Intranasal Delivery of Mesenchymal Stromal Cells Protects against Neonatal Hypoxic–Ischemic Brain Injury
Abstract
1. Introduction
2. Results
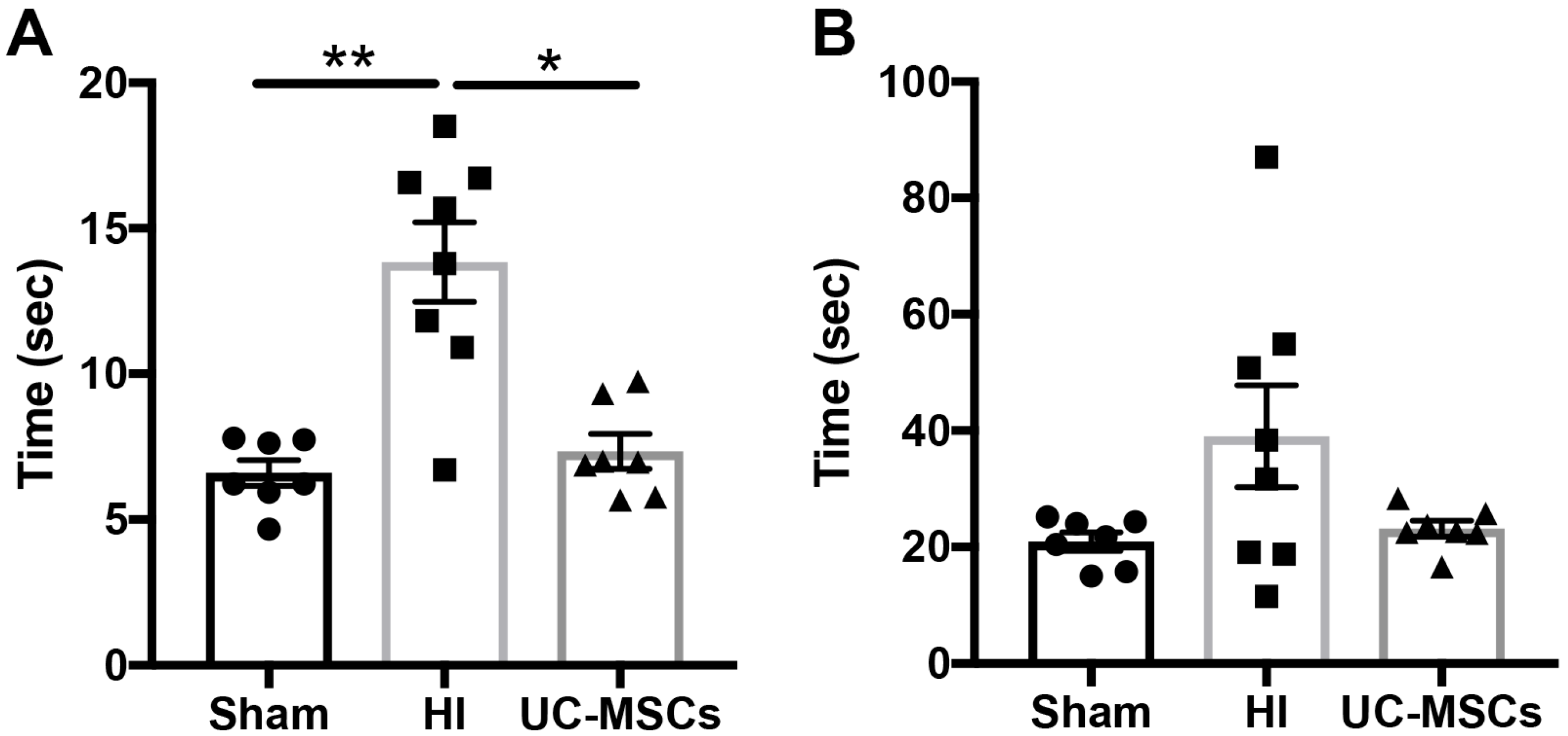
2.1. The Effect of Intranasal Umbilical Cord Mesenchymal Stromal Cells (UC-MSC) Treatment on Behavioural Outcomes
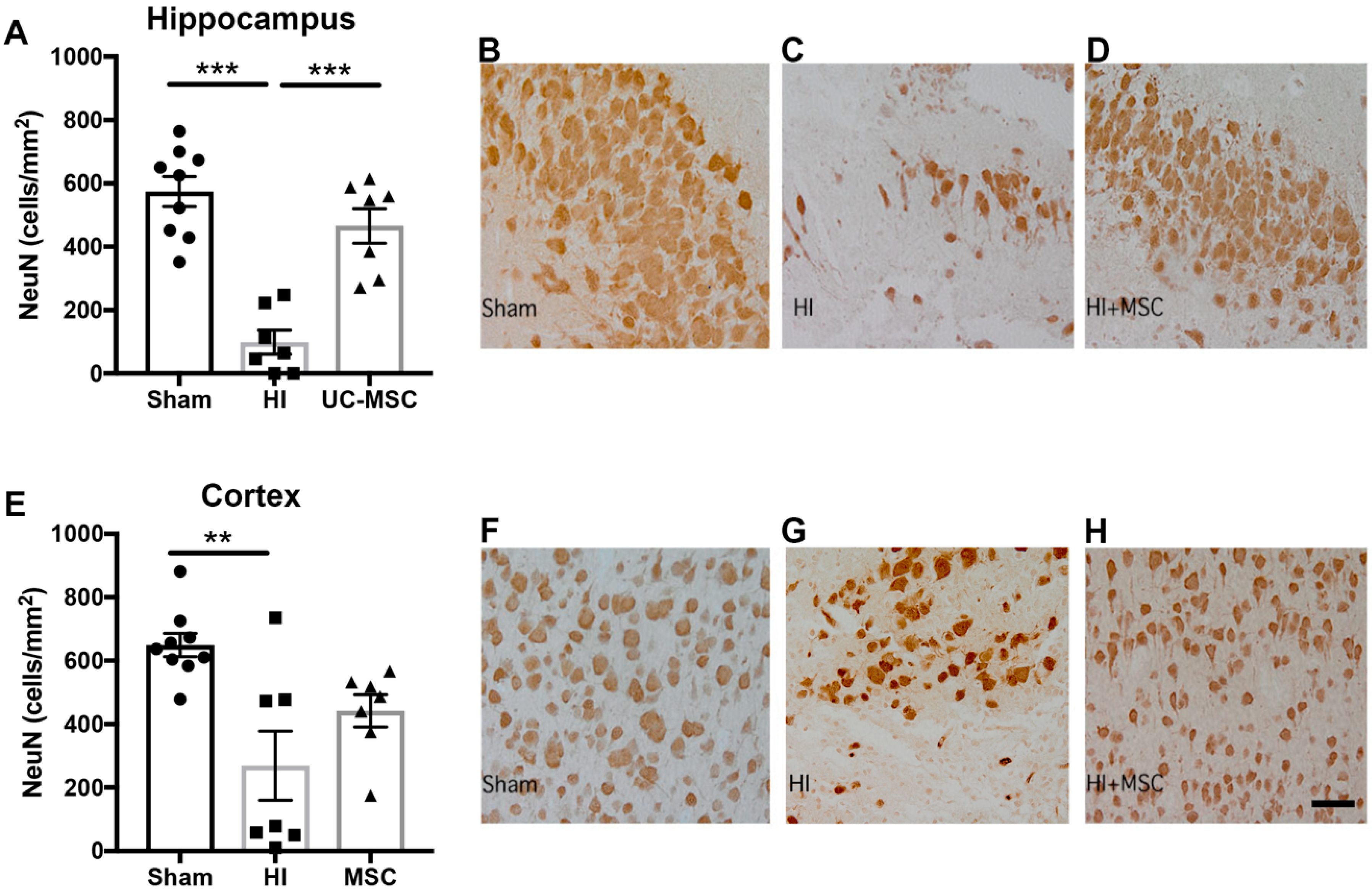
2.2. The Effect of Intranasal UC-MSC Treatment on Hypoxic–Ischemic (HI) Brain Injury
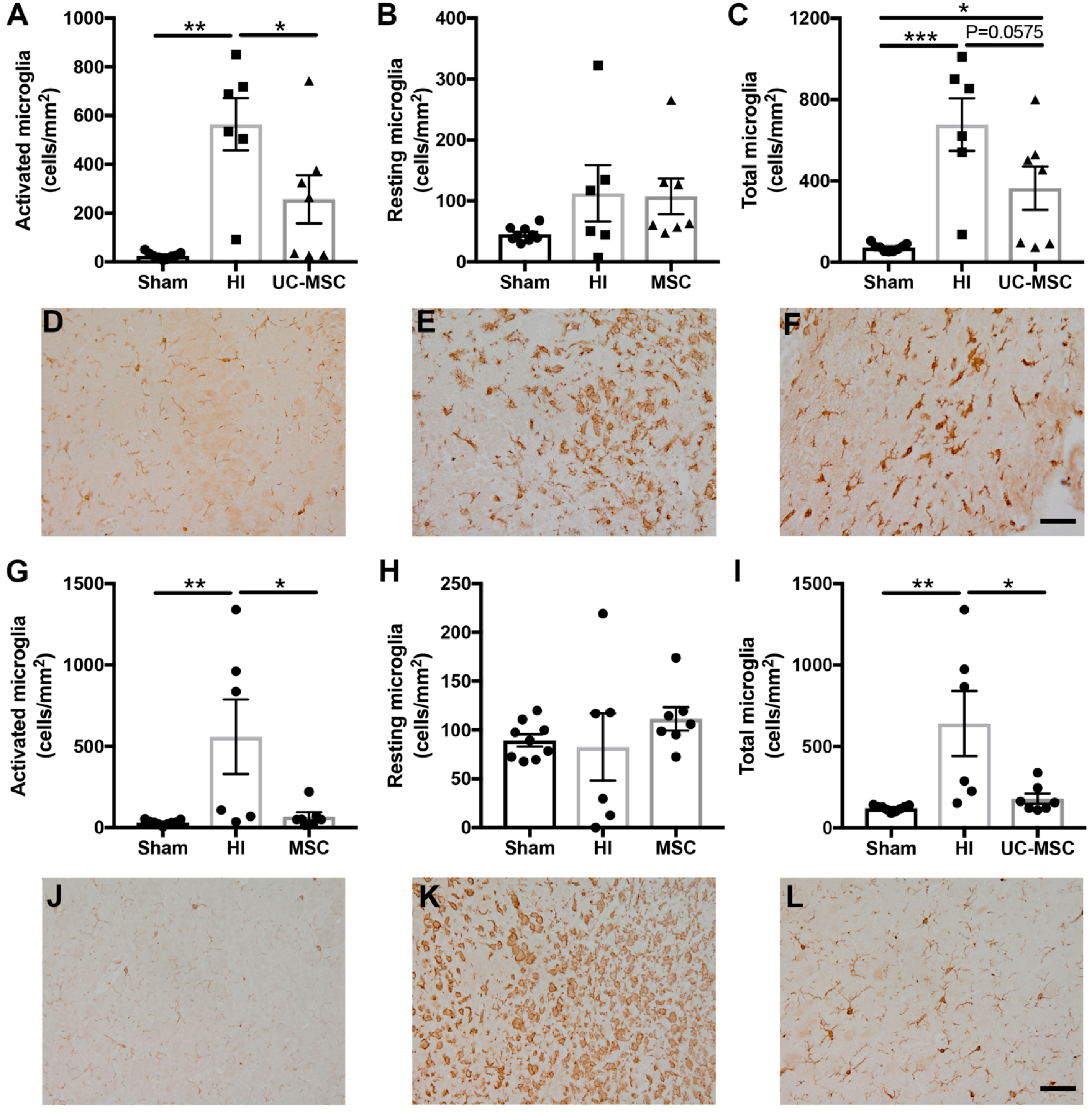
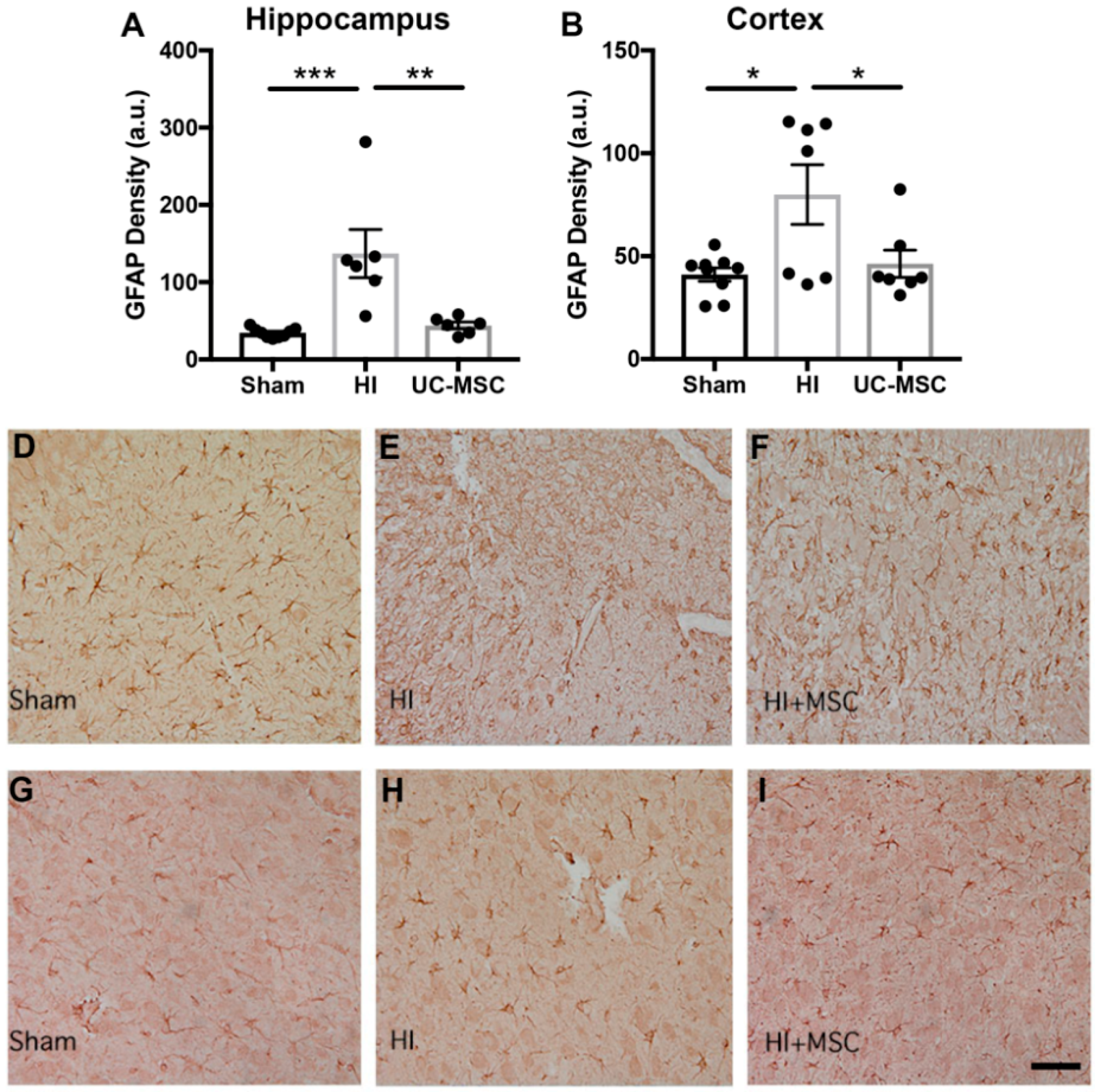
2.3. The Effect of Intranasal UC-MSC Treatment on Neuroinflammation
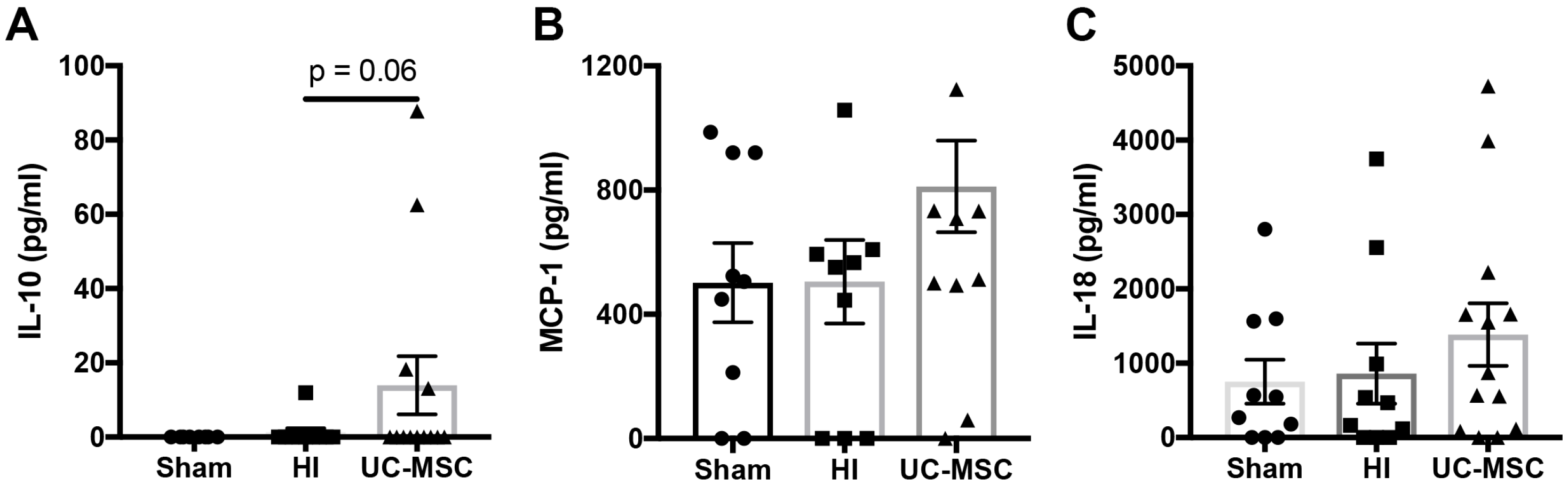
2.4. The Effect of Intranasal UC-MSC Treatment on Peripheral Cytokine Expression
2.5. The Effect of Intranasal UC-MSC Treatment on Gene Expression in the Injured Brain
3. Discussion
4. Materials and Methods
4.1. Ethics Approval
4.2. Cell Preparation
4.3. Animals
4.4. Animal Surgery and Cell Administration
4.5. Behavioural Testing: Negative Geotaxis
4.6. Cytokine Analysis
4.7. Gross Brain Morphology
4.8. Immunohistological Assessment
4.9. mRNA Expression
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BDNF | Brain derived neurotrophic factor |
| CP | Cerebral palsy |
| GDNF | Glial derived neurotrophic factor |
| GFAP | Glial fibrillary acidic protein |
| HI | Hypoxic–ischemic |
| IL | Interleukin |
| MCP-1 | Monocyte chemoattractant protein |
| MSC | Mesenchymal stromal cell |
| PND | Postnatal day |
| TNF | Tumour necrosis factor |
| UC | Umbilical cord |
| VEGF | Vascular endothelial growth factor |
References
