Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs)
Abstract
1. Introduction
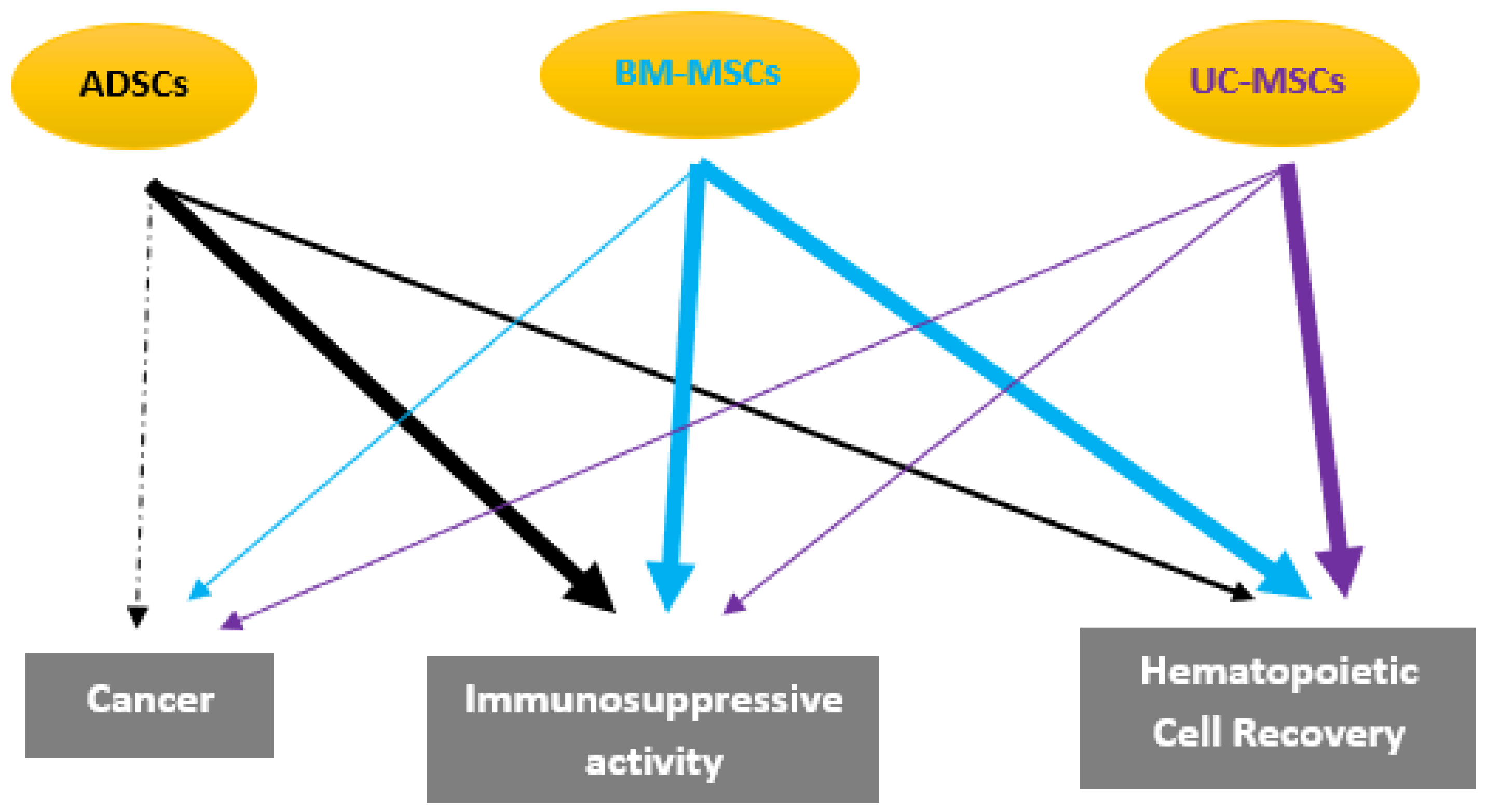
2. Mesenchymal Stem Cells Characteristics
3. Types of Stem Cells
3.1. Bone Marrow-Mesenchymal Stem Cells (BM-MSCs)
3.2. Umbilical Cord-Mesenchymal Stem Cells (UC-MSCs)
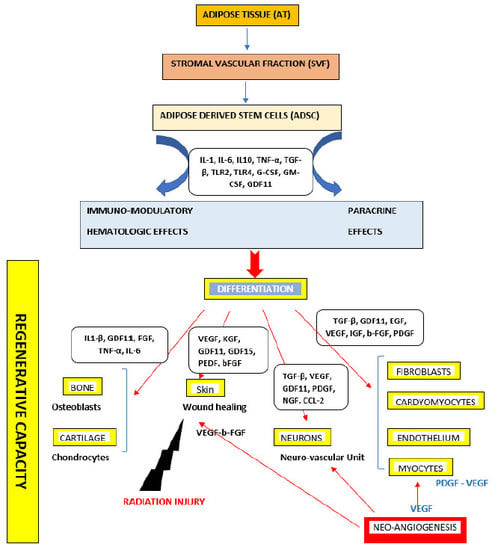
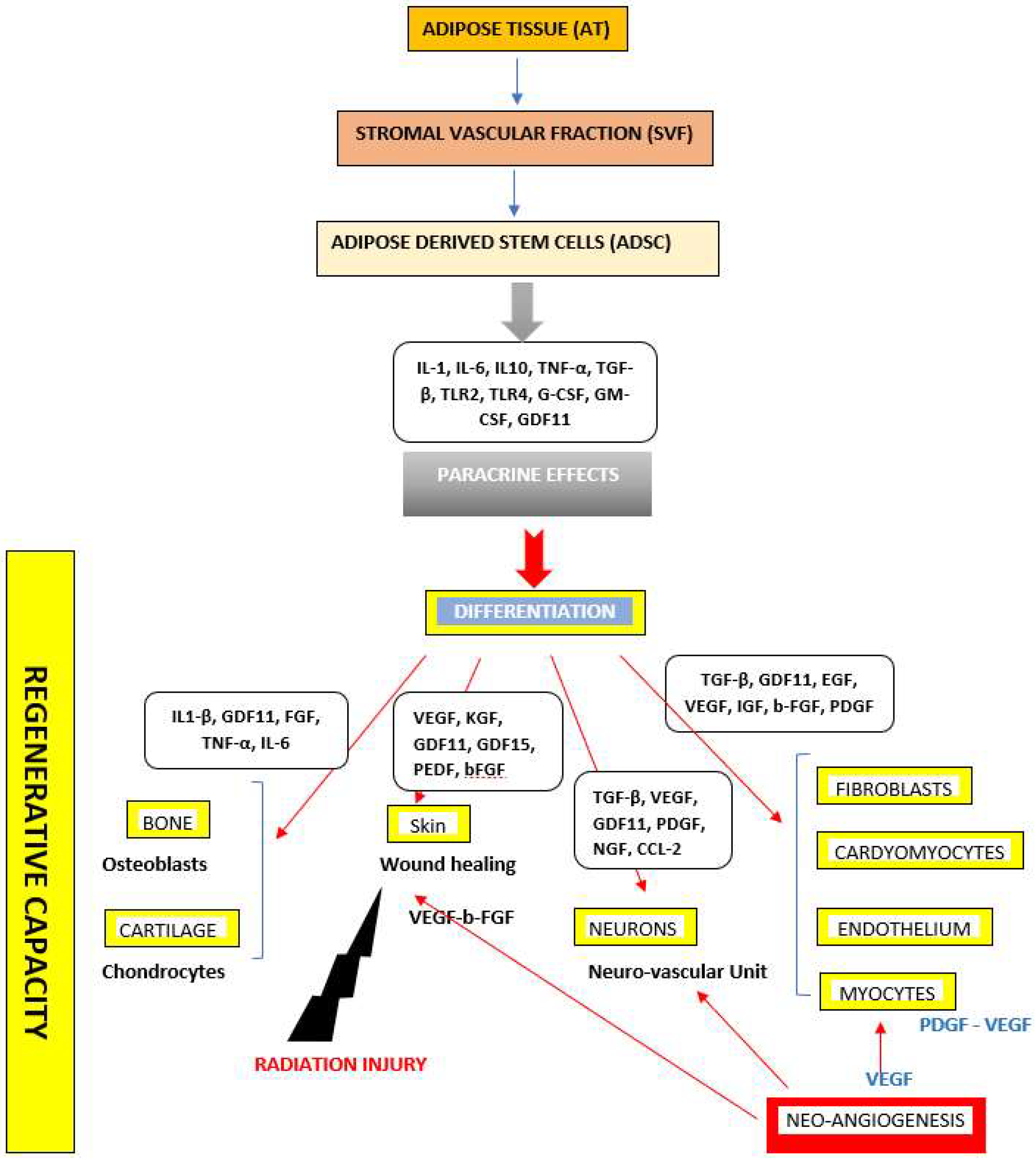
3.3. Adipose Derived Stem Cells (ADSCs)
4. Current Approaches in Adipose Derived Stem Cells (ADSCs)-Based Therapeutic Applications
4.1. The Effects of ADSCs on Wound Healing and Skin Regeneration
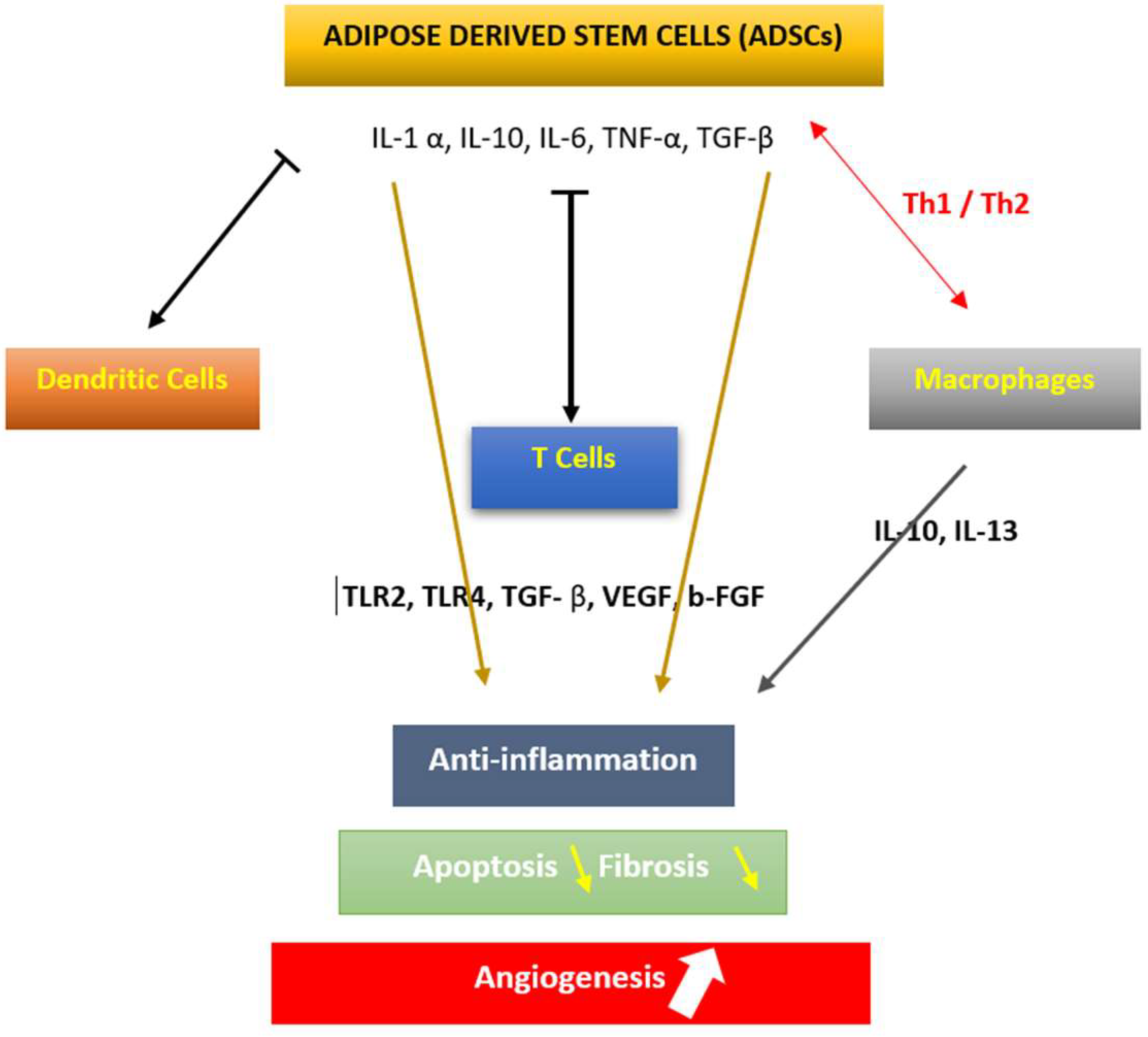
4.2. The Effects of ADSCs in Auto-Immune Disorders
4.3. The Effects of ADSCs on Hematological Disorders and Graft-Versus-Host Disease (GVHD)
4.4. The Effects of ADSCs on Bone/Cartilage Repair
4.5. The Effects of ADSCs on Cardiovascular and Muscular Diseases
4.6. The Effects of ADSC on Neuro-Degenerative Diseases
4.7. The Effects of ADSCs on Radiation Injuries
5. Issues Related to Autologous and Allogeneic Clinical Use of ADSCs
6. Summary and Conclusion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| ADSCs | adipose derived stem cells |
| ALS | amyotrophic lateral sclerosis |
| AT | adipose tissue |
| b-FGF | basic Fibroblast Growth Factor |
| BM | bone marrow |
| CCL-2 | Chemokine C-C Motif Ligand-2 |
| cGMP | current good manufacturing practices |
| ECM | extracellular matrix |
| EGF | Endothelial Growth Factor |
| G-CSF | Granulocyte-Colony Stimulating Factor |
| GDF-11, -15 | Growth Differentiation Factor-11, -15 |
| GM-CSF | Granulocyte Monocyte-Colony Stimulating Factor |
| GVHD | graft versus host disease |
| HSCs | hematopoietic stem cells |
| IFATS | International Federation of Adipose Therapeutics |
| IGF | Insulin Growth Factor |
| IL-1, -6, -10 | Interleukin-1, -6, -10 |
| ISCT | International Society for Cellular Therapy |
| KGF | Keratinocyte Growth Factor |
| MS | multiple sclerosis |
| MSCs | mesenchymal stem/stromal cells |
| NGF | Nerve Growth Factor |
| PDGF | Platelet Derived Growth Factor |
| PEDF | Pigment Epithelium Derived Factor |
| SLE | systemic lupus erythematosus |
| SS | systemic sclerosis |
| SVF | Stromal vascular fraction |
| TGF-β | Tumor growth factor-β |
| TLR2, 4 | Toll-like Receptor 2, 4 |
| TNF-α | Tumor necrosis factor-α |
| UC | umbilical cord |
| UCB | umbilical cord blood |
| VEGF | Vascular Endothelial Growth Factor |
References
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 2002, 13, 4279–4295. [Google Scholar] [CrossRef]
- Vermette, M.; Trottier, V.; Ménard, V.; Saint-Pierre, L.; Roy, A.; Fradette, J. Production of a new tissue-engineered adipose substitute from human adipose-derived stromal cells. Biomaterials 2007, 28, 2850–2860. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Dusting, G.J.; Stubbs, S.; Arunothayaraj, S.; Han, X.L.; Collas, P.; Morrison, W.A.; Dilley, R.J. Differentiation of human adipose-derived stem cells into beating cardiomyocytes. J. Cell. Mol. Med. 2010, 14, 878–889. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Bae, Y.K.; Kim, M.; Kwon, S.J.; Jeon, H.B.; Choi, S.J.; Kim, S.W.; Yang, Y.S.; Oh, W.; Chang, J.W. Comparative analysis of human mesenchymal stem cells from bone marrow, adipose tissue, and umbilical cord blood as sources of cell therapy. Int. J. Mol. Sci. 2013, 14, 17986–18001. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Martin, B.J. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ. Res. 2004, 95, 9–20. [Google Scholar] [CrossRef]
- Trottier, V.; Marceau-Fortier, G.; Germain, L.; Vincent, C.; Fradette, J. IFATS collection: Using human adipose-derived stem/stromal cells for the production of new skin substitutes. Stem Cells 2008, 26, 2713–2723. [Google Scholar] [CrossRef]
- Hyldig, K.; Riis, S.; Pennisi, C.P.; Zachar, V.; Fink, T. Implications of Extracellular Matrix Production by Adipose Tissue-Derived Stem Cells for Development of Wound Healing Therapies. Int. J. Mol. Sci. 2017, 18, 1167. [Google Scholar] [CrossRef] [PubMed]
- Eto, H.; Kato, H.; Suga, H.; Aoi, N.; Doi, K.; Kuno, S.; Yoshimura, K. The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes. Plast. Reconstr. Surg. 2012, 129, 1081–1092. [Google Scholar] [CrossRef] [PubMed]
- Koh, K.S.; Oh, T.S.; Kim, H.; Chung, I.W.; Lee, K.W.; Lee, H.B.; Park, E.J.; Jung, J.S.; Shin, I.S.; Ra, J.C.; et al. Clinical application of human adipose tissue-derived mesenchymal stem cells in progressive hemifacial atrophy (Parry-Romberg disease) with microfat grafting techniques using 3-dimensional computed tomography and 3-dimensional camera. Ann. Plast. Surg. 2012, 69, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Y.; Luo, X.; Fu, M.G.; Hu, X.; Dong, H.; Fan, Z.H. Cell-assisted lipotransfer for breast augmentation: A report of 18 patients. Chin. J. Plast. 2012, 28, 1–6. [Google Scholar]
- Yoshimura, K.; Sato, K.; Aoi, N.; Kurita, M.; Hirohi, T.; Harii, K. Cell-assisted lipotransfer for cosmetic breast augmentation: Supportive use of adipose-derived stem/stromal cells. Aesthetic Plast. Surg. 2008, 32, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, K.; Asano, Y.; Aoi, N.; Kurita, M.; Oshima, Y.; Sato, K.; Inoue, K.; Suga, H.; Eto, H.; Kato, H.; et al. Progenitor-enriched adipose tissue transplantation as rescue for breast implant complications. Breast J. 2010, 16, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Fang, B.; Song, Y.; Liao, L.; Zhang, Y.; Zhao, R.C. Favorable response to human adipose tissue-derived mesenchymal stem cells in steroid-refractory acute graft-versus-host disease. Transplant. Proc. 2007, 39, 3358–3362. [Google Scholar] [CrossRef] [PubMed]
- Stepien, A.; Dabrowska, N.L.; Maciagowska, M.; Macoch, R.P.; Zolocinska, A.; Mazur, S.; Siennicka, K.; Frankowska, E.; Kidzinski, R.; Chalimoniuk, M.; et al. Clinical Application of Autologous Adipose Stem Cells in Patients with Multiple Sclerosis: Preliminary Results. Mediat. Inflamm. 2016, 2016, 5302120. [Google Scholar] [CrossRef]
- Rigotti, G.; Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Takiya, C.M.; Amable, P.R.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A. Expanded Stem Cells, Stromal-Vascular Fraction, and Platelet-Rich Plasma Enriched Fat: Comparing Results of Different Facial Rejuvenation Approaches in a Clinical Trial. Aesthetic Surg. J. 2016, 36, 261–270. [Google Scholar] [CrossRef]
- Charles-de-Sá, L.; Gontijo-de-Amorim, N.F.; Maeda Takiya, C.; Borojevic, R.; Benati, D.; Bernardi, P.; Sbarbati, A.; Rigotti, G. Antiaging treatment of the facial skin by fat graft and adipose-derived stem cells. Plast. Reconstr. Surg. 2015, 135, 999–1009. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Pak, N.; Pak, Y.; Park, K.S.; Jeon, J.H.; Jeong, B.C.; Lee, S.H. Cartilage Regeneration in Humans with Adipose Tissue-Derived Stem Cells and Adipose Stromal Vascular Fraction Cells: Updated Status. Int. J. Mol. Sci. 2018, 19, 2146. [Google Scholar] [CrossRef]
- Gonzalez-Rey, E.; Gonzalez, M.A.; Varela, N.; O’Valle, F.; Hernandez-Cortes, P.; Rico, L.; Büscher, D.; Delgado, M. Human adipose-derived mesenchymal stem cells reduce inflammatory and T cell responses and induce regulatory T cells in vitro in rheumatoid arthritis. Ann. Rheum. Dis. 2010, 69, 241–248. [Google Scholar] [CrossRef]
- Assunção-Silva, R.C.; Mendes-Pinheiro, B.; Patrício, P.; Behie, L.A.; Teixeira, F.G.; Pinto, L.; Salgado, A.J. Exploiting the impact of the secretome of MSCs isolated from different tissue sources on neuronal differentiation and axonal growth. Biochimie 2018, 155, 83–91. [Google Scholar] [CrossRef]
- Widgerow, A.D.; Salibian, A.A.; Lalezari, S.; Evans, G.R.D. Neuromodulatory nerve regeneration: Adipose tissue-derived stem cells and neurotrophic mediation in peripheral nerve regeneration. J. Neurosci. Res. 2013, 91, 1517–1524. [Google Scholar] [CrossRef]
- Im, G.I. Regeneration of articular cartilage using adipose stem cells. J. Biomed. Mater. Res. A 2016, 104, 1830–1844. [Google Scholar] [CrossRef]
- Duckers, H.J.; Pinkernell, K.; Milstein, A.M.; Hedrick, M.H. The Bedside Celution system for isolation of adipose derived regenerative cells. EuroIntervention 2006, 2, 395–398. [Google Scholar]
- Katz, A.J.; Hedrick, M.H.; Llull, R.; Futrell, J.W. A novel device for the simple and efficient refinement of liposuctioned tissue. Plast. Reconstr. Surg. 2001, 107, 595–597. [Google Scholar] [CrossRef]
- Caplan, A.I. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J. Cell. Physiol. 2007, 213, 341–347. [Google Scholar] [CrossRef]
- Caplan, A.I. Mesenchymal stem cells. J. Orthop. Res. 1991, 9, 641–650. [Google Scholar] [CrossRef]
- Zomer, H.D.; Varela, G.K.D.S.; Delben, P.B.; Heck, D.; Jeremias, T.; Trentin, A.G. In vitro comparative study of human mesenchymal stromal cells from dermis and adipose tissue for application in skin wound healing. J. Tissue Eng. Regen. Med. 2019, 13. [Google Scholar] [CrossRef]
- Lei, M.; Li, K.; Li, B.; Gao, L.N.; Chen, F.M.; Jin, Y. Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials 2014, 35, 6332–6343. [Google Scholar] [CrossRef]
- Campagnoli, C.; Roberts, I.A.; Kumar, S.; Bennett, P.R.; Bellantuono, I.; Fisk, N.M. Identification of mesenchymal stem/progenitor cells in human first-trimester fetal blood, liver, and bone marrow. Blood 2001, 98, 2396–2402. [Google Scholar] [CrossRef]
- Vishnubalaji, R.; Al-Nbaheen, M.; Kadalmani, B.; Aldahmash, A.; Ramesh, T. Comparative investigation of the differentiation capability of bone-marrow- and adipose-derived mesenchymal stem cells by qualitative and quantitative analysis. Cell Tissue Res. 2012, 347, 419–427. [Google Scholar] [CrossRef]
