Nanoparticles and Nanomaterials as Plant Biostimulants
Abstract
1. Introduction
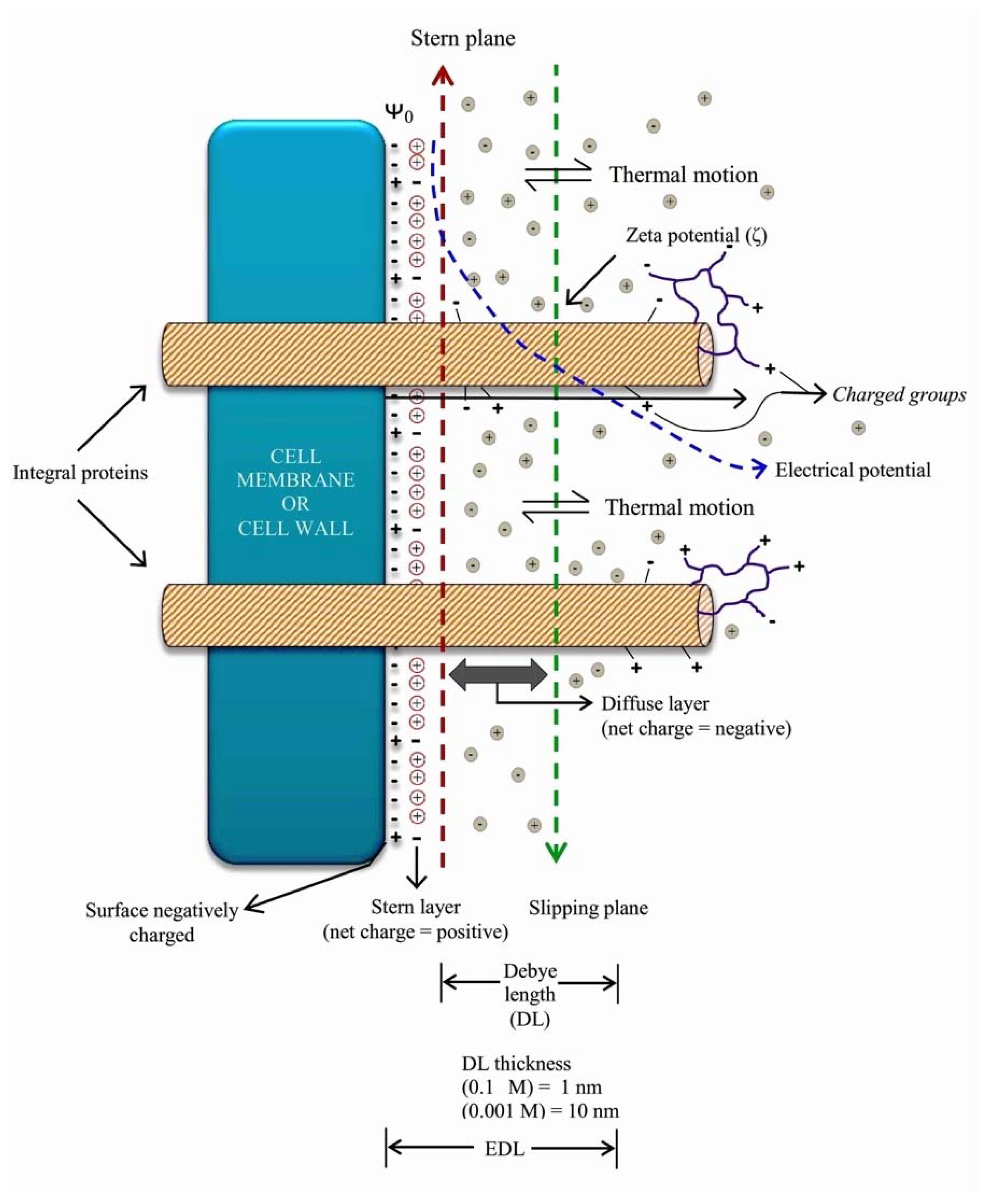
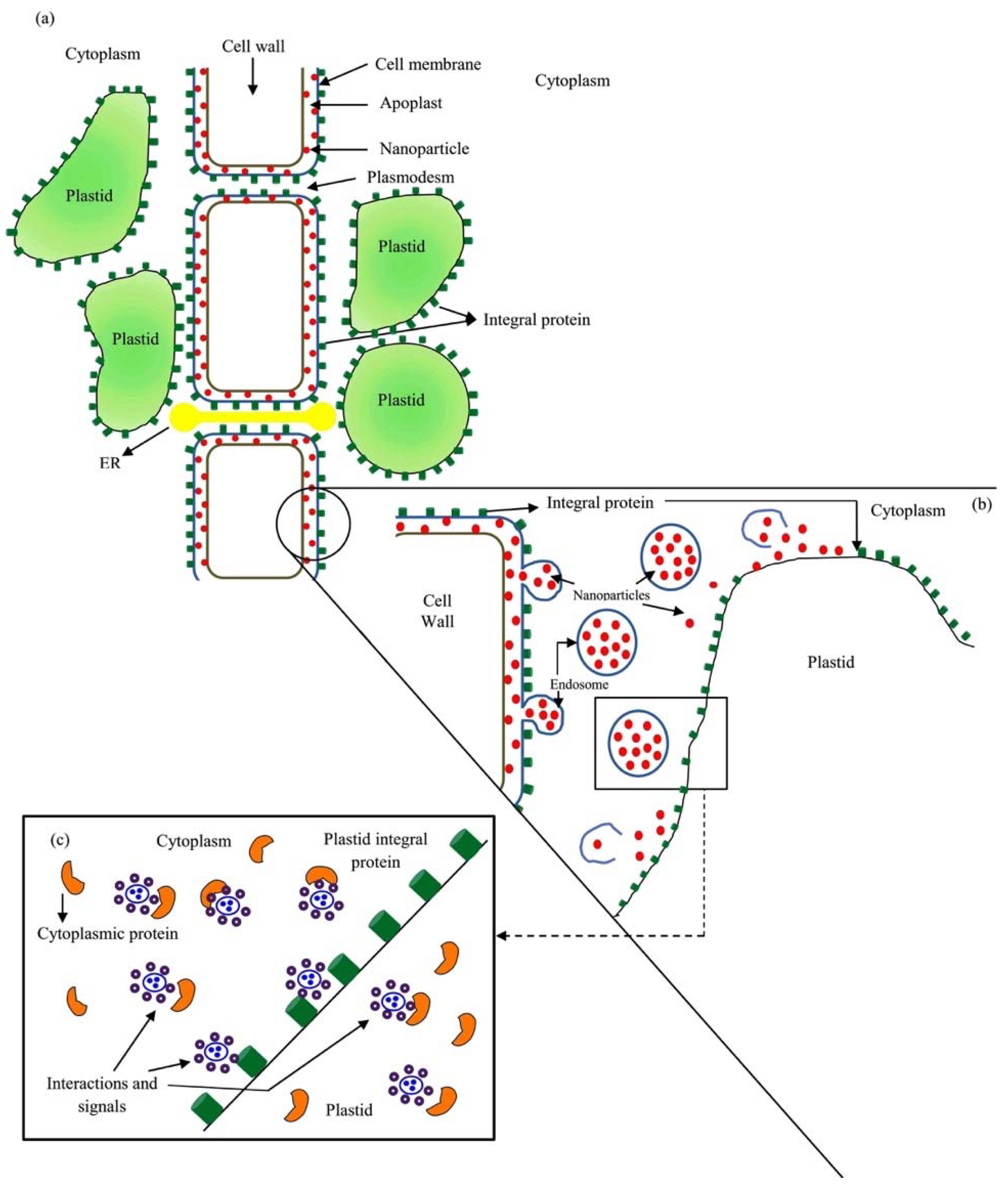
2. The Charges of the Cell’s Surface
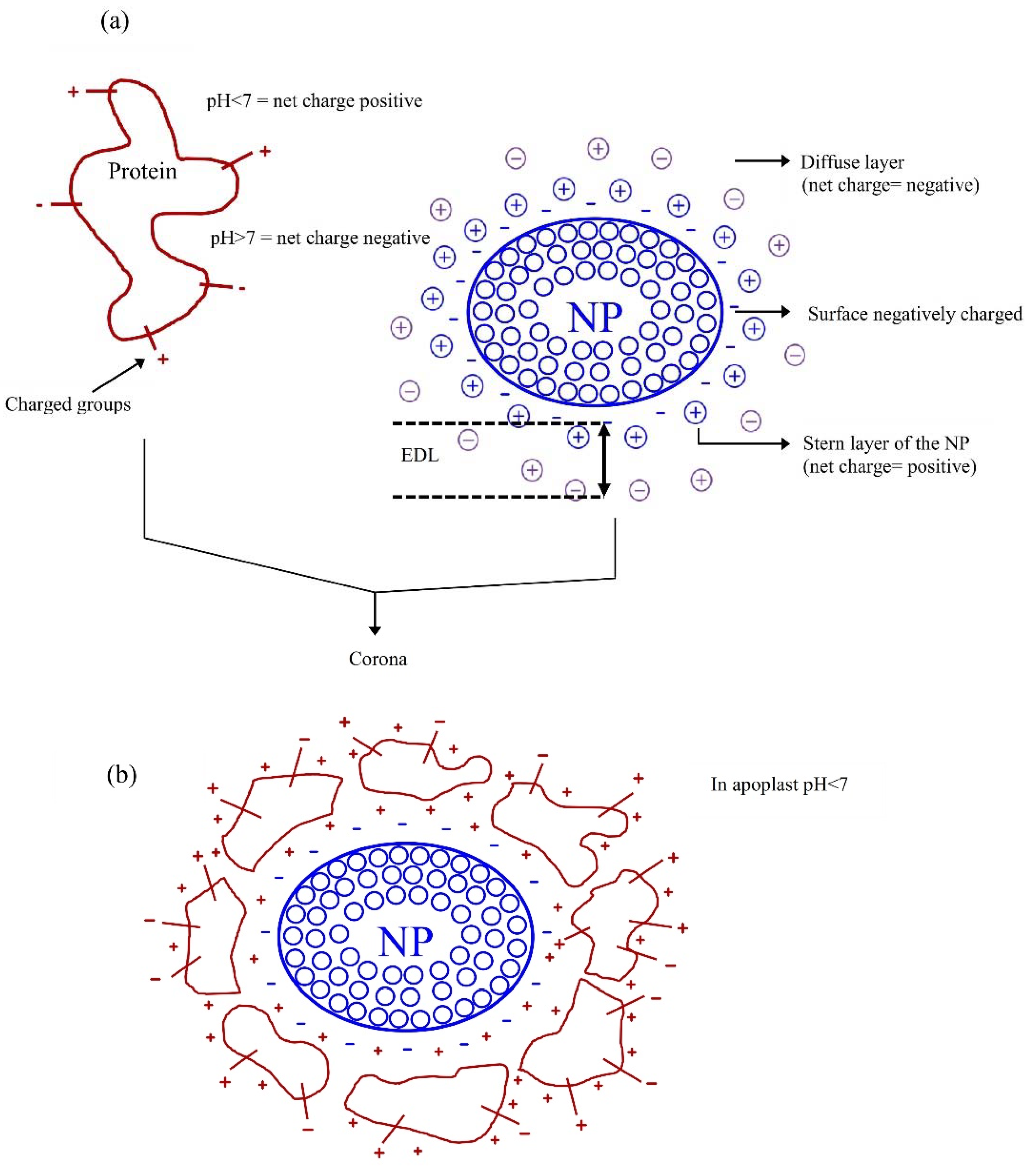
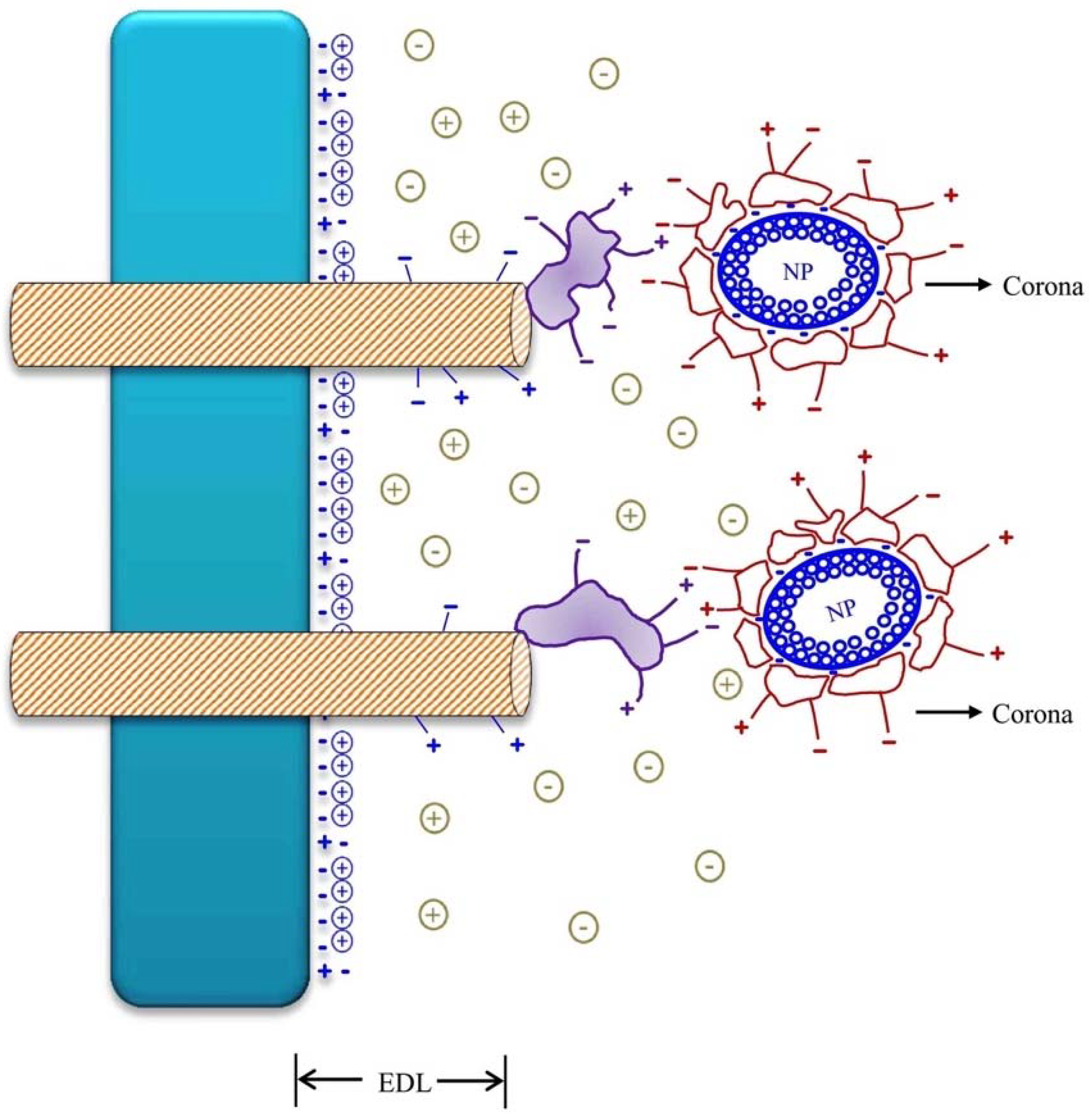
3. The Surface Charges of Nanoparticles (NPs) and Nanomaterials (NMs) and Its Interaction with Cell Surface Charges
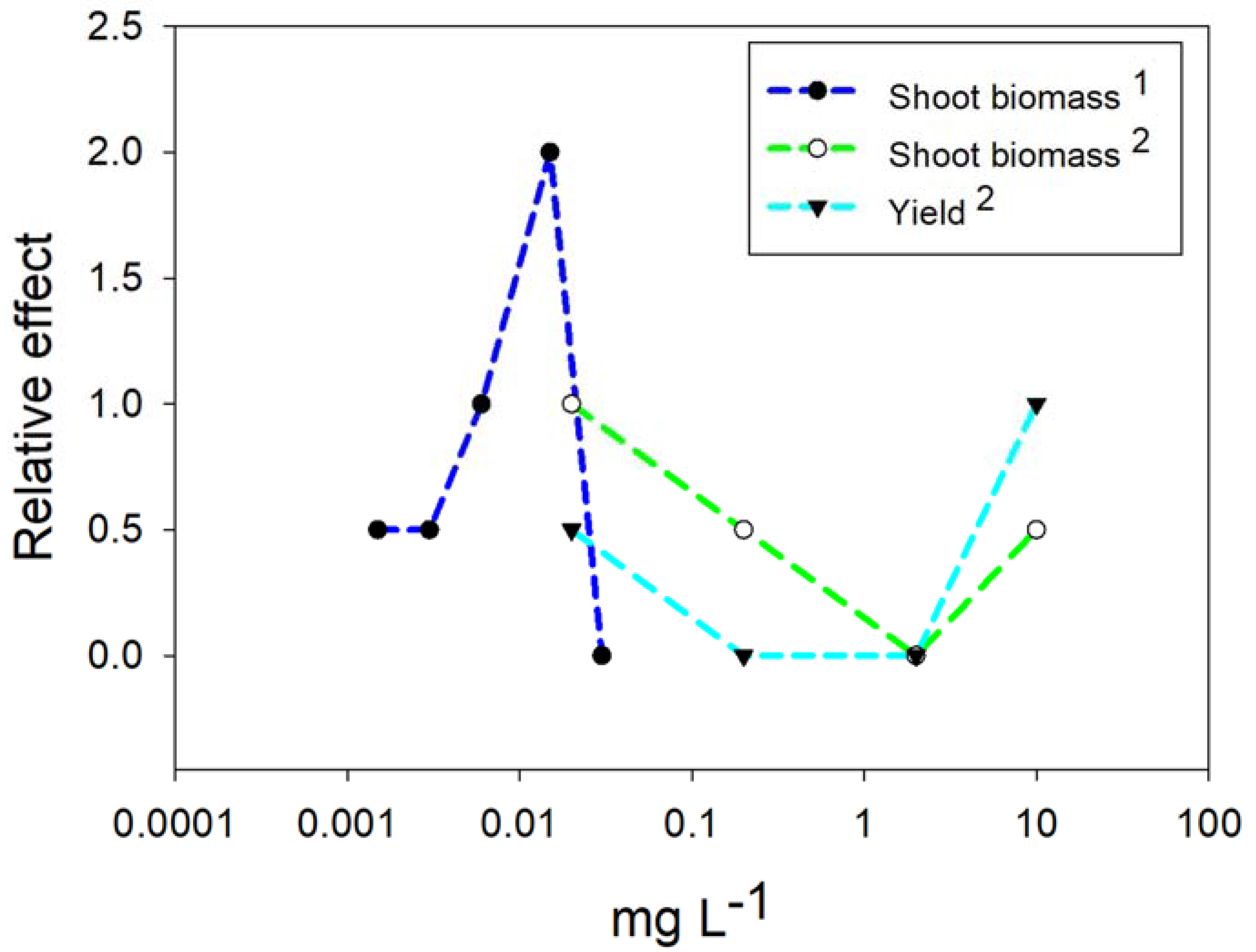
4. Responses of Plants to NPs and NMs
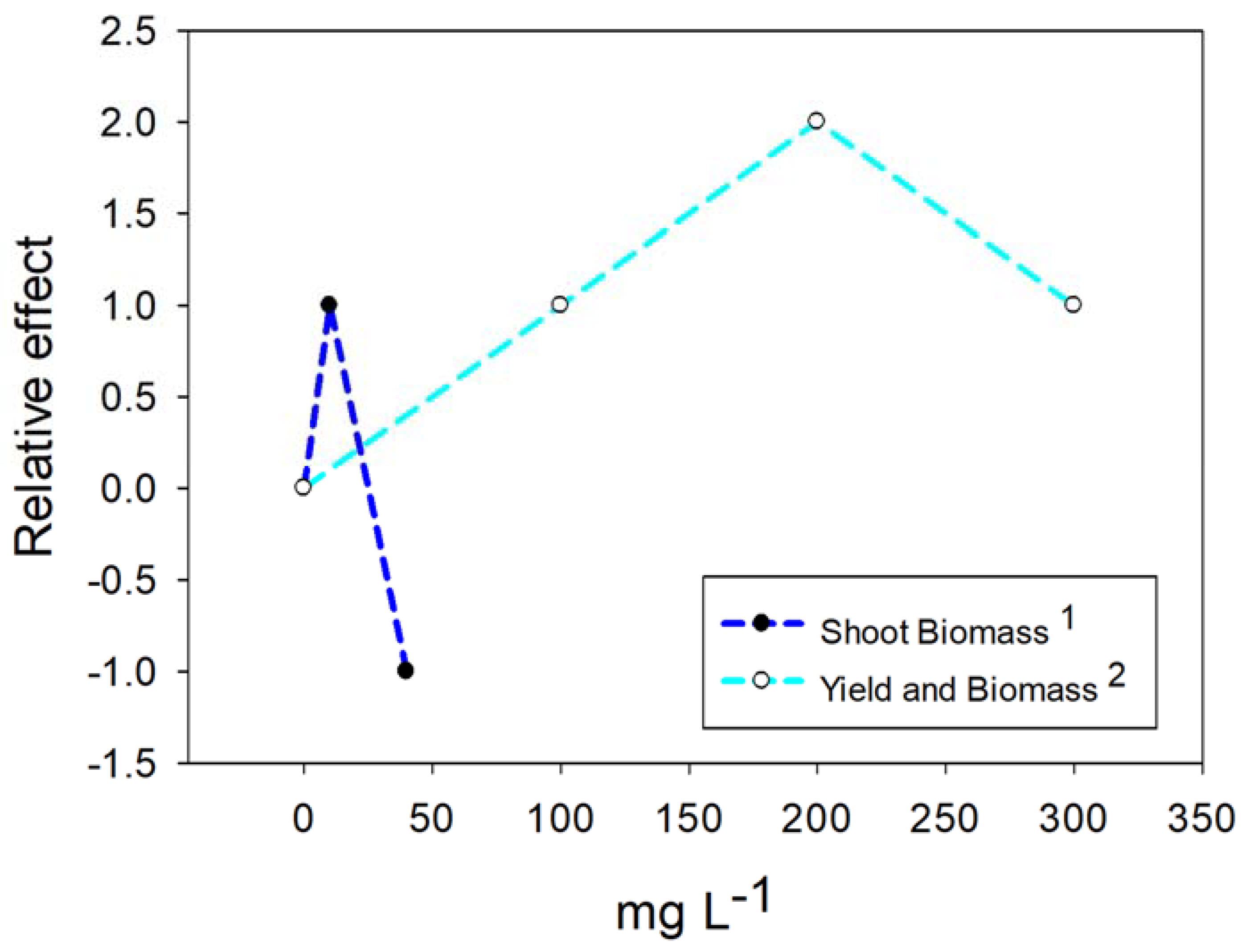
4.1. Nanoparticles of Titanium Dioxide (TiO2)
4.2. Nanoparticles of Copper (Cu)
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Vygovsky, Y.N.; Malov, A.N. The Physics of Laser Biostimulation; JSC MILTA-PKP GIT: Moscow, Russia, 2003. [Google Scholar]
- Cabrera-De la Fuente, M.; González-Morales, S.; Juárez-Maldonado, A.; Leija-Martínez, P.; Benavides-Mendoza, A. Plant nutrition and agronomic management to obtain crops with better nutritional and nutraceutical quality. In Therapeutic Foods; Holban, A.M., Grumezescu, A.M., Eds.; Academic Press: London, UK, 2018; pp. 99–140. ISBN 978-0-12-811517-6. [Google Scholar]
- Bell, I.R.; Ives, J.A.; Jonas, W.B. Nonlinear effects of nanoparticles: Biological variability from hormetic doses, small particle sizes, and dynamic adaptive interactions. Dose Resp. 2014, 12, 202–232. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Brown, P.; Saa, S. Biostimulants in agriculture. Front. Plant Sci. 2015, 6, 671. [Google Scholar] [CrossRef] [PubMed]
- Judy, J.D.; Bertsch, P.M. Bioavailability, toxicity, and fate of manufactured nanomaterials in terrestrial ecosystems. Adv. Agron. 2014, 123, 1–64. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; González-Morales, S.; Cabrera-De la Fuente, M.; Medrano-Macías, J.; Benavides-Mendoza, A. Nanometals as promoters of nutraceutical quality in crop plants. In Impact of Nanoscience in Food Industry; Grumezescu, A.M., Holban, A.M., Eds.; Academic Press: London, UK, 2018; pp. 277–310. ISBN 978-0-12-811441-4. [Google Scholar]
- Byczyńska, A. Nano-silver as a potential biostimulant for plant—A review. World Sci. News 2017, 86, 180–192. [Google Scholar]
- Campos, E.V.R.; de Oliveira, J.L.; Fernandes Fraceto, L.; Grillo, R. Global market of nanomaterials and colloidal formulations for agriculture: An overview. In Emerging Trends in Agri-nanotechnology: Fundamental and Applied Aspects; Singh, H.B., Mishra, S., Fernandes Fraceto, L., de Lima, R., Eds.; CAB International: Wallingford, UK, 2018; p. 302. ISBN 9781786391445. [Google Scholar]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zeng, G.; Xu, P.; Yan, M.; Xiong, W.; Zhou, S. Interaction of carbon nanotubes with microbial enzymes: Conformational transitions and potential toxicity. Environ. Sci. Nano 2017, 4, 1954–1960. [Google Scholar] [CrossRef]