- Kancherla, V.; Amendah, D.D.; Grosse, S.D.; Yeargin-Allsopp, M.; Van Naarden Braun, K. Medical expenditures attributable to cerebral palsy and intellectual disability among Medicaid-enrolled children. Res. Dev. Disabil. 2012, 33, 832–840. [Google Scholar] [CrossRef] [PubMed]
- Novak, I.; Walker, K.; Hunt, R.W.; Wallace, E.M.; Fahey, M.; Badawi, N. Concise Review: Stem Cell Interventions for People With Cerebral Palsy: Systematic Review With Meta-Analysis. Stem Cells Transl. Med. 2016, 5, 1014–1025. [Google Scholar] [CrossRef] [PubMed]
- Edwards, A.D.; Brocklehurst, P.; Gunn, A.J.; Halliday, H.; Juszczak, E.; Levene, M.; Strohm, B.; Thoresen, M.; Whitelaw, A.; Azzopardi, D. Neurological outcomes at 18 months of age after moderate hypothermia for perinatal hypoxic ischaemic encephalopathy: Synthesis and meta-analysis of trial data. BMJ 2010, 340, c363. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Can, A.; Karahuseyinoglu, S. Concise Review: Human Umbilical Cord Stroma with Regard to the Source of Fetus-Derived Stem Cells. Stem Cells 2007, 25, 2886–2895. [Google Scholar] [CrossRef] [PubMed]
- Donders, R.; Bogie, J.F.J.; Ravanidis, S.; Gervois, P.; Vanheusden, M.; Marée, R.; Schrynemackers, M.; Smeets, H.J.M.; Pinxteren, J.; Gijbels, K.; et al. Human Wharton’s Jelly-Derived Stem Cells Display a Distinct Immunomodulatory and Proregenerative Transcriptional Signature Compared to Bone Marrow-Derived Stem Cells. Stem Cells Dev. 2018, 27, 65–84. [Google Scholar] [CrossRef]
