Inhibition of Breast Cancer Cell Invasion by Ras Suppressor-1 (RSU-1) Silencing Is Reversed by Growth Differentiation Factor-15 (GDF-15)
Abstract
1. Introduction
2. Results
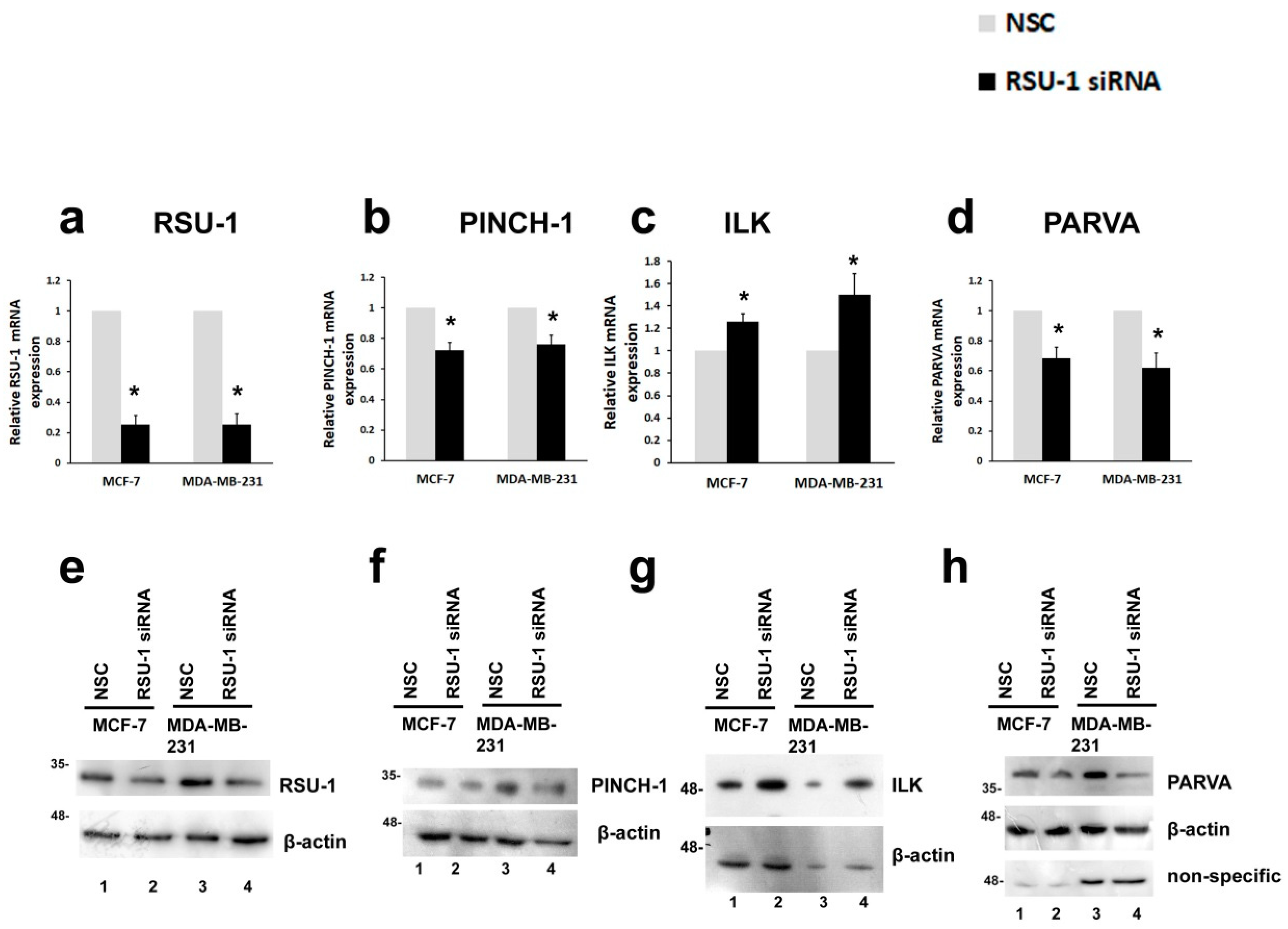
2.1. RSU-1 Silencing in MCF-7 and MDA-MB-231 Cells Downregulates PINCH-1 and PARVA, and Upregulates ILK
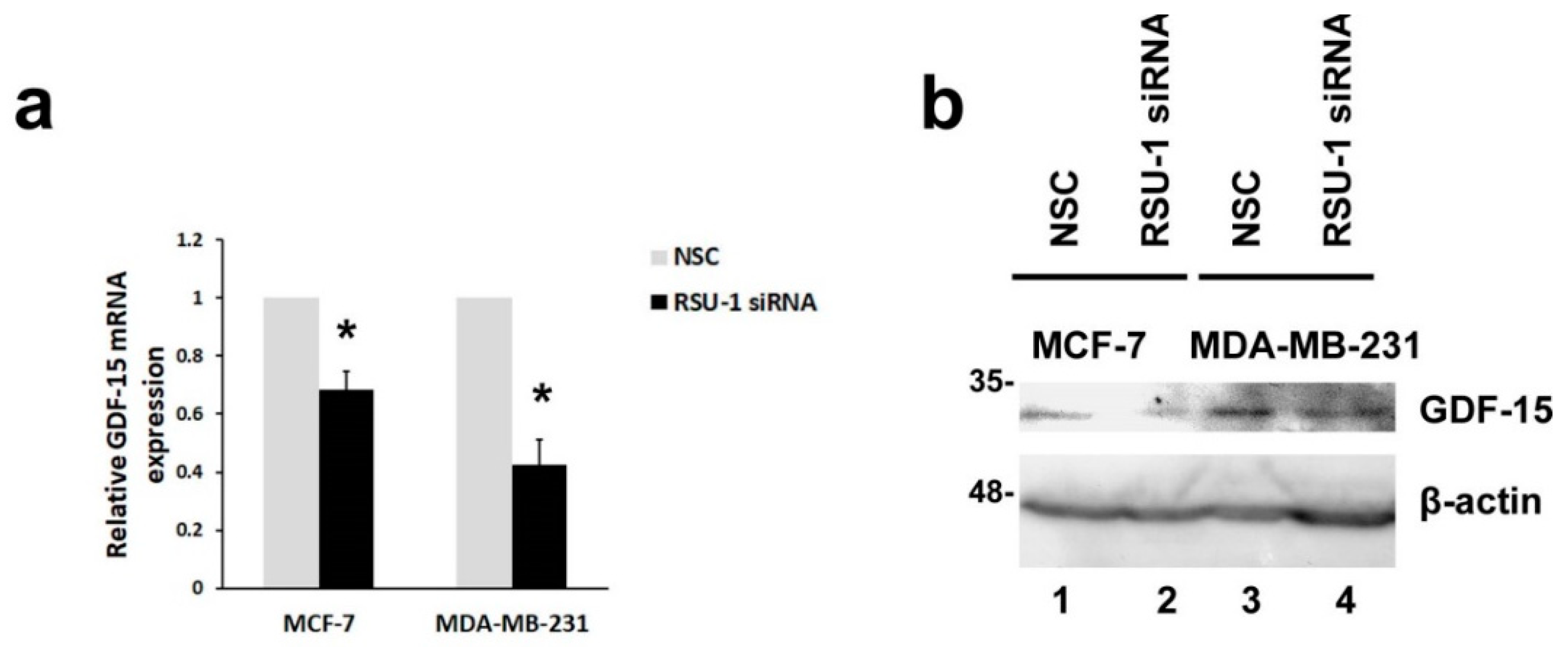
2.2. RSU-1 Depletion from MCF-7 and MDA-MB-231 Cells Leads to Downregulation of GDF-15
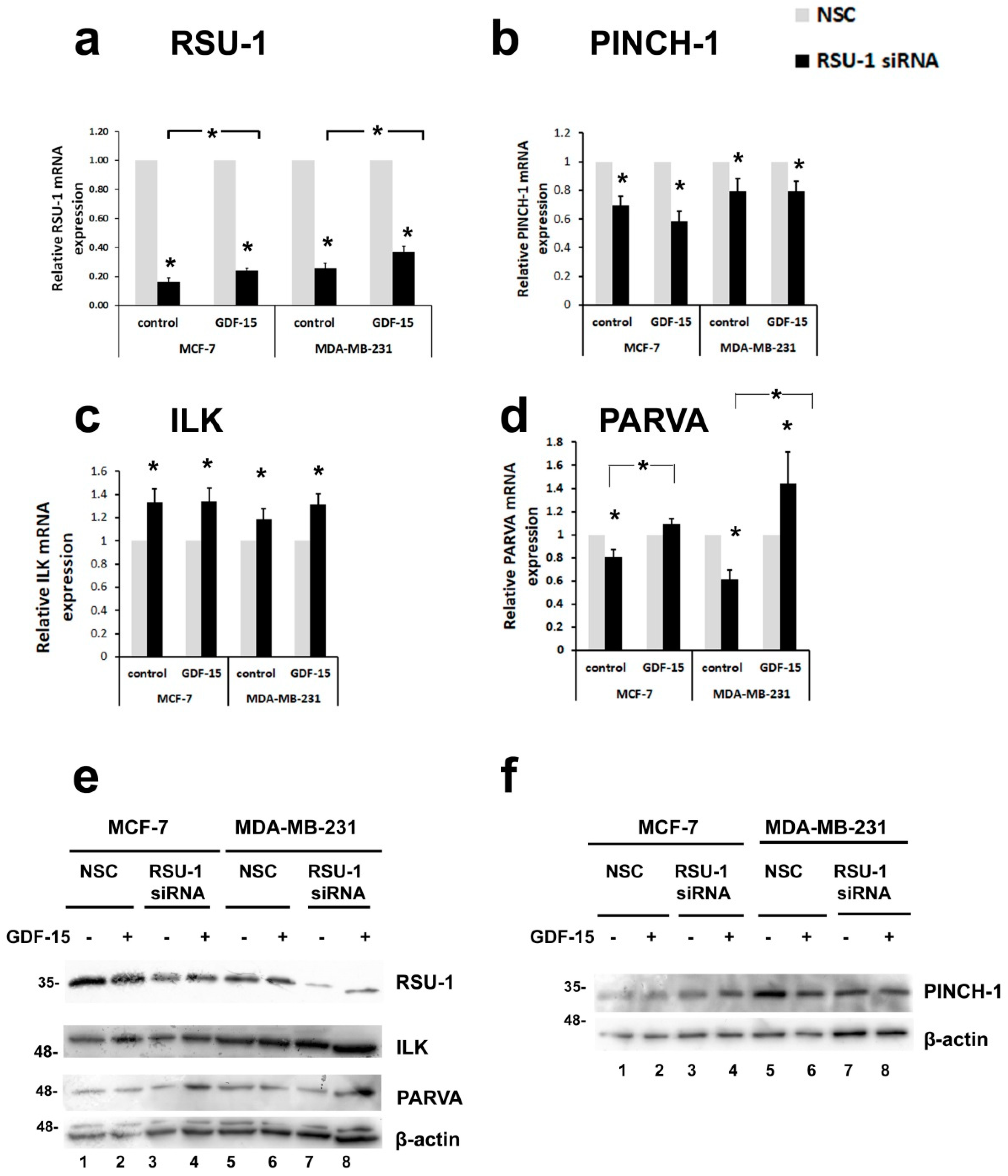
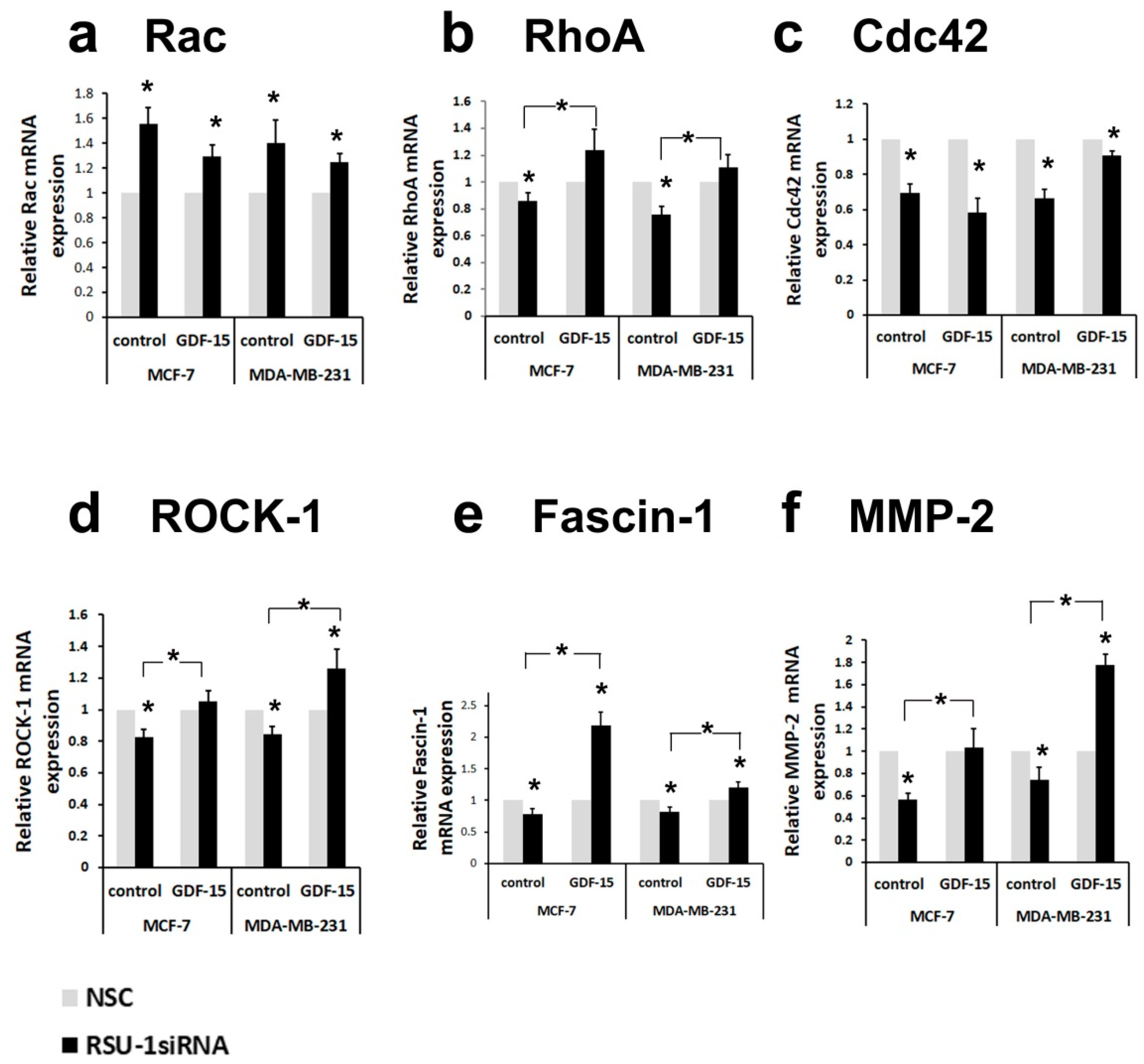
2.3. Human Recombinant GDF-15 (hrGDF-15) Protein Reverses the Effect of RSU-1 Silencing on Gene Expression of Actin-Related Genes
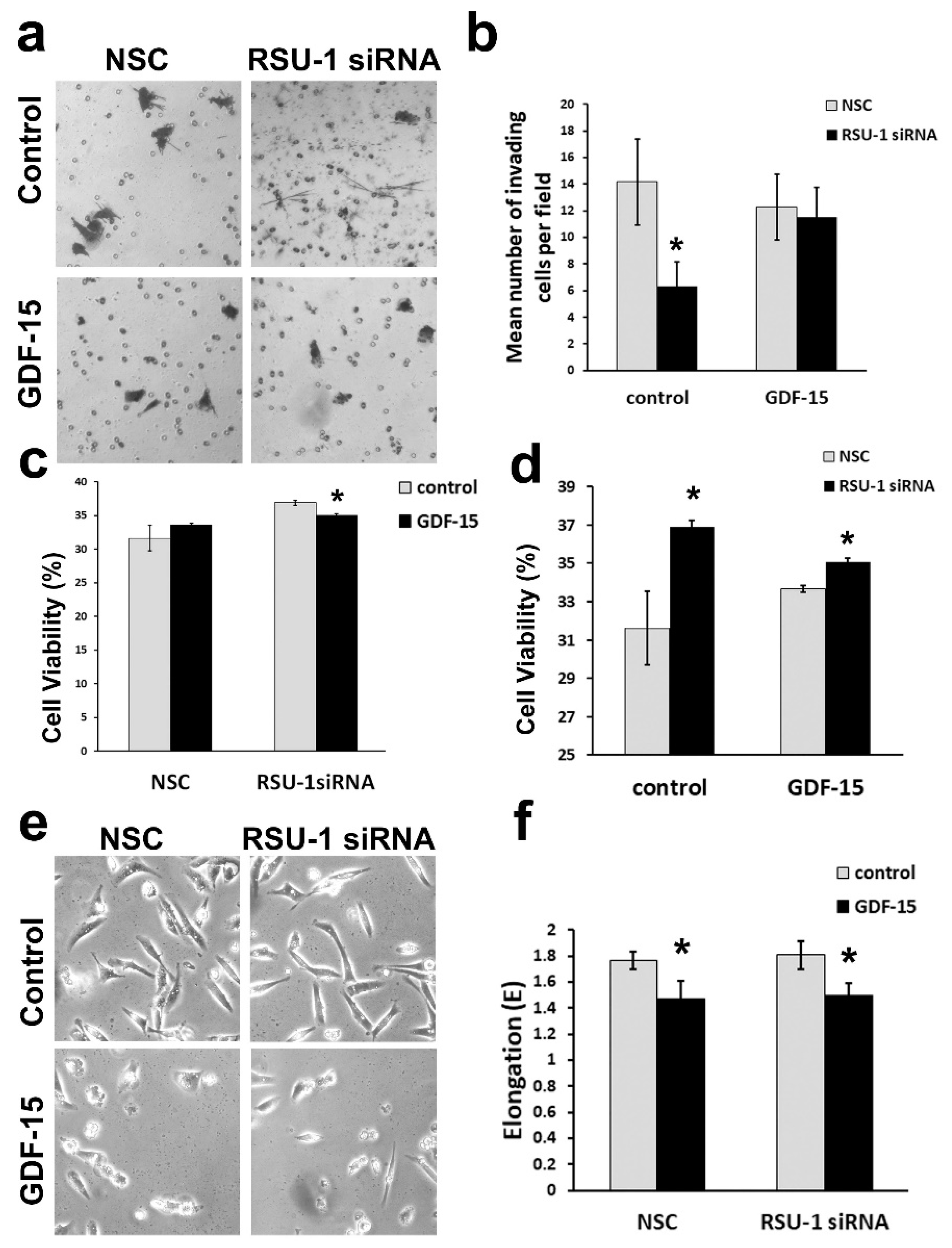
2.4. Treatment of BC Cells Lacking RSU-1 with GDF-15 Rescues the Inhibitory Effect of RSU-1 Silencing on Cell Invasion
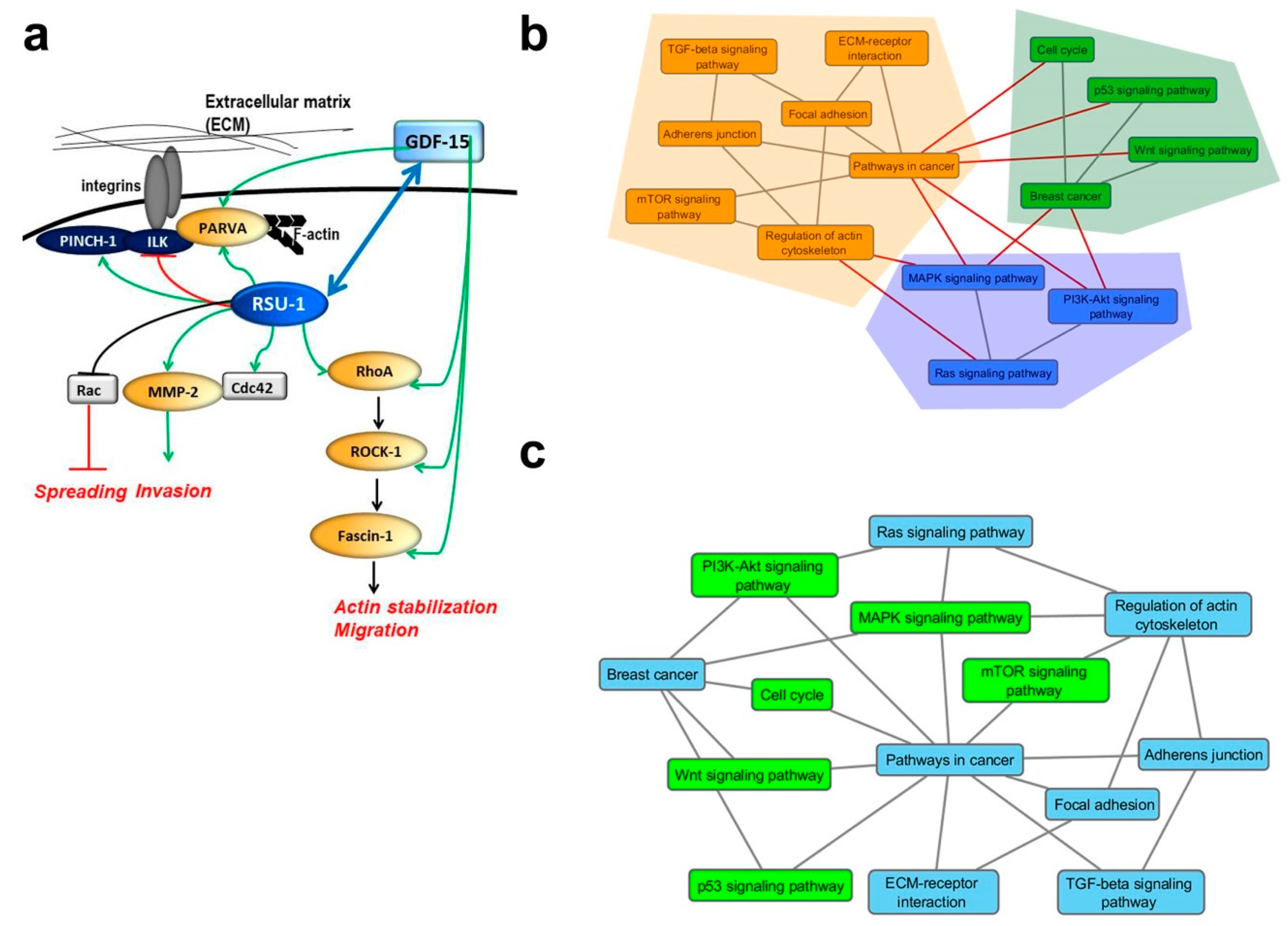
2.5. Graph Clustering Confirms a Connection between Ras Signaling, BC, Cell–ECM Adhesions, and Actin Cytoskeleton
3. Discussion
4. Materials and Methods
4.1. Antibodies and Reagents
4.2. BC Cell Lines
4.3. siRNA Transfection
4.4. GDF-15 Stimulation in siRNA-Transfected Cells
4.5. RNA Isolation and Real-Time PCR
4.6. Protein Extraction and Western Blot Analysis and Quantification
4.7. Cell Invasion Assays
4.8. Cell Viability
4.9. Cell Elongation
4.10. Graph Theory Analysis
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BC | Breast Cancer |
| Cdc42 | cell division control protein-42 |
| ECM | extracellular matrix |
| EMT | epithelial to mesenchymal transition |
| GDF-15 | Growth Differentiation Factor-15 |
| GFRAL | GDNF-family receptor a-like |
| ILK | integrin-linked kinase |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| MAPK | mitogen-activated protein kinase |
| MMP | Metalloproteinases |
| NSC | non-specific control |
| PARVA | alpha-parvin |
| PINCH-1 | Particularly Interesting New Cysteine-Histidine rich protein |
| ROCK-1 | Rho associated kinase-1 |
| RSU-1 | Ras suppressor-1 |
| TGF-β | Transforming Growth Factor-β |
References
- Giuliani, J.; Bonetti, A. Trends in survival for patients with metastatic breast cancer: Is survival improving? Tumori J. 2015, 101, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Bidirectional, allosteric signaling machines. Cell 2002, 110, 673–687. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Beavon, I.R. The E-cadherin-catenin complex in tumour metastasis: Structure, function and regulation. Eur. J. Cancer 2000, 36, 1607–1620. [Google Scholar] [CrossRef]
- Canel, M.; Serrels, A.; Frame, M.C.; Brunton, V.G. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J. Cell. Sci. 2013, 126, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Maller, O.; DuFort, C.C.; Weaver, V.M. YAP forces fibroblasts to feel the tension. Nat. Cell Biol. 2013, 15, 570–572. [Google Scholar] [CrossRef] [PubMed]
