Pharmacological Targeting of the ER-Resident Chaperones GRP94 or Cyclophilin B Induces Secretion of IL-22 Binding Protein Isoform-1 (IL-22BPi1)
Abstract
1. Introduction
2. Results
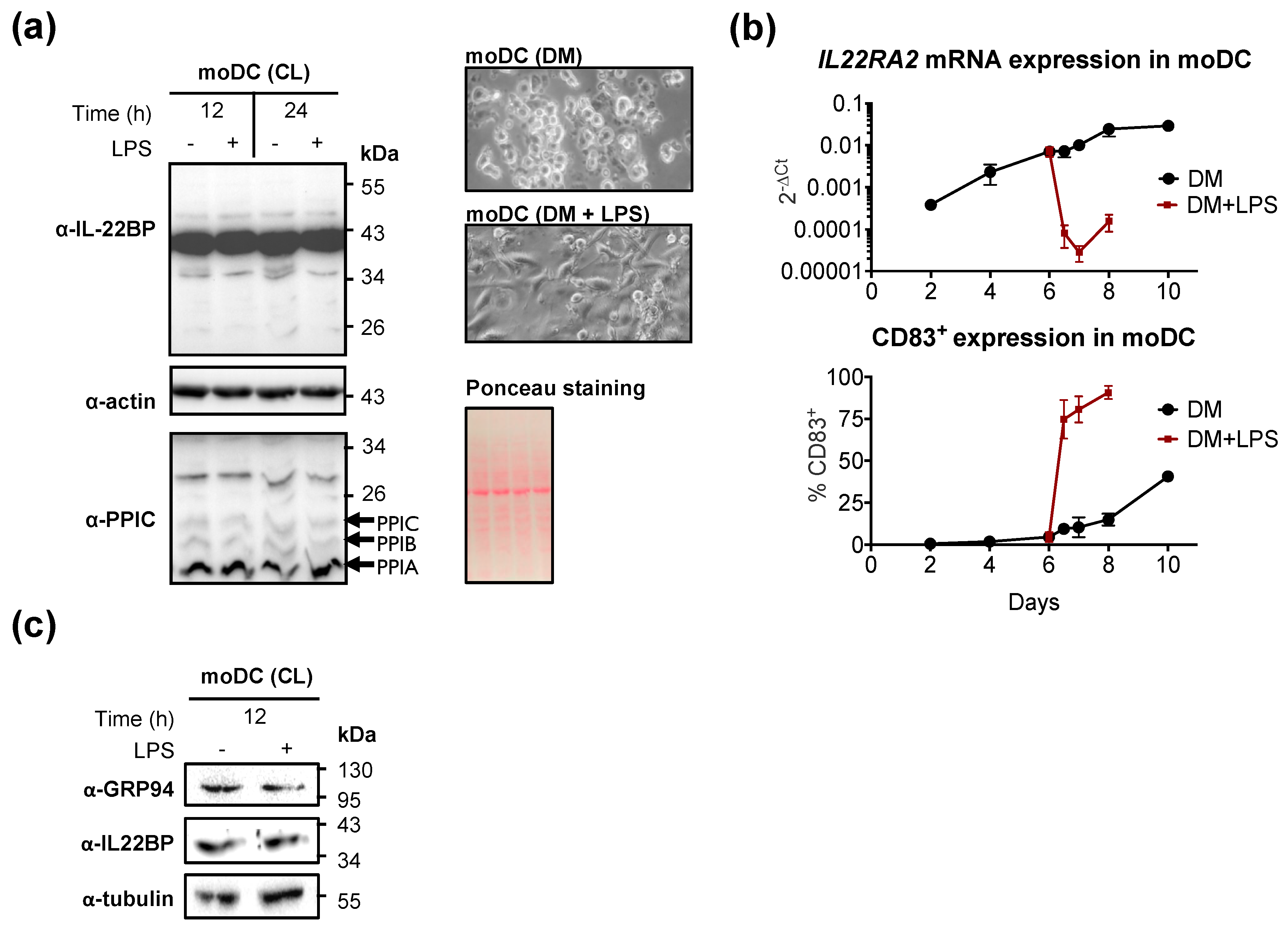
2.1. GRP94 and Cyclophilin B Are Co-Expressed with IL22RA2 in Monocyte-Derived Dendritic cells
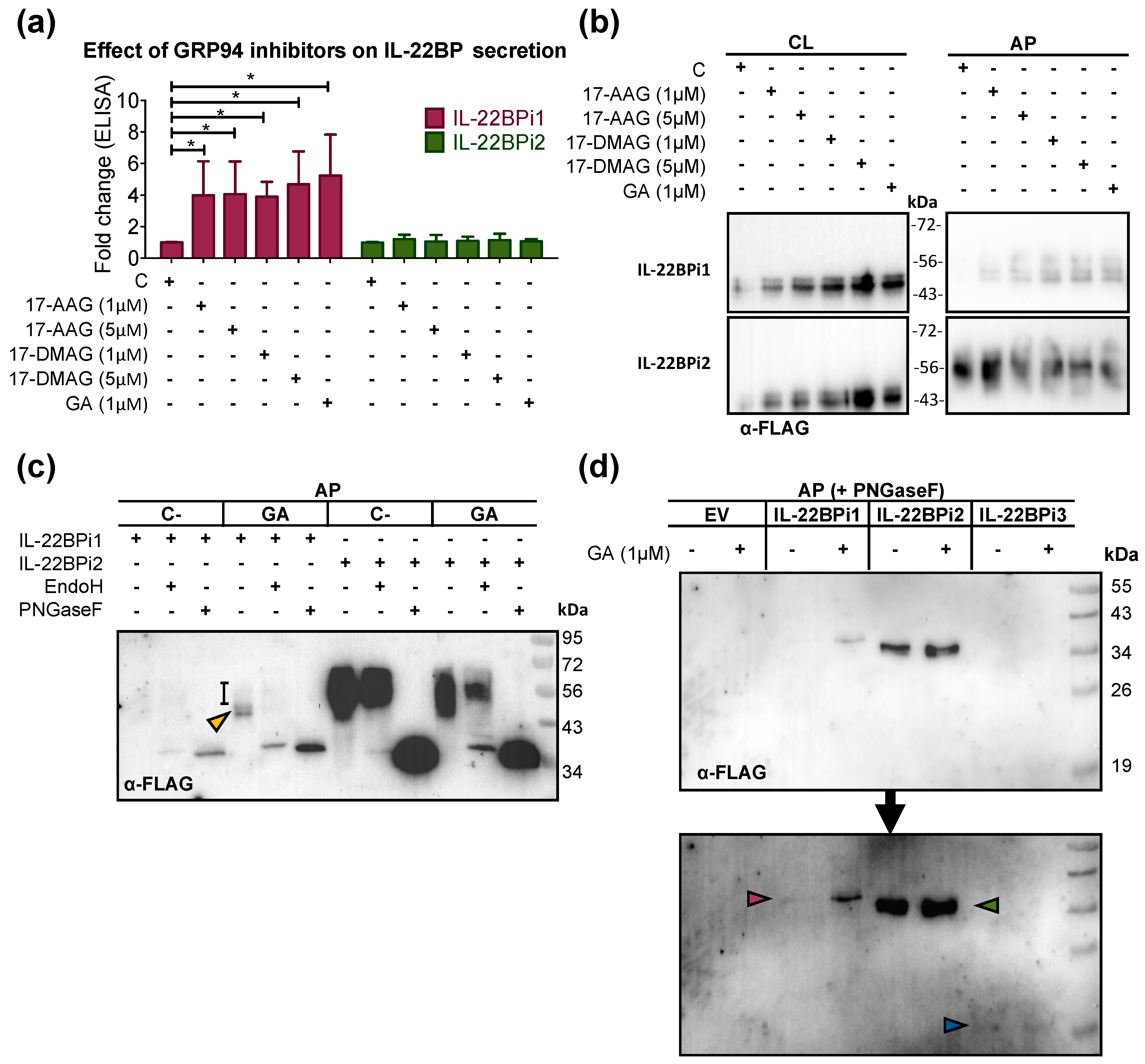
2.2. GRP94 Inhibitors Enhance IL-22BP1 Secretion
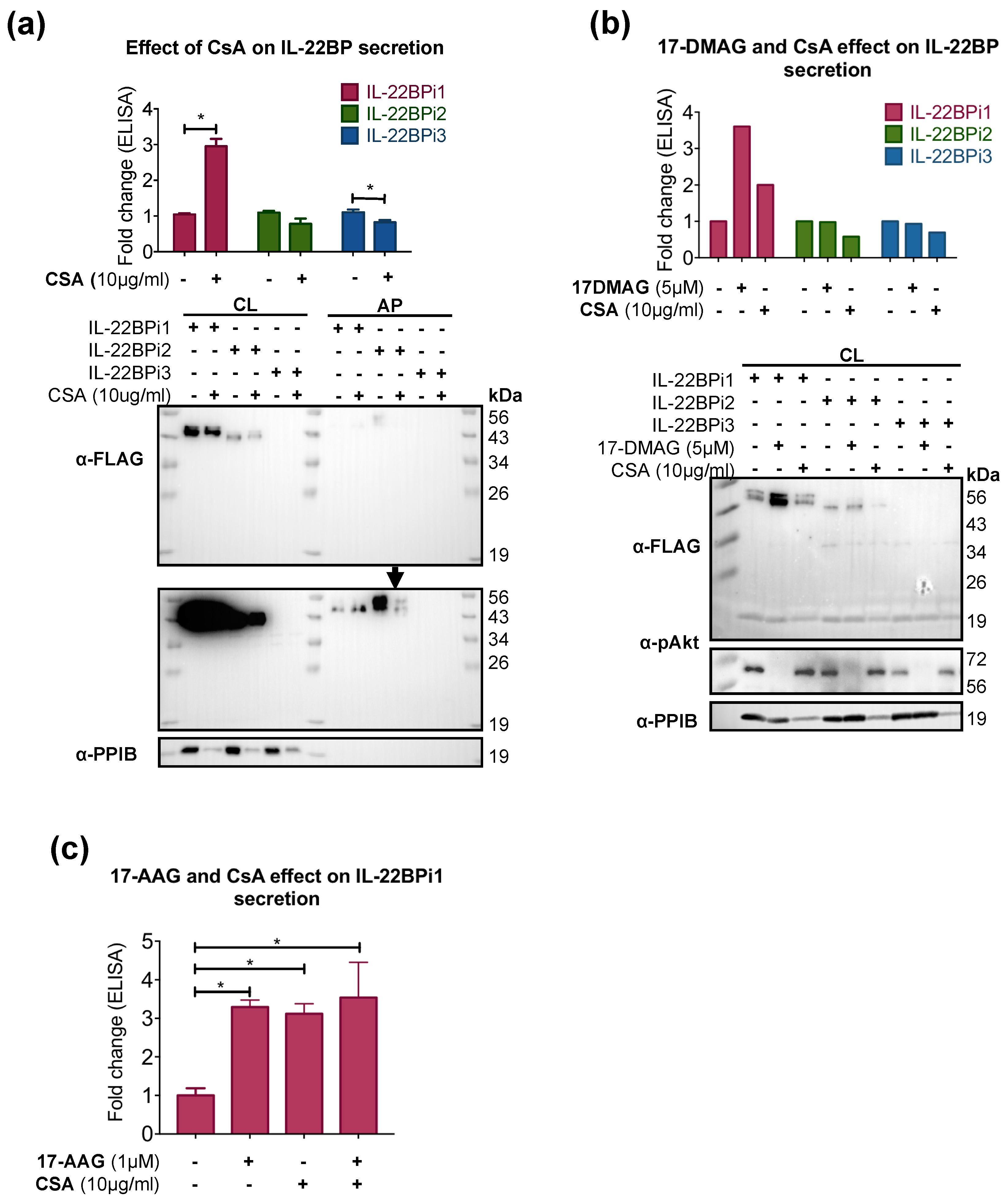
2.3. CsA Increases IL-22BPi1 Secretion
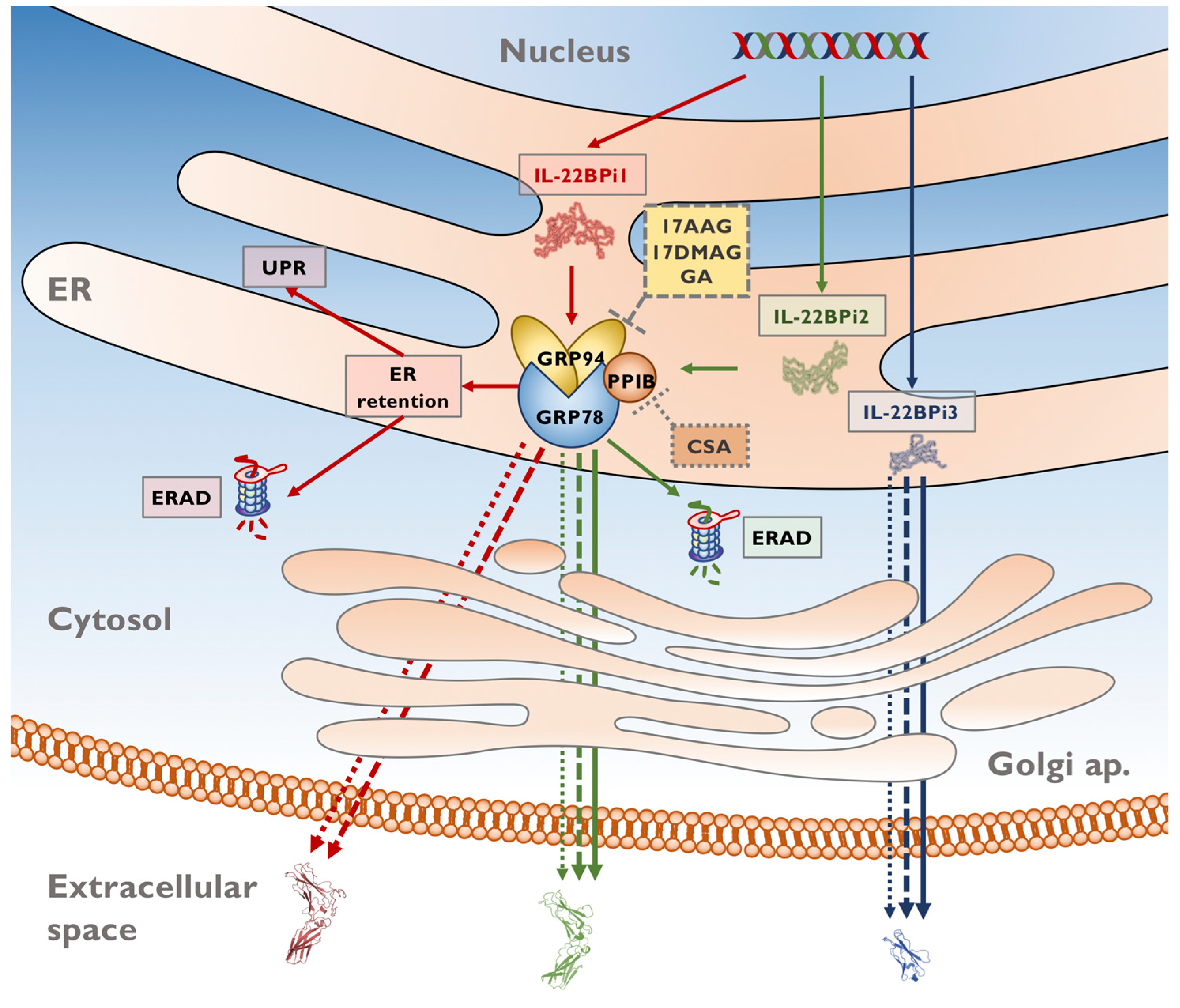
3. Discussion
4. Materials and Methods
4.1. Monocyte Isolation and Monocyte-Derived Dendritic Cell Differentiation
4.2. RNA Extraction and qPCR
4.3. Cell Culture and Transfection
4.4. Drug Treatment
4.5. Protein Extraction, Deglycosylation, Immunoblotting, and ELISA
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 17-AAG | 17-allylamino-17-demethoxygeldanamycin |
| 17-DMAG | 17-dimethylaminoethylamino-17-demethoxygeldanamycin |
| AP | acetone precipitates |
| CL | cell lysates |
| CM | conditioned medium |
| CsA | cyclosporin A |
| DM | differentiation medium |
| Endo H | endonuclease H |
| ER | endoplasmic reticulum |
| ERAD | ER-associated degradation |
| GA | geldanamycin |
| GRP94 | glucose-regulated protein 94 |
| GRP78 | glucose-regulated protein 78 |
| IL-22BP | interleukin-22 binding protein |
| IL22RA2 | interleukin-22 receptor alpha-chain 2 |
| moDC | monocyte-derived dendritic cell |
| PPIB | peptidylprolyl isomerase B (cyclophilin B) |
| PPIC | peptidylprolyl isomerase C (cyclophilin C) |
| SFM | serum-free medium |
| UPR | unfolded protein response |
References
- Sabat, R.; Ouyang, W.; Wolk, K. Therapeutic opportunities of the IL-22–IL-22R1 system. Nat. Rev. Drug Discov. 2013, 13, 21–38. [Google Scholar] [CrossRef]
