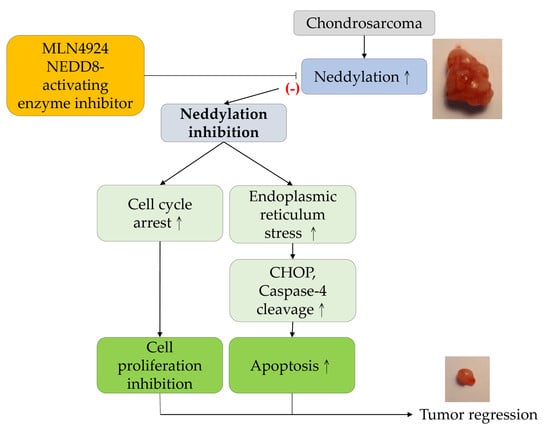
MLN4924, a Protein Neddylation Inhibitor, Suppresses the Growth of Human Chondrosarcoma through Inhibiting Cell Proliferation and Inducing Endoplasmic Reticulum Stress-Related Apoptosis
Abstract
:1. Introduction
2. Results
2.1. MLN4924 Reduces Cell Viability And Causes Cytotoxicity in Human Chondrosarcoma Cells
2.2. MLN4924 Suppresses Cell Proliferation by Hindering G2/M Cell Cycle Progression
2.3. MLN4924 Induces Cellular Apoptosis through Intrinsic and Extrinsic Apoptotic Pathways in Human Chondrosarcoma Cells
2.4. MLN4924 Promotes ER Stress-Related Signaling and Apoptosis in Human Chondrosarcoma Cells
2.5. MLN4924 Significantly Inhibits Chondrosarcoma Xenograft Growth in Vivo
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents and Antibodies
4.3. Measurement of Cell Viability
4.4. Lactate dehydrogenase Activity Assay
4.5. Apoptosis Assays in Vitro
4.6. Cell Proliferation Assay
4.7. Cell Cycle Analysis
4.8. Western Blot Analysis
4.9. In Vivo Xenograft Experiments
4.10. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Boer, H.H.H.; Maat, G. Dry Bone Histology of Bone Tumours. Int. J. Paleopathol. 2016. [Google Scholar] [CrossRef] [PubMed]
- Mery, B.; Espenel, S.; Guy, J.B.; Rancoule, C.; Vallard, A.; Aloy, M.T.; Rodriguez-Lafrasse, C.; Magne, N. Biological Aspects of Chondrosarcoma: Leaps and Hurdles. Crit. Rev. Oncol. Hematol. 2018, 126, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Herget, G.W.; Strohm, P.; Rottenburger, C.; Kontny, U.; Krauss, T.; Bohm, J.; Sudkamp, N.; Uhl, M. Insights into Enchondroma, Enchondromatosis and the Risk of Secondary Chondrosarcoma. Review of the Literature with an Emphasis on the Clinical Behaviour, Radiology, Malignant Transformation and the Follow Up. Neoplasma 2014, 61, 365–378. [Google Scholar] [CrossRef] [PubMed]
- Herget, G.W.; Uhl, M.; Opitz, O.G.; Adler, C.P.; Sudkamp, N.P.; Knoller, S. The Many Faces of Chondrosarcoma of Bone, Own Cases and Review of the Literature with an Emphasis on Radiology, Pathology and Treatment. Acta Chirurgiae Orthopaedicae et Traumatologiae Cechosl 2011, 78, 501–509. [Google Scholar]
- Chin, O.Y.; Dubal, P.M.; Sheikh, A.B.; Unsal, A.A.; Park, R.C.; Baredes, S.; Eloy, J.A. Laryngeal Chondrosarcoma: A Systematic Review of 592 Cases. Laryngoscope 2017, 127, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Schuetze, S.M.; Bolejack, V.; Choy, E.; Ganjoo, K.N.; Staddon, A.P.; Chow, W.A.; Tawbi, H.A.; Samuels, B.L.; Patel, S.R.; Von Mehren, M.; et al. Phase 2 Study of Dasatinib in Patients with Alveolar Soft Part Sarcoma, Chondrosarcoma, Chordoma, Epithelioid Sarcoma, or Solitary Fibrous Tumor. Cancer 2017, 123, 90–97. [Google Scholar] [CrossRef]