- Gkretsi, V.; Stylianopoulos, T. Cell Adhesion and Matrix Stiffness: Coordinating Cancer Cell Invasion and Metastasis. Front. Oncol. 2018, 8, 145. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielinska, W.; Grzanka, D.; Gagat, M. The Role of Actin Dynamics and Actin-Binding Proteins Expression in Epithelial-to-Mesenchymal Transition and Its Association with Cancer Progression and Evaluation of Possible Therapeutic Targets. BioMed Res. Int. 2018, 2018, 4578373. [Google Scholar] [CrossRef]
- Jain, R.K.; Martin, J.D.; Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 2014, 16, 321–346. [Google Scholar] [CrossRef]
- Tse, J.M.; Cheng, G.; Tyrrell, J.A.; Wilcox-Adelman, S.A.; Boucher, Y.; Jain, R.K.; Munn, L.L. Mechanical compression drives cancer cells toward invasive phenotype. Proc. Natl. Acad. Sci. USA 2012, 109, 911–916. [Google Scholar] [CrossRef]
- Demou, Z.N. Gene expression profiles in 3D tumor analogs indicate compressive strain differentially enhances metastatic potential. Ann. Biomed. Eng. 2010, 38, 3509–3520. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Tse, J.; Jain, R.K.; Munn, L.L. Micro-environmental mechanical stress controls tumor spheroid size and morphology by suppressing proliferation and inducing apoptosis in cancer cells. PLoS ONE 2009, 4, e4632. [Google Scholar] [CrossRef] [PubMed]
- Delarue, M.; Montel, F.; Vignjevic, D.; Prost, J.; Joanny, J.F.; Cappello, G. Compressive stress inhibits proliferation in tumor spheroids through a volume limitation. Biophys. J. 2014, 107, 1821–1828. [Google Scholar] [CrossRef] [PubMed]
- Helmlinger, G.; Netti, P.A.; Lichtenbeld, H.C.; Melder, R.J.; Jain, R.K. Solid stress inhibits the growth of multicellular tumor spheroids. Nat. Biotechnol. 1997, 15, 778–783. [Google Scholar] [CrossRef] [PubMed]
- Kaufman, L.J.; Brangwynne, C.P.; Kasza, K.E.; Filippidi, E.; Gordon, V.D.; Deisboeck, T.S.; Weitz, D.A. Glioma expansion in collagen I matrices: Analyzing collagen concentration-dependent growth and motility patterns. Biophys. J. 2005, 89, 635–650. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Stylianopoulos, T. Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis. Front. Oncol. 2018, 8. [Google Scholar] [CrossRef]
- Stylianopoulos, T.; Munn, L.L.; Jain, R.K. Reengineering the Physical Microenvironment of Tumors to Improve Drug Delivery and Efficacy: From Mathematical Modeling to Bench to Bedside. Trends Cancer 2018, 4, 292–319. [Google Scholar] [CrossRef]
- Gkretsi, V.; Stylianou, A.; Louca, M.; Stylianopoulos, T. Identification of Ras suppressor-1 (RSU-1) as a potential breast cancer metastasis biomarker using a three-dimensional in vitro approach. Oncotarget 2017, 8, 27364–27379. [Google Scholar] [CrossRef]
- Dougherty, G.W.; Chopp, T.; Qi, S.M.; Cutler, M.L. The Ras suppressor Rsu-1 binds to the LIM 5 domain of the adaptor protein PINCH1 and participates in adhesion-related functions. Exp. Cell Res. 2005, 306, 168–179. [Google Scholar] [CrossRef]
- Qin, J.; Wu, C. ILK: A pseudokinase in the center stage of cell-matrix adhesion and signaling. Curr. Opin. Cell Biol. 2012, 24, 607–613. [Google Scholar] [CrossRef]
