Neutrophil-to-Lymphocyte Ratio in Rectal Cancer—Novel Biomarker of Tumor Immunogenicity During Radiotherapy or Confounding Variable?
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mendenhall, W.M.; Bland, K.I.; Copeland, E.M.; Summers, G.E.; Pfaff, W.W.; Souba, W.W.; Million, R.R. Does preoperative radiation therapy enhance the probability of local control and survival in high-risk distal rectal cancer? Ann. Surg. 1992, 215, 696–705, discussion 705–706. [Google Scholar] [CrossRef]
- Rödel, C.; Graeven, U.; Fietkau, R.; Hohenberger, W.; Hothorn, T.; Arnold, D.; Hofheinz, R.-D.; Ghadimi, M.; Wolff, H.A.; Lang-Welzenbach, M.; et al. Oxaliplatin added to fluorouracil-based preoperative chemoradiotherapy and postoperative chemotherapy of locally advanced rectal cancer (the German CAO/ARO/AIO-04 study): final results of the multicentre, open-label, randomised, phase 3 trial. Lancet. Oncol. 2015, 16, 979–989. [Google Scholar] [CrossRef]
- Maas, M.; Nelemans, P.J.; Valentini, V.; Das, P.; Rödel, C.; Kuo, L.-J.; Calvo, F.A.; García-Aguilar, J.; Glynne-Jones, R.; Haustermans, K.; et al. Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data. Lancet Oncol. 2010, 11, 835–844. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: N1 versus N2 TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Souto, J.C.; Vila, L.; Brú, A. Polymorphonuclear neutrophils and cancer: Intense and sustained neutrophilia as a treatment against solid tumors. Med. Res. Rev. 2009, 31, 311–363. [Google Scholar] [CrossRef]
- Shen, J.; Zhu, Y.; Wu, W.; Zhang, L.; Ju, H.; Fan, Y.; Zhu, Y.; Luo, J.; Liu, P.; Zhou, N.; et al. Prognostic Role of Neutrophil-to-Lymphocyte Ratio in Locally Advanced Rectal Cancer Treated with Neoadjuvant Chemoradiotherapy. Med. Sci. Monit. 2017, 23, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Donskov, F. Immunomonitoring and prognostic relevance of neutrophils in clinical trials. Semin. Cancer Biol. 2013, 23, 200–207. [Google Scholar] [CrossRef]
- Dumitru, C.A.; Moses, K.; Trellakis, S.; Lang, S.; Brandau, S. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 2012, 61, 1155–1167. [Google Scholar] [CrossRef]