- Morales-Díaz, A.B.A.B.; Ortega-Ortíz, H.; Juárez-Maldonado, A.; Cadenas-Pliego, G.; González-Morales, S.; Benavides-Mendoza, A. Application of nanoelements in plant nutrition and its impact in ecosystems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2017, 8, 013001. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses-A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Cabot, C.; Martos, S.; Gallego, B.; Barceló, J. Do toxic ions induce hormesis in plants? Plant Sci. 2013, 212, 15–25. [Google Scholar] [CrossRef]
- Poschenrieder, C.; Tolrà, R.; Barceló, J. Can metals defend plants against biotic stress? Trends Plant Sci. 2006, 11, 288–295. [Google Scholar] [CrossRef] [PubMed]
- Hillegass, J.M.; Shukla, A.; Lathrop, S.A.; MacPherson, M.B.; Fukagawa, N.K.; Mossman, B.T. Assessing nanotoxicity in cells in vitro. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2010, 2, 219–231. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Benitez-Alfonso, Y. Roles and regulation of plant cell walls surrounding plasmodesmata. Curr. Opin. Plant Biol. 2014, 22, 93–100. [Google Scholar] [CrossRef]
- Kinraide, T.B.; Wang, P. The surface charge density of plant cell membranes (sigma): An attempt to resolve conflicting values for intrinsic sigma. J. Exp. Bot. 2010, 61, 2507–2518. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.; Weihs, G.F. Surface charge density. In Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1864–1866. [Google Scholar]
- Nel, A.E.; Mädler, L.; Velegol, D.; Xia, T.; Hoek, E.M.V.; Somasundaran, P.; Klaessig, F.; Castranova, V.; Thompson, M. Understanding biophysicochemical interactions at the nano–bio interface. Nat. Mater. 2009, 8, 543–557. [Google Scholar] [CrossRef] [PubMed]
- Milo, R.; Phillips, R. Cell Biology by the Numbers; Garland Science: New York, NY, USA, 2015; ISBN 978-0-8153-4163-5. [Google Scholar]
- Yoshinaga, M.Y.; Kellermann, M.Y.; Valentine, D.L.; Valentine, R.C. Phospholipids and glycolipids mediate proton containment and circulation along the surface of energy-transducing membranes. Prog. Lipid Res. 2016, 64, 1–15. [Google Scholar] [CrossRef]
- Fleischer, C.C.; Payne, C.K. Nanoparticle-cell interactions: Molecular structure of the protein corona and cellular outcomes. Acc. Chem. Res. 2014, 47, 2651–2659. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Zhao, J.; Stenzel, M.H. Entry of nanoparticles into cells: The importance of nanoparticle properties. Polym. Chem. 2018, 9, 259–272. [Google Scholar] [CrossRef]
- Abou Matar, T.; Karam, P. The role of hydrophobicity in the cellular uptake of negatively charged macromolecules. Macromol. Biosci. 2018, 18, 1700309. [Google Scholar] [CrossRef]
- Chang, Y.-N.; Zhang, M.; Xia, L.; Zhang, J.; Xing, G. The toxic effects and mechanisms of CuO and ZnO nanoparticles. Materials 2012, 5, 2850–2871. [Google Scholar] [CrossRef]
- Fischer, F.D.; Waitz, T.; Vollath, D.; Simha, N.K. On the role of surface energy and surface stress in phase-transforming nanoparticles. Prog. Mater. Sci. 2008, 53, 481–527. [Google Scholar] [CrossRef]