- Kern, S.; Eichler, H.; Stoeve, J.; Klüter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Bourin, P.; Bunnell, B.A.; Casteilla, L.; Dominici, M.; Katz, A.J.; March, K.L.; Redl, H.; Rubin, J.P.; Yoshimura, K.; Gimble, J.M. Stromal cells from the adipose tissue-derived stromal vascular fraction and culture expanded adipose tissue-derived stromal/stem cells: A joint statement of the International Federation for Adipose Therapeutics and Science (IFATS) and the International Society for Cellular Therapy (ISCT). Cytotherapy 2013, 15, 641–648. [Google Scholar]
- Puissant, B.; Barreau, C.; Bourin, P.; Clavel, C.; Corre, J.; Bousquet, C.; Taureau, C.; Cousin, B.; Abbal, M.; Laharrague, P.; et al. Immunomodulatory effect of human adipose tissue-derived adult stem cells: Comparison with bone marrow mesenchymal stem cells. Br. J. Haematol. 2005, 129, 118–129. [Google Scholar] [CrossRef]
- Musina, R.A.; Bekchanova, E.S.; Belyavskii, A.V.; Sukhikh, G.T. Differentiation potential of mesenchymal stem cells of different origin. Bull. Exp. Biol. Med. 2006, 141, 147–151. [Google Scholar] [CrossRef]
- Wagner, W.; Wein, F.; Seckinger, A.; Frankhauser, M.; Wirkner, U.; Krause, U.; Blake, J.; Schwager, C.; Eckstein, V.; Ansorge, W.; et al. Comparative characteristics of mesenchymal stem cells from human bone marrow, adipose tissue, and umbilical cord blood. Exp. Hematol. 2005, 33, 1402–1416. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Jacobs, S.A.; Pinxteren, J.; Roobrouck, V.D.; Luyckx, A.; van’t Hof, W.; Deans, R.; Verfaillie, C.M.; Waer, M.; Billiau, A.D.; Van Gool, S.W. Human multipotent adult progenitor cells are nonimmunogenic and exert potent immunomodulatory effects on alloreactive T-cell responses. Cell Transplant. 2013, 22, 1915–1928. [Google Scholar] [CrossRef]
- Spaggiari, G.M.; Abdelrazik, H.; Becchetti, F.; Moretta, L. MSCs inhibit monocyte-derived DC maturation and function by selectively interfering with the generation of immature DCs: Central role of MSC-derived prostaglandin E2. Blood 2009, 113, 6576–6583. [Google Scholar] [CrossRef]
- Jiang, X.X.; Zhang, Y.; Liu, B.; Zhang, S.X.; Wu, Y.; Yu, X.D.; Mao, N. Human mesenchymal stem cells inhibit differentiation and function of monocyte-derived dendritic cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef]
- Jiang, R.; Han, Z.; Zhuo, G.; Qu, X.; Li, X.; Wang, X.; Shao, Y.; Yang, S.; Han, Z.C. Transplantation of placenta-derived mesenchymal stem cells in type 2 diabetes: A pilot study. Front. Med. 2011, 5, 94–100. [Google Scholar] [CrossRef]
- Stagg, J.; Galipeau, J. Immune plasticity of bone marrow-derived mesenchymal stromal cells. In Handbook Experimental Pharmacology; Springer: Berlin, Germany, 2007; pp. 45–66. [Google Scholar]
- Bernardo, M.E.; Avanzini, M.A.; Ciccocioppo, R.; Perotti, C.; Cometa, A.M.; Moretta, A.; Marconi, M.; Valli, M.; Novara, F.; Bonetti, F.; et al. Phenotypical/functional characterization of in vitro-expanded mesenchymal stromal cells from patients with Crohn’s disease. Cytotherapy 2009, 11, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Bartholomew, A.; Sturgeon, C.; Siatskas, M.; Ferrer, K.; McIntosh, K.; Patil, S.; Hardy, W.; Devine, S.; Ucker, D.; Deans, R.; et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp. Hematol. 2002, 30, 42–48. [Google Scholar] [CrossRef]
- Meisel, R.; Zibert, A.; Laryea, M.; Göbel, U.; Däubener, W.; Dilloo, D. Human bone marrow stromal cells inhibit allogeneic T-cell responses by indoleamine 2,3-dioxygenase-mediated tryptophan degradation. Blood 2004, 103, 4619–4621. [Google Scholar] [CrossRef]
- Brooke, G.; Cook, M.; Blair, C.; Han, R.; Heazlewood, C.; Jones, B.; Kambouris, M.; Kollar, K.; McTaggart, S.; Pelekanos, R.; et al. Therapeutic applications of mesenchymal stromal cells. Semin. Cell Dev. Biol. 2007, 18, 846–858. [Google Scholar] [CrossRef]
- Pachón-Peña, G.; Yu, G.; Tucker, A.; Wu, X.; Vendrell, J.; Bunnell, B.A.; Gimble, J.M. Stromal stem cells from adipose tissue and bone marrow of age-matched female donors display distinct immunophenotypic profiles. J. Cell. Physiol. 2011, 226, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Shafiee, A.; Soleimani, M.; Chamheidari, G.A.; Seyedjafari, E.; Dodel, M.; Atashi, A.; Gheisari, Y. Electrospun nanofiber-based regeneration of cartilage enhanced by mesenchymal stem cells. J. Biomed. Mater. Res. A 2011, 99, 467–478. [Google Scholar] [CrossRef]
- Barlow, S.; Brooke, G.; Chatterjee, K.; Price, G.; Pelekanos, R.; Rossetti, T.; Doody, M.; Venter, D.; Pain, S.; Gilshenan, K.; et al. Comparison of human placenta- and bone marrow-derived multipotent mesenchymal stem cells. Stem Cells Dev. 2008, 17, 1095–1107. [Google Scholar] [CrossRef]
- Lee, R.H.; Kim, B.; Choi, I.; Kim, H.; Choi, H.S.; Suh, K.; Bae, Y.C.; Jung, J.S. Characterization and expression analysis of mesenchymal stem cells from human bone marrow and adipose tissue. Cell. Physiol. Biochem. 2004, 14, 311–324. [Google Scholar] [CrossRef]
- Doorn, J.; Moll, G.; Le Blanc, K.; van Blitterswijk, C.; de Boer, J. Therapeutic applications of mesenchymal stromal cells: Paracrine effects and potential improvements. Tissue Eng. Part B Rev. 2012, 18, 101–115. [Google Scholar] [CrossRef]
- Chatzistamatiou, T.K.; Papassavas, A.C.; Michalopoulos, E.; Gamaloutsos, C.; Mallis, P.; Gontika, I.; Panagouli, E.; Koussoulakos, S.L.; Stavropoulos-Giokas, C. Optimizing isolation culture and freezing methods to preserve Wharton’s jelly’s mesenchymal stem cell (MSC) properties: An MSC banking protocol validation for the Hellenic Cord Blood Bank. Transfusion 2014, 54, 3108–3120. [Google Scholar] [CrossRef]
- Weiss, M.L.; Troyer, D.L. Stem cells in the umbilical cord. Stem Cell Rev. 2006, 2, 155–162. [Google Scholar] [CrossRef]
- Fong, C.Y.; Subramanian, A.; Gauthaman, K.; Venugopal, J.; Biswas, A.; Ramakrishna, S.; Bongso, A. Human umbilical cord Wharton’s jelly stem cells undergo enhanced chondrogenic differentiation when grown on nanofibrous scaffolds and in a sequential two-stage culture medium environment. Stem Cell Rev. 2012, 8, 195–209. [Google Scholar] [CrossRef]
- Balzano, F.; Bellu, E.; Basoli, V.; Dei Giudici, S.; Santaniello, S.; Cruciani, S.; Facchin, F.; Oggiano, A.; Capobianco, G.; Dessole, F.; et al. Lessons from human umbilical cord: Gender differences in stem cells from Wharton’s jelly. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019, 234, 143–148. [Google Scholar] [CrossRef]
- Cheng, H.; Qiu, L.; Ma, J.; Zhang, H.; Cheng, M.; Li, W.; Zhao, X.; Liu, K. Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts. Mol. Biol. Rep. 2011, 38, 5161–5168. [Google Scholar] [CrossRef]
- Zhang, X.; Hirai, M.; Cantero, S.; Ciubotariu, R.; Dobrila, L.; Hirsh, A.; Igura, K.; Satoh, H.; Yokomi, I.; Nishimura, T.; et al. Isolation and characterization of mesenchymal stem cells from human umbilical cord blood: Reevaluation of critical factors for successful isolation and high ability to proliferate and differentiate to chondrocytes as compared to mesenchymal stem cells from bone marrow and adipose tissue. J. Cell. Biochem. 2011, 112, 1206–1218. [Google Scholar]
- De Miguel, M.P.; Fuentes-Julián, S.; Blázquez-Martínez, A.; Pascual, C.Y.; Aller, M.A.; Arias, J.; Arnalich-Montiel, F. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr. Mol. Med. 2012, 12, 574–591. [Google Scholar] [CrossRef]
- Krupa, P.; Vackova, I.; Ruzicka, J.; Zaviskova, K.; Dubisova, J.; Koci, Z.; Turnovcova, K.; Urdzikova, L.M.; Kubinova, S.; Rehak, S.; et al. The Effect of Human Mesenchymal Stem Cells Derived from Wharton’s Jelly in Spinal Cord Injury Treatment Is Dose-Dependent and Can Be Facilitated by Repeated Application. Int. J. Mol. Sci. 2018, 19, 1503. [Google Scholar] [CrossRef]
- Weiss, M.L.; Medicetty, S.; Bledsoe, A.R.; Rachakatla, R.S.; Choi, M.; Merchav, S.; Luo, Y.; Rao, M.S.; Velagaleti, G.; Troyer, D. Human umbilical cord matrix stem cells: Preliminary characterization and effect of transplantation in a rodent model of Parkinson’s disease. Stem Cells 2006, 24, 781–792. [Google Scholar] [CrossRef]
- López, Y.; Lutjemeier, B.; Seshareddy, K.; Trevino, E.M.; Hageman, K.S.; Musch, T.I.; Borgarelli, M.; Weiss, M.L. Wharton’s jelly or bone marrow mesenchymal stromal cells improve cardiac function following myocardial infarction for more than 32 weeks in a rat model: A preliminary report. Curr. Stem Cell Res. Ther. 2013, 8, 46–59. [Google Scholar] [CrossRef]
- Wang, H.S.; Shyu, J.F.; Shen, W.S.; Hsu, H.C.; Chi, T.C.; Chen, C.P.; Huang, S.W.; Shyr, Y.M.; Tang, K.T.; Chen, T.H. Transplantation of insulin-producing cells derived from umbilical cord stromal mesenchymal stem cells to treat NOD mice. Cell Transplant. 2011, 20, 455–466. [Google Scholar] [CrossRef]
- Palumbo, P.; Miconi, G.; Cinque, B.; La Torre, C.; Lombardi, F.; Zoccali, G.; Orsini, G.; Leocata, P.; Giuliani, M.; Cifone, M.G. In vitro evaluation of different methods of handling human liposuction aspirate and their effect on adipocytes and adipose derived stem cells. J. Cell. Physiol. 2015, 230, 1974–1981. [Google Scholar] [CrossRef]
- Miyagi-Shiohira, C.; Kurima, K.; Kobayashi, N.; Saitoh, I.; Watanabe, M.; Noguchi, Y.; Matsushita, M.; Noguchi, H. Cryopreservation of Adipose-Derived Mesenchymal Stem Cells. Cell Med. 2015, 8, 3–7. [Google Scholar] [CrossRef]
- Vallée, M.; Côté, J.F.; Fradette, J. Adipose-tissue engineering: Taking advantage of the properties of human adipose-derived stem/stromal cells. Pathol. Biol. 2009, 57, 309–317. [Google Scholar] [CrossRef]
- Strem, B.M.; Hicok, K.C.; Zhu, M.; Wulur, I.; Alfonso, Z.; Schreiber, R.E.; Fraser, J.K.; Hedrick, M.H. Multipotential differentiation of adipose tissue-derived stem cells. Keio J. Med. 2005, 54, 132–141. [Google Scholar] [CrossRef]
- Lataillade, J.J.; Magne, B.; Bey, E.; Leclerc, T.; Trouillas, M. Skin engineering for severe burns. Transfus. Clin. Biol. 2017, 24, 245–250. [Google Scholar] [CrossRef]