- Uccelli, A.; Prockop, D.J. Why should mesenchymal stem cells (MSCs) cure autoimmune diseases? Curr. Opin. Immunol. 2010, 22, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Payne, N.L.; Sun, G.; McDonald, C.; Layton, D.; Moussa, L.; Emerson-Webber, A.; Veron, N.; Siatskas, C.; Herszfeld, D.; Price, J.; et al. Distinct immunomodulatory and migratory mechanisms underpin the therapeutic potential of human mesenchymal stem cells in autoimmune demyelination. Cell Transplant. 2013, 22, 1409–1425. [Google Scholar] [CrossRef]
- Payne, N.L.; Sun, G.; McDonald, C.; Moussa, L.; Emerson-Webber, A.; Loisel-Meyer, S.; Medin, J.A.; Siatskas, C.; Bernard, C.C. Human adipose-derived mesenchymal stem cells engineered to secrete IL-10 inhibit APC function and limit CNS autoimmunity. Brain Behav. Immun. 2013, 30, 103–114. [Google Scholar] [CrossRef]
- Li, J.; Yawno, T.; Sutherland, A.E.; Gurung, S.; Paton, M.; McDonald, C.; Tiwari, A.; Pham, Y.; Castillo-Melendez, M.; Jenkin, G.; et al. Preterm umbilical cord blood derived mesenchymal stem/stromal cells protect preterm white matter brain development against hypoxia-ischemia. Exp. Neurol. 2018, 308, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.S.; Ahn, S.Y.; Im, G.H.; Sung, D.K.; Park, Y.R.; Choi, S.H.; Choi, S.J.; Chang, Y.S.; Oh, W.; Lee, J.H.; et al. Human umbilical cord blood-derived mesenchymal stem cell transplantation attenuates severe brain injury by permanent middle cerebral artery occlusion in newborn rats. Pediatr. Res. 2012, 72, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary passage is a major obstacle for intravenous stem cell delivery: The pulmonary first-pass effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Chang, Y.S.; Ahn, S.Y.; Yoo, H.S.; Sung, S.I.; Choi, S.J.; Oh, W.I.; Park, W.S. Mesenchymal stem cells for bronchopulmonary dysplasia: Phase 1 dose-escalation clinical trial. J. Pediatr. 