Analysis of bZIP Transcription Factor Family and Their Expressions under Salt Stress in Chlamydomonas reinhardtii
Abstract
1. Introduction
2. Results and Discussion
2.1. Identification of C. reinhardtii bZIP Family Members
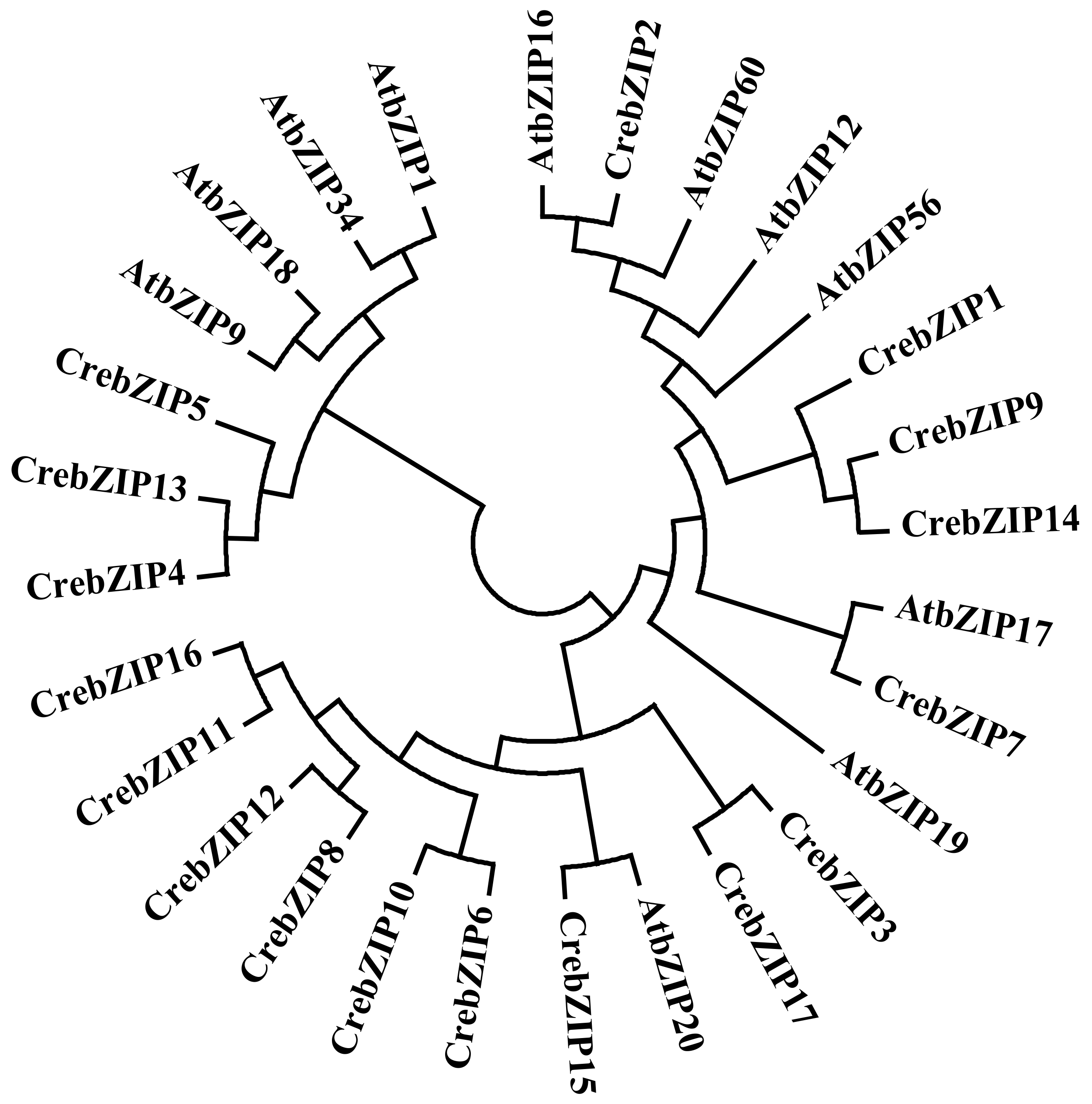
2.2. Phylogenetic and Motif Analysis of CrebZIP Proteins
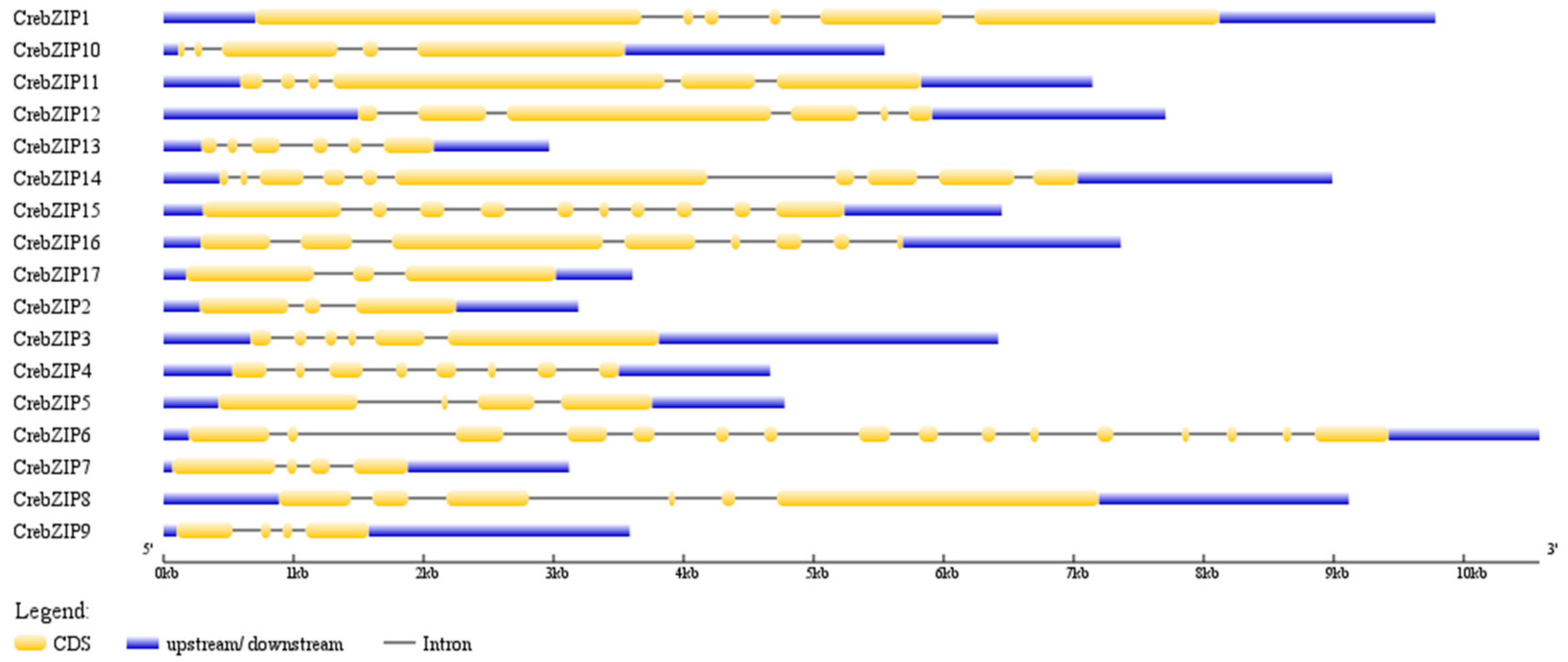
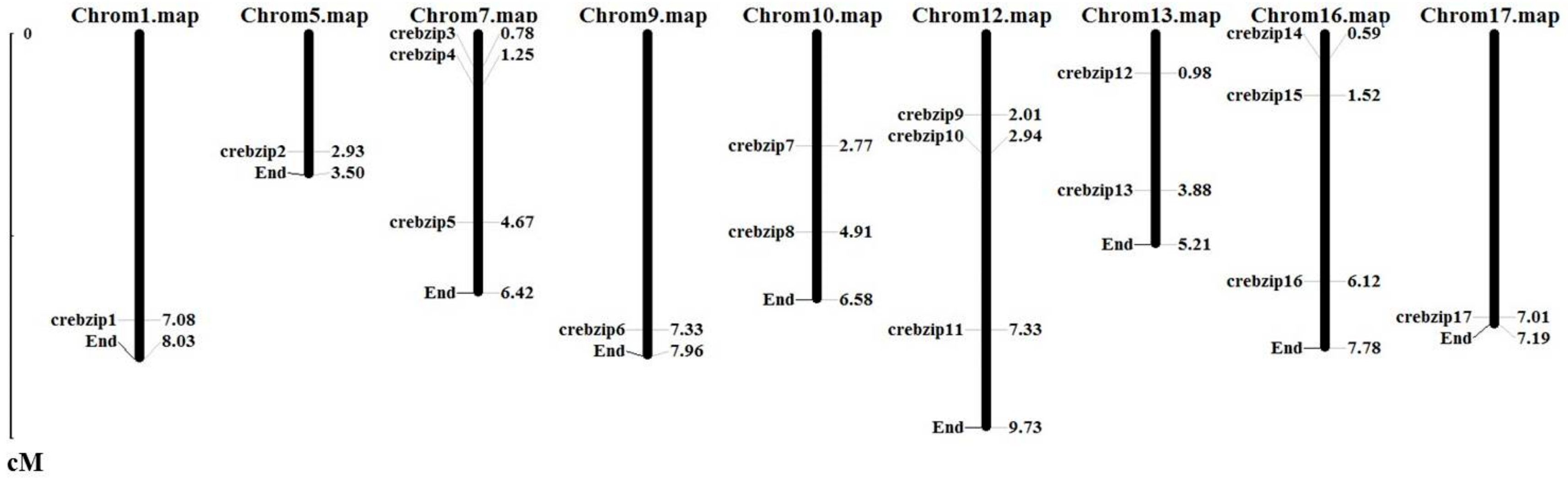
2.3. Analysis of CrebZIP Gene Structure and Their Chromosomal Assignment
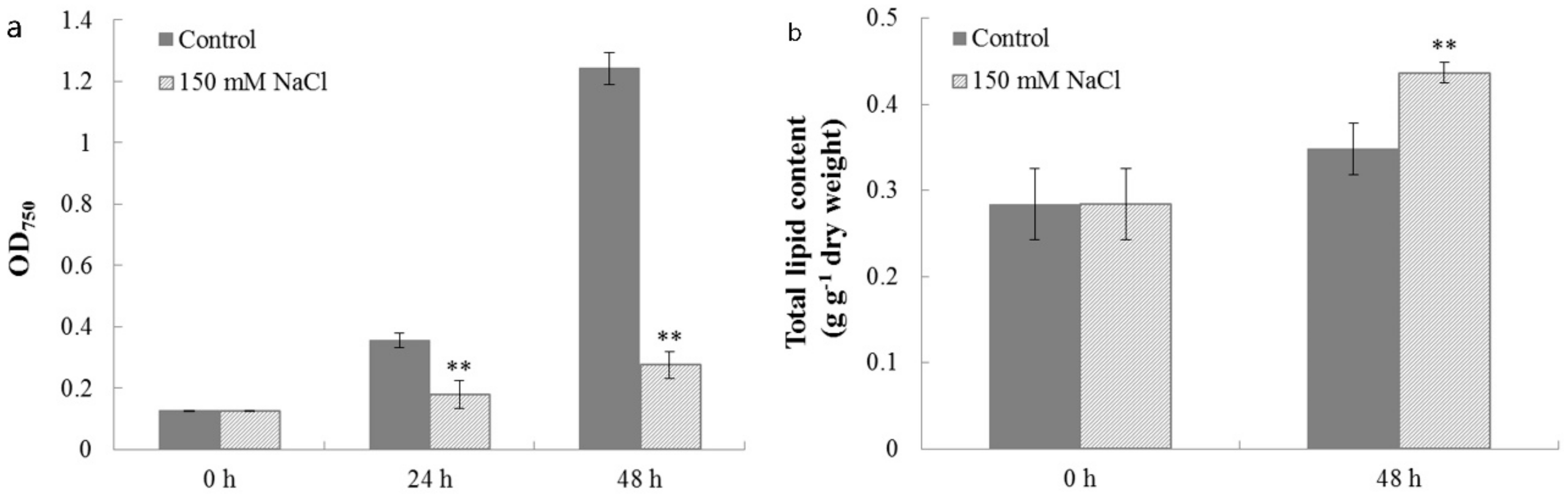
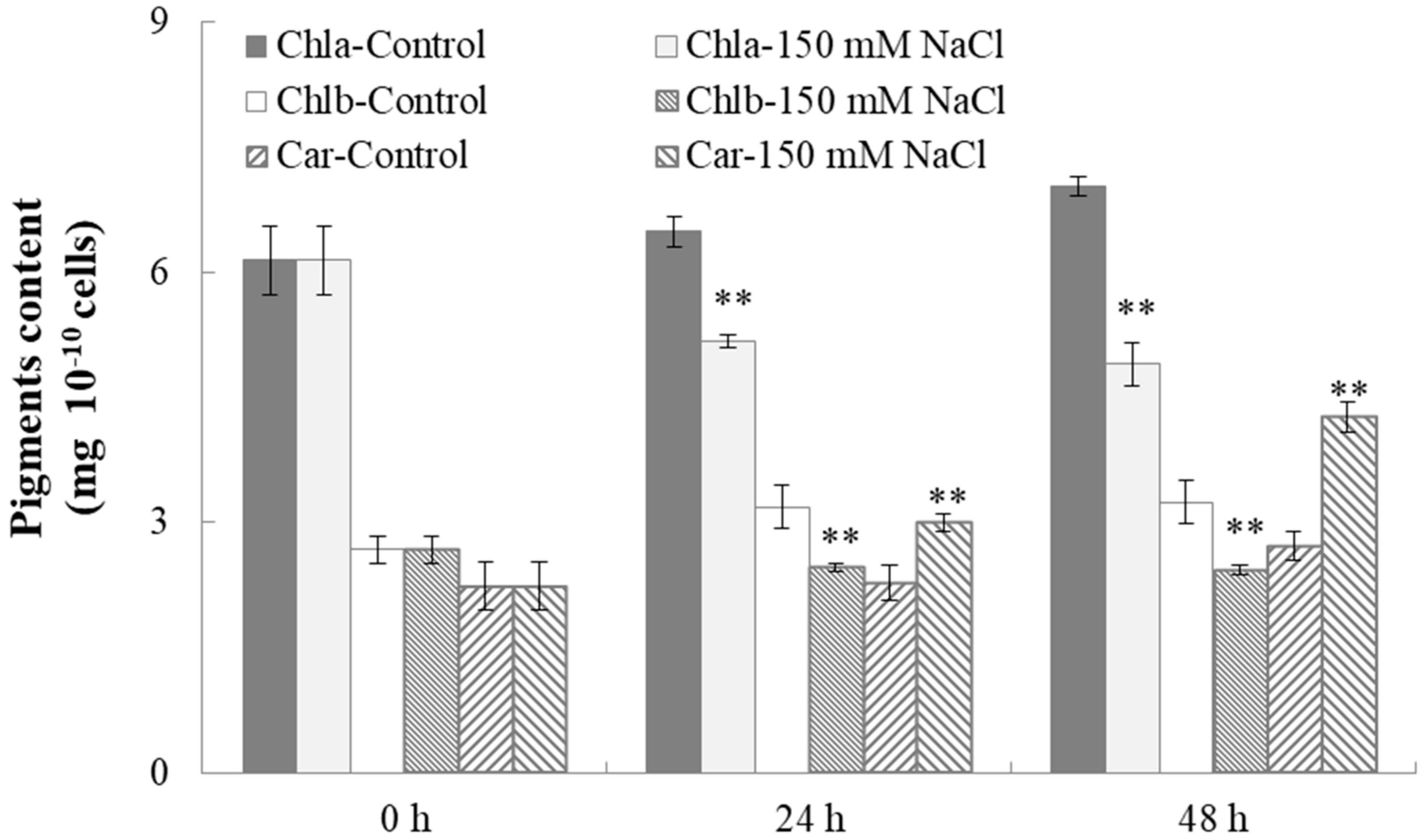
2.4. Characterization of Cell Growth and Lipid Accumulation in C. reinhardtii Under Salt Stress
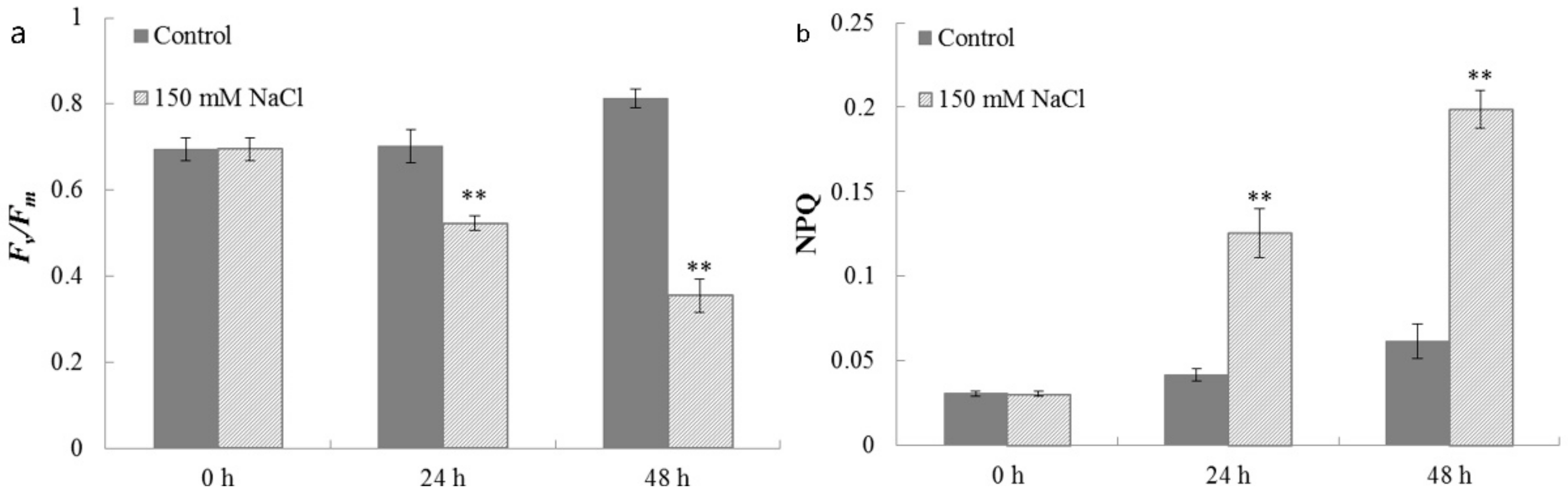
2.5. Photosynthetic Properties of C. reinhardtii Cells under Salt Stress
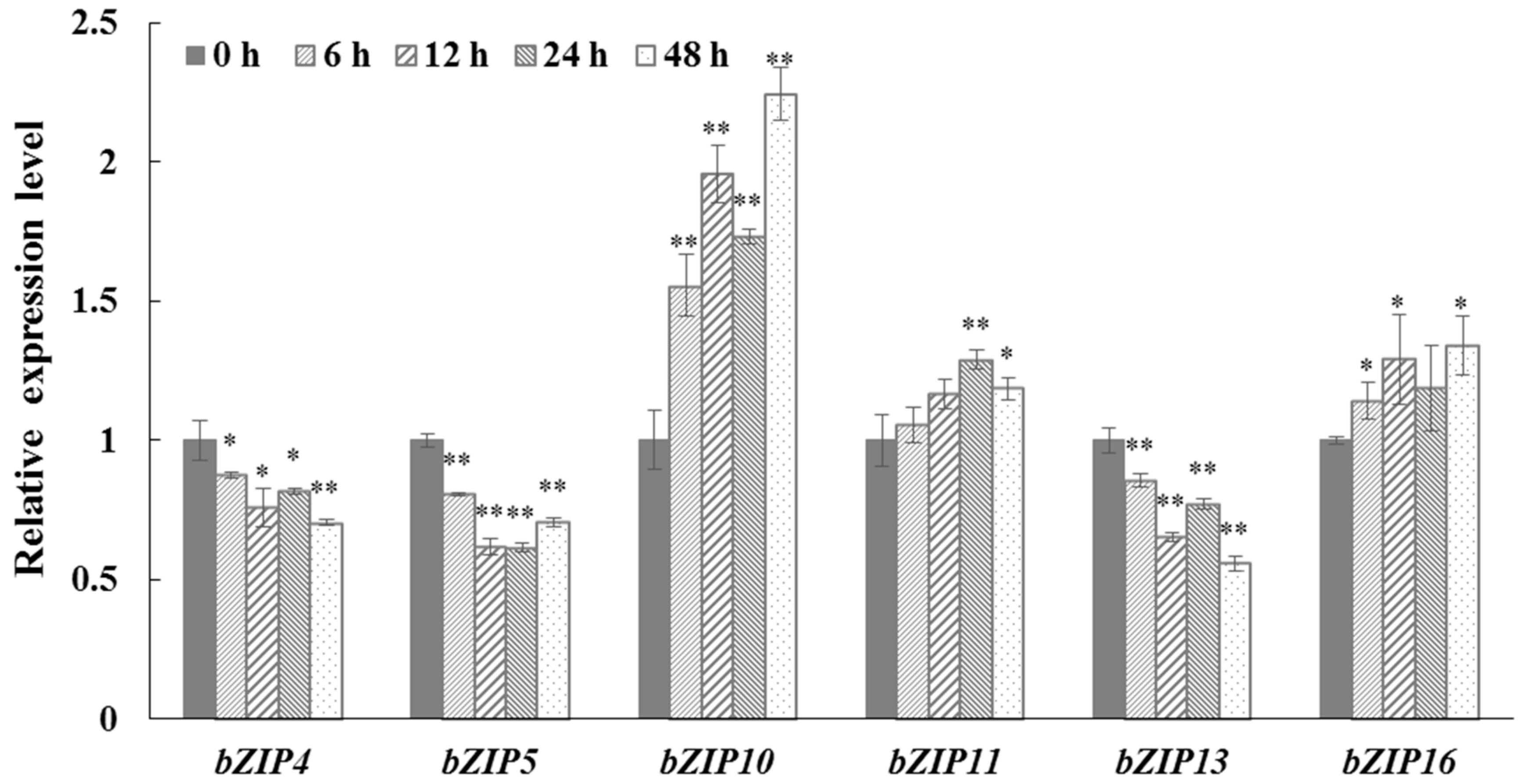
2.6. The Expression of CrebZIP Genes under Salt Stress
3. Materials and Methods
3.1. Microalgae Strains and Growth Conditions
3.2. Genome-Wide Identification of C. reinhardtii bZIP Gene Family
3.3. Motif Recognition and Phylogenetic Analysis of C. reinhardtii bZIP Gene Family
3.4. bZIP Protein Secondary Structure Prediction
3.5. bZIP Gene Structure and Chromosomal Assignment Analysis
3.6. Measure of Microalgae Biomass Concentration
3.7. Analysis of Total Lipid Content in Microalgae Cells
3.8. Nile Red Staining and Microscopy for Assessment of Oil Accumulation in Microalgae Cells
3.9 Determination of the Pigment Contents in Microalgae Cells
3.10 Chlorophyll Fluorescence Measurements
3.11 RNA Isolation and Quantitative Real-Time (qRT)-PCR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Wijffels, R.H.; Barbosa, M.J. An outlook on microalgal biofuels. Science 2010, 329, 796–799. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.H.; Cui, Y.B.; Wan, M.X.; Wang, W.L.; Li, Y.G. Lipid accumulation and biosynthesis genes response of the oleaginous Chlorella pyrenoidosa under three nutrition stressors. Biotechnol. Biofuels 2014, 7, 17. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.T.; Ullrich, N.; Joo, S.; Waffenschmidt, S.; Goodenough, U. Algal lipid bodies: Stress induction, purification, and biochemical characterization in wild-type and starchless Chlamydomonas reinhardtii. Eukaryot. Cell 2009, 8, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Cakmak, T.; Angun, P.; Demiray, Y.E.; Ozkan, A.D.; Elibol, Z.; Tekinay, T. Differential effects of nitrogen and sulfur deprivation on growth and biodiesel feedstock production of Chlamydomonas reinhardtii. Biotechnol. Bioeng. 2012, 109, 1947–1957. [Google Scholar] [CrossRef] [PubMed]