- Mitchell, J.B.; McIntosh, K.; Zvonic, S.; Garrett, S.; Floyd, Z.E.; Kloster, A.; Di Halvorsen, Y.; Storms, R.W.; Goh, B.; Kilroy, G.; et al. Immunophenotype of human adipose-derived cells: Temporal changes in stromal-associated and stem cell-associated markers. Stem Cells 2006, 24, 376–385. [Google Scholar] [CrossRef]
- Maumus, M.; Peyrafitte, J.A.; D’Angelo, R.; Fournier-Wirth, C.; Bouloumié, A.; Casteilla, L.; Sengenès, C.; Bourin, P. Native human adipose stromal cells: Localization, morphology and phenotype. Int. J. Obes. 2011, 35, 1141–1153. [Google Scholar] [CrossRef]
- Zhao, D.; Liu, L.; Chen, Q.; Wang, F.; Li, Q.; Zeng, Q.; Huang, J.; Luo, M.; Li, W.; Zheng, Y.; et al. Hypoxia with Wharton’s jelly mesenchymal stem cell coculture maintains stemness of umbilical cord blood-derived CD34+ cells. Stem Cell Res. Ther. 2018, 9, 158. [Google Scholar] [CrossRef]
- Sengenès, C.; Lolmède, K.; Zakaroff-Girard, A.; Busse, R.; Bouloumié, A. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells. J. Cell. Physiol. 2005, 205, 114–122. [Google Scholar] [CrossRef]
- McIntosh, K.; Zvonic, S.; Garrett, S.; Mitchell, J.B.; Floyd, Z.E.; Hammill, L.; Kloster, A.; Di Halvorsen, Y.; Ting, J.P.; Storms, R.W.; et al. The immunogenicity of human adipose-derived cells: Temporal changes in vitro. Stem Cells 2006, 24, 1246–1253. [Google Scholar] [CrossRef]
- Scherberich, A.; Di Maggio, N.D.; McNagny, K.M. A familiar stranger: CD34 expression and putative functions in SVF cells of adipose tissue. World J. Stem Cells 2013, 5, 1–8. [Google Scholar] [CrossRef]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef]
- Cuevas-Diaz Duran, R.; González-Garza, M.T.; Cardenas-Lopez, A.; Chavez-Castilla, L.; Cruz-Vega, D.E.; Moreno-Cuevas, J.E. Age-related yield of adipose-derived stem cells bearing the low-affinity nerve growth factor receptor. Stem Cells Int. 2013, 2013, 372164. [Google Scholar] [CrossRef]
- Calabrese, G.; Giuffrida, R.; Lo Furno, D.; Parrinello, N.L.; Forte, S.; Gulino, R.; Colarossi, C.; Schinocca, L.R.; Giuffrida, R.; Cardile, V.; et al. Potential Effect of CD271 on Human Mesenchymal Stromal Cell Proliferation and Differentiation. Int. J. Mol. Sci. 2015, 16, 15609–15624. [Google Scholar] [CrossRef]
- Rydén, M.; Dicker, A.; Götherström, C.; Aström, G.; Tammik, C.; Arner, P.; Le Blanc, K. Functional characterization of human mesenchymal stem cell-derived adipocytes. Biochem. Biophys. Res. Commun. 2003, 311, 391–397. [Google Scholar] [CrossRef]
- Dubey, N.K.; Mishra, V.K.; Dubey, R.; Deng, Y.H.; Tsai, F.C.; Deng, W.P. Revisiting the Advances in Isolation, Characterization and Secretome of Adipose-Derived Stromal/Stem Cells. Int. J. Mol. Sci. 2018, 19, 2200. [Google Scholar] [CrossRef]
- Shi, Y.Y.; Nacamuli, R.P.; Salim, A.; Longaker, M.T. The osteogenic potential of adipose-derived mesenchymal cells is maintained with aging. Plast. Reconstr. Surg. 2005, 116, 1686–1696. [Google Scholar] [CrossRef]
- McIntosh, K.R. Evaluation of cellular and humoral immune responses to allogeneic adipose-derived stem/stromal cells. Methods Mol. Biol. 2011, 702, 133–150. [Google Scholar]
- Dhar, S.; Yoon, E.S.; Kachgal, S.; Evans, G.R.D. Long-term maintenance of neuronally differentiated human adipose tissue-derived stem cells. Tissue Eng. 2007, 13, 2625–2632. [Google Scholar] [CrossRef]
- Othmani, A.E.; Rouam, S.; Abbad, A.; Erraoui, C.; Harriba, S.; Boukind, H.; Nourlil, J.; Malka, G.; Mazini, L. Cryopreservation Impacts Cell Functionality of Long Term Expanded Adipose-Derived Stem Cells. J. Stem Cell Res. Ther. 2019, 9, 445. [Google Scholar] [CrossRef]
- Klar, A.S.; Zimoch, J.; Biedermann, T. Skin Tissue Engineering: Application of Adipose-Derived Stem Cells. Biomed. Res. Int. 2017, 2017, 9747010. [Google Scholar] [CrossRef]
- Lee, H.C.; An, S.G.; Lee, H.W.; Park, J.S.; Cha, K.S.; Hong, T.J.; Park, J.H.; Lee, S.Y.; Kim, S.P.; Kim, Y.D.; et al. Safety and effect of adipose tissue-derived stem cell implantation in patients with critical limb ischemia: A pilot study. Circ. J. 2012, 76, 1750–1760. [Google Scholar] [CrossRef]
- Lataillade, J.J.; Doucet, C.; Bey, E.; Carsin, H.; Huet, C.; Clairand, I.; Bottollier-Depois, J.F.; Chapel, A.; Ernou, I.; Gourven, M.; et al. New approach to radiation burn treatment by dosimetry-guided surgery combined with autologous mesenchymal stem cell therapy. Regen. Med. 2007, 2, 785–794. [Google Scholar] [CrossRef]
- García-Olmo, D.; Herreros, D.; De-La-Quintana, P.; Guadalajara, H.; Trébol, J.; Georgiev-Hristov, T.; García-Arranz, M. Adipose-derived stem cells in Crohn’s rectovaginal fistula. Case Rep. Med. 2010, 2010, 961758. [Google Scholar] [CrossRef] [PubMed]
- García-Arranz, M.; Herreros, M.D.; González-Gómez, C.; de la Quintana, P.; Guadalajara, H.; Georgiev-Hristov, T.; Trébol, J.; Garcia-Olmo, D. Treatment of Crohn’s-Related Rectovaginal Fistula with Allogeneic Expanded-Adipose Derived Stem Cells: A Phase I-IIa Clinical Trial. Stem Cells Transl. Med. 2016, 5, 1441–1446. [Google Scholar] [CrossRef]
- Panes, J. Stem Cell Therapy for Perianal Fistulas in Crohn’s Disease. Gastroenterol. Hepatol. 2016, 12, 637–640. [Google Scholar]
- Shi, D.; Wang, D.; Li, X.; Zhang, H.; Che, N.; Lu, Z.; Sun, L. Allogeneic transplantation of umbilical cord-derived mesenchymal stem cells for diffuse alveolar hemorrhage in systemic lupus erythematosus. Clin. Rheumatol. 2012, 31, 841–846. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Kong, W.; Deng, W.; Wang, D.; Feng, X.; Zhao, C.; Hua, B.; Wang, H.; Sun, L. Safety analysis in patients with autoimmune disease receiving allogeneic mesenchymal stem cells infusion: A long-term retrospective study. Stem Cell Res. Ther. 2018, 9, 312. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Bernardo, M.E.; Sgarella, A.; Maccario, R.; Avanzini, M.A.; Ubezio, C.; Minelli, A.; Alvisi, C.; Vanoli, A.; Calliada, F.; et al. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn’s disease. Gut 2011, 60, 788–798. [Google Scholar] [CrossRef]
- Duijvestein, M.; Vos, A.C.W.; Roelofs, H.; Wildenberg, M.E.; Wendrich, B.B.; Verspaget, H.W.; Kooy-Winkelaar, E.M.C.; Koning, F.; Zwaginga, J.J.; Fidder, H.H.; et al. Autologous bone marrow-derived mesenchymal stromal cell treatment for refractory luminal Crohn’s disease: Results of a phase I study. Gut 2010, 59, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Yocum, D.E. Combination therapy: The risks of infection and tumor induction. Springer Semin. Immunopathol. 2001, 23, 63–72. [Google Scholar] [CrossRef]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar] [CrossRef]
- Ra, J.C.; Kang, S.K.; Shin, I.S.; Park, H.G.; Joo, S.A.; Kim, J.G.; Kang, B.C.; Lee, Y.S.; Nakama, K.; Piao, M.; et al. Stem cell treatment for patients with autoimmune disease by systemic infusion of culture-expanded autologous adipose tissue derived mesenchymal stem cells. J. Transl. Med. 2011, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.D.; Vanikar, A.V.; Trivedi, H.L.; Thakkar, U.G.; Gopal, S.C.; Chandra, T. Novel therapy for insulin-dependent diabetes mellitus: Infusion of in vitro-generated insulin-secreting cells. Clin. Exp. Med. 2015, 15, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Dander, E.; Lucchini, G.; Vinci, P.; Introna, M.; Masciocchi, F.; Perseghin, P.; Balduzzi, A.; Bonanomi, S.; Longoni, D.; Gaipa, G.; et al. Mesenchymal stromal cells for the treatment of graft-versus-host disease: Understanding the in vivo biological effect through patient immune monitoring. Leukemia 2012, 26, 1681–1684. [Google Scholar] [CrossRef]
- Bernardo, M.E.; Cometa, A.M.; Pagliara, D.; Vinti, L.; Rossi, F.; Cristantielli, R.; Palumbo, G.; Locatelli, F. Ex vivo expansion of mesenchymal stromal cells. Best Pract. Res. Clin. Haematol. 2011, 24, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Bernardo, M.E.; Fibbe, W.E. Mesenchymal stromal cells and hematopoietic stem cell transplantation. Immunol. Lett. 2015, 168, 215–221. [Google Scholar] [CrossRef]
- Ball, L.M.; Bernardo, M.E.; Roelofs, H.; Lankester, A.; Cometa, A.; Egeler, R.M.; Locatelli, F.; Fibbe, W.E. Cotransplantation of ex vivo expanded mesenchymal stem cells accelerates lymphocyte recovery and may reduce the risk of graft failure in haploidentical hematopoietic stem-cell transplantation. Blood 2007, 110, 2764–2767. [Google Scholar] [CrossRef]
- Wu, Y.M.; Cao, Y.B.; Li, X.H.; Xu, L.X.; Liu, Z.Y.; Liu, B.; Yan, B.; Yang, X.L.; Li, S.W.; Da, W.M.; et al. Transplantation of umbilical cord mesenchymal stem cells combined with haploidentical hematopoietic stem cells for 36 patients with refractory/relapsed myeloid leukemia. J. Exp. Hematol. 2014, 22, 1053–1057. [Google Scholar]
- Wu, Y.; Wang, Z.; Cao, Y.; Xu, L.; Li, X.; Liu, P.; Yan, P.; Liu, Z.; Zhao, D.; Wang, J.; et al. Cotransplantation of haploidentical hematopoietic and umbilical cord mesenchymal stem cells with a myeloablative regimen for refractory/relapsed hematologic malignancy. Ann. Hematol. 2013, 92, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Koç, O.N.; Gerson, S.L.; Cooper, B.W.; Dyhouse, S.M.; Haynesworth, S.E.; Caplan, A.I.; Lazarus, H.M. Rapid hematopoietic recovery after coinfusion of autologous-blood stem cells and culture-expanded marrow mesenchymal stem cells in advanced breast cancer patients receiving high-dose chemotherapy. J. Clin. Oncol. 2000, 18, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Corre, J.; Barreau, C.; Cousin, B.; Chavoin, J.P.; Caton, D.; Fournial, G.; Penicaud, L.; Casteilla, L.; Laharrague, P. Human subcutaneous adipose cells support complete differentiation but not self-renewal of hematopoietic progenitors. J. Cell. Physiol. 2006, 208, 282–288. [Google Scholar] [CrossRef] [PubMed]