2014, 164, 966–972e6. [Google Scholar] [CrossRef] [PubMed]
- Rice, J.E., 3rd; Vannucci, R.C.; Brierley, J.B. The influence of immaturity on hypoxic-ischemic brain damage in the rat. Ann. Neurol. 1981, 9, 131–141. [Google Scholar] [CrossRef]
- Gunn, A.J.; Thoresen, M. Hypothermic neuroprotection. NeuroRx 2006, 3, 154–169. [Google Scholar] [CrossRef] [PubMed]
- Donega, V.; Nijboer, C.H.; Braccioli, L.; Slaper-Cortenbach, I.; Kavelaars, A.; van Bel, F.; Heijnen, C.J. Intranasal administration of human MSC for ischemic brain injury in the mouse: In vitro and in vivo neuroregenerative functions. PLoS ONE 2014, 9, e112339. [Google Scholar] [CrossRef]
- Donega, V.; Nijboer, C.H.; van Velthoven, C.T.; Youssef, S.A.; de Bruin, A.; van Bel, F.; Kavelaars, A.; Heijnen, C.J. Assessment of long-term safety and efficacy of intranasal mesenchymal stem cell treatment for neonatal brain injury in the mouse. Pediatr. Res. 2015, 78, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Van Velthoven, C.T.; Dzietko, M.; Wendland, M.F.; Derugin, N.; Faustino, J.; Heijnen, C.J.; Ferriero, D.M.; Vexler, Z.S. Mesenchymal stem cells attenuate MRI-identifiable injury, protect white matter, and improve long-term functional outcomes after neonatal focal stroke in rats. J. Neurosci. Res. 2017, 95, 1225–1236. [Google Scholar] [CrossRef]