- Haram, A.; Boland, M.R.; Kelly, M.E.; Bolger, J.C.; Waldron, R.M.; Kerin, M.J. The prognostic value of neutrophil-to-lymphocyte ratio in colorectal cancer: A systematic review. J. Surg. Oncol. 2017, 115, 470–479. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, S.B.; Beak, S.K.; Han, Y.D.; Cho, M.S.; Hur, H.; Lee, K.Y.; Kim, N.K.; Min, B.S. Temporal changes in immune cell composition and cytokines in response to chemoradiation in rectal cancer. Sci. Rep. 2018, 8, 7565. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.G.; Park, W.; Kim, H.; Choi, D.H.; Park, H.C.; Kim, S.-H.; Cho, Y.B.; Yun, S.H.; Kim, H.C.; Lee, W.Y.; et al. Baseline neutrophil–lymphocyte ratio and platelet–lymphocyte ratio in rectal cancer patients following neoadjuvant chemoradiotherapy. Tumori J. 2018. [Google Scholar] [CrossRef]
- Zhang, X.; Li, J.; Peng, Q.; Huang, Y.; Tang, L.; Zhuang, Q.; Lin, F.; Lin, X.; Du, K.; Wu, J. Association of markers of systemic and local inflammation with prognosis of patients with rectal cancer who received neoadjuvant radiotherapy. Cancer Manag. Res. 2019, 11, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Ward, W.H.; Goel, N.; Ruth, K.J.; Esposito, A.C.; Lambreton, F.; Sigurdson, E.R.; Meyer, J.E.; Farma, J.M. Predictive value of leukocyte- and platelet-derived ratios in rectal adenocarcinoma. J. Surg. Res. 2018, 232, 275–282. [Google Scholar] [CrossRef]
- Vallard, A.; Garcia, M.-A.; Diao, P.; Espenel, S.; De Laroche, G.; Guy, J.-B.; Mrad, M.B.; Rancoule, C.; Kaczmarek, D.; Muron, T.; et al. Outcomes prediction in pre-operative radiotherapy locally advanced rectal cancer: leucocyte assessment as immune biomarker. Radiat. Oncol. 2018, 102. [Google Scholar] [CrossRef][Green Version]
- Shen, L.; Zhang, H.; Liang, L.; Li, G.; Fan, M.; Wu, Y.; Zhu, J.; Zhang, Z. Baseline neutrophil-lymphocyte ratio (≥2.8) as a prognostic factor for patients with locally advanced rectal cancer undergoing neoadjuvant chemoradiation. Radiat. Oncol. 2014, 9, 295. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R. A Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The Hallmarks of Cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Liu, H.; Wang, H.; Wu, J.; Wang, Y.; Zhao, L.; Li, G.; Zhou, M. Lymphocyte nadir predicts tumor response and survival in locally advanced rectal cancer after neoadjuvant chemoradiotherapy: Immunologic relevance. Radiother. Oncol. 