- De Toni, F.; Poglio, S.; Youcef, A.B.; Cousin, B.; Pflumio, F.; Bourin, P.; Casteilla, L.; Laharrague, P. Human adipose-derived stromal cells efficiently support hematopoiesis in vitro and in vivo: A key step for therapeutic studies. Stem Cells Dev. 2011, 20, 2127–2138. [Google Scholar] [CrossRef] [PubMed]
- Morgan, E.F.; De Giacomo, A.; Gerstenfeld, L.C. Overview of skeletal repair (fracture healing and its assessment). Methods Mol. Biol. 2014, 1130, 13–31. [Google Scholar]
- Ude, C.C.; Sulaiman, S.B.; Min-Hwei, N.; Hui-Cheng, C.; Ahmad, J.; Yahaya, N.M.; Saim, A.B.; Idrus, R.B.H. Cartilage regeneration by chondrogenic induced adult stem cells in osteoarthritic sheep model. PLoS ONE 2014, 9, e98770. [Google Scholar] [CrossRef]
- Park, Y.B.; Ha, C.W.; Lee, C.H.; Yoon, Y.C.; Park, Y.G. Cartilage Regeneration in Osteoarthritic Patients by a Composite of Allogeneic Umbilical Cord Blood-Derived Mesenchymal Stem Cells and Hyaluronate Hydrogel: Results from a Clinical Trial for Safety and Proof-of-Concept with 7 Years of Extended Follow-Up. Stem Cells Transl. Med. 2017, 6, 613–621. [Google Scholar] [CrossRef]
- Mesimäki, K.; Lindroos, B.; Törnwall, J.; Mauno, J.; Lindqvist, C.; Kontio, R.; Miettinen, S.; Suuronen, R. Novel maxillary reconstruction with ectopic bone formation by GMP adipose stem cells. Int. J. Oral Maxillofac. Surg. 2009, 38, 201–209. [Google Scholar] [CrossRef]
- Lendeckel, S.; Jödicke, A.; Christophis, P.; Heidinger, K.; Wolff, J.; Fraser, J.K.; Hedrick, M.H.; Berthold, L.; Howaldt, H.P. Autologous stem cells (adipose) and fibrin glue used to treat widespread traumatic calvarial defects: Case report. J. Craniomaxillofac. Surg. 2004, 32, 370–373. [Google Scholar] [CrossRef]
- Pak, J.; Lee, J.H.; Park, K.S.; Jeong, B.C.; Lee, S.H. Regeneration of Cartilage in Human Knee Osteoarthritis with Autologous Adipose Tissue-Derived Stem Cells and Autologous Extracellular Matrix. Biores. Open Access 2016, 5, 192–200. [Google Scholar] [CrossRef]
- Ganey, T.; Hutton, W.C.; Moseley, T.; Hedrick, M.; Meisel, H.J. Intervertebral disc repair using adipose tissue-derived stem and regenerative cells: Experiments in a canine model. Spine 2009, 34, 2297–2304. [Google Scholar] [CrossRef]
- Uysal, A.C.; Mizuno, H.; Tobita, M.; Ogawa, R.; Hyakusoku, H. The effect of adipose-derived stem cells on ischemia-reperfusion injury: Immunohistochemical and ultrastructural evaluation. Plast. Reconstr. Surg. 2009, 124, 804–815. [Google Scholar] [CrossRef]
- Ryu, H.H.; Lim, J.H.; Byeon, Y.E.; Park, J.R.; Seo, M.S.; Lee, Y.W.; Kim, W.H.; Kang, K.S.; Kweon, O.K. Functional recovery and neural differentiation after transplantation of allogenic adipose-derived stem cells in a canine model of acute spinal cord injury. J. Vet. Sci. 2009, 10, 273–284. [Google Scholar] [CrossRef]
- Dragoo, J.L.; Carlson, G.; McCormick, F.; Khan-Farooqi, H.; Zhu, M.; Zuk, P.A.; Benhaim, P. Healing full-thickness cartilage defects using adipose-derived stem cells. Tissue Eng. 2007, 13, 1615–1621. [Google Scholar] [CrossRef]
- Puente, B.N.; Kimura, W.; Muralidhar, S.A.; Moon, J.; Amatruda, J.F.; Phelps, K.L.; Grinsfelder, D.; Rothermel, B.A.; Chen, R.; Garcia, J.A.; et al. The oxygen-rich postnatal environment induces cardiomyocyte cell-cycle arrest through DNA damage response. Cell 2014, 157, 565–579. [Google Scholar] [CrossRef]
- Tani, H.; Sadahiro, T.; Ieda, M. Direct Cardiac Reprogramming: A Novel Approach for Heart Regeneration. Int. J. Mol. Sci. 2018, 19, 2629. [Google Scholar] [CrossRef]
- Maioli, M.; Rinaldi, S.; Santaniello, S.; Castagna, A.; Pigliaru, G.; Gualini, S.; Cavallini, C.; Fontani, V.; Ventura, C. Radio electric conveyed fields directly reprogram human dermal skin fibroblasts toward cardiac, neuronal, and skeletal muscle-like lineages. Cell Transplant. 2013, 22, 1227–1235. [Google Scholar] [CrossRef]
- Yun, C.W.; Lee, S.H. Enhancement of Functionality and Therapeutic Efficacy of Cell-Based Therapy Using Mesenchymal Stem Cells for Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 982. [Google Scholar] [CrossRef]
- Maioli, M.; Contini, G.; Santaniello, S.; Bandiera, P.; Pigliaru, G.; Sanna, R.; Rinaldi, S.; Delitala, A.P.; Montella, A.; Bagella, L.; et al. Amniotic fluid stem cells morph into a cardiovascular lineage: Analysis of a chemically induced cardiac and vascular commitment. Drug Des. Dev. Ther. 2013, 7, 1063–1073. [Google Scholar]
- Li, N.; Rochette, L.; Wu, Y.; Rosenblatt-Velin, N. New Insights into the Role of Exosomes in the Heart After Myocardial Infarction. J. Cardiovasc. Transl. Res. 2019, 12, 18–27. [Google Scholar] [CrossRef]
- Moghaddam, A.S.; Afshari, J.T.; Esmaeili, S.A.; Saburi, E.; Joneidi, Z.; Momtazi-Borojeni, A.A. Cardioprotective microRNAs: Lessons from stem cell-derived exosomal microRNAs to treat cardiovascular disease. Atherosclerosis 2019, 285, 1–9. [Google Scholar] [CrossRef]
- Makino, S.; Fukuda, K.; Miyoshi, S.; Konishi, F.; Kodama, H.; Pan, J.; Sano, M.; Takahashi, T.; Hori, S.; Abe, H.; et al. Cardiomyocytes can be generated from marrow stromal cells in vitro. J. Clin. Investig. 1999, 103, 697–705. [Google Scholar] [CrossRef]
- Assmann, A.; Heke, M.; Kröpil, P.; Ptok, L.; Hafner, D.; Ohmann, C.; Martens, A.; Karluβ, A.; Emmert, M.Y.; Kutschka, I.; et al. Laser-supported CD133+ cell therapy in patients with ischemic cardiomyopathy: Initial results from a prospective phase I multicenter trial. PLoS ONE 2014, 9, e101449. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Fang, W.; Ye, F.; Liu, Y.H.; Qian, J.; Shan, S.; Zhang, J.; Chunhua, R.Z.; Liao, L.; Lin, S.; et al. Effect on left ventricular function of intracoronary transplantation of autologous bone marrow mesenchymal stem cell in patients with acute myocardial infarction. Am. J. Cardiol. 2004, 94, 92–95. [Google Scholar] [CrossRef]
- Hare, J.M.; Traverse, J.H.; Henry, T.D.; Dib, N.; Strumpf, R.K.; Schulman, S.P.; Gerstenblith, G.; DeMaria, A.N.; Denktas, A.E.; Gammon, R.S.; et al. A randomized, double-blind, placebo-controlled, dose-escalation study of intravenous adult human mesenchymal stem cells (prochymal) after acute myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2277–2286. [Google Scholar] [CrossRef]
- Li, X.; Hu, Y.; Guo, Y.; Chen, Y.; Guo, D.; Zhou, H.; Zhang, F.; Zhao, Q. Safety and efficacy of intracoronary human umbilical cord-derived mesenchymal stem cell treatment for very old patients with coronary chronic total occlusion. Curr. Pharm. Des. 2015, 21, 1426–1432. [Google Scholar] [CrossRef]
- Bartolucci, J.; Verdugo, F.J.; González, P.L.; Larrea, R.E.; Abarzua, E.; Goset, C.; Rojo, P.; Palma, I.; Lamich, R.; Pedreros, P.A.; et al. Safety and Efficacy of the Intravenous Infusion of Umbilical Cord Mesenchymal Stem Cells in Patients with Heart Failure: A Phase 1/2 Randomized Controlled Trial (RIMECARD Trial [Randomized Clinical Trial of Intravenous Infusion Umbilical Cord Mesenchymal Stem Cells on Cardiopathy]). Circ. Res. 2017, 121, 1192–1204. [Google Scholar]
- Kastrup, J.; Haack-Sørensen, M.; Juhl, M.; Harary Søndergaard, R.; Follin, B.; Drozd Lund, L.; Mønsted Johansen, E.; Ali Qayyum, A.; Bruun Mathiasen, A.; Jørgensen, E.; et al. Cryopreserved Off-the-Shelf Allogeneic Adipose-Derived Stromal Cells for Therapy in Patients with Ischemic Heart Disease and Heart Failure-A Safety Study. Stem Cells Transl. Med. 2017, 6, 1963–1971. [Google Scholar] [CrossRef]
- Henry, T.D.; Pepine, C.J.; Lambert, C.R.; Traverse, J.H.; Schatz, R.; Costa, M.; Povsic, T.J.; David Anderson, R.; Willerson, J.T.; Kesten, S.; et al. The Athena trials: Autologous adipose-derived regenerative cells for refractory chronic myocardial ischemia with left ventricular dysfunction. Catheter. Cardiovasc. Interv. 2017, 89, 169–177. [Google Scholar] [CrossRef]
- Kastrup, J.; Schou, M.; Gustafsson, I.; Nielsen, O.W.; Møgelvang, R.; Kofoed, K.F.; Kragelund, C.; Hove, J.D.; Fabricius-Bjerre, A.; Heitman, M.; et al. Rationale and Design of the First Double-Blind, Placebo-Controlled Trial with Allogeneic Adipose Tissue-Derived Stromal Cell Therapy in Patients with Ischemic Heart Failure: A Phase II Danish Multicentre Study. Stem Cells Int. 2017, 2017, 8506370. [Google Scholar] [CrossRef]
- Yamada, Y.; Wang, X.D.; Yokoyama, S.; Fukuda, N.; Takakura, N. Cardiac progenitor cells in brown adipose tissue repaired damaged myocardium. Biochem. Biophys. Res. Commun. 2006, 342, 662–670. [Google Scholar] [CrossRef]
- Li, P.; Cui, K.; Zhang, B.; Wang, Z.; Shen, Y.; Wang, X.; Zhang, J.; Tong, F.; Li, S. Transplantation of human umbilical cord-derived mesenchymal stems cells for the treatment of Becker muscular dystrophy in affected pedigree members. Int. J. Mol. Med. 2015, 35, 1051–1057. [Google Scholar] [CrossRef]
- Rajput, B.S.; Chakrabarti, S.K.; Dongare, V.S.; Ramirez, C.M.; Deb, K.D. Human Umbilical Cord Mesenchymal Stem Cells in the Treatment of Duchenne Muscular Dystrophy: Safety and Feasibility Study in India. J. Stem Cells 2015, 10, 141–156. [Google Scholar]
- Siegel, G.; Krause, P.; Wöhrle, S.; Nowak, P.; Ayturan, M.; Kluba, T.; Brehm, B.R.; Neumeister, B.; Köhler, D.; Rosenberger, P.; et al. Bone marrow-derived human mesenchymal stem cells express cardiomyogenic proteins but do not exhibit functional cardiomyogenic differentiation potential. Stem Cells Dev. 2012, 21, 2457–2470. [Google Scholar] [CrossRef]
- Sato, T.; Iso, Y.; Uyama, T.; Kawachi, K.; Wakabayashi, K.; Omori, Y.; Soda, T.; Shoji, M.; Koba, S.; Yokoyama, S.I.; et al. Coronary vein infusion of multipotent stromal cells from bone marrow preserves cardiac function in swine ischemic cardiomyopathy via enhanced neovascularization. Lab. Investig. 2011, 91, 553–564. [Google Scholar] [CrossRef]
- Vela, D.C.; Silva, G.V.; Assad, J.A.R.; Sousa, A.L.S.; Coulter, S.; Fernandes, M.R.; Perin, E.C.; Willerson, J.T.; Buja, L.M. Histopathological study of healing after allogenic mesenchymal stem cell delivery in myocardial infarction in dogs. J. Histochem. Cytochem. 2009, 57, 167–176. [Google Scholar] [CrossRef]