- He, Q.; Yang, H.; Wu, L.; Hu, C. Effect of light intensity on physiological changes, carbon allocation and neutral lipid accumulation in oleaginous microalgae. Bioresour. Technol. 2015, 191, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Merchant, S.S.; Prochnik, S.E.; Vallon, O.; Harris, E.H.; Karpowicz, S.J.; Witman, G.B.; Terry, A.; Salamov, A.; Fritz-Laylin, L.K.; Marechal-Drouard, L.; et al. The Chlamydomonas genome reveals the evolution of key animal and plant functions. Science 2007, 318, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Qu, G.; Qi, X.; Lu, L.; Tian, C.; Ma, Y. Transcriptome analysis of Chlamydomonas reinhardtii during the process of lipid accumulation. Genomics 2013, 101, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Adrián, L.G.D.L.; Sascha, S.; Jacob, V.; Saheed, I.; Warren, C.; Bilgin, D.D.; Yohn, C.B.; Serdar, T.; Reiss, D.J.; Orellana, M.V. Transcriptional program for nitrogen starvation-induced lipid accumulation in Chlamydomonas reinhardtii. Biotechnol. Biofuels 2015, 8, 207. [Google Scholar] [CrossRef]
- Rolland, N.; Atteia, A.; Decottignies, P.; Garin, J.; Hippler, M.; Kreimer, G.; Lemaire, S.D.; Mittag, M.; Wagner, V. Chlamydomonas proteomics. Curr. Opin. Microbiol. 2009, 12, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Wienkoop, S.; Weiss, J.; May, P.; Kempa, S.; Irgang, S.; Recuenco-Munoz, L.; Pietzke, M.; Schwemmer, T.; Rupprecht, J.; Egelhofer, V.; et al. Targeted proteomics for Chlamydomonas reinhardtii combined with rapid subcellular protein fractionation, metabolomics and metabolic flux analyses. Mol. Biosyst. 2010, 6, 1018–1031. [Google Scholar] [CrossRef] [PubMed]
- Mastrobuoni, G.; Irgang, S.; Pietzke, M.; Aßmus, H.E.; Wenzel, M.; Schulze, W.X.; Kempa, S. Proteome dynamics and early salt stress response of the photosynthetic organism Chlamydomonas reinhardtii. BMC Genom. 2012, 13, 215. [Google Scholar] [CrossRef] [PubMed]
- May, P.; Wienkoop, S.; Kempa, S.; Usadel, B.; Christian, N.; Rupprecht, J.; Weiss, J.; Recuenco-Munoz, L.; Ebenhöh, O.; Weckwerth, W.; et al. Metabolomics- and proteomics-assisted genome annotation and analysis of the draft metabolic network of Chlamydomonas reinhardtii. Genetics 2008, 179, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Valledor, L.; Furuhashi, T.; Recuencomuñoz, L.; Wienkoop, S.; Weckwerth, W. System-level network analysis of nitrogen starvation and recovery in Chlamydomonas reinhardtii reveals potential new targets for increased lipid accumulation. Biotechnol. Biofuels 2014, 7, 171. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Wang, D.; Jing, L.; Jing, G.; Kang, N.; Jian, X. Genome-wide identification of transcription factors and transcription-factor binding sites in oleaginous microalgae Nannochloropsis. Sci. Rep. 2014, 4, 5454. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, L.G.; Riaño-Pachón, D.M.; Schrago, C.G.; dos Santos, R.V.; Mueller-Roeber, B.; Vincentz, M. The role of bZIP transcription factors in green plant evolution: Adaptive features emerging from four founder genes. PLoS ONE 2008, 3, e2944. [Google Scholar] [CrossRef] [PubMed]