- Wu, C. The PINCH-ILK-parvin complexes: Assembly, functions and regulation. Biochim. Biophys. Acta 2004, 1692, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Chen, K.; Tu, Y.; Velyvis, A.; Yang, Y.; Qin, J.; Wu, C. Assembly of the PINCH-ILK-CH-ILKBP complex precedes and is essential for localization of each component to cell-matrix adhesion sites. J. Cell Sci. 2002, 115, 4777–4786. [Google Scholar] [CrossRef] [PubMed]
- Cutler, M.L.; Bassin, R.H.; Zanoni, L.; Talbot, N. Isolation of rsp-1, a novel cDNA capable of suppressing v-Ras transformation. Mol. Cell. Biol. 1992, 12, 3750–3756. [Google Scholar] [CrossRef] [PubMed]
- Tsuda, T.; Marinetti, M.R.; Masuelli, L.; Cutler, M.L. The Ras suppressor RSU-1 localizes to 10p13 and its expression in the U251 glioblastoma cell line correlates with a decrease in growth rate and tumorigenic potential. Oncogene 1995, 11, 397–403. [Google Scholar]
- Vasaturo, F.; Dougherty, G.W.; Cutler, M.L. Ectopic expression of Rsu-1 results in elevation of p21CIP and inhibits anchorage-independent growth of MCF7 breast cancer cells. Breast Cancer Res. Treat. 2000, 61, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Donthamsetty, S.; Bhave, V.S.; Mars, W.M.; Bowen, W.C.; Orr, A.; Haynes, M.M.; Wu, C.; Michalopoulos, G.K. Role of PINCH and its partner tumor suppressor Rsu-1 in regulating liver size and tumorigenesis. PLoS ONE 2013, 8, e74625. [Google Scholar] [CrossRef]
- Qiu, R.G.; Chen, J.; McCormick, F.; Symons, M. A role for Rho in Ras transformation. Proc. Natl. Acad. Sci. USA 1995, 92, 11781–11785. [Google Scholar] [CrossRef] [PubMed]
- Bar-Sagi, D.; Hall, A. Ras and Rho GTPases: A family reunion. Cell 2000, 103, 227–238. [Google Scholar] [CrossRef]
- Barbazan, J.; Alonso-Alconada, L.; Muinelo-Romay, L.; Vieito, M.; Abalo, A.; Alonso-Nocelo, M.; Candamio, S.; Gallardo, E.; Fernandez, B.; Abdulkader, I.; et al. Molecular characterization of circulating tumor cells in human metastatic colorectal cancer. PLoS ONE 2012, 7, e40476. [Google Scholar] [CrossRef] [PubMed]
- Giotopoulou, N.; Valiakou, V.; Papanikolaou, V.; Dubos, S.; Athanassiou, E.; Tsezou, A.; Zacharia, L.C.; Gkretsi, V. Ras suppressor-1 promotes apoptosis in breast cancer cells by inhibiting PINCH-1 and activating p53-upregulated-modulator of apoptosis (PUMA); verification from metastatic breast cancer human samples. Clin. Exp. Metastasis 2015, 32, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Gkretsi, V.; Bogdanos, D.P. Elimination of Ras Suppressor-1 from hepatocellular carcinoma cells hinders their in vitro metastatic properties. Anticancer Res. 2015, 35, 1509–1512. [Google Scholar]
- Bootcov, M.R.; Bauskin, A.R.; Valenzuela, S.M.; Moore, A.G.; Bansal, M.; He, X.Y.; Zhang, H.P.; Donnellan, M.; Mahler, S.; Pryor, K.; et al. MIC-1, a novel macrophage inhibitory cytokine, is a divergent member of the TGF-beta superfamily. Proc. Natl. Acad. Sci. USA 1997, 94, 11514–11519. [Google Scholar] [CrossRef] [PubMed]
- Hromas, R.; Hufford, M.; Sutton, J.; Xu, D.; Li, Y.; Lu, L. PLAB, a novel placental bone morphogenetic protein. Biochim. Biophys. Acta 1997, 1354, 40–44. [Google Scholar] [CrossRef]