- Donega, V.; van Velthoven, C.T.; Nijboer, C.H.; van Bel, F.; Kas, M.J.; Kavelaars, A.; Heijnen, C.J. Intranasal mesenchymal stem cell treatment for neonatal brain damage: Long-term cognitive and sensorimotor improvement. PLoS ONE 2013, 8, e51253. [Google Scholar] [CrossRef]
- Archambault, J.; Moreira, A.; McDaniel, D.; Winter, L.; Sun, L.; Hornsby, P. Therapeutic potential of mesenchymal stromal cells for hypoxic ischemic encephalopathy: A systematic review and meta-analysis of preclinical studies. PLoS ONE 2017, 12, e0189895. [Google Scholar] [CrossRef] [PubMed]
- Marlow, N.; Rose, A.S.; Rands, C.E.; Draper, E.S. Neuropsychological and educational problems at school age associated with neonatal encephalopathy. Arch. Dis. Child. Fetal. Neonatal. Ed. 2005, 90, F380–F387. [Google Scholar] [CrossRef] [PubMed]
- Azzopardi, D.; Strohm, B.; Marlow, N.; Brocklehurst, P.; Deierl, A.; Eddama, O.; Goodwin, J.; Halliday, H.L.; Juszczak, E.; Kapellou, O.; et al. Effects of hypothermia for perinatal asphyxia on childhood outcomes. N. Engl. J. Med. 2014, 371, 140–149. [Google Scholar] [CrossRef]
- Chavez-Valdez, R.; Emerson, P.; Goffigan-Holmes, J.; Kirkwood, A.; Martin, L.J.; Northington, F.J. Delayed injury of hippocampal interneurons after neonatal hypoxia-ischemia and therapeutic hypothermia in a murine model. Hippocampus 2018, 28, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Penny, T.; Sutherland, A.E.; Mihelakis, J.G.; Paton, M.C.B.; Pham, Y.; Lee, J.; Jones, N.M.; Jenkin, G.; Fahey, M.C.; Miller, S.L.; et al. Human umbilical cord therapy improves long-term behavioural outcomes following neonatal hypoxic ischemic brain injury. Front. Physiol. 2019, 10, 283. [Google Scholar] [CrossRef]