- Alexeev, V.; Arita, M.; Donahue, A.; Bonaldo, P.; Chu, M.L.; Igoucheva, O. Human adipose-derived stem cell transplantation as a potential therapy for collagen VI-related congenital muscular dystrophy. Stem Cell Res. Ther. 2014, 5, 21. [Google Scholar] [CrossRef]
- DiRocco, J.D.; Pavone, L.A.; Carney, D.E.; Lutz, C.J.; Gatto, L.A.; Landas, S.K.; Nieman, G.F. Dynamic alveolar mechanics in four models of lung injury. Intensive Care Med. 2006, 32, 140–148. [Google Scholar] [CrossRef]
- Rodriguez, A.M.; Pisani, D.; Dechesne, C.A.; Turc-Carel, C.; Kurzenne, J.Y.; Wdziekonski, B.; Villageois, A.; Bagnis, C.; Breittmayer, J.P.; Groux, H.; et al. Transplantation of a multipotent cell population from human adipose tissue induces dystrophin expression in the immunocompetent mdx mouse. J. Exp. Med. 2005, 201, 1397–1405. [Google Scholar] [CrossRef]
- Merkle, F.T.; Fuentealba, L.C.; Sanders, T.A.; Magno, L.; Kessaris, N.; Alvarez-Buylla, A. Adult neural stem cells in distinct microdomains generate previously unknown interneuron types. Nat. Neurosci. 2014, 17, 207–214. [Google Scholar] [CrossRef]
- Kim, Y.J.; Hwang, S.H.; Cho, H.H.; Shin, K.K.; Bae, Y.C.; Jung, J.S. MicroRNA 21 regulates the proliferation of human adipose tissue-derived mesenchymal stem cells and high-fat diet-induced obesity alters microRNA 21 expression in white adipose tissues. J. Cell. Physiol. 2012, 227, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Weimann, J.M.; Charlton, C.A.; Brazelton, T.R.; Hackman, R.C.; Blau, H.M. Contribution of transplanted bone marrow cells to Purkinje neurons in human adult brains. Proc. Natl. Acad. Sci. USA 2003, 100, 2088–2093. [Google Scholar] [CrossRef]
- Canesi, M.; Giordano, R.; Lazzari, L.; Isalberti, M.; Isaias, I.U.; Benti, R.; Rampini, P.; Marotta, G.; Colombo, A.; Cereda, E.; et al. Finding a new therapeutic approach for no-option Parkinsonisms: Mesenchymal stromal cells for progressive supranuclear palsy. J. Transl. Med. 2016, 14, 127. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, G.K.; Kondziolka, D.; Wechsler, L.R.; Lunsford, L.D.; Coburn, M.L.; Billigen, J.B.; Kim, A.S.; Johnson, J.N.; Bates, D.; King, B.; et al. Clinical Outcomes of Transplanted Modified Bone Marrow-Derived Mesenchymal Stem Cells in Stroke: A Phase 1/2a Study. Stroke 2016, 47, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O.; et al. Safety and Clinical Effects of Mesenchymal Stem Cells Secreting Neurotrophic Factor Transplantation in Patients with Amyotrophic Lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Harris, V.K.; Stark, J.; Vyshkina, T.; Blackshear, L.; Joo, G.; Stefanova, V.; Sara, G.; Sadiq, S.A. Phase I Trial of Intrathecal Mesenchymal Stem Cell-derived Neural Progenitors in Progressive Multiple Sclerosis. EBioMedicine 2018, 29, 23–30. [Google Scholar] [CrossRef]
- Yamout, B.; Hourani, R.; Salti, H.; Barada, W.; El-Hajj, T.; Al-Kutoubi, A.; Herlopian, A.; Baz, E.K.; Mahfouz, R.; Khalil-Hamdan, R.; et al. Bone marrow mesenchymal stem cell transplantation in patients with multiple sclerosis: A pilot study. J. Neuroimmunol. 2010, 227, 185–189. [Google Scholar] [CrossRef]
- Dahbour, S.; Jamali, F.; Alhattab, D.; Al-Radaideh, A.; Ababneh, O.; Al-Ryalat, N.; Al-Bdour, M.; Hourani, B.; Msallam, M.; Rasheed, M.; et al. Mesenchymal stem cells and conditioned media in the treatment of multiple sclerosis patients: Clinical, ophthalmological and radiological assessments of safety and efficacy. CNS Neurosci. Ther. 2017, 23, 866–874. [Google Scholar] [CrossRef]
- Li, X.; Bai, J.; Ji, X.; Li, R.; Xuan, Y.; Wang, Y. Comprehensive characterization of four different populations of human mesenchymal stem cells as regards their immune properties, proliferation and differentiation. Int. J. Mol. Med. 2014, 34, 695–704. [Google Scholar] [CrossRef]
- Kalbermatten, D.F.; Schaakxs, D.; Kingham, P.J.; Wiberg, M. Neurotrophic activity of human adipose stem cells isolated from deep and superficial layers of abdominal fat. Cell Tissue Res. 2011, 344, 251–260. [Google Scholar] [CrossRef]
- Rehman, J.; Traktuev, D.; Li, J.; Merfeld-Clauss, S.; Temm-Grove, C.J.; Bovenkerk, J.E.; Pell, C.L.; Johnstone, B.H.; Considine, R.V.; March, K.L. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation 2004, 109, 1292–1298. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, L.; Zhong, J.; Gu, H.; Feng, D.; Johnstone, B.H.; March, K.L.; Farlow, M.R.; Du, Y. Adipose stromal cells-secreted neuroprotective media against neuronal apoptosis. Neurosci. Lett. 2009, 462, 76–79. [Google Scholar] [CrossRef]
- Ikegame, Y.; Yamashita, K.; Hayashi, S.I.; Mizuno, H.; Tawada, M.; You, F.; Yamada, K.; Tanaka, Y.; Egashira, Y.; Nakashima, S.; et al. Comparison of mesenchymal stem cells from adipose tissue and bone marrow for ischemic stroke therapy. Cytotherapy 2011, 13, 675–685. [Google Scholar] [CrossRef]
- Leu, S.; Lin, Y.C.; Yuen, C.M.; Yen, C.H.; Kao, Y.H.; Sun, C.K.; Yip, H.K. Adipose-derived mesenchymal stem cells markedly attenuate brain infarct size and improve neurological function in rats. J. Transl. Med. 2010, 8, 63. [Google Scholar] [CrossRef]
- Qiao, L.; Huang, F.; Zhao, M.; Xie, J.; Shi, J.; Wang, J.; Lin, X.; Zuo, H.; Wang, Y.; Geng, T. A two-year follow-up study of cotransplantation with neural stem/progenitor cells and mesenchymal stromal cells in ischemic stroke patients. Cell Transplant. 2014, 23, 65–72. [Google Scholar] [CrossRef]
- Kim, H.Y.; Kim, H.; Oh, K.W.; Oh, S.I.; Koh, S.H.; Baik, W.; Noh, M.Y.; Kim, K.S.; Kim, S.H. Biological markers of mesenchymal stromal cells as predictors of response to autologous stem cell transplantation in patients with amyotrophic lateral sclerosis: An investigator-initiated trial and in vivo study. Stem Cells 2014, 32, 2724–2731. [Google Scholar] [CrossRef]
- San-Marina, S.; Voss, S.; Crespo-Diaz, R.; Wyles, C.; Behfar, A.; Stalboeger, P.; Janus, J.R. Adipose-Derived Mesenchymal Stem Cell Features in Patients with a History of Head and Neck Radiation. Laryngoscope Investig. Otolaryngol. 2016, 1, 36–41. [Google Scholar] [CrossRef]
- Maria, O.M.; Shalaby, M.; Syme, A.; Eliopoulos, N.; Muanza, T. Adipose mesenchymal stromal cells minimize and repair radiation-induced oral mucositis. Cytotherapy 2016, 18, 1129–1145. [Google Scholar] [CrossRef]
- Danan, D.; Lehman, C.E.; Mendez, R.E.; Langford, B.; Koors, P.D.; Dougherty, M.I.; Peirce, S.M.; Gioeli, D.G.; Jameson, M.J. Effect of Adipose-Derived Stem Cells on Head and Neck Squamous Cell Carcinoma. Otolaryngol. Head Neck Surg. 2018, 158, 882–888. [Google Scholar] [CrossRef]
- Riccobono, D.; Nikovics, K.; François, S.; Favier, A.L.; Jullien, N.; Schrock, G.; Scherthan, H.; Drouet, M. First Insights into the M2 Inflammatory Response After Adipose-Tissue-Derived Stem Cell Injections in Radiation-Injured Muscles. Health Phys. 2018, 115, 37–48. [Google Scholar] [CrossRef]
- Van de Putte, D.; Demarquay, C.; Van Daele, E.; Moussa, L.; Vanhove, C.; Benderitter, M.; Ceelen, W.; Pattyn, P.; Mathieu, N. Adipose-Derived Mesenchymal Stromal Cells Improve the Healing of Colonic Anastomoses Following High Dose of Irradiation Through Anti-Inflammatory and Angiogenic Processes. Cell Transplant. 2017, 26, 1919–1930. [Google Scholar] [CrossRef]
- Grønhøj, C.; Jensen, D.H.; Vester-Glowinski, P.; Jensen, S.B.; Bardow, A.; Oliveri, R.S.; Fog, L.M.; Specht, L.; Thomsen, C.; Darkner, S.; et al. Safety and Efficacy of Mesenchymal Stem Cells for Radiation-Induced Xerostomia: A Randomized, Placebo-Controlled Phase 1/2 Trial (MESRIX). Int. J. Radiat. Oncol. Biol. Phys. 2018, 101, 581–592. [Google Scholar] [CrossRef]
- Ebrahimian, T.G.; Pouzoulet, F.; Squiban, C.; Buard, V.; André, M.; Cousin, B.; Gourmelon, P.; Benderitter, M.; Casteilla, L.; Tamarat, R. Cell therapy based on adipose tissue-derived stromal cells promotes physiological and pathological wound healing. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 503–510. [Google Scholar] [CrossRef]
- Haubner, F.; Gassner, H.G. Potential of adipose-derived stem cells concerning the treatment of wound healing complications after radiotherapy. HNO 2015, 63, 111–117. [Google Scholar] [CrossRef]
- Singh, M.; Alavi, A.; Wong, R.; Akita, S. Radiodermatitis: A Review of Our Current Understanding. Am. J. Clin. Dermatol. 2016, 17, 277–292. [Google Scholar] [CrossRef]
- Mashiko, T.; Takada, H.; Wu, S.H.; Kanayama, K.; Feng, J.; Tashiro, K.; Asahi, R.; Sunaga, A.; Hoshi, K.; Kurisaki, A.; et al. Therapeutic effects of a recombinant human collagen peptide bioscaffold with human adipose-derived stem cells on impaired wound healing after radiotherapy. J. Tissue Eng. Regen. Med. 2018, 12, 1186–1194. [Google Scholar] [CrossRef]
- Wu, S.H.; Shirado, T.; Mashiko, T.; Feng, J.; Asahi, R.; Kanayama, K.; Mori, M.; Chi, D.; Sunaga, A.; Sarukawa, S.; et al. Therapeutic Effects of Human Adipose-Derived Products on Impaired Wound Healing in Irradiated Tissue. Plast. Reconstr. Surg. 2018, 142, 383–391. [Google Scholar] [CrossRef]
- Rochette, L.; Guenancia, C.; Gudjoncik, A.; Hachet, O.; Zeller, M.; Cottin, Y.; Vergely, C. Anthracyclines/trastuzumab: New aspects of cardiotoxicity and molecular mechanisms. Trends Pharmacol. Sci. 2015, 36, 326–348. [Google Scholar] [CrossRef]
- Dalloz, F.; Maingon, P.; Cottin, Y.; Briot, F.; Horiot, J.C.; Rochette, L. Effects of combined irradiation and doxorubicin treatment on cardiac function and antioxidant defenses in the rat. Free Radic. Biol. Med. 1999, 26, 785–800. [Google Scholar] [CrossRef]
- Bey, E.; Prat, M.; Duhamel, P.; Benderitter, M.; Brachet, M.; Trompier, F.; Battaglini, P.; Ernou, I.; Boutin, L.; Gourven, M.; et al. Emerging therapy for improving wound repair of severe radiation burns using local bone marrow-derived stem cell administrations. Wound Repair Regen. 2010, 18, 50–58. [Google Scholar] [CrossRef]