- Lawton, L.N.; Bonaldo, M.F.; Jelenc, P.C.; Qiu, L.; Baumes, S.A.; Marcelino, R.A.; de Jesus, G.M.; Wellington, S.; Knowles, J.A.; Warburton, D.; et al. Identification of a novel member of the TGF-beta superfamily highly expressed in human placenta. Gene 1997, 203, 17–26. [Google Scholar] [CrossRef]
- Baek, S.J.; Kim, K.S.; Nixon, J.B.; Wilson, L.C.; Eling, T.E. Cyclooxygenase inhibitors regulate the expression of a TGF-beta superfamily member that has proapoptotic and antitumorigenic activities. Mol. Pharmacol. 2001, 59, 901–908. [Google Scholar] [CrossRef] [PubMed]
- Kalli, M.; Papageorgis, P.; Gkretsi, V.; Stylianopoulos, T. Solid Stress Facilitates Fibroblasts Activation to Promote Pancreatic Cancer Cell Migration. Ann. Biomed. Eng. 2018, 46, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Aw Yong, K.M.; Zeng, Y.; Vindivich, D.; Phillip, J.M.; Wu, P.H.; Wirtz, D.; Getzenberg, R.H. Morphological effects on expression of growth differentiation factor 15 (GDF15), a marker of metastasis. J. Cell. Physiol. 2014, 229, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Unsicker, K.; Spittau, B.; Krieglstein, K. The multiple facets of the TGF-beta family cytokine growth/differentiation factor-15/macrophage inhibitory cytokine-1. Cytokine Growth Factor Rev. 2013, 24, 373–384. [Google Scholar] [CrossRef] [PubMed]
- Wallin, U.; Glimelius, B.; Jirstrom, K.; Darmanis, S.; Nong, R.Y.; Ponten, F.; Johansson, C.; Pahlman, L.; Birgisson, H. Growth differentiation factor 15: A prognostic marker for recurrence in colorectal cancer. Br. J. Cancer 2011, 104, 1619–1627. [Google Scholar] [CrossRef] [PubMed]
- Fairlie, W.D.; Moore, A.G.; Bauskin, A.R.; Russell, P.K.; Zhang, H.P.; Breit, S.N. MIC-1 is a novel TGF-beta superfamily cytokine associated with macrophage activation. J. Leukoc. Biol. 1999, 65, 2–5. [Google Scholar] [CrossRef]
- Lee, D.H.; Yang, Y.; Lee, S.J.; Kim, K.Y.; Koo, T.H.; Shin, S.M.; Song, K.S.; Lee, Y.H.; Kim, Y.J.; Lee, J.J.; et al. Macrophage inhibitory cytokine-1 induces the invasiveness of gastric cancer cells by up-regulating the urokinase-type plasminogen activator system. Cancer Res. 2003, 63, 4648–4655. [Google Scholar]
- Li, C.; Wang, J.; Kong, J.; Tang, J.; Wu, Y.; Xu, E.; Zhang, H.; Lai, M. GDF15 promotes EMT and metastasis in colorectal cancer. Oncotarget 2016, 7, 860–872. [Google Scholar] [CrossRef]
- Paralkar, V.M.; Vail, A.L.; Grasser, W.A.; Brown, T.A.; Xu, H.; Vukicevic, S.; Ke, H.Z.; Qi, H.; Owen, T.A.; Thompson, D.D. Cloning and characterization of a novel member of the transforming growth factor-beta/bone morphogenetic protein family. J. Biol. Chem. 1998, 273, 13760–13767. [Google Scholar] [CrossRef]
- Strelau, J.; Schmeer, C.; Peterziel, H.; Sackmann, T.; Herold-Mende, C.; Steiner, H.; Weller, M.; Unsicker, K. Expression and putative functions of GDF-15, a member of the TGF-beta superfamily, in human glioma and glioblastoma cell lines. Cancer Lett. 2008, 270, 30–39. [Google Scholar] [CrossRef]
- Zimmers, T.A.; Jin, X.; Gutierrez, J.C.; Acosta, C.; McKillop, I.H.; Pierce, R.H.; Koniaris, L.G. Effect of in vivo loss of GDF-15 on hepatocellular carcinogenesis. J. Cancer Res. Clin. Oncol. 2008, 134, 753–759. [Google Scholar] [CrossRef]