- Gao, C.P.; Liu, J.H.; Hou, F.; Liu, H.; Xu, W.J. Low-Grade Chondrosarcoma of the Cricoid Cartilage: A Case Report and Review of the Literature. Skeletal Radiol. 2017, 46, 1597–1601. [Google Scholar] [CrossRef]
- Rothman, S. How Is the Balance between Protein Synthesis and Degradation Achieved? Theor. Biol. Med. Model. 2010, 7, 25. [Google Scholar] [CrossRef]
- Mccubrey, J.A.; Abrams, S.L.; Fitzgerald, T.L.; Cocco, L.; Martelli, A.M.; Montalto, G.; Cervello, M.; Scalisi, A.; Candido, S.; Libra, M.; et al. Roles of Signaling Pathways in Drug Resistance, Cancer Initiating Cells and Cancer Progression and Metastasis. Adv. Biol. Regul. 2015, 57, 75–101. [Google Scholar] [CrossRef]
- Myung, J.; Kim, K.B.; Crews, C.M. The Ubiquitin-Proteasome Pathway and Proteasome Inhibitors. Med. Res. Rev. 2001, 21, 245–273. [Google Scholar] [CrossRef]
- Metzger, M.B.; Hristova, V.A.; Weissman, A.M. Hect and Ring Finger Families of E3 Ubiquitin Ligases at a Glance. J. Cell Sci. 2012, 125 Pt 3, 531–537. [Google Scholar] [CrossRef]
- Wu, J.T.; Lin, H.C.; Hu, Y.C.; Chien, C.T. Neddylation and Deneddylation Regulate Cul1 and Cul3 Protein Accumulation. Nat. Cell Biol. 2005, 7, 1014–1020. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.S.; Lukashchuk, N.; Sczaniecka-Clift, M.; Britton, S.; Le Sage, C.; Calsou, P.; Beli, P.; Galanty, Y.; Jackson, S.P. Neddylation Promotes Ubiquitylation and Release of Ku From Dna-Damage Sites. Cell Rep. 2015, 11, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Walden, H.; Podgorski, M.S.; Huang, D.T.; Miller, D.W.; Howard, R.J.; Minor, D.L., Jr.; Holton, J.M.; Schulman, B.A. The Structure of the Appbp1-Uba3-Nedd8-Atp Complex Reveals the Basis for Selective Ubiquitin-Like Protein Activation by an E1. Mol. Cell 2003, 12, 1427–1437. [Google Scholar] [CrossRef]
- Li, L.; Wang, M.; Yu, G.; Chen, P.; Li, H.; Wei, D.; Zhu, J.; Xie, L.; Jia, H.; Shi, J.; et al. Overactivated Neddylation Pathway as a Therapeutic Target in Lung Cancer. J. Natl. Cancer Inst. 2014, 106, Dju083. [Google Scholar] [CrossRef] [PubMed]
- Hua, W.; Li, C.; Yang, Z.; Li, L.; Jiang, Y.; Yu, G.; Zhu, W.; Liu, Z.; Duan, S.; Chu, Y.; et al. Suppression of Glioblastoma by Targeting the Overactivated Protein Neddylation Pathway. Neuro-Oncology 2015, 17, 1333–1343. [Google Scholar] [CrossRef] [PubMed]
- Bulatov, E.; Ciulli, A. Targeting Cullin-Ring E3 Ubiquitin Ligases for Drug Discovery: Structure, Assembly and Small-Molecule Modulation. Biochem. J. 2015, 467, 365–386. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Morgan, M.A.; Sun, Y. Targeting Neddylation Pathways to Inactivate Cullin-Ring Ligases for Anticancer Therapy. Antioxid. Redox. Signal. 2014, 21, 2383–2400. [Google Scholar] [CrossRef] [PubMed]