- Chen, M.; Zhou, S.; Zhu, Y.; Sun, Y.; Zeng, G.; Yang, C.; Xu, P.; Yan, M.; Liu, Z.; Zhang, W. Toxicity of carbon nanomaterials to plants, animals and microbes: Recent progress from 2015-present. Chemosphere 2018, 206, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Loosli, F.; Vitorazi, L.; Berret, J.-F.; Stoll, S. Towards a better understanding on agglomeration mechanisms and thermodynamic properties of TiO2 nanoparticles interacting with natural organic matter. Water Res. 2015, 80, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Docter, D.; Westmeier, D.; Markiewicz, M.; Stolte, S.; Knauer, S.K.; Stauber, R.H. The nanoparticle biomolecule corona: Lessons learned—Challenge accepted? Chem. Soc. Rev. 2015, 44, 6094–6121. [Google Scholar] [CrossRef] [PubMed]
- Aramesh, M.; Shimoni, O.; Ostrikov, K.; Prawer, S.; Cervenka, J. Surface charge effects in protein adsorption on nanodiamonds. Nanoscale 2015, 7, 5726–5736. [Google Scholar] [CrossRef]
- Gessner, A.; Lieske, A.; Paulke, B.R.; Müller, R.H. Influence of surface charge density on protein adsorption on polymeric nanoparticles: Analysis by two-dimensional electrophoresis. Eur. J. Pharm. Biopharm. 2002, 54, 165–170. [Google Scholar] [CrossRef]
- Gunawan, C.; Lim, M.; Marquis, C.P.; Amal, R. Nanoparticle–protein corona complexes govern the biological fates and functions of nanoparticles. J. Mater. Chem. B 2014, 2, 2060. [Google Scholar] [CrossRef]
- Mout, R.; Moyano, D.F.; Rana, S.; Rotello, V.M. Surface functionalization of nanoparticles for nanomedicine. Chem. Soc. Rev. 2012, 41, 2539. [Google Scholar] [CrossRef]
- Gitlin, I.; Carbeck, J.D.; Whitesides, G.M. Why are proteins charged? Networks of charge–charge interactions in proteins measured by charge ladders and capillary electrophoresis. Angew. Chem. Int. Ed. 2006, 45, 3022–3060. [Google Scholar] [CrossRef]
- Lundqvist, M.; Stigler, J.; Cedervall, T.; Berggård, T.; Flanagan, M.B.; Lynch, I.; Elia, G.; Dawson, K. The evolution of the protein corona around nanoparticles: A test study. ACS Nano 2011, 5, 7503–7509. [Google Scholar] [CrossRef]
- Monopoli, M.P.; Walczyk, D.; Campbell, A.; Elia, G.; Lynch, I.; Bombelli, F.B.; Dawson, K.A. Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. J. Am. Chem. Soc. 2011, 133, 2525–2534. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.; Laurent, S.; Tawil, N.; Yahia, L.; Mahmoudi, M. Nanoparticle and protein corona. In Protein-Nanoparticle Interactions. The Bio-Nano Interface; Rahman, M., Laurent, S., Tawil, N., Yahia, L., Mahmoud, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 21–44. ISBN 978-3-642-37555-2. [Google Scholar]
- Jackson, T.C.; Patani, B.O.; Israel, M.B. Nanomaterials and cell interactions: A review. J. Biomater. Nanobiotechnol. 2017, 8, 1–9. [Google Scholar] [CrossRef]
- Kozlowski, L.P. Proteome-pI: Proteome isoelectric point database. Nucleic Acids Res. 2017, 45, D1112–D1116. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zeng, Y.; Zhuang, X.; Sun, L.; Yao, X.; Pimpl, P.; Jiang, L. Organelle pH in the Arabidopsis endomembrane system. Mol. Plant 2013, 6, 1419–1437. [Google Scholar] [CrossRef] [PubMed]