- Prudnikova, T.Y.; Rawat, S.J.; Chernoff, J. Molecular pathways: Targeting the kinase effectors of RHO-family GTPases. Clin. Cancer Res. 2015, 21, 24–29. [Google Scholar] [CrossRef]
- Dougherty, G.W.; Jose, C.; Gimona, M.; Cutler, M.L. The Rsu-1-PINCH1-ILK complex is regulated by Ras activation in tumor cells. Eur. J. Cell. Biol. 2008, 87, 721–734. [Google Scholar] [CrossRef]
- Kadrmas, J.L.; Smith, M.A.; Clark, K.A.; Pronovost, S.M.; Muster, N.; Yates, J.R.; 3rd Beckerle, M.C. The integrin effector PINCH regulates JNK activity and epithelial migration in concert with Ras suppressor 1. J. Cell. Biol. 2004, 167, 1019–1024. [Google Scholar] [CrossRef]
- Chen, E.Y.; Tan, C.M.; Kou, Y.; Duan, Q.; Wang, Z.; Meirelles, G.V.; Clark, N.R.; Ma’ayan, A. Enrichr: Interactive and collaborative HTML5 gene list enrichment analysis tool. BMC Bioinform. 2013, 14, 128. [Google Scholar] [CrossRef]
- Kuleshov, M.V.; Jones, M.R.; Rouillard, A.D.; Fernandez, N.F.; Duan, Q.; Wang, Z.; Koplev, S.; Jenkins, S.L.; Jagodnik, K.M.; Lachmann, A.; et al. Enrichr: A comprehensive gene set enrichment analysis web server 2016 update. Nucleic Acids Res. 2016, 44, W90-7. [Google Scholar] [CrossRef]
- Newman, M.E.; Girvan, M. Finding and evaluating community structure in networks. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 2004, 69, 026113. [Google Scholar] [CrossRef]
- Zacharia, L.C.; Stylianopoulos, T.; Gkretsi, V. Ras Suppressor-1 (RSU-1) in Cancer Cell Metastasis: Friend or Foe? Crit. Rev. Oncog. 2017, 22, 249–253. [Google Scholar] [CrossRef]
- Zacharia, L.C.; Gkretsi, V. The Ras suppressor-1 (RSU-1) in cancer. Adv. Mod. Oncol. Res. 2017, 3. [Google Scholar] [CrossRef]
- Zhang, J.T.; Li, Q.X.; Wang, D.; Zhu, Z.L.; Yang, Y.H.; Cui, D.S.; Wang, M.W.; Sun, X.F. Up-regulation of PINCH in the stroma of oral squamous cell carcinoma predicts nodal metastasis. Oncol. Rep. 2005, 14, 1519–1522. [Google Scholar] [CrossRef]
- Tsinias, G.; Nikou, S.; Papadas, T.; Pitsos, P.; Papadaki, H.; Bravou, V. High PINCH1 Expression in Human Laryngeal Carcinoma Associates with Poor Prognosis. Anal. Cell. Pathol. (Amst) 2018, 2018, 2989635. [Google Scholar] [CrossRef]
- Yoganathan, N.; Yee, A.; Zhang, Z.; Leung, D.; Yan, J.; Fazli, L.; Kojic, D.L.; Costello, P.C.; Jabali, M.; Dedhar, S.; et al. Integrin-linked kinase, a promising cancer therapeutic target: Biochemical and biological properties. Pharmacol. Ther. 2002, 93, 233–242. [Google Scholar] [CrossRef]
- Chen, D.; Zhang, Y.; Zhang, X.; Li, J.; Han, B.; Liu, S.; Wang, L.; Ling, Y.; Mao, S.; Wang, X. Overexpression of integrin-linked kinase correlates with malignant phenotype in non-small cell lung cancer and promotes lung cancer cell invasion and migration via regulating epithelial-mesenchymal transition (EMT)-related genes. Acta Histochem. 2013, 115, 128–136. [Google Scholar] [CrossRef]