- Soucy, T.A.; Smith, P.G.; Milhollen, M.A.; Berger, A.J.; Gavin, J.M.; Adhikari, S.; Brownell, J.E.; Burke, K.E.; Cardin, D.P.; Critchley, S.; et al. An Inhibitor of Nedd8-Activating Enzyme as a New Approach to Treat Cancer. Nature 2009, 458, 732–736. [Google Scholar] [CrossRef] [PubMed]
- Duncan, K.; Schafer, G.; Vava, A.; Parker, M.I.; Zerbini, L.F. Targeting Neddylation in Cancer Therapy. Future Oncol. 2012, 8, 1461–1470. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Yu, G.; Lee, H.W.; Li, L.; Wang, L.; Yang, D.; Pan, Y.; Ding, C.; Qian, J.; Wu, L.; et al. The Nedd8-Activating Enzyme Inhibitor Mln4924 Induces Autophagy and Apoptosis to Suppress Liver Cancer Cell Growth. Cancer Res. 2012, 72, 3360–3371. [Google Scholar] [CrossRef] [PubMed]
- Ho, I.L.; Kuo, K.L.; Liu, S.H.; Chang, H.C.; Hsieh, J.T.; Wu, J.T.; Chiang, C.K.; Lin, W.C.; Tsai, Y.C.; Chou, C.T.; et al. Mln4924 Synergistically Enhances Cisplatin-Induced Cytotoxicity via Jnk and Bcl-Xl Pathways in Human Urothelial Carcinoma. Sci. Rep. 2015, 5, 16948. [Google Scholar] [CrossRef] [PubMed]
- Swords, R.T.; Kelly, K.R.; Smith, P.G.; Garnsey, J.J.; Mahalingam, D.; Medina, E.; Oberheu, K.; Padmanabhan, S.; O’dwyer, M.; Nawrocki, S.T.; et al. Inhibition of Nedd8-Activating Enzyme: A Novel Approach for the Treatment of Acute Myeloid Leukemia. Blood 2010, 115, 3796–3800. [Google Scholar] [CrossRef] [PubMed]
- Kuo, K.L.; Ho, I.L.; Shi, C.S.; Wu, J.T.; Lin, W.C.; Tsai, Y.C.; Chang, H.C.; Chou, C.T.; Hsu, C.H.; Hsieh, J.T.; et al. Mln4924, a Novel Protein Neddylation Inhibitor, Suppresses Proliferation and Migration of Human Urothelial Carcinoma: In Vitro and In Vivo Studies. Cancer Lett. 2015, 363, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.C.; Kuo, K.L.; Shi, C.S.; Wu, J.T.; Hsieh, J.T.; Chang, H.C.; Liao, S.M.; Chou, C.T.; Chiang, C.K.; Chiu, W.S.; et al. Mln4924, a Novel Nedd8-Activating Enzyme Inhibitor, Exhibits Antitumor Activity and Enhances Cisplatin-Induced Cytotoxicity in Human Cervical Carcinoma: In Vitro and In Vivo Study. Am. J. Cancer Res. 2015, 5, 3350–3362. [Google Scholar] [PubMed]
- Paiva, C.; Godbersen, J.C.; Berger, A.; Brown, J.R.; Danilov, A.V. Targeting Neddylation Induces Dna Damage and Checkpoint Activation and Sensitizes Chronic Lymphocytic Leukemia B Cells to Alkylating Agents. Cell Death Dis. 2015, 6, E1807. [Google Scholar] [CrossRef] [PubMed]
- Polychronidou, G.; Karavasilis, V.; Pollack, S.M.; Huang, P.H.; Lee, A.; Jones, R.L. Novel Therapeutic Approaches in Chondrosarcoma. Future Oncol. 2017, 13, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, S.T.; Griffin, P.; Kelly, K.R.; Carew, J.S. Mln4924: A Novel First-In-Class Inhibitor of Nedd8-Activating Enzyme for Cancer Therapy. Expert Opin. Investig. Drugs 2012, 21, 1563–1573. [Google Scholar] [CrossRef]