- McDonald, C.A.; Penny, T.R.; Paton, M.C.B.; Sutherland, A.E.; Nekkanti, L.; Yawno, T.; Castillo-Melendez, M.; Fahey, M.C.; Jones, N.M.; Jenkin, G.; et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J. Neuroinflamm. 2018, 15, 47. [Google Scholar] [CrossRef]
- McDonald, C.A.; Payne, N.L.; Sun, G.; Moussa, L.; Siatskas, C.; Lim, R.; Wallace, E.M.; Jenkin, G.; Bernard, C.C. Immunosuppressive potential of human amnion epithelial cells in the treatment of experimental autoimmune encephalomyelitis. J. Neuroinflamm. 2015, 12, 112. [Google Scholar] [CrossRef]
- Paton, M.C.B.; Allison, B.J.; Fahey, M.C.; Li, J.; Sutherland, A.E.; Pham, Y.; Nitsos, I.; Bischof, R.J.; Moss, T.J.; Polglase, G.R.; et al. Umbilical cord blood versus mesenchymal stem cells for inflammation-induced preterm brain injury in fetal sheep. Pediatr. Res. 2019. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; McDonald, C.A.; Fahey, M.C.; Jenkin, G.; Miller, S.L. Could cord blood cell therapy reduce preterm brain injury? Front. Neurol. 2014, 5, 200. [Google Scholar] [CrossRef]
- Harting, M.T.; Jimenez, F.; Xue, H.; Fischer, U.M.; Baumgartner, J.; Dash, P.K.; Cox, C.S. Intravenous mesenchymal stem cell therapy for traumatic brain injury. J. Neurosurg. 2009, 110, 1189–1197. [Google Scholar] [CrossRef]