- Zenewicz, L.A. IL-22: There Is a Gap in Our Knowledge. ImmunoHorizons 2018, 2, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Zenewicz, L.A.; Flavell, R.A. Recent advances in IL-22 biology. Int. Immunol. 2011, 23, 159–163. [Google Scholar] [CrossRef] [PubMed]
- Lim, C.; Savan, R. The role of the IL-22/IL-22R1 axis in cancer. Cytokine Growth Factor Rev. 2014, 25, 257–271. [Google Scholar] [CrossRef]
- Markota, A.; Endres, S.; Kobold, S. Targeting interleukin-22 for cancer therapy. Hum. Vaccin. Immunother. 2018, 14, 2012–2015. [Google Scholar] [CrossRef]
- Sabat, R.; Witte, E.; Witte, K.; Wolk, K. IL-22 and IL-17: An Overview. In IL-17, IL-22 and Their Producing Cells: Role in Inflammation and Autoimmunity; Quesniaux, V., Ryffel, B., Padova, F., Eds.; Springer: Basel, Switzerland, 2013; pp. 11–35. ISBN 978-3-0348-0521-6. [Google Scholar]
- Dumoutier, L.; Lejeune, D.; Colau, D.; Renauld, J.-C. Cloning and Characterization of IL-22 Binding Protein, a Natural Antagonist of IL-10-Related T Cell-Derived Inducible Factor/IL-22. J. Immunol. 2001, 166, 7090–7095. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Presnell, S.R.; Parrish-Novak, J.; Kindsvogel, W.; Jaspers, S.; Chen, Z.; Dillon, S.R.; Gao, Z.; Gilbert, T.; Madden, K.; et al. A soluble class II cytokine receptor, IL-22RA2, is a naturally occurring IL-22 antagonist. Proc. Natl. Acad. Sci. USA 2001, 98, 9511–9516. [Google Scholar] [CrossRef]
- Kotenko, S.V.; Izotova, L.S.; Mirochnitchenko, O.V.; Esterova, E.; Dickensheets, H.; Donnelly, R.P.; Pestka, S. Identification, Cloning, and Characterization of a Novel Soluble Receptor That Binds IL-22 and Neutralizes Its Activity. J. Immunol. 2001, 166, 7096–7103. [Google Scholar] [CrossRef]