- Riechmann, J.L.; Heard, J.; Martin, G.; Reuber, L.; Jiang, C.; Keddie, J.; Adam, L.; Pineda, O.; Ratcliffe, O.J.; Samaha, R.R. Arabidopsis transcription factors: Genome-wide comparative analysis among eukaryotes. Science 2000, 290, 2105–2110. [Google Scholar] [CrossRef] [PubMed]
- Jakoby, M.; Weisshaar, B.; Drögelaser, W.; Vicentecarbajosa, J.; Tiedemann, J.; Kroj, T.; Parcy, F. bZIP transcription factors in Arabidopsis. Trends Plant Sci. 2002, 7, 106–111. [Google Scholar] [CrossRef]
- Zou, M.; Guan, Y.; Ren, H.; Zhang, F.; Chen, F. A bZIP transcription factor, OsABI5, is involved in rice fertility and stress tolerance. Plant Mol. Biol. 2008, 66, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Yanez, M.; Caceres, S.; Orellana, S.; Bastias, A.; Verdugo, I.; Ruiz-Lara, S.; Casaretto, J.A. An abiotic stress-responsive bZIP transcription factor from wild and cultivated tomatoes regulates stress-related genes. Plant Cell Rep. 2009, 28, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Ying, S.; Zhang, D.F.; Fu, J.; Shi, Y.S.; Song, Y.C.; Wang, T.Y.; Li, Y. Cloning and characterization of a maize bZIP transcription factor, ZmbZIP72, confers drought and salt tolerance in transgenic Arabidopsis. Planta 2012, 235, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, J.; Zhang, B.; Vanitha, J.; Ramachandran, S.; Jiang, S.Y. Genome-wide expansion and expression divergence of the basic leucine zipper transcription factors in higher plants with an emphasis on sorghum. J. Integr. Plant Biol. 2011, 53, 212–231. [Google Scholar] [CrossRef] [PubMed]
- Que, F.; Wang, G.L.; Huang, Y.; Xu, Z.S.; Wang, F.; Xiong, A.S. Genomic identification of group A bZIP transcription factors and their responses to abiotic stress in carrot. Genet. Mol. Res. 2015, 14, 13274–13288. [Google Scholar] [CrossRef] [PubMed]
- Fukazawa, J.; Sakai, T.; Ishida, S.; Yamaguchi, I.; Kamiya, Y.; Takahashi, Y. Repression of shoot growth, a bZIP transcriptional activator, regulates cell elongation by controlling the level of gibberellins. Plant Cell 2000, 12, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Kobayashi, Y.; Yamamoto, S.; Daimon, Y.; Yamaguchi, A.; Ikeda, Y.; Ichinoki, H.; Notaguchi, M.; Goto, K.; Araki, T. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science 2005, 309, 1052–1056. [Google Scholar] [CrossRef] [PubMed]
- Silveira, A.B.; Gauer, L.; Tomaz, J.P.; Cardoso, P.R.; Carmelloguerreiro, S.; Vincentz, M. The Arabidopsis AtbZIP9 protein fused to the VP16 transcriptional activation domain alters leaf and vascular development. Plant Sci. 2007, 172, 1148–1156. [Google Scholar] [CrossRef]