- Scabini, M.; Stellari, F.; Cappella, P.; Rizzitano, S.; Texido, G.; Pesenti, E. In Vivo Imaging of Early Stage Apoptosis by Measuring Real-Time Caspase-3/7 Activation. Apoptosis 2011, 16, 198–207. [Google Scholar] [CrossRef]
- Cusimano, A.; Azzolina, A.; Iovanna, J.L.; Bachvarov, D.; Mccubrey, J.A.; D’alessandro, N.; Montalto, G.; Cervello, M. Novel Combination of Celecoxib and Proteasome Inhibitor Mg132 Provides Synergistic Antiproliferative and Proapoptotic Effects in Human Liver Tumor Cells. Cell Cycle 2010, 9, 1399–1410. [Google Scholar] [CrossRef]
- Staals, E.L.; Bacchini, P.; Bertoni, F. Dedifferentiated Central Chondrosarcoma. Cancer 2006, 106, 2682–2691. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, D.; Xie, L.; Tang, S.; Guo, W. Mesenchymal Chondrosarcoma of Bone and Soft Tissue: A Systematic Review of 107 Patients in the Past 20 Years. PLoS ONE 2015, 10, E0122216. [Google Scholar] [CrossRef] [PubMed]
- Amichetti, M.; Amelio, D.; Cianchetti, M.; Enrici, R.M.; Minniti, G. A Systematic Review of Proton Therapy In the Treatment of Chondrosarcoma of the Skull Base. Neurosurg. Rev. 2010, 33, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Boehme, K.A.; Schleicher, S.B.; Traub, F.; Rolauffs, B. Chondrosarcoma: A Rare Misfortune in Aging Human Cartilage? The Role of Stem and Progenitor Cells in Proliferation, Malignant Degeneration and Therapeutic Resistance. Int. J. Mol. Sci. 2018, 19, 311. [Google Scholar] [CrossRef] [PubMed]
- Campbell, V.T.; Nadesan, P.; Ali, S.A.; Wang, C.Y.; Whetstone, H.; Poon, R.; Wei, Q.; Keilty, J.; Proctor, J.; Wang, L.W.; et al. Hedgehog Pathway Inhibition in Chondrosarcoma Using the Smoothened Inhibitor Ipi-926 Directly Inhibits Sarcoma Cell Growth. Mol. Cancer Ther. 2014, 13, 1259–1269. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wan, Q.; Na, S.; Yokota, H.; Yan, J.L.; Hamamura, K. Suppressed Invasive And Migratory Behaviors of Sw1353 Chondrosarcoma Cells through the Regulation of Src, Rac1 Gtpase, and Mmp13. Cell. Signal. 2015, 27, 2332–2342. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, S.; Kanbara, K.; Neo, M. Synergistic Anti-Proliferative Effects of Mtor And Mek Inhibitors in High-Grade Chondrosarcoma Cell Line Oums-27. Acta Histochem. 2018, 120, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Ma, W.; He, X.; Jha, R.K. Review of Therapeutic Strategies for Osteosarcoma, Chondrosarcoma, and Ewing’s Sarcoma. Med. Sci. Monit. 2011, 17, Ra177. [Google Scholar] [CrossRef] [PubMed]
- Lohberger, B.; Steinecker-Frohnwieser, B.; Stuendl, N.; Kaltenegger, H.; Leithner, A.; Rinner, B. The Proteasome Inhibitor Bortezomib Affects Chondrosarcoma Cells via the Mitochondria-Caspase Dependent Pathway and Enhances Death Receptor Expression and Autophagy. PLoS ONE 2016, 11, E0168193. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Schmitt, S.; Buac, D.; Dou, Q.P. Targeting The Ubiquitin-Proteasome System for Cancer Therapy. Expert Opin. Ther. Targets 2013, 17, 1091–1108. [Google Scholar] [CrossRef] [PubMed]