- Wei, C.-C.; Ho, T.-W.; Liang, W.-G.; Chen, G.-Y.; Chang, M.-S. Cloning and characterization of mouse IL-22 binding protein. Genes Immun. 2003, 4, 204–211. [Google Scholar] [CrossRef]
- Weiss, B.; Wolk, K.; Grünberg, B.H.; Volk, H.-D.; Sterry, W.; Asadullah, K.; Sabat, R. Cloning of murine IL-22 receptor alpha 2 and comparison with its human counterpart. Genes Immun. 2004, 5, 330–336. [Google Scholar] [CrossRef]
- Jones, B.C.; Logsdon, N.J.; Walter, M.R. Structure of IL-22 Bound to Its High-Affinity IL-22R1 Chain. Structure 2008, 16, 1333–1344. [Google Scholar] [CrossRef]
- de Moura, P.R.; Watanabe, L.; Bleicher, L.; Colau, D.; Dumoutier, L.; Lemaire, M.M.; Renauld, J.-C.; Polikarpov, I. Crystal structure of a soluble decoy receptor IL-22BP bound to interleukin-22. FEBS Lett. 2009, 583, 1072–1077. [Google Scholar] [CrossRef]
- Wolk, K.; Witte, E.; Hoffmann, U.; Doecke, W.-D.; Endesfelder, S.; Asadullah, K.; Sterry, W.; Volk, H.-D.; Wittig, B.M.; Sabat, R. IL-22 Induces Lipopolysaccharide-Binding Protein in Hepatocytes: A Potential Systemic Role of IL-22 in Crohn’s Disease. J. Immunol. 2007, 178, 5973–5981. [Google Scholar] [CrossRef]
- Lim, C.; Hong, M.; Savan, R. Human IL-22 binding protein isoforms act as a rheostat for IL-22 signaling. Sci. Signal. 2016, 9, ra95. [Google Scholar] [CrossRef]
- Gómez-Fernández, P.; Urtasun, A.; Paton, A.W.; Paton, J.C.; Borrego, F.; Dersh, D.; Argon, Y.; Alloza, I.; Vandenbroeck, K. Long Interleukin-22 Binding Protein Isoform-1 Is an Intracellular Activator of the Unfolded Protein Response. Front. Immunol. 2018, 9, 2934. [Google Scholar] [CrossRef]
- Martin, J.C.; Bériou, G.; Heslan, M.; Bossard, C.; Jarry, A.; Abidi, A.; Hulin, P.; Ménoret, S.; Thinard, R.; Anegon, I.; et al. IL-22BP is produced by eosinophils in human gut and blocks IL-22 protective actions during colitis. Mucosal Immunol. 2016, 9, 539–549. [Google Scholar] [CrossRef]