- Shen, H.; Cao, K.; Wang, X. A conserved proline residue in the leucine zipper region of AtbZIP34 and AtbZIP61 in Arabidopsis thaliana interferes with the formation of homodimer. Biochem. Biophys. Res. Commun. 2007, 362, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Baena-González, E.; Rolland, F.; Thevelein, J.M.; Sheen, J. A central integrator of transcription networks in plant stress and energy signalling. Nature 2007, 448, 938–942. [Google Scholar] [CrossRef] [PubMed]
- Lara, P.; Oñatesánchez, L.; Abraham, Z.; Ferrándiz, C.; Díaz, I.; Carbonero, P.; Vicentecarbajosa, J. Synergistic activation of seed storage protein gene expression in Arabidopsis by ABI3 and two bZIPs related to OPAQUE2. J. Biol. Chem. 2003, 278, 21003–21011. [Google Scholar] [CrossRef] [PubMed]
- Thurow, C.; Schiermeyer, A.S.; Butterbrodt, T.; Nickolov, K.; Gatz, C. Tobacco bZIP transcription factor TGA2.2 and related factor TGA2.1 have distinct roles in plant defense responses and plant development. Plant J. 2005, 44, 100–113. [Google Scholar] [CrossRef] [PubMed]
- Kaminaka, H.; Näke, C.; Epple, P.; Dittgen, J.; Schütze, K.; Chaban, C.; Holt, B.F.; Merkle, T.; Schäfer, E.; Harter, K. bZIP10-LSD1 antagonism modulates basal defense and cell death in Arabidopsis following infection. EMBO J. 2006, 25, 4400–4411. [Google Scholar] [CrossRef] [PubMed]
- Nieva, C.; Busk, P.K.; Domínguez-Puigjaner, E.; Lumbreras, V.; Testillano, P.S.; Risueño, M.C.; Pagès, M. Isolation and functional characterisation of two new bZIP maize regulators of the ABA responsive gene rab28. Plant Mol. Biol. 2005, 58, 899–914. [Google Scholar] [CrossRef] [PubMed]
- Uno, Y.; Furihata, T.; Abe, H.; Yoshida, R.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Arabidopsis basic leucine zipper transcription factors involved in an abscisic acid-dependent signal transduction pathway under drought and high-salinity conditions. Proc. Natl. Acad. Sci. USA 2000, 97, 11632–11637. [Google Scholar] [CrossRef] [PubMed]
- Wellmer, F.; Kircher, S.; Rügner, A.; Frohnmeyer, H.; Schäfer, E.; Harter, K. Phosphorylation of the parsley bZIP transcription factor CRPF2 is regulated by light. J. Biol. Chem. 1999, 274, 29476–29482. [Google Scholar] [CrossRef] [PubMed]
- Ulm, R.; Baumann, A.; Oravecz, A.; Máté, Z.; Adám, E.; Oakeley, E.J.; Schäfer, E.; Nagy, F. Genome-wide analysis of gene expression reveals function of the bZIP transcription factor HY5 in the UV-B response of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 1397–1402. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Mao, B.; Ou, S.; Wang, W.; Liu, L.; Wu, Y.; Chu, C.; Wang, X. OsbZIP71, a bZIP transcription factor, confers salinity and drought tolerance in rice. Plant Mol. Biol. 2014, 84, 19–36. [Google Scholar] [CrossRef] [PubMed]