2019, 131, 52–59. [Google Scholar] [CrossRef]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Nakamura, K.; Smyth, M.J. Targeting cancer-related inflammation in the era of immunotherapy. Immunol. Cell Biol. 2017, 95, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Rao, H.-L.; Chen, J.-W.; Li, M.; Xiao, Y.-B.; Fu, J.; Zeng, Y.-X.; Cai, M.-Y.; Xie, D. Increased intratumoral neutrophil in colorectal carcinomas correlates closely with malignant phenotype and predicts patients’ adverse prognosis. PLoS ONE 2012, 7, e30806. [Google Scholar] [CrossRef]
- Balkwill, F.R.; Mantovani, A. Cancer-related inflammation: Common themes and therapeutic opportunities. Semin. Cancer Biol. 2012, 22, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Carruthers, R.; Tho, L.M.; Brown, J.; Kakumanu, S.; Mccartney, E.; Mcdonald, A.C. Systemic inflammatory response is a predictor of outcome in patients undergoing preoperative chemoradiation for locally advanced rectal cancer. Color. Dis. 2012, 14, e701–e707. [Google Scholar] [CrossRef]
- Krauthamer, M.; Rouvinov, K.; Ariad, S.; Man, S.; Walfish, S.; Pinsk, I.; Sztarker, I.; Charkovsky, T.; Lavrenkov, K. A study of inflammation-based predictors of tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Oncology 2013, 85, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Ren, D.-L.; Li, J.; Yu, H.-C.; Peng, S.-Y.; Lin, W.-D.; Wang, X.-L.; Ghoorun, A.; Luo, Y.-X. Nomograms for predicting pathological response to neoadjuvant treatments in patients with rectal cancer. World J. Gastroenterol. 2019, 25, 118–137. [Google Scholar] [CrossRef]
- Kim, I.Y.; You, S.H.; Kim, Y.W. Neutrophil-lymphocyte ratio predicts pathologic tumor response and survival after preoperative chemoradiation for rectal cancer. BMC Surg. 2014, 14, 94. [Google Scholar] [CrossRef] [PubMed]
- Gani, C.; Bonomo, P.; Zwirner, K.; Schroeder, C.; Menegakis, A.; Rödel, C.; Zips, D. Organ preservation in rectal cancer—Challenges and future strategies. Clin. Transl. Radiat. Oncol. 2017, 3, 9–15. [Google Scholar] [CrossRef][Green Version]