- Rejiya, C.S.; Kumar, J.; Raji, V.; Vibin, M.; Abraham, A. Laser immunotherapy with gold nanorods causes selective killing of tumour cells. Pharmacol. Res. 2012, 65, 261–269. [Google Scholar] [CrossRef]
- Kinraide, T.B. Plasma membrane surface potential (ψpm) as a determinant of ion bioavailability: A critical analysis of new and published toxicological studies and a simplified method for the computation of plant ψpm. Environ. Toxicol. Chem. 2006, 25, 3188–3198. [Google Scholar] [CrossRef] [PubMed]
- Haak, D.C.; Fukao, T.; Grene, R.; Hua, Z.; Ivanov, R.; Perrella, G.; Li, S.; Verslues, P.E.; Nakashima, K.; Batelli, G.; et al. Multilevel regulation of abiotic stress responses in plants. Front. Plant Sci. 2017, 8, 1564. [Google Scholar] [CrossRef]
- Stanley, S. Biological nanoparticles and their influence on organisms. Curr. Opin. Biotechnol. 2014, 28, 69–74. [Google Scholar] [CrossRef]
- Alberts, B.; Johnson, A.; Lewis, J.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 4th ed.; Garland Science: New York, NY, USA, 2002; ISBN 978-0815332183. [Google Scholar]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of chitosan–PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef]
- Septiadi, D.; Crippa, F.; Moore, T.L.; Rothen-Rutishauser, B.; Petri-Fink, A. Nanoparticle-cell interaction: A cell mechanics perspective. Adv. Mater. 2018, 30, 1704463. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-X.; Srivastava, R.; Che, P.; Howell, S.H. Salt stress responses in Arabidopsis utilize a signal transduction pathway related to endoplasmic reticulum stress signaling. Plant J. 2007, 51, 897–909. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. The role of surface charge in cellular uptake and cytotoxicity of medical nanoparticles. Int. J. Nanomed. 2012, 7, 5577–5591. [Google Scholar] [CrossRef]
- Ruenraroengsak, P.; Tetley, T.D. Differential bioreactivity of neutral, cationic and anionic polystyrene nanoparticles with cells from the human alveolar compartment: Robust response of alveolar type 1 epithelial cells. Part. Fibre Toxicol. 2015, 12, 19. [Google Scholar] [CrossRef] [PubMed]
- Fleischer, C.C.; Payne, C.K. Nanoparticle surface charge mediates the cellular receptors used by protein-nanoparticle complexes. J. Phys. Chem. B 2012, 116, 8901–8907. [Google Scholar] [CrossRef]
- Yue, Z.-G.; Wei, W.; Lv, P.-P.; Yue, H.; Wang, L.-Y.; Su, Z.-G.; Ma, G.-H. Surface charge affects cellular uptake and intracellular trafficking of chitosan-based nanoparticles. Biomacromolecules 2011, 12, 2440–2446. [Google Scholar] [CrossRef]
- Wang, J. Engineered Nanomaterials and Plant Interactions: Uptake, Translocation, Transformation and Physiological Effects. Ph.D. Thesis, Rice University, Houston, TX, USA, 2014. [Google Scholar]
- Nair, R.; Mohamed, M.S.; Gao, W.; Maekawa, T.; Yoshida, Y.; Ajayan, P.M.; Kumar, D.S. Effect of carbon nanomaterials on the germination and growth of rice plants. J. Nanosci. Nanotechnol. 2012, 12, 2212–2220. [Google Scholar] [CrossRef]