- Riano-Pachon, D.M.; Correa, L.G.; Trejos-Espinosa, R.; Mueller-Roeber, B. Green transcription factors: A Chlamydomonas overview. Genetics 2008, 179, 31–39. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Zou, H.F.; Wei, W.; Hao, Y.J.; Tian, A.G.; Huang, J.; Liu, Y.F.; Zhang, J.S.; Chen, S.Y. Soybean GmbZIP44, GmbZIP62 and GmbZIP78 genes function as negative regulator of aba signaling and confer salt and freezing tolerance in transgenic Arabidopsis. Planta 2008, 228, 225–240. [Google Scholar] [CrossRef] [PubMed]
- Chuang, C.F.; Running, M.P.; Williams, R.W.; Meyerowitz, E.M. The PERIANTHIA gene encodes a bZIP protein involved in the determination of floral organ number in Arabidopsis thaliana. Genes Dev. 1999, 13, 334–344. [Google Scholar] [CrossRef] [PubMed]
- Mikami, K.; Sakamoto, A.; Iwabuchi, M. The HBP-1 family of wheat basic/leucine zipper proteins interacts with overlapping cis-acting hexamer motifs of plant histone genes. J. Biol. Chem. 1994, 269, 9974–9985. [Google Scholar] [PubMed]
- Cheong, Y.H.; Park, J.M.; Yoo, C.M.; Bahk, J.D.; Cho, M.J.; Hong, J.C. Isolation and characterization of STGA1, a member of the TGA1 family of bZIP transcription factors from soybean. Mol. Cells 1994, 4, 405–412. [Google Scholar]
- Nijhawan, A.; Jain, M.; Tyagi, A.K.; Khurana, J.P. Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol. 2008, 146, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, Y.; Shi, H.; Guo, M.; Chai, M.; He, Q.; Yan, M.; Cao, D.; Zhao, L.; Cai, H. Evolutionary and expression analyses of soybean basic leucine zipper transcription factor family. BMC Genom. 2018, 19, 159. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.L.; Chen, X.; Yang, T.B.; Cheng, Q.; Cheng, Z.M. Genome-wide identification of bZIP family genes involved in drought and heat stresses in strawberry (Fragaria vesca). Int. J. Genom. 2017, 2017, 1–14. [Google Scholar] [CrossRef]
- Kato, Y.; Ho, S.H.; Vavricka, C.J.; Chang, J.S.; Hasunuma, T.; Kondo, A. Evolutionary engineering of salt-resistant Chlamydomonas sp. strains reveals salinity stress-activated starch-to-lipid biosynthesis switching. Bioresour. Technol. 2017, 245, 1484–1490. [Google Scholar] [CrossRef] [PubMed]
- Kalita, N.; Baruah, G.; Chandra, R.; Goswami, D.; Talukdar, J.; Kalita, M.C. Ankistrodesmus falcatus: A promising candidate for lipid production, its biochemical analysis and strategies to enhance lipid productivity. J. Microbiol. Biotechnol. Res. 2011, 1, 148–157. [Google Scholar]
- Lu, N.; Wei, D.; Chen, F.; Yang, S.T. Lipidomic profiling and discovery of lipid biomarkers in snow alga Chlamydomonas nivalis under salt stress. Eur. J. Lipid Sci. Technol. 2012, 114, 253–265. [Google Scholar] [CrossRef]
- Mohan, S.V.; Devi, M.P. Salinity stress induced lipid synthesis to harness biodiesel during dual mode cultivation of mixotrophic microalgae. Bioresour. Technol. 2014, 165, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Gong, H.; Wen, X.; Lu, C. Salt stress induces a decrease in excitation energy transfer from phycobilisomes to photosystem II but an increase to photosystem I in the cyanobacterium Spirulina platensis. J. Plant Physiol. 2010, 167, 951–958. [Google Scholar] [CrossRef] [PubMed]