- Wen, B.; Zhang, L.; Wang, C.; Huang, R.; Peng, H.; Zhang, T.; Dong, J.; Xiao, W.; Zeng, Z.; Liu, M.; et al. Prognostic significance of clinical and pathological stages on locally advanced rectal carcinoma after neoadjuvant chemoradiotherapy. Radiat. Oncol. 2015, 10, 124. [Google Scholar] [CrossRef]
- Gabrilovich, D.I.; Ostrand-Rosenberg, S.; Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 2012, 12, 253–268. [Google Scholar] [CrossRef] [PubMed]
- Diefenhardt, M.; Hofheinz, R.-D.; Martin, D.; Beißbarth, T.; Arnold, D.; Hartmann, A.; von der Grün, J.; Grützmann, R.; Liersch, T.; Ströbel, P.; et al. Leukocytosis and neutrophilia as independent prognostic immunological biomarkers for clinical outcome in the CAO/ARO/AIO-04 randomized phase 3 rectal cancer trial. Int. J. Cancer 2019. [Google Scholar] [CrossRef]
- Diaz-Montero, C.M.; Salem, M.L.; Nishimura, M.I.; Garrett-Mayer, E.; Cole, D.J.; Montero, A.J. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin-cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 2009, 58, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Ohki, S.; Shibata, M.; Gonda, K.; Machida, T.; Shimura, T.; Nakamura, I.; Ohtake, T.; Koyama, Y.; Suzuki, S.; Ohto, H.; et al. Circulating myeloid-derived suppressor cells are increased and correlate to immune suppression, inflammation and hypoproteinemia in patients with cancer. Oncol. Rep. 2012, 28, 453–458. [Google Scholar] [CrossRef] [PubMed]
- Gani, C.; Schroeder, C.; Heinrich, V.; Spillner, P.; Lamprecht, U.; Berger, B.; Zips, D. Long-term local control and survival after preoperative radiochemotherapy in combination with deep regional hyperthermia in locally advanced rectal cancer. Int. J. Hyperth. 2016, 32, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Schemper, M.; Smith, T.L. A note on quantifying follow-up in studies of failure time. Control. Clin. Trials 1996, 17, 343–346. [Google Scholar] [CrossRef]
- Thies, S.; Langer, R. Tumor Regression Grading of Gastrointestinal Carcinomas after Neoadjuvant Treatment. Front. Oncol. 2013, 3, 262. [Google Scholar] [CrossRef] [PubMed]