- Wang, D.; Lin, Z.; Wang, T.; Yao, Z.; Qin, M.; Zheng, S.; Lu, W. Where does the toxicity of metal oxide nanoparticles come from: The nanoparticles, the ions, or a combination of both? J. Hazard. Mater. 2016, 308, 328–334. [Google Scholar] [CrossRef]
- Nair, R.; Varghese, S.H.; Nair, B.G.; Maekawa, T.; Yoshida, Y.; Kumar, D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010, 179, 154–163. [Google Scholar] [CrossRef]
- Wang, P.; Lombi, E.; Zhao, F.-J.; Kopittke, P.M. Nanotechnology: A new opportunity in plant sciences. Trends Plant Sci. 2016, 21, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Uzun, O.; Hu, Y.; Hu, Y.; Han, H.-S.; Watson, N.; Chen, S.; Irvine, D.J.; Stellacci, F. Surface-structure-regulated cell-membrane penetration by monolayer-protected nanoparticles. Nat. Mater. 2008, 7, 588–595. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, A.; Salvati, A.; Santos-Martinez, M.J.; Radomski, M.W.; Dawson, K.A.; Åberg, C. Nanoparticle adhesion to the cell membrane and Its effect on nanoparticle uptake efficiency. J. Am. Chem. Soc. 2013, 135, 1438–1444. [Google Scholar] [CrossRef]
- Verma, A.; Stellacci, F. Effect of surface properties on nanoparticle-cell interactions. Small 2010, 6, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Saquib, Q.; Alatar, A.A.; Al-Khedhairy, A.A.; Hegazy, A.K.; Musarrat, J. Phytotoxic hazards of NiO-nanoparticles in tomato: A study on mechanism of cell death. J. Hazard. Mater. 2013, 250–251, 318–332. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Leyva, C.E.; Bacópulos-Mejía, E.; Ruiz-Torres, N.A.; Ibarra-Jiménez, L.; González-Morales, S.; Benavides-Mendoza, A. Irradiation of tomato seeds with Uv-B and UV-C: Impact on germination, vigor and growth. Rev. Mex. Ciencias Agrícolas 2017, 8, 103–116. [Google Scholar]
- Clément, L.; Hurel, C.; Marmier, N. Toxicity of TiO2 nanoparticles to cladocerans, algae, rotifers and plants—Effects of size and crystalline structure. Chemosphere 2013, 90, 1083–1090. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Acharya, K. Toxicological effect of metal oxide nanoparticles on soil and aquatic habitats. Arch. Environ. Contam. Toxicol. 2018, 75, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Buzea, C.; Pacheco, I.I.; Robbie, K. Nanomaterials and nanoparticles: Sources and toxicity. Biointerphases 2007, 2, MR17. [Google Scholar] [CrossRef]
- Capaldi Arruda, S.C.; Diniz Silva, A.L.; Moretto Galazzi, R.; Antunes Azevedo, R.; Zezzi Arruda, M.A.; Arruda, S.C.C.; Silva, A.L.D.; Galazzi, R.M.; Azevedo, R.A.; Arruda, M.A.Z.; et al. Nanoparticles applied to plant science: A review. Talanta 2015, 131, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Faizan, M.; Faraz, A.; Yusuf, M.; Khan, S.T.; Hayat, S. Zinc oxide nanoparticle-mediated changes in photosynthetic efficiency and antioxidant system of tomato plants. Photosynthetica 2018, 56, 678–686. [Google Scholar] [CrossRef]