- Santos, C.V. Regulation of chlorophyll biosynthesis and degradation by salt stress in sunflower leaves. Sci. Hortic. 2004, 103, 93–99. [Google Scholar] [CrossRef]
- Rao, A.R.; Dayananda, C.; Sarada, R.; Shamala, T.R.; Ravishankar, G.A. Effect of salinity on growth of green alga Botryococcus braunii and its constituents. Bioresour. Technol. 2007, 98, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Fedina, I.S.; Grigorova, I.D.; Georgieva, K.M. Response of barley seedlings to UV-B radiation as affected by NaCl. J. Plant Physiol. 2003, 160, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Finazzi, G.; Johnson, G.N.; Dall’Osto, L.; Zito, F.; Bonente, G.; Bassi, R.; Wollman, F.A. Nonphotochemical quenching of chlorophyll fluorescence in Chlamydomonas reinhardtii. Biochemistry 2006, 45, 1490–1498. [Google Scholar] [CrossRef] [PubMed]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [PubMed]
- Mou, S.; Zhang, X.; Ye, N.; Dong, M.; Liang, C.; Qiang, L.; Miao, J.; Dong, X.; Zhou, Z. Cloning and expression analysis of two different LhcSR genes involved in stress adaptation in an Antarctic microalga, Chlamydomonas sp. ICE-L. Extremophiles 2012, 16, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Kan, G.; Shi, C.; Wang, X.; Xie, Q.; Wang, M.; Wang, X.; Miao, J. Acclimatory responses to high-salt stress in Chlamydomonas (Chlorophyta, Chlorophyceae) from Antarctica. Acta Oceanol. Sin. 2012, 31, 116–124. [Google Scholar] [CrossRef]
- Wang, T.; Ge, H.; Liu, T.; Tian, X.; Wang, Z.; Guo, M.; Chu, J.; Zhuang, Y. Salt stress induced lipid accumulation in heterotrophic culture cells of Chlorella protothecoides: Mechanisms based on the multi-level analysis of oxidative response, key enzyme activity and biochemical alteration. J. Biotechnol. 2016, 228, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Meng, X.; Cai, J.; Li, G.; Dong, T.; Li, Z. Basic leucine zipper transcription factor SlbZIP1 mediates salt and drought stress tolerance in tomato. BMC Plant Biol. 2018, 18, 83. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.; Wu, G.; Deshpande, R.R.; Vieler, A.; Gärtner, K.; Li, X.; Moellering, E.R.; Zäuner, S.; Cornish, A.J.; Liu, B. Changes in transcript abundance in Chlamydomonas reinhardtii following nitrogen deprivation predict diversion of metabolism. Plant Physiol. 2010, 154, 1737–1752. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, F.; Yamagata, D.; Ishikawa, M.; Fukamatsu, Y.; Ogura, Y.; Kasahara, M.; Kiyosue, T.; Kikuyama, M.; Wada, M.; Kataoka, H. AUREOCHROME, a photoreceptor required for photomorphogenesis in stramenopiles. Proc. Natl. Acad. Sci. USA 2007, 104, 19625–19630. [Google Scholar] [CrossRef] [PubMed]