- Falanga, V.; Iwamoto, S.; Chartier, M.; Yufit, T.; Butmarc, J.; Kouttab, N.; Shrayer, D.; Carson, P. Autologous bone marrow-derived cultured mesenchymal stem cells delivered in a fibrin spray accelerate healing in murine and human cutaneous wounds. Tissue Eng. 2007, 13, 1299–1312. [Google Scholar] [CrossRef]
- Qin, H.L.; Zhu, X.H.; Zhang, B.; Zhou, L.; Wang, W.Y. Clinical Evaluation of Human Umbilical Cord Mesenchymal Stem Cell Transplantation After Angioplasty for Diabetic Foot. Exp. Clin. Endocrinol. Diabetes 2016, 124, 497–503. [Google Scholar] [CrossRef]
- Valbonesi, M.; Giannini, G.; Migliori, F.; Dalla Costa, R.; Dejana, A.M. Cord blood (CB) stem cells for wound repair. Preliminary report of 2 cases. Transfus. Apher. Sci. 2004, 30, 153–156. [Google Scholar] [CrossRef]
- Anderi, R.; Makdissy, N.; Azar, A.; Rizk, F.; Hamade, A. Cellular therapy with human autologous adipose-derived adult cells of stromal vascular fraction for alopecia areata. Stem Cell Res. Ther. 2018, 9, 141. [Google Scholar] [CrossRef]
- Trojahn Kølle, S.F.; Oliveri, R.S.; Glovinski, P.V.; Elberg, J.J.; Fischer-Nielsen, A.; Drzewiecki, K.T. Importance of mesenchymal stem cells in autologous fat grafting: A systematic review of existing studies. J. Plast. Surg. Hand Surg. 2012, 46, 59–68. [Google Scholar] [CrossRef]
- Shin, H.; Ryu, H.H.; Kwon, O.; Park, B.S.; Jo, S.J. Clinical use of conditioned media of adipose tissue-derived stem cells in female pattern hair loss: A retrospective case series study. Int. J. Dermatol. 2015, 54, 730–735. [Google Scholar] [CrossRef]
- Foubert, P.; Barillas, S.; Gonzalez, A.D.; Alfonso, Z.; Zhao, S.; Hakim, I.; Meschter, C.; Tenenhaus, M.; Fraser, J.K. Uncultured adipose-derived regenerative cells (ADRCs) seeded in collagen scaffold improves dermal regeneration, enhancing early vascularization and structural organization following thermal burns. Burns 2015, 41, 1504–1516. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Jang, K.A.; Sung, J.H.; Park, J.S.; Kwon, Y.H.; Kim, K.J.; Kim, W.S. Adipose-derived stem cells and their secretory factors as a promising therapy for skin aging. Dermatol. Surg. 2008, 34, 1323–1326. [Google Scholar] [PubMed]
- Rigotti, G.; Marchi, A.; Galiè, M.; Baroni, G.; Benati, D.; Krampera, M.; Pasini, A.; Sbarbati, A. Clinical treatment of radiotherapy tissue damage by lipoaspirate transplant: A healing process mediated by adipose-derived adult stem cells. Plast. Reconstr. Surg. 2007, 119, 1409–1422. [Google Scholar] [CrossRef] [PubMed]
- Claudio-da-Silva, C.; Baptista, L.S.; Carias, R.B.V.; Menezes Neto, H. da C.; Borojevic, R. Autologous mesenchymal stem cells culture from adipose tissue for treatment of facial rhytids. Rev. Col. Bras. Cir. 2009, 36, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Sterodimas, A.; de Faria, J.; Nicaretta, B.; Boriani, F. Autologous fat transplantation versus adipose-derived stem cell-enriched lipografts: A study. Aesthetic Surg. J. 2011, 31, 682–693. [Google Scholar] [CrossRef]
- Satti, H.S.; Waheed, A.; Ahmed, P.; Ahmed, K.; Akram, Z.; Aziz, T.; Satti, T.M.; Shahbaz, N.; Khan, M.A.; Malik, S.A. Autologous mesenchymal stromal cell transplantation for spinal cord injury: A Phase I pilot study. Cytotherapy 2016, 18, 518–522. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Bonilla, C.; Fernández, C.; Rubio, J.J.; Mucientes, J.; Rodriguez, B.; Blanco, E.; Donis, L. Progressive increase in brain glucose metabolism after intrathecal administration of autologous mesenchymal stromal cells in patients with diffuse axonal injury. Cytotherapy 2017, 19, 88–94. [Google Scholar] [CrossRef]
- Vaquero, J.; Zurita, M.; Rico, M.A.; Bonilla, C.; Aguayo, C.; Montilla, J.; Bustamante, S.; Carballido, J.; Marin, E.; Martinez, F.; et al. An approach to personalized cell therapy in chronic complete paraplegia: The Puerta de Hierro phase I/II clinical trial. Cytotherapy 2016, 18, 1025–1036. [Google Scholar] [CrossRef]
- Mendonça, M.V.P.; Larocca, T.F.; de Freitas Souza, B.S.; Villarreal, C.F.; Silva, L.F.M.; Matos, A.C.; Novaes, M.A.; Bahia, C.M.P.; de Oliveira Melo Martinez, A.C.; Kaneto, C.M.; et al. Safety and neurological assessments after autologous transplantation of bone marrow mesenchymal stem cells in subjects with chronic spinal cord injury. Stem Cell Res. Ther. 2014, 5, 126. [Google Scholar] [CrossRef]
- Llufriu, S.; Sepúlveda, M.; Blanco, Y.; Marín, P.; Moreno, B.; Berenguer, J.; Gabilondo, I.; Martínez-Heras, E.; Sola-Valls, N.; Arnaiz, J.A.; et al. Randomized placebo-controlled phase II trial of autologous mesenchymal stem cells in multiple sclerosis. PLoS ONE 2014, 9, e113936. [Google Scholar] [CrossRef]
- Shichinohe, H.; Kawabori, M.; Iijima, H.; Teramoto, T.; Abumiya, T.; Nakayama, N.; Kazumata, K.; Terasaka, S.; Arato, T.; Houkin, K. Research on advanced intervention using novel bone marrOW stem cell (RAINBOW): A study protocol for a phase I, open-label, uncontrolled, dose-response trial of autologous bone marrow stromal cell transplantation in patients with acute ischemic stroke. BMC Neurol. 2017, 17, 179. [Google Scholar] [CrossRef]
- Hlebokazov, F.; Dakukina, T.; Ihnatsenko, S.; Kosmacheva, S.; Potapnev, M.; Shakhbazau, A.; Goncharova, N.; Makhrov, M.; Korolevich, P.; Misyuk, N.; et al. Treatment of refractory epilepsy patients with autologous mesenchymal stem cells reduces seizure frequency: An open label study. Adv. Med. Sci. 2017, 62, 273–279. [Google Scholar] [CrossRef]
- Liu, X.; Fu, X.; Dai, G.; Wang, X.; Zhang, Z.; Cheng, H.; Zheng, P.; An, Y. Comparative analysis of curative effect of bone marrow mesenchymal stem cell and bone marrow mononuclear cell transplantation for spastic cerebral palsy. J. Transl. Med. 2017, 15, 48. [Google Scholar] [CrossRef]
- Karussis, D.; Karageorgiou, C.; Vaknin-Dembinsky, A.; Gowda-Kurkalli, B.; Gomori, J.M.; Kassis, I.; Bulte, J.W.M.; Petrou, P.; Ben-Hur, T.; Abramsky, O.; et al. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol. 2010, 67, 1187–1194. [Google Scholar] [CrossRef]
- Mazzini, L.; Vercelli, A.; Ferrero, I.; Boido, M.; Cantello, R.; Fagioli, F. Transplantation of mesenchymal stem cells in ALS. Prog. Brain Res. 2012, 201, 333–359. [Google Scholar] [PubMed]
- Wang, L.; Lu, M. Regulation and direction of umbilical cord blood mesenchymal stem cells to adopt neuronal fate. Int. J. Neurosci. 2014, 124, 149–159. [Google Scholar] [CrossRef]
- Oh, K.W.; Moon, C.; Kim, H.Y.; Oh, S.I.; Park, J.; Lee, J.H.; Chang, I.Y.; Kim, K.S.; Kim, S.H. Phase I trial of repeated intrathecal autologous bone marrow-derived mesenchymal stromal cells in amyotrophic lateral sclerosis. Stem Cells Transl. Med. 2015, 4, 590–597. [Google Scholar] [CrossRef]
- Moviglia, G.A.; Fernandez Viña, R.; Brizuela, J.A.; Saslavsky, J.; Vrsalovic, F.; Varela, G.; Bastos, F.; Farina, P.; Etchegaray, G.; Barbieri, M.; et al. Combined protocol of cell therapy for chronic spinal cord injury. Report on the electrical and functional recovery of two patients. Cytotherapy 2006, 8, 202–209. [Google Scholar] [CrossRef]
- Syková, E.; Rychmach, P.; Drahorádová, I.; Konrádová, Š.; Růžičková, K.; Voříšek, I.; Forostyak, S.; Homola, A.; Bojar, M. Transplantation of Mesenchymal Stromal Cells in Patients with Amyotrophic Lateral Sclerosis: Results of Phase I/IIa Clinical Trial. Cell Transplant. 2017, 26, 647–658. [Google Scholar] [CrossRef]
- Geffner, L.F.; Santacruz, P.; Izurieta, M.; Flor, L.; Maldonado, B.; Auad, A.H.; Montenegro, X.; Gonzalez, R.; Silva, F. Administration of autologous bone marrow stem cells into spinal cord injury patients via multiple routes is safe and improves their quality of life: Comprehensive case studies. Cell Transplant. 2008, 17, 1277–1293. [Google Scholar] [CrossRef] [PubMed]
- Pal, R.; Venkataramana, N.K.; Bansal, A.; Balaraju, S.; Jan, M.; Chandra, R.; Dixit, A.; Rauthan, A.; Murgod, U.; Totey, S. Ex vivo-expanded autologous bone marrow-derived mesenchymal stromal cells in human spinal cord injury/paraplegia: A pilot clinical study. Cytotherapy 2009, 11, 897–911. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Guan, L.X.; Zhang, K.; Zhang, Q.; Dai, L.J. A combined procedure to deliver autologous mesenchymal stromal cells to patients with traumatic brain injury. Cytotherapy 2008, 10, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.J.; Kim, J.; Kim, M.Y.; Bae, Y.S.; Ryu, S.H.; Lee, T.G.; Kim, J.H. Proteomic analysis of tumor necrosis factor-alpha-induced secretome of human adipose tissue-derived mesenchymal stem cells. J. Proteome Res. 2010, 9, 1754–1762. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Liu, X.; Hua, R.; Dai, G.; Wang, X.; Gao, J.; An, Y. Clinical observation of umbilical cord mesenchymal stem cell transplantation in treatment for sequelae of thoracolumbar spinal cord injury. J. Transl. Med. 2014, 12, 253. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhu, W.; Zhu, J.; Wu, L.; Xu, G.; Liu, X. Feasibility of delivering mesenchymal stem cells via catheter to the proximal end of the lesion artery in patients with stroke in the territory of the middle cerebral artery. Cell Transplant. 2013, 22, 2291–2298. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Cheng, H.; Dai, G.; Wang, X.; Hua, R.; Liu, X.; Wang, P.; Chen, G.; Yue, W.; An, Y. Umbilical cord mesenchymal stem cell transplantation significantly improves neurological function in patients with sequelae of traumatic brain injury. Brain Res. 2013, 1532, 76–84. [Google Scholar] [CrossRef]
- Li, J.F.; Zhang, D.J.; Geng, T.; Chen, L.; Huang, H.; Yin, H.L.; Zhang, Y.; Lou, J.Y.; Cao, B.; Wang, Y.L. The potential of human umbilical cord-derived mesenchymal stem cells as a novel cellular therapy for multiple sclerosis. Cell Transplant. 2014, 23, 113–122. [Google Scholar] [CrossRef]