- Hernández-Hernández, H.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Hernández-Fuentes, A.D.; Juárez-Maldonado, A. Cu Nanoparticles in chitosan-PVA hydrogels as promoters of growth, productivity and fruit quality in tomato. Emir. J. Food Agric. 2017, 29. [Google Scholar] [CrossRef]
- Juárez-Maldonado, A.; Ortega-Ortíz, H.; Pérez-Labrada, F.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Cu Nanoparticles absorbed on chitosan hydrogels positively alter morphological, production, and quality characteristics of tomato. J. Appl. Bot. Food Qual. 2016, 89, 183–189. [Google Scholar] [CrossRef]
- Boonyanitipong, P.; Kositsup, B.; Kumar, P.; Baruah, S.; Dutta, J. Toxicity of ZnO and TiO2 Nanoparticles on germinating Rice seed Oryza sativa L. Int. J. Biosci. Biochem. Bioinform. 2011, 1, 4–7. [Google Scholar] [CrossRef]
- Begum, P.; Ikhtiari, R.; Fugetsu, B. Graphene phytotoxicity in the seedling stage of cabbage, tomato, red spinach, and lettuce. Carbon N. Y. 2011, 49, 3907–3919. [Google Scholar] [CrossRef]
- Cheng, F.; Liu, Y.-F.; Lu, G.-Y.; Zhang, X.-K.; Xie, L.-L.; Yuan, C.-F.; Xu, B.-B. Graphene oxide modulates root growth of Brassica napus L. and regulates ABA and IAA concentration. J. Plant Physiol. 2016, 193, 57–63. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Hu, R.; Zhong, Y.; Zhao, X.; Chen, Q.; Zhu, H. Graphene oxide as a water transporter promoting germination of plants in soil. Nano Res. 2018, 11, 1928–1937. [Google Scholar] [CrossRef]
- de la Rosa, G.; García-Castañeda, C.; Vázquez-Núñez, E.; Alonso-Castro, Á.J.; Basurto-Islas, G.; Mendoza, Á.; Cruz-Jiménez, G.; Molina, C. Physiological and biochemical response of plants to engineered NMs: Implications on future design. Plant Physiol. Biochem. 2017, 110, 226–235. [Google Scholar] [CrossRef]
- Reyes-Coronado, D.; Rodríguez-Gattorno, G.; Espinosa-Pesqueira, M.E.; Cab, C.; de Coss, R.; Oskam, G. Phase-pure TiO2 nanoparticles: Anatase, brookite and rutile. Nanotechnology 2008, 19, 145605. [Google Scholar] [CrossRef]
- Lyu, S.; Wei, X.; Chen, J.; Wang, C.; Wang, X.; Pan, D. Titanium as a beneficial element for crop production. Front. Plant Sci. 2017, 8, 597. [Google Scholar] [CrossRef]
- Mattiello, A.; Marchiol, L. Application of nanotechnology in agriculture: Assessment of TiO2 nanoparticle effects on barley. In Application of Titanium Dioxide; Janus, M., Ed.; InTech: London, UK, 2017; pp. 23–39. [Google Scholar]
- Jaberzadeh, A.; Moaveni, P.; Tohidi Moghadam, H.R.; Zahedi, H. Influence of bulk and nanoparticles titanium foliar application on some agronomic traits, seed gluten and starch contents of wheat subjected to water deficit stress. Not. Bot. Horti Agrobot. Cluj-Napoca 2013, 41, 201–207. [Google Scholar] [CrossRef]
- Wallace, A.; Alexander, G.V.; Chaudhry, F.M. Phytotoxicity of cobalt, vanadium, titanium, silver, and chromium. Commun. Soil Sci. Plant Anal. 1977, 8, 751–756. [Google Scholar] [CrossRef]