- Zhao, M.; Gao, Y.; Wang, L.; Liu, S.; Han, B.; Ma, L.; Ling, Y.; Mao, S.; Wang, X. Overexpression of integrin-linked kinase promotes lung cancer cell migration and invasion via NF-kappaB-mediated upregulation of matrix metalloproteinase-9. Int. J. Med. Sci. 2013, 10, 995–1002. [Google Scholar] [CrossRef]
- Chen, P.; Shen, W.Z.; Karnik, P. Suppression of malignant growth of human breast cancer cells by ectopic expression of integrin-linked kinase. Int. J. Cancer 2004, 111, 881–891. [Google Scholar] [CrossRef]
- Pignatelli, J.; LaLonde, S.E.; LaLonde, D.P.; Clarke, D.; Turner, C.E. Actopaxin (alpha-parvin) phosphorylation is required for matrix degradation and cancer cell invasion. J. Biol. Chem. 2012, 287, 37309–37320. [Google Scholar] [CrossRef]
- Ito, M.; Hagiyama, M.; Mimae, T.; Inoue, T.; Kato, T.; Yoneshige, A.; Nakanishi, J.; Kondo, T.; Okada, M.; Ito, A. alpha-Parvin, a pseudopodial constituent, promotes cell motility and is associated with lymph node metastasis of lobular breast carcinoma. Breast Cancer Res. Treat. 2014, 144, 59–69. [Google Scholar] [CrossRef]
- Srinivasan, S.; Ashok, V.; Mohanty, S.; Das, A.; Das, S.; Kumar, S.; Sen, S.; Purwar, R. Blockade of Rho-associated protein kinase (ROCK) inhibits the contractility and invasion potential of cancer stem like cells. Oncotarget 2017, 8, 21418–21428. [Google Scholar] [CrossRef]
- Li, D.; Jin, L.; Alesi, G.N.; Kim, Y.M.; Fan, J.; Seo, J.H.; Wang, D.; Tucker, M.; Gu, T.L.; Lee, B.H.; et al. The prometastatic ribosomal S6 kinase 2-cAMP response element-binding protein (RSK2-CREB) signaling pathway up-regulates the actin-binding protein fascin-1 to promote tumor metastasis. J. Biol. Chem. 2013, 288, 32528–32538. [Google Scholar] [CrossRef]
- Fritz, G.; Brachetti, C.; Bahlmann, F.; Schmidt, M.; Kaina, B. Rho GTPases in human breast tumours: Expression and mutation analyses and correlation with clinical parameters. Br. J. Cancer 2002, 87, 635–644. [Google Scholar] [CrossRef]
- Peake, B.F.; Eze, S.M.; Yang, L.; Castellino, R.C.; Nahta, R. Growth differentiation factor 15 mediates epithelial mesenchymal transition and invasion of breast cancers through IGF-1R-FoxM1 signaling. Oncotarget 2017, 8, 94393–94406. [Google Scholar] [CrossRef]
- Breit, S.N.; Johnen, H.; Cook, A.D.; Tsai, V.W.; Mohammad, M.G.; Kuffner, T.; Zhang, H.P.; Marquis, C.P.; Jiang, L.; Lockwood, G.; et al. The TGF-beta superfamily cytokine, MIC-1/GDF15: A pleotrophic cytokine with roles in inflammation, cancer and metabolism. Growth Factors 2011, 29, 187–195. [Google Scholar] [CrossRef]
- Codo, P.; Weller, M.; Kaulich, K.; Schraivogel, D.; Silginer, M.; Reifenberger, G.; Meister, G.; Roth, P. Control of glioma cell migration and invasiveness by GDF-15. Oncotarget 2016, 7, 7732–7746. [Google Scholar] [CrossRef]
- Czuchra, A.; Wu, X.; Meyer, H.; van Hengel, J.; Schroeder, T.; Geffers, R.; Rottner, K.; Brakebusch, C. Cdc42 is not essential for filopodium formation, directed migration, cell polarization, and mitosis in fibroblastoid cells. Mol. Biol. Cell 2005, 16, 4473–4484. [Google Scholar] [CrossRef]