- Voutsadakis, I.A. The Ubiquitin-Proteasome System in Colorectal Cancer. Biochim. Biophys. Acta 2008, 1782, 800–808. [Google Scholar] [CrossRef] [PubMed]
- Dawson, S.P. Hepatocellular Carcinoma and the Ubiquitin-Proteasome System. Biochim. Biophys. Acta 2008, 1782, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Fong, Y.C.; Li, T.M.; Wu, C.M.; Hsu, S.F.; Kao, S.T.; Chen, R.J.; Lin, C.C.; Liu, S.C.; Wu, C.L.; Tang, C.H. Bmp-2 Increases Migration of Human Chondrosarcoma Cells via Pi3k/Akt Pathway. J. Cell. Physiol. 2008, 217, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Oshiro, Y.; Chaturvedi, V.; Hayden, D.; Nazeer, T.; Johnson, M.; Johnston, D.A.; Ordonez, N.G.; Ayala, A.G.; Czerniak, B. Altered P53 Is Associated with Aggressive Behavior of Chondrosarcoma: A Long Term Follow-Up Study. Cancer 1998, 83, 2324–2334. [Google Scholar] [CrossRef]
- Santalla Martinez, M.; Blanco Cid, N.; Dacal Quintas, R. Bortezomib-Induced Lung Toxicity. Arch. Bronconeumol. 2014, 50, 564–565. [Google Scholar] [CrossRef]
- Brownell, J.E.; Sintchak, M.D.; Gavin, J.M.; Liao, H.; Bruzzese, F.J.; Bump, N.J.; Soucy, T.A.; Milhollen, M.A.; Yang, X.; Burkhardt, A.L.; et al. Substrate-Assisted Inhibition of Ubiquitin-Like Protein-Activating Enzymes: The Nedd8 E1 Inhibitor Mln4924 Forms a Nedd8-Amp Mimetic In Situ. Mol. Cell 2010, 37, 102–111. [Google Scholar] [CrossRef]
- Liakopoulos, D.; Busgen, T.; Brychzy, A.; Jentsch, S.; Pause, A. Conjugation of the Ubiquitin-Like Protein Nedd8 to Cullin-2 Is Linked to Von Hippel-Lindau Tumor Suppressor Function. Proc. Natl. Acad. Sci. USA 1999, 96, 5510–5515. [Google Scholar] [CrossRef]
- Fan, M.; Bigsby, R.M.; Nephew, K.P. The Nedd8 Pathway Is Required for Proteasome-Mediated Degradation of Human Estrogen Receptor (Er)-Alpha and Essential for the Antiproliferative Activity of Ici 182,780 in Eralpha-Positive Breast Cancer Cells. Mol. Endocrinol. 2003, 17, 356–365. [Google Scholar] [CrossRef]
- Dou, Q.P.; Zonder, J.A. Overview of Proteasome Inhibitor-Based Anti-Cancer Therapies: Perspective on Bortezomib and Second Generation Proteasome Inhibitors versus Future Generation Inhibitors of Ubiquitin-Proteasome System. Curr. Cancer Drug Targets 2014, 14, 517–536. [Google Scholar] [CrossRef]
- Reihe, C.A.; Pekas, N.; Wu, P.; Wang, X. Systemic Inhibition Of Neddylation By 3-Day Mln4924 Treatment Regime Does Not Impair Autophagic Flux in Mouse Hearts and Brains. Am. J. Cardiovasc. Dis. 2017, 7, 134–150. [Google Scholar]