- Nakamura, K.; Mieda, T.; Suto, N.; Matsuura, S.; Hirai, H. Mesenchymal stem cells as a potential therapeutic tool for spinocerebellar ataxia. Cerebellum 2015, 14, 165–170. [Google Scholar] [CrossRef]
- Cordes, A.L.; Jahn, K.; Hass, R.; Schwabe, K.; Weissinger, E.M.; Ganser, A.; Götz, F.; Dengler, R.; Krauss, J.K.; Petri, S. Intramedullary spinal cord implantation of human CD34+ umbilical cord-derived cells in ALS. Amyotroph. Lateral Scler. 2011, 12, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Hur, J.W.; Cho, T.H.; Park, D.H.; Lee, J.B.; Park, J.Y.; Chung, Y.G. Intrathecal transplantation of autologous adipose-derived mesenchymal stem cells for treating spinal cord injury: A human trial. J. Spinal Cord Med. 2016, 39, 655–664. [Google Scholar] [CrossRef]
- Díez-Tejedor, E.; Gutiérrez-Fernández, M.; Martínez-Sánchez, P.; Rodríguez-Frutos, B.; Ruiz-Ares, G.; Lara, M.L.; Gimeno, B.F. Reparative therapy for acute ischemic stroke with allogeneic mesenchymal stem cells from adipose tissue: A safety assessment: A phase II randomized, double-blind, placebo-controlled, single-center, pilot clinical trial. J. Stroke Cerebrovasc. Dis. 2014, 23, 2694–2700. [Google Scholar] [CrossRef]
- Tsai, Y.A.; Liu, R.S.; Lirng, J.F.; Yang, B.H.; Chang, C.H.; Wang, Y.C.; Wu, Y.S.; Ho, J.H.C.; Lee, O.K.; Soong, B.W. Treatment of Spinocerebellar Ataxia with Mesenchymal Stem Cells: A Phase I/IIa Clinical Study. Cell Transplant. 2017, 26, 503–512. [Google Scholar] [CrossRef]
- Fernández, O.; Izquierdo, G.; Fernández, V.; Leyva, L.; Reyes, V.; Guerrero, M.; León, A.; Arnaiz, C.; Navarro, G.; Páramo, M.D.; et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS ONE 2018, 13, e0195891. [Google Scholar] [CrossRef]
- Di Summa, P.G.; Kingham, P.J.; Raffoul, W.; Wiberg, M.; Terenghi, G.; Kalbermatten, D.F. Adipose-derived stem cells enhance peripheral nerve regeneration. J. Plast. Reconstr. Aesthetic Surg. 2010, 63, 1544–1552. [Google Scholar] [CrossRef]
- Staff, N.P.; Madigan, N.N.; Morris, J.; Jentoft, M.; Sorenson, E.J.; Butler, G.; Gastineau, D.; Dietz, A.; Windebank, A.J. Safety of intrathecal autologous adipose-derived mesenchymal stromal cells in patients with ALS. Neurology 2016, 87, 2230–2234. [Google Scholar] [CrossRef]
- Gu, F.; Wang, D.; Zhang, H.; Feng, X.; Gilkeson, G.S.; Shi, S.; Sun, L. Allogeneic mesenchymal stem cell transplantation for lupus nephritis patients refractory to conventional therapy. Clin. Rheumatol. 2014, 33, 1611–1619. [Google Scholar] [CrossRef]
- Sun, L.; Wang, D.; Liang, J.; Zhang, H.; Feng, X.; Wang, H.; Hua, B.; Liu, B.; Ye, S.; Hu, X.; et al. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010, 62, 2467–2475. [Google Scholar] [CrossRef]
- Kong, D.; Zhuang, X.; Wang, D.; Qu, H.; Jiang, Y.; Li, X.; Wu, W.; Xiao, J.; Liu, X.; Liu, J.; et al. Umbilical cord mesenchymal stem cell transfusion ameliorated hyperglycemia in patients with type 2 diabetes mellitus. Clin. Lab. 2014, 60, 1969–1976. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Wu, Z.; Xu, X.; Liao, L.; Chen, J.; Huang, L.; Wu, W.; Luo, F.; Wu, C.; Pugliese, A.; et al. Umbilical Cord Mesenchymal Stromal Cell with Autologous Bone Marrow Cell Transplantation in Established Type 1 Diabetes: A Pilot Randomized Controlled Open-Label Clinical Study to Assess Safety and Impact on Insulin Secretion. Diabetes Care 2016, 39, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Dave, S.D.; Vanikar, A.V.; Trivedi, H.L. Co-infusion of adipose tissue derived mesenchymal stem cell-differentiated insulin-making cells and haematopoietic cells with renal transplantation: A novel therapy for type 1 diabetes mellitus with end-stage renal disease. BMJ Case Rep. 2013, 2013, 009901. [Google Scholar] [CrossRef]
- Riordan, N.H.; Ichim, T.E.; Min, W.P.; Wang, H.; Solano, F.; Lara, F.; Alfaro, M.; Rodriguez, J.P.; Harman, R.J.; Patel, A.N.; et al. Non-expanded adipose stromal vascular fraction cell therapy for multiple sclerosis. J. Transl. Med. 2009, 7, 29. [Google Scholar] [CrossRef]
- Liang, J.; Zhang, H.; Hua, B.; Wang, H.; Lu, L.; Shi, S.; Hou, Y.; Zeng, X.; Gilkeson, G.S.; Sun, L. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann. Rheum. Dis. 2010, 69, 1423–1429. [Google Scholar] [CrossRef]
- Fodor, P.B.; Paulseth, S.G. Adipose Derived Stromal Cell (ADSC) Injections for Pain Management of Osteoarthritis in the Human Knee Joint. Aesthetic Surg. J. 2016, 36, 229–236. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, H.Y.; Lyu, Z.S.; Cao, X.N.; Shi, M.M.; Wen, Q.; Tang, F.F.; Wang, Y.; Xu, L.P.; Zhang, X.H.; et al. Dysfunctional Bone Marrow Mesenchymal Stem Cells in Patients with Poor Graft Function after Allogeneic Hematopoietic Stem Cell Transplantation. Biol. Blood Marrow Transplant. 2018, 24, 1981–1989. [Google Scholar] [CrossRef]
- Koh, Y.G.; Kwon, O.R.; Kim, Y.S.; Choi, Y.J.; Tak, D.H. Adipose-Derived Mesenchymal Stem Cells with Microfracture Versus Microfracture Alone: 2-Year Follow-up of a Prospective Randomized Trial. Arthroscopy 2016, 32, 97–109. [Google Scholar] [CrossRef] [PubMed]
- Koh, Y.G.; Choi, Y.J.; Kwon, S.K.; Kim, Y.S.; Yeo, J.E. Clinical results and second-look arthroscopic findings after treatment with adipose-derived stem cells for knee osteoarthritis. Knee Surg. Sports Traumatol. Arthrosc. 2015, 23, 1308–1316. [Google Scholar] [CrossRef] [PubMed]
- Marcacci, M.; Kon, E.; Moukhachev, V.; Lavroukov, A.; Kutepov, S.; Quarto, R.; Mastrogiacomo, M.; Cancedda, R. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007, 13, 947–955. [Google Scholar] [CrossRef]
- Yamada, Y.; Nakamura, S.; Ito, K.; Umemura, E.; Hara, K.; Nagasaka, T.; Abe, A.; Baba, S.; Furuichi, Y.; Izumi, Y.; et al. Injectable bone tissue engineering using expanded mesenchymal stem cells. Stem Cells 2013, 31, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Quarto, R.; Mastrogiacomo, M.; Cancedda, R.; Kutepov, S.M.; Mukhachev, V.; Lavroukov, A.; Kon, E.; Marcacci, M. Repair of large bone defects with the use of autologous bone marrow stromal cells. N. Engl. J. Med. 2001, 344, 385–386. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Qu, Z.; Yin, X.; Shang, C.; Ao, Q.; Gu, Y.; Liu, Y. Efficacy of umbilical cord-derived mesenchymal stem cell-based therapy for osteonecrosis of the femoral head: A three-year follow-up study. Mol. Med. Rep. 2016, 14, 4209–4215. [Google Scholar] [CrossRef] [PubMed]
- Qu, Z.; Fang, G.; Cui, Z.; Liu, Y. Cell therapy for bone nonunion: A retrospective study. Minerva Med. 2015, 106, 315–321. [Google Scholar]
- Castillo-Cardiel, G.; López-Echaury, A.C.; Saucedo-Ortiz, J.A.; Fuentes-Orozco, C.; Michel-Espinoza, L.R.; Irusteta-Jiménez, L.; Salazar-Parra, M.; González-Ojeda, A. Bone regeneration in mandibular fractures after the application of autologous mesenchymal stem cells, a randomized clinical trial. Dent Traumatol. 2017, 33, 38–44. [Google Scholar] [CrossRef]
- Kebriaei, P.; Isola, L.; Bahceci, E.; Holland, K.; Rowley, S.; McGuirk, J.; Devetten, M.; Jansen, J.; Herzig, R.; Schuster, M.; et al. Adult human mesenchymal stem cells added to corticosteroid therapy for the treatment of acute graft-versus-host disease. Biol. Blood Marrow Transplant. 2009, 15, 804–811. [Google Scholar] [CrossRef]
- Lazarus, H.M.; Koc, O.N.; Devine, S.M.; Curtin, P.; Maziarz, R.T.; Holland, H.K.; Shpall, E.J.; McCarthy, P.; Atkinson, K.; Cooper, B.W.; et al. Cotransplantation of HLA-identical sibling culture-expanded mesenchymal stem cells and hematopoietic stem cells in hematologic malignancy patients. Biol. Blood Marrow Transplant. 2005, 11, 389–398. [Google Scholar] [CrossRef]
- Ringdén, O.; Uzunel, M.; Rasmusson, I.; Remberger, M.; Sundberg, B.; Lönnies, H.; Marschall, H.U.; Dlugosz, A.; Szakos, A.; Hassan, Z.; et al. Mesenchymal stem cells for treatment of therapy-resistant graft-versus-host disease. Transplantation 2006, 81, 1390–1397. [Google Scholar] [CrossRef]
- Le Blanc, K.; Rasmusson, I.; Sundberg, B.; Götherström, C.; Hassan, M.; Uzunel, M.; Ringdén, O. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet 2004, 363, 1439–1441. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Hu, B.; Liu, J.; Kong, P.; Lou, S.; Su, Y.; Yang, T.; Li, H.; Liu, Y.; et al. Phase II Multicenter, Randomized, Double-Blind Controlled Study of Efficacy and Safety of Umbilical Cord-Derived Mesenchymal Stromal Cells in the Prophylaxis of Chronic Graft-Versus-Host Disease After HLA-Haploidentical Stem-Cell Transplantation. J. Clin. Oncol. 2016, 34, 2843–2850. [Google Scholar] [CrossRef]
- Chen, G.; Yang, T.; Tian, H.; Qiao, M.; Liu, H.; Fu, C.; Miao, M.; Jin, Z.; Tang, X.; Han, Y.; et al. Clinical study of umbilical cord-derived mesenchymal stem cells for treatment of nineteen patients with steroid-resistant severe acute graft-versus-host disease. Chin. J. Hematol. 2012, 33, 303–306. [Google Scholar] [CrossRef]
- Gonzalo-Daganzo, R.; Regidor, C.; Martín-Donaire, T.; Rico, M.A.; Bautista, G.; Krsnik, I.; Forés, R.; Ojeda, E.; Sanjuán, I.; García-Marco, J.A.; et al. Results of a pilot study on the use of third-party donor mesenchymal stromal cells in cord blood transplantation in adults. Cytotherapy 2009, 11, 278–288. [Google Scholar] [CrossRef]
- Macmillan, M.L.; Blazar, B.R.; DeFor, T.E.; Wagner, J.E. Transplantation of ex-vivo culture-expanded parental haploidentical mesenchymal stem cells to promote engraftment in pediatric recipients of unrelated donor umbilical cord blood: Results of a phase I-II clinical trial. Bone Marrow Transplant. 2009, 43, 447–454. [Google Scholar] [CrossRef]
- Vanikar, A.V.; Trivedi, H.L.; Kumar, A.; Gopal, S.C.; Patel, H.V.; Gumber, M.R.; Kute, V.B.; Shah, P.R.; Dave, S.D. Co-infusion of donor adipose tissue-derived mesenchymal and hematopoietic stem cells helps safe minimization of immunosuppression in renal transplantation—Single center experience. Ren. Fail. 2014, 36, 1376–1384. [Google Scholar] [CrossRef]