- Dworak, O.; Keilholz, L.; Hoffmann, A. Pathological features of rectal cancer after preoperative RCT. Int. J. Colorectal Dis. 1997, 12, 19–23. [Google Scholar] [CrossRef]
| Median | IQR | ||
|---|---|---|---|
| Age (years) | 65.5 | 15 | |
| Tumor location (cm) | 6 | 6 | |
| CEA (mg/dL) | 3.88 | 8,7 | |
| Primary tumor volume (cc) | 33.86 | 48 | |
| n | % | ||
| Gender | Male | 142 | 64.5 |
| Female | 78 | 35.5 | |
| T-stage | T1 | 1 | 0.5 |
| T2 | 7 | 3.2 | |
| T3 | 193 | 87.7 | |
| T4 | 19 | 8.6 | |
| N-stage | cN0 | 39 | 17.7 |
| cN+ | 181 | 82.3 | |
| Grading | G1 | 17 | 7.7 |
| G2 | 173 | 78.6 | |
| G3 | 24 | 10.9 | |
| Missing | 6 | 2.7 | |
| Location | Lower third | 92 | 41.8 |
| Mid third | 115 | 52.3 | |
| Upper third | 13 | 5.9 | |
| Subgroups | n | % | |
|---|---|---|---|
| Chemotherapy dose | Complete | 208 | 94.5 |
| Incomplete | 12 | 5.5 | |
| Type of surgery | LAR | 158 | 71.8 |
| APR | 62 | 28.2 | |
| TME Quality | Perfect | 66 | 30.0 |
| Intermediate | 17 | 7.7 | |
| Poor | 7 | 3.2 | |
| Missing | 130 | 59.1 | |
| Postoperative T-stage | ypT0 | 36 | 16.4 |
| ypT1 | 16 | 7.3 | |
| ypT2 | 66 | 30.0 | |
| ypT3 | 94 | 42.7 | |
| ypT4 | 8 | 3.6 | |
| Postoperative N-stage | ypN0 | 164 | 74.5 |
| ypN1 | 38 | 17.3 | |
| ypN2 | 18 | 8.2 | |
| Resection status | R0 | 214 | 97.3 |
| R1 | 5 | 2.3 | |
| Rx | 1 | 0.5 | |
| Dworak regression | 0 | 27 | 12.3 |
| 1 | 70 | 31.8 | |
| 2 | 1 | 0.5 | |
| 3 | 44 | 20.0 | |
| 4 | 35 | 15.9 | |
| missing | 43 | 19.5 | |
| Postoperative Chemotherapy | yes | 122 | 55.5 |
| no | 40 | 18.2 | |
| missing | 58 | 26.4 |
| n | Median | Min | Max | IQR | |
|---|---|---|---|---|---|
| CRP | 191 | 0.26 | 0.01 | 11.79 | 0.63 |
| White cell count | 217 | 7840 | 4080 | 18,920 | 2570 |
| Platelets | 216 | 297 | 147 | 810 | 123.25 |
| Neutrophils | 109 | 5368 | 808 | 15,673 | 2062.69 |
| Lymphocytes | 108 | 1536 | 521 | 4438 | 778.49 |
| Monocytes | 108 | 486 | 185 | 1091 | 186.32 |
| NLR | 108 | 3.11 | 1.23 | 18.85 | 1.71 |
| LMR | 108 | 3.32 | 1.14 | 8.08 | 1.97 |
| NMR | 108 | 11.04 | 6.35 | 28.67 | 4.45 |
| PLR | 108 | 0.18 | 0.05 | 0.98 | 0.13 |
| Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|