- Laaksonen, H.; Guerreiro-Cacais, A.O.; Adzemovic, M.Z.; Parsa, R.; Zeitelhofer, M.; Jagodic, M.; Olsson, T. The multiple sclerosis risk gene IL22RA2 contributes to a more severe murine autoimmune neuroinflammation. Genes Immun. 2014, 15, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.C.; Wolk, K.; Bériou, G.; Abidi, A.; Witte-Händel, E.; Louvet, C.; Kokolakis, G.; Drujont, L.; Dumoutier, L.; Renauld, J.-C.; et al. Limited Presence of IL-22 Binding Protein, a Natural IL-22 Inhibitor, Strengthens Psoriatic Skin Inflammation. J. Immunol. 2017, 198, 3671–3678. [Google Scholar] [CrossRef]
- Huber, S.; Gagliani, N.; Zenewicz, L.A.; Huber, F.J.; Bosurgi, L.; Hu, B.; Hedl, M.; Zhang, W.; O’Connor, W.; Murphy, A.J.; et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 2012, 491, 259–263. [Google Scholar] [CrossRef] [PubMed]
- Park, S.W.; Ozcan, U. Potential for therapeutic manipulation of the UPR in disease. Semin. Immunopathol. 2013, 35, 351–373. [Google Scholar] [CrossRef]
- Rivas, A.; Vidal, R.L.; Hetz, C. Targeting the unfolded protein response for disease intervention. Expert Opin. Ther. Targets 2015, 19, 1203–1218. [Google Scholar] [CrossRef]
- Liu, M.; Chen, Z.; Chen, L. Endoplasmic reticulum stress: A novel mechanism and therapeutic target for cardiovascular diseases. Acta Pharmacol. Sin. 2016, 37, 425–443. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Vandenbroeck, K. The endoplasmic reticulum protein folding factory and its chaperones: New targets for drug discovery? Br. J. Pharmacol. 2011, 162, 328–345. [Google Scholar] [CrossRef] [PubMed]
- Eletto, D.; Dersh, D.; Argon, Y. GRP94 in ER quality control and stress responses. Semin. Cell Dev. Biol. 2010, 21, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Gewirth, D.T. Paralog Specific Hsp90 Inhibitors—A Brief History and a Bright Future. Curr. Top. Med. Chem. 2016, 16, 2779–2791. [Google Scholar] [CrossRef] [PubMed]