- Huysman, M.J.; Fortunato, A.E.; Matthijs, M.; Costa, B.S.; Vanderhaeghen, R.; Van den Daele, H.; Sachse, M.; Inzé, D.; Bowler, C.; Kroth, P.G.; et al. AUREOCHROME1a-mediated induction of the diatom-specific cyclin dsCYC2 controls the onset of cell division in diatoms (Phaeodactylum tricornutum). Plant Cell 2013, 25, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.B.; Niyogi, K.K. SINGLET OXYGEN RESISTANT 1 links reactive electrophile signaling to singlet oxygen acclimation in Chlamydomonas reinhardtii. Proc. Natl. Acad. Sci. USA 2012, 109, E1302–E1311. [Google Scholar] [CrossRef] [PubMed]
- Harris, E. The Chlamydomonas sourcebook: Introduction into Chlamydomonas and Its Laboratory Use, 2nd ed.; Elsevier Science Publishing Co. Inc.: New York, NY, USA, 2008; ISBN 978-012-370-874-8. [Google Scholar]
- Chen, L.; Liu, T.Z.; Zhang, W.; Chen, X.L.; Wang, J.F. Biodiesel production from algae oil high in free fatty acids by two-step catalytic conversion. Bioresour. Technol. 2012, 111, 208–214. [Google Scholar] [CrossRef] [PubMed]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]


| Gene Number | NCBI Accession Number | Phytozome Identifier | Chromosome Localization (bp) | Protein Length (aa) | Molecular Weight (Da) | Theoretical pI | Hydrophility | Instability Index |
|---|---|---|---|---|---|---|---|---|
| CrebZIP1 | PNW88934.1 | Cre01.g051174 | Chr.1: 7078516–7088297 | 2018 | 198,080.64 | 6.00 | −0.422 | 68.82 |
| CrebZIP2 | PNW83651.1 | Cre05.g238250 | Chr.5: 2933060–2936249 | 524 | 53,624.16 | 5.47 | −0.045 | 58.98 |
| CrebZIP3 | PNW80451.1 | Cre07.g318050 | Chr.7: 782941–789359 | 802 | 82,929.15 | 6.16 | −0.406 | 59.38 |
| CrebZIP4 | PNW80535.1 | Cre07.g321550 | Chr.7: 1255642–1260306 | 393 | 41,429.96 | 5.48 | −0.510 | 44.72 |
| CrebZIP5 | PNW81157.1 | Cre07.g344668 | Chr.7: 4675427–4680202 | 750 | 72,176.41 | 6.18 | −0.046 | 65.59 |
| CrebZIP6 | PNW79382.1 | Cre09.g413050 | Chr.9: 7331940–7342521 | 1053 | 106,414.13 | 6.42 | −0.401 | 54.24 |
| CrebZIP7 | PNW77489.1 | Cre10.g438850 | Chr.10: 2769177–2772294 | 485 | 49,874.20 | 6.64 | −0.678 | 58.82 |
| CrebZIP8 | PNW77864.1 | Cre10.g454850 | Chr.10: 4910181–4919296 | 1363 | 128,662.31 | 8.75 | −0.249 | 64.61 |
| CrebZIP9 | PNW74780.1 | Cre12.g510200 | Chr.12: 2010086–2013670 | 353 | 35, 933.29 | 6.36 | −0. 398 | 58.58 |
| CrebZIP10 | PNW74984.1 | Cre12.g501600 | Chr.12: 2939321–2944866 | 902 | 89,920.31 | 6.25 | −0.274 | 56.84 |
| CrebZIP11 | PNW75863.1 | Cre12.g557300 | Chr.12: 7330581–7337724 | 1526 | 154,908.24 | 9.40 | −0.719 | 65.07 |
| CrebZIP12 | PNW73681.1 | Cre13.g568350 | Chr.13: 981891–989596 | 1150 | 114,710.75 | 9.55 | −0.563 | 68.90 |
| CrebZIP13 | XP_001693067.1 | Cre13.g590350 | Chr.13: 3885273–3888238 | 334 | 34,514.92 | 4.96 | −0.172 | 58.83 |