- Senapati, S.; Rachagani, S.; Chaudhary, K.; Johansson, S.L.; Singh, R.K.; Batra, S.K. Overexpression of macrophage inhibitory cytokine-1 induces metastasis of human prostate cancer cells through the FAK-RhoA signaling pathway. Oncogene 2010, 29, 1293–1302. [Google Scholar] [CrossRef]
- Breit, S.N.; Tsai, V.W.; Brown, D.A. Targeting Obesity and Cachexia: Identification of the GFRAL Receptor-MIC-1/GDF15 Pathway. Trends Mol. Med. 2017, 23, 1065–1067. [Google Scholar] [CrossRef]
- Emmerson, P.J.; Wang, F.; Du, Y.; Liu, Q.; Pickard, R.T.; Gonciarz, M.D.; Coskun, T.; Hamang, M.J.; Sindelar, D.K.; Ballman, K.K.; et al. The metabolic effects of GDF15 are mediated by the orphan receptor GFRAL. Nat. Med. 2017, 23, 1215–1219. [Google Scholar] [CrossRef]
- Mullican, S.E.; Lin-Schmidt, X.; Chin, C.N.; Chavez, J.A.; Furman, J.L.; Armstrong, A.A.; Beck, S.C.; South, V.J.; Dinh, T.Q.; Cash-Mason, T.D.; et al. GFRAL is the receptor for GDF15 and the ligand promotes weight loss in mice and nonhuman primates. Nat. Med. 2017, 23, 1150–1157. [Google Scholar] [CrossRef]
- Yang, L.; Chang, C.C.; Sun, Z.; Madsen, D.; Zhu, H.; Padkjaer, S.B.; Wu, X.; Huang, T.; Hultman, K.; Paulsen, S.J.; et al. GFRAL is the receptor for GDF15 and is required for the anti-obesity effects of the ligand. Nat. Med. 2017, 23, 1158–1166. [Google Scholar] [CrossRef]
- Gkretsi, V.; Bogdanos, D.P. Experimental evidence of Migfilin as a new therapeutic target of hepatocellular carcinoma metastasis. Exp. Cell Res. 2015, 334, 219–227. [Google Scholar] [CrossRef]
- Al-Nasiry, S.; Geusens, N.; Hanssens, M.; Luyten, C.; Pijnenborg, R. The use of Alamar Blue assay for quantitative analysis of viability, migration and invasion of choriocarcinoma cells. Hum. Reprod. 2007, 22, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, A.; Gkretsi, V.; Stylianopoulos, T. Transforming growth factor-beta modulates pancreatic cancer associated fibroblasts cell shape, stiffness and invasion. Biochim. Biophys. Acta 2018. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D.; Alexandratou, E. Nanotopography of collagen thin films in correlation with fibroblast response. J. Nanophotonics 2013, 7, 073590. [Google Scholar] [CrossRef]
- Stylianou, A.; Yova, D. Atomic force microscopy investigation of the interaction of low-level laser irradiation of collagen thin films in correlation with fibroblast response. Lasers Med. Sci. 2015, 30, 2369–2379. [Google Scholar] [CrossRef]
- Yim, E.K.; Reano, R.M.; Pang, S.W.; Yee, A.F.; Chen, C.S.; Leong, K.W. Nanopattern-induced changes in morphology and motility of smooth muscle cells. Biomaterials 2005, 26, 5405–5413. [Google Scholar] [CrossRef]
- Zachariou, M.; Minadakis, G.; Oulas, A.; Afxenti, S.; Spyrou, G.M. Integrating multi-source information on a single network to detect disease-related clusters of molecular mechanisms. J. Proteomics 2018. [Google Scholar] [CrossRef]
- Kakouri, A.; Christodoulou, C.; Zachariou, M.; Oulas, A.; Minadakis, G.; Demetriou, C.; Votsi, C.; Papanicolaou-Zamba, E.; Kyproula, C.; Spyrou, G. Revealing Clusters of Connected Pathways through Multisource Data Integration in Huntington’s disease and Spastic Ataxia. IEEE J. Biomed. Health Inform. 2018. [Google Scholar] [CrossRef] [PubMed]