- Duerfeldt, A.S.; Peterson, L.B.; Maynard, J.C.; Ng, C.L.; Eletto, D.; Ostrovsky, O.; Shinogle, H.E.; Moore, D.S.; Argon, Y.; Nicchitta, C.V; et al. Development of a Grp94 inhibitor. J. Am. Chem. Soc. 2012, 134, 9796–9804. [Google Scholar] [CrossRef]
- Randow, F.; Seed, B. Endoplasmic reticulum chaperone gp96 is required for innate immunity but not cell viability. Nat. Cell Biol. 2001, 3, 891–896. [Google Scholar] [CrossRef]
- Liu, J.; Farmer, J.D.; Lane, W.S.; Friedman, J.; Weissman, I.; Schreiber, S.L. Calcineurin is a common target of cyclophilin-cyclosporin A and FKBP-FK506 complexes. Cell 1991, 66, 807–815. [Google Scholar] [CrossRef]
- Price, E.R.; Jin, M.; Lim, D.; Pati, S.; Walsh, C.T.; McKeon, F.D. Cyclophilin B trafficking through the secretory pathway is altered by binding of cyclosporin A. Proc. Natl. Acad. Sci. USA 1994, 91, 3931–3935. [Google Scholar] [CrossRef]
- Martin, J.C.; Bériou, G.; Heslan, M.; Chauvin, C.; Utriainen, L.; Aumeunier, A.; Scott, C.L.; Mowat, A.; Cerovic, V.; Houston, S.A.; et al. Interleukin-22 binding protein (IL-22BP) is constitutively expressed by a subset of conventional dendritic cells and is strongly induced by retinoic acid. Mucosal Immunol. 2014, 7, 101–113. [Google Scholar] [CrossRef]
- Jinnohara, T.; Kanaya, T.; Hase, K.; Sakakibara, S.; Kato, T.; Tachibana, N.; Sasaki, T.; Hashimoto, Y.; Sato, T.; Watarai, H.; et al. IL-22BP dictates characteristics of Peyer’ s patch follicle-associated epithelium for antigen uptake. J. Exp. Med 2017, 214, 1607–1618. [Google Scholar] [CrossRef]