- Li, Y.H.; Feng, L.; Zhang, G.X.; Ma, C.G. Intranasal delivery of stem cells as therapy for central nervous system disease. Exp. Mol. Pathol. 2015, 98, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Fransson, M.; Piras, E.; Wang, H.; Burman, J.; Duprez, I.; Harris, R.A.; LeBlanc, K.; Magnusson, P.U.; Brittebo, E.; Loskog, A.S. Intranasal delivery of central nervous system-retargeted human mesenchymal stromal cells prolongs treatment efficacy of experimental autoimmune encephalomyelitis. Immunology 2014, 142, 431–441. [Google Scholar] [CrossRef] [PubMed]
- Teo, J.D.; Morris, M.J.; Jones, N.M. Hypoxic postconditioning improves behavioural deficits at 6 weeks following hypoxic-ischemic brain injury in neonatal rats. Behav. Brain Res. 2017, 333, 27–34. [Google Scholar] [CrossRef]
- Teo, J.D.; Morris, M.J.; Jones, N.M. Maternal obesity increases inflammation and exacerbates damage following neonatal hypoxic-ischaemic brain injury in rats. Brain Behav. Immun. 2017, 63, 186–196. [Google Scholar] [CrossRef]

| Gene | Sham | HI | UC-MSC |
|---|---|---|---|
| BDNF | 1.00 ± 0.07 | 0.59 ± 0.14 * | 0.60 ± 0.04 * |
| VEGF | 1.00 ± 0.34 | 0.39 ± 0.07 | 0.51 ± 0.07 |
| IGF-1 | 1.00 ± 0.23 | 16.07 ± 4.72 | 13.36 ± 8.06 |
| Claudin 5 | 1.00 ± 0.15 | 0.72 ± 0.13 | 0.74 ± 0.14 |
| Occludin | 1.00 ± 0.10 | 0.76 ± 0.05 | 1.07 ± 0.30 |
| GDNF | 1.00 ± 0.22 | 0.69 ± 0.15 | 1.47 ± 0.61 |
| Gene | Sequence | |
|---|---|---|
| VEGFa | F | AGCGACAAGGCAGACTATTA |
| R | AATCCCAGAGCACAGACTCC | |
| Claudin 5 | F | TTGTGAGGACTTGACCGACC |
| R | CTGTTAGCGGCAGTTTGGTG | |
| Occludin | F | TATGCTGACCGTAGTACAGAAAGT |
| R | TTCCACTCGGGCTCAATCC | |
| BDNF | F | AGCAGTCAAGTGCCTTTGGA |
| R | CGCTAATACTGTCACACACGC | |
| GDNF | F | AAGTTATGGGATGTCGTGGCT |
| R | AGAAGCCTCTTACCGGCG | |
| IGF | F | CGGGACGTACCAAAATGAGC |