| Primary tumor location | Lower third vs. upper/mid third | p | HR | 95% CI | p |
| 0.725 | - | - | - | ||
| T-stage | cT1/cT2/cT3 vs. cT4 | 0.087 | 1.19 | 0.43–3.30 | 0.733 |
| N-stage | cN0 vs. cN+ | 0.571 | - | - | - |
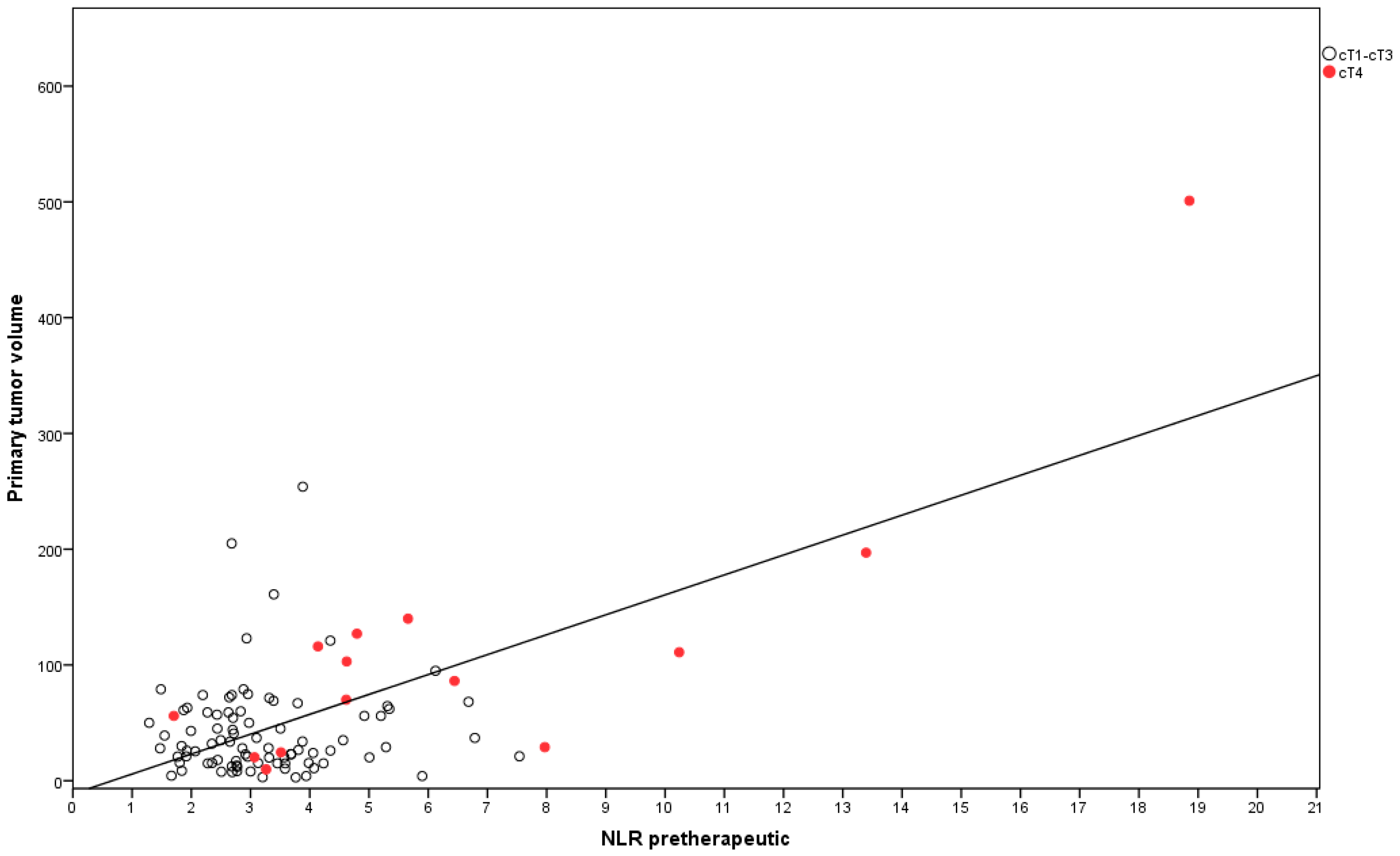
| GTV | ≤33.86 cc vs. >33.86 cc | 0.003 | 0.338 | 0.14–0.83 | 0.017 |
| Age | ≤ 65.5 vs. > 65.5 | 0.139 | - | - | - |
| CEA | ≤ 3.88 vs. > 3.88 | 0.16 | - | - | - |
| Grading | G3 vs. G1/G2 | 0.133 | - | - | - |
| CRP | ≤ 0.26 vs. > 0.26 | 0.324 | - | - | - |
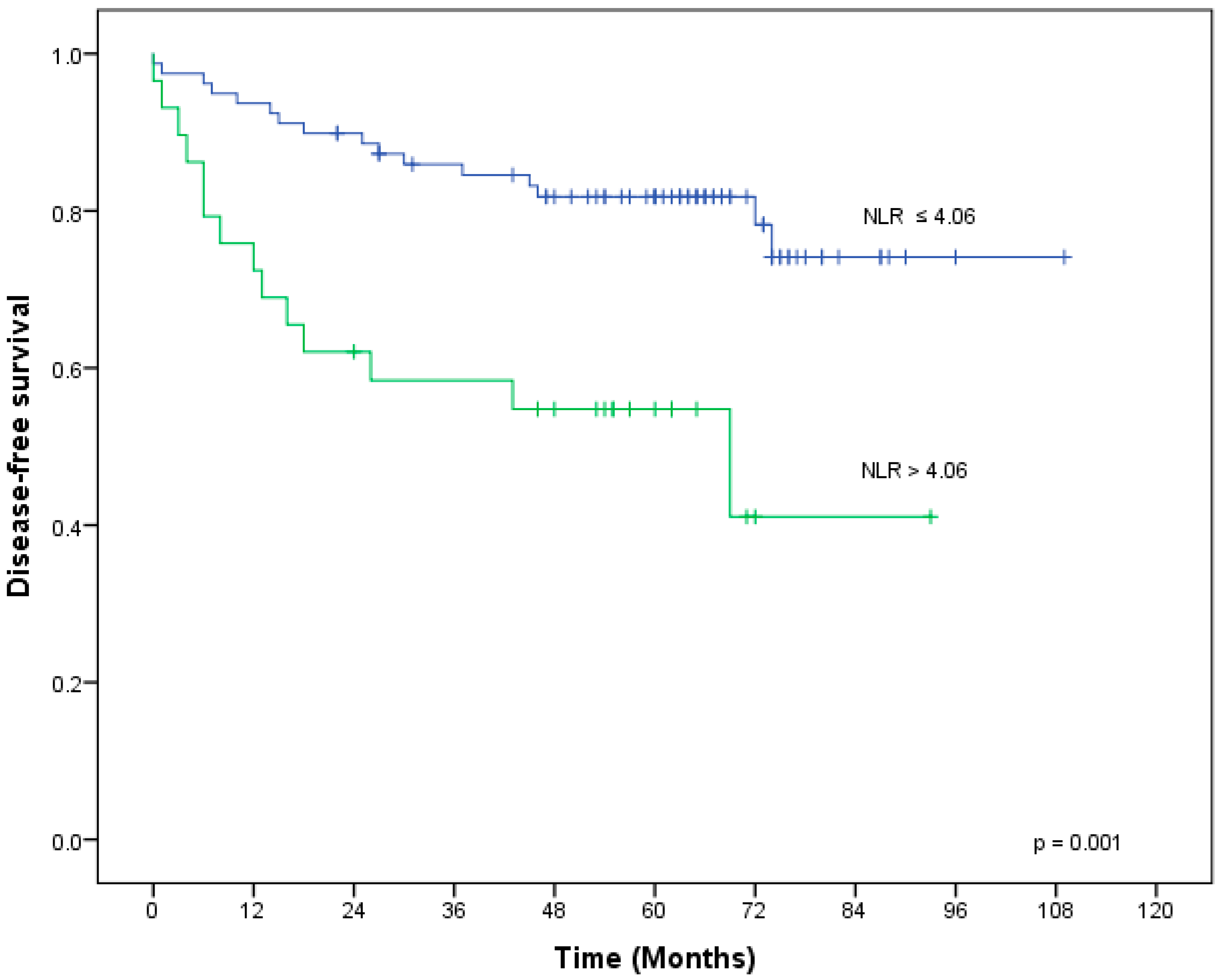
| NLR | ≤ 4.06 vs. > 4.06 | 0.001 | 0.3 | 0.11–0.81 | 0.017 |
| Neutrophil count | ≤ 6021 vs. > 6021 | 0.019 | 2.195 | 0.61–7.86 | 0.227 |
| Leukocyte count | ≤ 8120 vs. > 8120 | 0.023 | 0.375 | 0.13–1.10 | 0.074 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Braun, L.H.; Baumann, D.; Zwirner, K.; Eipper, E.; Hauth, F.; Peter, A.; Zips, D.; Gani, C. Neutrophil-to-Lymphocyte Ratio in Rectal Cancer—Novel Biomarker of Tumor Immunogenicity During Radiotherapy or Confounding Variable? Int. J. Mol. Sci. 2019, 20, 2448. https://doi.org/10.3390/ijms20102448
Braun LH, Baumann D, Zwirner K, Eipper E, Hauth F, Peter A, Zips D, Gani C. Neutrophil-to-Lymphocyte Ratio in Rectal Cancer—Novel Biomarker of Tumor Immunogenicity During Radiotherapy or Confounding Variable? International Journal of Molecular Sciences. 2019; 20(10):2448. https://doi.org/10.3390/ijms20102448
Chicago/Turabian StyleBraun, Lore Helene, David Baumann, Kerstin Zwirner, Ewald Eipper, Franziska Hauth, Andreas Peter, Daniel Zips, and Cihan Gani. 2019. "Neutrophil-to-Lymphocyte Ratio in Rectal Cancer—Novel Biomarker of Tumor Immunogenicity During Radiotherapy or Confounding Variable?" International Journal of Molecular Sciences 20, no. 10: 2448. https://doi.org/10.3390/ijms20102448
APA StyleBraun, L. H., Baumann, D., Zwirner, K., Eipper, E., Hauth, F., Peter, A., Zips, D., & Gani, C. (2019). Neutrophil-to-Lymphocyte Ratio in Rectal Cancer—Novel Biomarker of Tumor Immunogenicity During Radiotherapy or Confounding Variable? International Journal of Molecular Sciences, 20(10), 2448. https://doi.org/10.3390/ijms20102448