- Voglis, S.; Moos, S.; Kloos, L.; Wanke, F.; Zayoud, M.; Pelczar, P.; Giannou, A.D.; Pezer, S.; Albers, M.; Luessi, F.; et al. Regulation of IL-22BP in psoriasis. Sci. Rep. 2018, 8, 5085. [Google Scholar] [CrossRef]
- Stocki, P.; Chapman, D.C.; Beach, L.A.; Williams, D.B. Depletion of cyclophilins B and C Leads to dysregulation of endoplasmic reticulum redox homeostasis. J. Biol. Chem. 2014, 289, 23086–23096. [Google Scholar] [CrossRef]
- Le Naour, F.; Hohenkirk, L.; Grolleau, A.; Misek, D.E.; Lescure, P.; Geiger, J.D.; Hanash, S.; Beretta, L. Profiling Changes in Gene Expression during Differentiation and Maturation of Monocyte-derived Dendritic Cells Using Both Oligonucleotide Microarrays and Proteomics. J. Biol. Chem. 2001, 276, 17920–17931. [Google Scholar] [CrossRef] [PubMed]
- Bullwinkel, J.; Lüdemann, A.; Debarry, J.; Singh, P.B. Epigenotype switching at the CD14 and CD209 genes during differentiation of human monocytes to dendritic cells. Epigenetics 2011, 6, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Fearon, P.; Lonsdale-Eccles, A.A.; Ross, O.K.; Todd, C.; Sinha, A.; Allain, F.; Reynolds, N.J. Keratinocyte Secretion of Cyclophilin B via the Constitutive Pathway Is Regulated through Its Cyclosporin-Binding Site. J. Investig. Dermatol. 2011, 131, 1085–1094. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Wang, K.; Groenendyk, J.; Barakat, K.; Mizianty, M.J.; Ruan, J.; Michalak, M.; Kurgan, L. Human structural proteome-wide characterization of Cyclosporine a targets. Bioinformatics 2014, 30, 3561–3566. [Google Scholar] [CrossRef]
- Meunier, L.; Usherwood, Y.-K.; Chung, K.T.; Hendershot, L.M. A Subset of Chaperones and Folding Enzymes Form Multiprotein Complexes in Endoplasmic Reticulum to Bind Nascent Proteins. Mol. Biol. Cell 2002, 13, 4456–4469. