | 1.12 (4.43), [0.10–19.39] | 25.49 (38.31), [0.10–154.01] | 0.008 | 0.013 |
| HSIL-10 (pg/mL) | 1.41 (2.84), [0.14–12.17] | 1.03 (2.40), [0.05–16.27] | 0.597 | 0.835 |
| sRAGE (pg/mL) | 146.8 (89.63), [13.38–327.40] | 232.22 (163.7), [10.8–970.00] | 0.041 | 0.046 |
| SF-sVAP-1 (ng/mL) | 38.12 (22.98), [6.20–88.35] | 107.94 (41.42), [7.38–05.47] | <0.0001 | <0.0001 |
| sVAP-1 (ng/mL)—serum | Not available | 482.5 (132.5), [180.6–695.6] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bournazou, E.; Samuels, J.; Zhou, H.; Krasnokutsky, S.; Patel, J.; Han, T.; Bencardino, J.; Rybak, L.; Abramson, S.B.; Junker, U.; et al. Vascular Adhesion Protein-1 (VAP-1) as Predictor of Radiographic Severity in Symptomatic Knee Osteoarthritis in the New York University Cohort. Int. J. Mol. Sci. 2019, 20, 2642. https://doi.org/10.3390/ijms20112642
Bournazou E, Samuels J, Zhou H, Krasnokutsky S, Patel J, Han T, Bencardino J, Rybak L, Abramson SB, Junker U, et al. Vascular Adhesion Protein-1 (VAP-1) as Predictor of Radiographic Severity in Symptomatic Knee Osteoarthritis in the New York University Cohort. International Journal of Molecular Sciences. 2019; 20(11):2642. https://doi.org/10.3390/ijms20112642
Chicago/Turabian StyleBournazou, Eirini, Jonathan Samuels, Hua Zhou, Svetlana Krasnokutsky, Jyoti Patel, Tianzhen Han, Jenny Bencardino, Leon Rybak, Steven B. Abramson, Uwe Junker, and et al. 2019. "Vascular Adhesion Protein-1 (VAP-1) as Predictor of Radiographic Severity in Symptomatic Knee Osteoarthritis in the New York University Cohort" International Journal of Molecular Sciences 20, no. 11: 2642. https://doi.org/10.3390/ijms20112642
APA StyleBournazou, E., Samuels, J., Zhou, H., Krasnokutsky, S., Patel, J., Han, T., Bencardino, J., Rybak, L., Abramson, S. B., Junker, U., Brown, K. S., & Attur, M. (2019). Vascular Adhesion Protein-1 (VAP-1) as Predictor of Radiographic Severity in Symptomatic Knee Osteoarthritis in the New York University Cohort. International Journal of Molecular Sciences, 20(11), 2642. https://doi.org/10.3390/ijms20112642