- Nesteruk, J.; Voronina, N.; Kundt, G.; Donndorf, P.; Klopsch, C.; Kaminski, A.; Duckers, H.J.; Steinhoff, G. Stem cell registry programme for patients with ischemic cardiomyopathy undergoing coronary artery bypass grafting: What benefits does it derive? ESC Heart Fail. 2017, 4, 105–111. [Google Scholar] [CrossRef]
- Houtgraaf, J.H.; de Jong, R.; Kazemi, K.; de Groot, D.; van der Spoel, T.I.G.; Arslan, F.; Hoefer, I.; Pasterkamp, G.; Itescu, S.; Zijlstra, F.; et al. Intracoronary infusion of allogeneic mesenchymal precursor cells directly after experimental acute myocardial infarction reduces infarct size, abrogates adverse remodeling, and improves cardiac function. Circ. Res. 2013, 113, 153–166. [Google Scholar] [CrossRef]
- Panfilov, I.A.; de Jong, R.; Takashima, S.I.; Duckers, H.J. Clinical study using adipose-derived mesenchymal-like stem cells in acute myocardial infarction and heart failure. Methods Mol. Biol. 2013, 1036, 207–212. [Google Scholar]
- Larijani, B.; Aghayan, H.; Goodarzi, P.; Mohamadi-Jahani, F.; Norouzi-Javidan, A.; Dehpour, A.R.; Fallahzadeh, K.; Azam Sayahpour, F.; Bidaki, K.; Arjmand, B. Clinical Grade Human Adipose Tissue-Derived Mesenchymal Stem Cell Banking. Acta Med. Iran. 2015, 53, 540–546. [Google Scholar] [PubMed]
- Zanata, F.; Shaik, S.; Devireddy, R.V.; Wu, X.; Ferreira, L.M.; Gimble, J.M. Cryopreserved Adipose Tissue-Derived Stromal/Stem Cells: Potential for Applications in Clinic and Therapy. Adv. Exp. Med. Biol. 2016, 951, 137–146. [Google Scholar]
- Roato, I.; Alotto, D.; Belisario, D.C.; Casarin, S.; Fumagalli, M.; Cambieri, I.; Piana, R.; Stella, M.; Ferracini, R.; Castagnoli, C. Adipose Derived-Mesenchymal Stem Cells Viability and Differentiating Features for Orthopaedic Reparative Applications: Banking of Adipose Tissue. Stem Cells Int. 2016, 2016, 4968724. [Google Scholar] [CrossRef]
- MacRae, J.W.; Tholpady, S.S.; Ogle, R.C.; Morgan, R.F. Ex vivo fat graft preservation: Effects and implications of cryopreservation. Ann. Plast. Surg. 2004, 52, 281–282. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, O.; Ullmann, Y.; Shupak, A.; Ramon, Y.; Gilhar, A.; Kehat, I.; Peled, I.J. The role of frozen storage in preserving adipose tissue obtained by suction-assisted lipectomy for repeated fat injection procedures. Dermatol. Surg. 2001, 27, 645–647. [Google Scholar]
- Palumbo, P.; Lombardi, F.; Siragusa, G.; Cifone, M.G.; Cinque, B.; Giuliani, M. Methods of Isolation, Characterization and Expansion of Human Adipose-Derived Stem Cells (ASCs): An Overview. Int. J. Mol. Sci. 2018, 19, 1897. [Google Scholar] [CrossRef]
- Kode, J.A.; Mukherjee, S.; Joglekar, M.V.; Hardikar, A.A. Mesenchymal stem cells: Immunobiology and role in immunomodulation and tissue regeneration. Cytotherapy 2009, 11, 377–391. [Google Scholar] [CrossRef]
- Qayyum, A.A.; Kaur, K.P.; Mathiasen, A.B.; Haack-Sørensen, M.; Ekblond, A.; Kastrup, J. Influence of patient related factors on number of mesenchymal stromal cells reached after in vitro culture expansion for clinical treatment. Scand. J. Clin. Lab. Investig. 2017, 77, 541–548. [Google Scholar] [CrossRef]
- Varghese, J.; Griffin, M.; Mosahebi, A.; Butler, P. Systematic review of patient factors affecting adipose stem cell viability and function: Implications for regenerative therapy. Stem Cell Res. Ther. 2017, 8, 45. [Google Scholar] [CrossRef]
- Gimble, J.M.; Katz, A.J.; Bunnell, B.A. Adipose-derived stem cells for regenerative medicine. Circ. Res. 2007, 100, 1249–1260. [Google Scholar] [CrossRef]
| Stem Cell-Based Therapy | References | Cell Origin | Auto/Allo | Associated Effects | Application |
|---|---|---|---|---|---|
| Skin | [84,85,86] | BM-MSCs | Auto | Modulation of inflammation, wound repair | Radiation burns, burns |
| [87,88,89]] | UC-MSCs | Allo | Wound healing, skin improvement, neoangiogenesis and ulcer healing, decrease in features associated with AD | Burns, wound healing, severe diabetic foot, Atopic Dermatitis (AD) | |
| [10,11,12,16,17,90,91,92,93,94,95,96,97,98] | ADSCs | Auto | Wound healing, traumatic scars, facial tissue defects, post-mastectomy radiation, breast augmentation, facial rejuvenation, hair loss and growth, breast reconstitution, neogenesis across affected arteries | Soft tissue augmentation, esthetic remodeling, anti-aging, ulcers, burns, alopecia, lipodystrophies, critical limb ischemia | |
| Nerve system | [99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119] | BM-MSCs | Auto Allo | Safety and efficacy, increased functional recovery, improvement, slowdown of Amyotrophic Lateral Sclerosis (ALS) progression, safety in ALS, spinal cord repair, expanded disability scale score improvement, improvement treatment of MS | Ischemic stroke, traumatic brain injury, spinal cord injury, ALS, chronic spinal cord lesions, multiple sclerosis (MS), Parkinson, spastic cerebral palsy, refractory epilepsy, chronic complete paraplegia |
| [5,120,121,122,123,124,125] | UC-MSCs | Allo | Recovery of neurologic function, neuroprotection from sclerosis, delay SCA progression, safety and effectiveness, feasible and safety approach in stroke, self-care in patients | ALS, hereditary spinocerebellar ataxia (SCA), MS, Stroke in Middle Cerebral Artery, Traumatic Brain Injury sequelae | |
| [126,127,128,129,130,131] | ADSCs | Auto | Recovery from ischemia, safety | Spinal cord injury, ALS, MS | |
| Autoimmune disorders | [132,133,134,135] | BM-MSCs | Auto | improvement the clinical condition, reduction of Crohn’s disease and perianal diseases activity | Crohn’s diseases, perianal diseases, SLE |
| [112,132,136,137,138,139,140] | UCB-MSCs | Allo Allo | Safety and improvement of insulin secretion after HSC transplantation, renal remission for LN patients, safety and increase T cell level, partial disease remission, Safety with adverse events | Diabete I, Diabete II, Lupus Nephritis (LN), Systemic Lupus Erythematosus (SLE), systemic sclerosis (SS), Sjörgren’s syndrome | |
| [15,141,142,143,144,145,146,147,148] | ADSCs | Auto Allo | Proinflammatory cytokine decrease, improvement safety and efficacy treatment, Generation of Insulin-secreting cells, improvement in disease activity and safety | perianal and rectovaginal fistules, Crohn’s diseases, Type 1 Diabete Mellitus, refractory SLE Polymyolitis, SS | |
| Cartilage | [63,117] | BM-MSCs | Auto | Good integration in bone | knee chondral lesions, cartilage defects |
| [149] | UC-MSCs | Allo | Cartilage regeneration | Osteoarthritis | |
| [19,22,150,151,152,153,154,155,156,157,158] | ADSCs | Allo Auto | Tendon fibers arrangement, suppressive activity and decrease inflammatory responses, Safety, Proinflammatory cytokine decrease, improvement of knee joint | Intervertebral disc damage, Rheumatoid arthritis disease, Osteoarthritis | |
| Bone | [159,160,161] | BM-MSCs | Auto | Large bone diaphysis, good scaffold integration with host bone | Durable bone regeneration, bone defects, bone segment loss, bone diaphysis defects |
| [162,163] | UCB-MSCs | Allo | Decreased in healing time in bone callus formation and marrow flow | Bone nonunion, Necrosis of Femoral Heads | |
| [164,165,166] | ADSCs | Auto Allo | Bone regeneration, calvarial continuity | Maxillary reconstitution, traumatic calvarial defects | |
| Immune and Hematological Disorders | [167,168,169,170,171,172,173,174] | BM-MSCs | Allo, Auto | Improvement of HSC engraftment, Lymphocyte recovery, Hematopoietic recovery, Safety and no adverse events | Acute and severe GVHD, Hematopoietic malignancy |
| [175,176,177,178,179,180] | UC-MSCs | Allo | Neutrophil and platelet engraftment, successful engraftment, Safety, Reduced graft failure, Decrease in GVHD symptoms | Acute leukemia, Hematologic malignancy, GVHD, Steroid-resistant severe and acute GVHD | |
| [14,181,182] | ADSCs | Auto, | Improvement of HSC engraftment, immunosuppressive activity, short term hematopoiesis, Renal transplantation immuno-suppressive minimization | Acute and chronic GVHD, Hematopoiesis support, engraftment | |
| Cardio-vascular and Muscle | [183,184,185,186] | BM-MSCs | Auto, Allo | improved left ventricular function, decreased cardiac arrhythmias, improvement myocardial function, Decrease in infarct size | Heart infarct, Ischemic cardiomyopathy |
| [187,188,189,190] | UCB-MSCs | Allo | Improvement of muscle size and activity, stabilize muscle power in DMD, decrease in infarct size, improve left ventricular function | Becker muscular disease (BMD), Duchene Muscular Disease (DMD), Coronary chronic total occlusion | |
| [191,192,193,194,195] | ADSCs | Auto Allo | Reduction in myocardial scar formation, improvement of cardiac function, safe and efficient | Myocardial infarction, ischemic heart disease, Heart failure |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazini, L.; Rochette, L.; Amine, M.; Malka, G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). Int. J. Mol. Sci. 2019, 20, 2523. https://doi.org/10.3390/ijms20102523
Mazini L, Rochette L, Amine M, Malka G. Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). International Journal of Molecular Sciences. 2019; 20(10):2523. https://doi.org/10.3390/ijms20102523
Chicago/Turabian StyleMazini, Loubna, Luc Rochette, Mohamed Amine, and Gabriel Malka. 2019. "Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs)" International Journal of Molecular Sciences 20, no. 10: 2523. https://doi.org/10.3390/ijms20102523
APA StyleMazini, L., Rochette, L., Amine, M., & Malka, G. (2019). Regenerative Capacity of Adipose Derived Stem Cells (ADSCs), Comparison with Mesenchymal Stem Cells (MSCs). International Journal of Molecular Sciences, 20(10), 2523. https://doi.org/10.3390/ijms20102523