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Herscovitz, H. Nascent lipidated apolipoprotein B is transported to the Golgi as an incompletely folded intermediate as probed by its association with network of endoplasmic reticulum molecular chaperones, GRP94, ERp72, BiP, calreticulin, and cyclophilin B. J. Biol. Chem. 2003, 278, 7459–7468. [Google Scholar] [CrossRef]
- Jansen, G.; Määttänen, P.; Denisov, A.Y.; Scarffe, L.; Schade, B.; Balghi, H.; Dejgaard, K.; Chen, L.Y.; Muller, W.J.; Gehring, K.; et al. An Interaction Map of Endoplasmic Reticulum Chaperones and Foldases. Mol. Cell. Proteom. 2012, 11, 710–723. [Google Scholar] [CrossRef]
- Bernasconi, R.; Soldà, T.; Galli, C.; Pertel, T.; Luban, J.; Molinari, M. Cyclosporine A-Sensitive, Cyclophilin B-Dependent Endoplasmic Reticulum-Associated Degradation. PLoS ONE 2010, 5, e13008. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Choi, T.G.; Ding, Y.; Kim, Y.; Ha, K.S.; Lee, K.H.; Kang, I.; Ha, J.; Kaufman, R.J.; Lee, J.; et al. Overexpressed cyclophilin B suppresses apoptosis associated with ROS and Ca2+ homeostasis after ER stress. J. Cell Sci. 2008, 121, 3636–3648. [Google Scholar] [CrossRef]
- Christianson, J.C.; Shaler, T.A.; Tyler, R.E.; Kopito, R.R. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat. Cell Biol. 2008, 10, 272–282. [Google Scholar] [CrossRef]
- Di, X.-J.; Wang, Y.-J.; Han, D.-Y.; Fu, Y.-L.; Duerfeldt, A.S.; Blagg, B.S.J.; Mu, T.-W. Grp94 Protein Delivers γ-Aminobutyric Acid Type A (GABA A) Receptors to Hrd1 Protein-mediated Endoplasmic Reticulum-associated Degradation. J. Biol. Chem. 2016, 291, 9526–9539. [Google Scholar] [CrossRef]
- Yang, Y.; Li, Z. Roles of heat shock protein gp96 in the ER quality control: Redundant or unique function? Mol. Cells 2005, 20, 173–182. [Google Scholar] [PubMed]