- Tlustoš, P.; Cígler, P.; Hrubý, M.; Kužel, S.; Száková, J.; Balík, J. The role of titanium in biomass production and its influence on essential elements’ contents in field growing crops. Plant Soil Environ. 2005, 51, 19–25. [Google Scholar] [CrossRef]
- Mohammadi, R.; Maali-Amiri, R.; Mantri, N.L. Effect of TiO2 nanoparticles on oxidative damage and antioxidant defense systems in chickpea seedlings during cold stress. Russ. J. Plant Physiol. 2014, 61, 768–775. [Google Scholar] [CrossRef]
- Qi, M.; Liu, Y.; Li, T. Nano- TiO2 improve the photosynthesis of tomato leaves under mild heat stress. Biol. Trace Elem. Res. 2013, 156, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, H.; Esmailpour, M.; Gheranpaye, A. Effects of TiO2 nanoparticles and water-deficit stress on morpho-physiological characteristics of dragonhead (Dracocephalum moldavica L.) plants. Acta Agric. Slov. 2016, 107, 385–396. [Google Scholar] [CrossRef]
- Aghdam, M.T.B.; Mohammadi, H.; Ghorbanpour, M. Effects of nanoparticulate anatase titanium dioxide on physiological and biochemical performance of Linum usitatissimum (Linaceae) under well-watered and drought stress conditions. Braz. J. Bot. 2016, 39, 139–146. [Google Scholar] [CrossRef]
- Kiapour, H.; Moaveni, P.; Habibi, D.; Sani, B. Evaluation of the application of gibbrellic acid and titanium dioxide nanoparticles under drought stress on some traits of basil (Ocimum basilicum L.). Int. J. Agron. Agric. Res. 2015, 6, 138–150. [Google Scholar]
- Khan, M.N. Nano-titanium Dioxide (Nano-TiO2) mitigates NaCl stress by enhancing antioxidative enzymes and accumulation of compatible solutes in tomato (Lycopersicon esculentum Mill.). J. Plant Sci. 2016, 11, 1–11. [Google Scholar] [CrossRef]
- Zheng, L.; Mingyu, S.; Xiao, W.; Chao, L.; Chunxiang, Q.; Liang, C.; Hao, H.; Xiaoqing, L.; Fashui, H. Antioxidant stress is promoted by nano-anatase in spinach chloroplasts under UV-B radiation. Biol. Trace Elem. Res. 2008, 121, 69–79. [Google Scholar] [CrossRef]
- Mahakham, W.; Sarmah, A.K.; Maensiri, S.; Theerakulpisut, P. Nanopriming technology for enhancing germination and starch metabolism of aged rice seeds using phytosynthesized silver nanoparticles. Sci. Rep. 2017, 7, 8263. [Google Scholar] [CrossRef]
- Zhang, M.; Gao, B.; Chen, J.; Li, Y. Effects of graphene on seed germination and seedling growth. J. Nanoparticle Res. 2015, 17, 78. [Google Scholar] [CrossRef]
- Gao, F.; Liu, C.; Qu, C.; Zheng, L.; Yang, F.; Su, M.; Hong, F. Was improvement of spinach growth by nano- TiO2 treatment related to the changes of Rubisco activase? Biometals 2008, 21, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Hong, F.; You, W.; Liu, C.; Gao, F.; Wu, C.; Yang, P. Influences of nano anatase TiO2 on nitrogen metabolism of growing spinach. Biol. Trace Elem. Res. 2006, 110, 179–190. [Google Scholar] [CrossRef]
- Ingle, A.P.; Duran, N.; Rai, M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014, 98, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Ebbs, S.D.; Kochian, L.V. Toxicity of Zinc and Copper to Brassica species: Implications for phytoremediation. J. Environ. Qual. 1997, 26, 776–781. [Google Scholar] [CrossRef]