| R | CAAGCAGAGTGCCAGGTAGAA | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McDonald, C.A.; Djuliannisaa, Z.; Petraki, M.; Paton, M.C.B.; Penny, T.R.; Sutherland, A.E.; Castillo-Melendez, M.; Novak, I.; Jenkin, G.; Fahey, M.C.; et al. Intranasal Delivery of Mesenchymal Stromal Cells Protects against Neonatal Hypoxic–Ischemic Brain Injury. Int. J. Mol. Sci. 2019, 20, 2449. https://doi.org/10.3390/ijms20102449
McDonald CA, Djuliannisaa Z, Petraki M, Paton MCB, Penny TR, Sutherland AE, Castillo-Melendez M, Novak I, Jenkin G, Fahey MC, et al. Intranasal Delivery of Mesenchymal Stromal Cells Protects against Neonatal Hypoxic–Ischemic Brain Injury. International Journal of Molecular Sciences. 2019; 20(10):2449. https://doi.org/10.3390/ijms20102449
Chicago/Turabian StyleMcDonald, Courtney A., Zlatikha Djuliannisaa, Maria Petraki, Madison C. B. Paton, Tayla R. Penny, Amy E. Sutherland, Margie Castillo-Melendez, Iona Novak, Graham Jenkin, Michael C. Fahey, and et al. 2019. "Intranasal Delivery of Mesenchymal Stromal Cells Protects against Neonatal Hypoxic–Ischemic Brain Injury" International Journal of Molecular Sciences 20, no. 10: 2449. https://doi.org/10.3390/ijms20102449
APA StyleMcDonald, C. A., Djuliannisaa, Z., Petraki, M., Paton, M. C. B., Penny, T. R., Sutherland, A. E., Castillo-Melendez, M., Novak, I., Jenkin, G., Fahey, M. C., & Miller, S. L. (2019). Intranasal Delivery of Mesenchymal Stromal Cells Protects against Neonatal Hypoxic–Ischemic Brain Injury. International Journal of Molecular Sciences, 20(10), 2449. https://doi.org/10.3390/ijms20102449