- Nganga, A.; Bruneau, N.; Sbarra, V.; Lombardo, D.; Le Petit-Thevenin, J. Control of pancreatic bile-salt-dependent-lipase secretion by the glucose-regulated protein of 94 kDa (Grp94). Biochem. J. 2000, 352 Pt 3, 865–874. [Google Scholar]
- Mimnaugh, E.G.; Chavany, C.; Neckers, L. Polyubiquitination and Proteasomal Degradation of the p185 c-erb B-2 Receptor Protein-tyrosine Kinase Induced by Geldanamycin. J. Biol. Chem. 1996, 271, 22796–22801. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gómez-Fernández, P.; Urtasun, A.; Astobiza, I.; Mena, J.; Alloza, I.; Vandenbroeck, K. Pharmacological Targeting of the ER-Resident Chaperones GRP94 or Cyclophilin B Induces Secretion of IL-22 Binding Protein Isoform-1 (IL-22BPi1). Int. J. Mol. Sci. 2019, 20, 2440. https://doi.org/10.3390/ijms20102440
Gómez-Fernández P, Urtasun A, Astobiza I, Mena J, Alloza I, Vandenbroeck K. Pharmacological Targeting of the ER-Resident Chaperones GRP94 or Cyclophilin B Induces Secretion of IL-22 Binding Protein Isoform-1 (IL-22BPi1). International Journal of Molecular Sciences. 2019; 20(10):2440. https://doi.org/10.3390/ijms20102440
Chicago/Turabian StyleGómez-Fernández, Paloma, Andoni Urtasun, Ianire Astobiza, Jorge Mena, Iraide Alloza, and Koen Vandenbroeck. 2019. "Pharmacological Targeting of the ER-Resident Chaperones GRP94 or Cyclophilin B Induces Secretion of IL-22 Binding Protein Isoform-1 (IL-22BPi1)" International Journal of Molecular Sciences 20, no. 10: 2440. https://doi.org/10.3390/ijms20102440
APA StyleGómez-Fernández, P., Urtasun, A., Astobiza, I., Mena, J., Alloza, I., & Vandenbroeck, K. (2019). Pharmacological Targeting of the ER-Resident Chaperones GRP94 or Cyclophilin B Induces Secretion of IL-22 Binding Protein Isoform-1 (IL-22BPi1). International Journal of Molecular Sciences, 20(10), 2440. https://doi.org/10.3390/ijms20102440