- Minadakis, G.; Zachariou, M.; Oulas, A.; Spyrou, G.M. PathwayConnector: Finding complementary pathways to enhance functional analysis. Bioinformatics 2018. [Google Scholar] [CrossRef] [PubMed]
- Suesskind, D.; Schatz, A.; Schnichels, S.; Coupland, S.E.; Lake, S.L.; Wissinger, B.; Bartz-Schmidt, K.U.; Henke-Fahle, S. GDF-15: A novel serum marker for metastases in uveal melanoma patients. Graefes Arch. Clin. Exp. Ophthalmol. 2012, 250, 887–895. [Google Scholar] [CrossRef] [PubMed]
| Primer Name | Sequence |
|---|---|
| Cdc42 | Forward: 5′-GCCCGTGACCTGAAGGCTGTCA-3′ |
| Reverse: 5′-TGCTTTTAGTATGATGCCGACACCA-3′ | |
| Fascin-1 | Forward: 5′-AGCTGCTACTTTGACATCGA-3′ |
| Reverse: 5′-TCATGAGGAAGAGCTCTGAGT-3′ | |
| GDF-15 | Forward: 5′-TCAAGGTCGTGGGACGTGACA-3′ |
| Reverse: 5′-GCCGTGCGGACGAAGATTCT-3′ | |
| ILK | Forward: 5′-GACATGACTGCCCGAATTAG-3′ |
| Reverse: 5′-CTGAGCGTCTGTTTGTGTCT-3′ | |
| MMP-2 | Forward: 5′-ATGACAGCTGCACCACTGAG-3′ |
| Reverse: 5′-AGTTCCCACCAACAGTGGAC-3′ | |
| PARVA | Forward: 5′-CAATTCGACTCCCAGACCAT-3′ |
| Reverse: 5′-TGGTCGAACAAGGTGTCAAA-3′ | |
| PINCH-1 | Forward: 5′-CCGCTGAGAAGATCGTGAAC-3′ |
| Reverse: 5′-GGGCAAAGAGCATCTGAAAG-3′ | |
| Rac1 | Forward: 5′-AACCAATGCATTTCCTGGAG-3′ |
| Reverse: 5′-CAGATTCACCGGTTTTCCAT-3′ | |
| RhoA | Forward: 5′-CGGGAGCTAGCCAAGATGAAG-3′ |
| Reverse: 5′-CCTTGCAGAGCAGCTCTCGTA-3′ | |
| ROCK-1 | Forward: 5′-ACCTGTAACCCAAGGAGATGTG-3′ |
| Reverse: 5′-CACAATTGGCAGGAAAGTGG-3′ | |
| RSU-1 | Forward: 5′-AGGCCACAGAGCAAGGTCTA-3′ |
| Reverse: 5′-CGTGCAATCTCAAAAGCTCA-3′ | |
| β-actin | Forward: 5′-CGAGCACAGAGCCTCGCCTTTGCC-3′ |
| Reverse: 5′-TGTCGACGACGAGCGCGGCGATAT-3′ |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gkretsi, V.; Louca, M.; Stylianou, A.; Minadakis, G.; Spyrou, G.M.; Stylianopoulos, T. Inhibition of Breast Cancer Cell Invasion by Ras Suppressor-1 (RSU-1) Silencing Is Reversed by Growth Differentiation Factor-15 (GDF-15). Int. J. Mol. Sci. 2019, 20, 163. https://doi.org/10.3390/ijms20010163
Gkretsi V, Louca M, Stylianou A, Minadakis G, Spyrou GM, Stylianopoulos T. Inhibition of Breast Cancer Cell Invasion by Ras Suppressor-1 (RSU-1) Silencing Is Reversed by Growth Differentiation Factor-15 (GDF-15). International Journal of Molecular Sciences. 2019; 20(1):163. https://doi.org/10.3390/ijms20010163
Chicago/Turabian StyleGkretsi, Vasiliki, Maria Louca, Andreas Stylianou, George Minadakis, George M. Spyrou, and Triantafyllos Stylianopoulos. 2019. "Inhibition of Breast Cancer Cell Invasion by Ras Suppressor-1 (RSU-1) Silencing Is Reversed by Growth Differentiation Factor-15 (GDF-15)" International Journal of Molecular Sciences 20, no. 1: 163. https://doi.org/10.3390/ijms20010163
APA StyleGkretsi, V., Louca, M., Stylianou, A., Minadakis, G., Spyrou, G. M., & Stylianopoulos, T. (2019). Inhibition of Breast Cancer Cell Invasion by Ras Suppressor-1 (RSU-1) Silencing Is Reversed by Growth Differentiation Factor-15 (GDF-15). International Journal of Molecular Sciences, 20(1), 163. https://doi.org/10.3390/ijms20010163