- Atha, D.H.; Wang, H.; Petersen, E.J.; Cleveland, D.; Holbrook, R.D.; Jaruga, P.; Dizdaroglu, M.; Xing, B.; Nelson, B.C. Copper oxide nanoparticle mediated DNA damage in terrestrial plant models. Environ. Sci. Technol. 2012, 46, 1819–1827. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Guo, H.; Li, J.; Wang, Y.; Xiao, L.; Xing, B. Interaction of γ-Fe2O3 nanoparticles with Citrus maxima leaves and the corresponding physiological effects via foliar application. J. Nanobiotechnol. 2017, 15, 51. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Fuentes, A.; López-Vargas, E.; Pinedo-Espinoza, J.; Campos-Montiel, R.; Valdés-Reyna, J.; Juárez-Maldonado, A. Postharvest behavior of bioactive compounds in tomato fruits treated with Cu nanoparticles and NaCl stress. Appl. Sci. 2017, 7, 980. [Google Scholar] [CrossRef]
- Hong, J.; Wang, L.; Sun, Y.; Zhao, L.; Niu, G.; Tan, W.; Rico, C.M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Foliar applied nanoscale and microscale CeO2 and CuO alter cucumber (Cucumis sativus) fruit quality. Sci. Total Environ. 2016, 563, 904–911. [Google Scholar] [CrossRef] [PubMed]
- Pradhan, S.; Patra, P.; Mitra, S.; Dey, K.K.; Basu, S.; Chandra, S.; Palit, P.; Goswami, A. Copper nanoparticle (CuNP) nanochain arrays with a reduced toxicity response: A biophysical and biochemical outlook on Vigna radiata. J. Agric. Food Chem. 2015, 63, 2606–2617. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Juárez-Maldonado, A.; Ortega-Ortíz, H.; Morales-Díaz, A.B.; González-Morales, S.; Morelos-Moreno, Á.; Cabrera-De la Fuente, M.; Sandoval-Rangel, A.; Cadenas-Pliego, G.; Benavides-Mendoza, A. Nanoparticles and Nanomaterials as Plant Biostimulants. Int. J. Mol. Sci. 2019, 20, 162. https://doi.org/10.3390/ijms20010162
Juárez-Maldonado A, Ortega-Ortíz H, Morales-Díaz AB, González-Morales S, Morelos-Moreno Á, Cabrera-De la Fuente M, Sandoval-Rangel A, Cadenas-Pliego G, Benavides-Mendoza A. Nanoparticles and Nanomaterials as Plant Biostimulants. International Journal of Molecular Sciences. 2019; 20(1):162. https://doi.org/10.3390/ijms20010162
Chicago/Turabian StyleJuárez-Maldonado, Antonio, Hortensia Ortega-Ortíz, América Berenice Morales-Díaz, Susana González-Morales, Álvaro Morelos-Moreno, Marcelino Cabrera-De la Fuente, Alberto Sandoval-Rangel, Gregorio Cadenas-Pliego, and Adalberto Benavides-Mendoza. 2019. "Nanoparticles and Nanomaterials as Plant Biostimulants" International Journal of Molecular Sciences 20, no. 1: 162. https://doi.org/10.3390/ijms20010162
APA StyleJuárez-Maldonado, A., Ortega-Ortíz, H., Morales-Díaz, A. B., González-Morales, S., Morelos-Moreno, Á., Cabrera-De la Fuente, M., Sandoval-Rangel, A., Cadenas-Pliego, G., & Benavides-Mendoza, A. (2019). Nanoparticles and Nanomaterials as Plant Biostimulants. International Journal of Molecular Sciences, 20(1), 162. https://doi.org/10.3390/ijms20010162