- Tong, S.; Si, Y.; Yu, H.; Zhang, L.; Xie, P.; Jiang, W. Mln4924 (Pevonedistat), a Protein Neddylation Inhibitor, Suppresses Proliferation and Migration of Human Clear Cell Renal Cell Carcinoma. Sci. Rep. 2017, 7, 5599. [Google Scholar] [CrossRef] [PubMed]
- Stringer, D.K.; Piper, R.C. Terminating Protein Ubiquitination: Hasta La Vista, Ubiquitin. Cell Cycle 2011, 10, 3067–3071. [Google Scholar] [CrossRef] [PubMed]
- Kyriakis, J.M.; Avruch, J. Mammalian Mitogen-Activated Protein Kinase Signal Transduction Pathways Activated by Stress and Inflammation. Physiol. Rev. 2001, 81, 807–869. [Google Scholar] [CrossRef] [PubMed]
- Schonthal, A.H. Endoplasmic Reticulum Stress and Autophagy as Targets for Cancer Therapy. Cancer Lett. 2009, 275, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.C.; Chen, Y.J.; Huang, K.H.; Kuo, K.L.; Yang, T.H.; Huang, K.Y.; Wang, C.C.; Tang, C.H.; Yang, R.S.; Liu, S.H. Induction of Sirtuin-1 Signaling by Resveratrol Induces Human Chondrosarcoma Cell Apoptosis and Exhibits Antitumor Activity. Sci. Rep. 2017, 7, 3180. [Google Scholar] [CrossRef] [PubMed]






© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.-H.; Lee, C.-Y.; Huang, T.-J.; Huang, K.-Y.; Tang, C.-H.; Liu, S.-H.; Kuo, K.-L.; Kuan, F.-C.; Lin, W.-C.; Shi, C.-S. MLN4924, a Protein Neddylation Inhibitor, Suppresses the Growth of Human Chondrosarcoma through Inhibiting Cell Proliferation and Inducing Endoplasmic Reticulum Stress-Related Apoptosis. Int. J. Mol. Sci. 2019, 20, 72. https://doi.org/10.3390/ijms20010072
Wu M-H, Lee C-Y, Huang T-J, Huang K-Y, Tang C-H, Liu S-H, Kuo K-L, Kuan F-C, Lin W-C, Shi C-S. MLN4924, a Protein Neddylation Inhibitor, Suppresses the Growth of Human Chondrosarcoma through Inhibiting Cell Proliferation and Inducing Endoplasmic Reticulum Stress-Related Apoptosis. International Journal of Molecular Sciences. 2019; 20(1):72. https://doi.org/10.3390/ijms20010072
Chicago/Turabian StyleWu, Meng-Huang, Ching-Yu Lee, Tsung-Jen Huang, Kuo-Yuan Huang, Chih-Hsin Tang, Shing-Hwa Liu, Kuan-Lin Kuo, Feng-Che Kuan, Wei-Chou Lin, and Chung-Sheng Shi. 2019. "MLN4924, a Protein Neddylation Inhibitor, Suppresses the Growth of Human Chondrosarcoma through Inhibiting Cell Proliferation and Inducing Endoplasmic Reticulum Stress-Related Apoptosis" International Journal of Molecular Sciences 20, no. 1: 72. https://doi.org/10.3390/ijms20010072
APA StyleWu, M.-H., Lee, C.-Y., Huang, T.-J., Huang, K.-Y., Tang, C.-H., Liu, S.-H., Kuo, K.-L., Kuan, F.-C., Lin, W.-C., & Shi, C.-S. (2019). MLN4924, a Protein Neddylation Inhibitor, Suppresses the Growth of Human Chondrosarcoma through Inhibiting Cell Proliferation and Inducing Endoplasmic Reticulum Stress-Related Apoptosis. International Journal of Molecular Sciences, 20(1), 72. https://doi.org/10.3390/ijms20010072