| CrebZIP14 | PNW71231.1 | Cre16.g692250 | Chr.16: 591049–600037 | 1525 | 153,084.80 | 5.30 | −0.558 | 72.50 |
| CrebZIP15 | PNW71414.1 | Cre16.g653300 | Chr.16: 1524635–1531081 | 867 | 90,393.04 | 6.11 | −0.374 | 55.17 |
| CrebZIP16 | PNW72253.1 | Cre16.g675700 | Chr.16: 6123854–6131215 | 1172 | 115,285.37 | 6.48 | −0.512 | 46.46 |
| CrebZIP17 | PNW71098.1 | Cre17.g746547 | Chr.17: 7009739–7013345 | 767 | 75,438.72 | 6.01 | −0.379 | 55.72 |
| Gene Number | Alpha Helix (%) | Extended Strand (%) | Beta Bridge (%) | Random Coil (%) |
|---|---|---|---|---|
| CrebZIP1 | 24.58 | 2.68 | 0 | 72.75 |
| CrebZIP2 | 53.24 | 1.53 | 0 | 45.23 |
| CrebZIP3 | 36.41 | 8.73 | 0 | 54.86 |
| CrebZIP4 | 45.29 | 1.53 | 0 | 53.18 |
| CrebZIP5 | 41.60 | 0.80 | 0 | 57.60 |
| CrebZIP6 | 43.49 | 2.94 | 0 | 53.56 |
| CrebZIP7 | 45.80 | 0.42 | 0 | 53.78 |
| CrebZIP8 | 30.01 | 3.67 | 0 | 66.32 |
| CrebZIP9 | 36.54 | 8.22 | 0 | 55.24 |
| CrebZIP10 | 38.03 | 3.77 | 0 | 58.20 |
| CrebZIP11 | 26.34 | 4.59 | 0 | 69.07 |
| CrebZIP12 | 39.57 | 3.22 | 0 | 57.22 |
| CrebZIP13 | 50.30 | 3.59 | 0 | 46.11 |
| CrebZIP14 | 39.08 | 1.70 | 0 | 59.21 |
| CrebZIP15 | 46.25 | 1.85 | 0 | 51.90 |
| CrebZIP16 | 33.19 | 1.88 | 0 | 64.93 |
| CrebZIP17 | 27.38 | 3.00 | 0 | 69.62 |
| Gene Number | Forward Primer (5′-3′) | Reverse Primer (5′-3′) |
|---|---|---|
| CrebZIP1 | CGGTCGATGACGCTAAGGC | TGGTCGGTGTCGGAGGAGT |
| CrebZIP2 | GAGCGCAAGAAGCAGTACGTGACCT | GATGTTCCGCAGTGCCTCGTTCT |
| CrebZIP3 | ATGCACCAAACGCCAAATCG | ATCTGCTGTAGGTCCGCCAGGGTA |
| CrebZIP4 | GACAGCGGAGACTCAGACAT | CCCTTCTTACGCTGCCTGTA |
| CrebZIP5 | CCGTCATCCATGCACCTACT | GCTGAAGATCAAGACCGCTG |
| CrebZIP6 | CCCGTTTCCAGCCCATCAGAC | TGCAAGGCCGAGGGTGTTGATGAC |
| CrebZIP7 | TGGGAGGCTCTGGCTTTGG | TGGCTGCTGCTGCTGCTGATG |
| CrebZIP8 | GCAAAGGCAAGGGCAAGG | CGCAGGAGTTCTCAGCCGATT |
| CrebZIP9 | GCGCTTCTTCCTCGCTACCA | CGCAAGCCCTTGTGCTGTTA |
| CrebZIP10 | CCCTGACGTCCACCTTAACT | TCCCTCAGACAGCAACTCAG |
| CrebZIP11 | ACGGACGTATGACATGAGCA | ACTGCTGGAACTGGTGGAA |
| CrebZIP12 | CACAGCGACCGCAGCCATAA | GCCCAGCTTGTCCGAGAAGGA |
| CrebZIP13 | GAGCTGCCTTCGACAAGC | CTGTGCTAGGCGATTCAGC |
| CrebZIP14 | GATGGCGGGCTTATGTCGG | CAAGGCGTCCACGTTGTG |
| CrebZIP15 | TGACTCATCCACGCACTTCCTC | TGATGTTGCACCAGCCCTGA |
| CrebZIP16 | GGTTTCCTTCCCTACCCA | ACGGCACGGTTGTCAGCA |
| CrebZIP17 | AAGCGCATTGTTGACGGAG | CGCAGCAGGTCTAATAAGTCG |
| Creα-tubulin | CTCGCTTCGCTTTGACGGTG | CGTGGTACGCCTTCTCGGC |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, C.; Mao, X.; Hao, J.; Wang, X.; Xue, J.; Cui, H.; Li, R. Analysis of bZIP Transcription Factor Family and Their Expressions under Salt Stress in Chlamydomonas reinhardtii. Int. J. Mol. Sci. 2018, 19, 2800. https://doi.org/10.3390/ijms19092800
Ji C, Mao X, Hao J, Wang X, Xue J, Cui H, Li R. Analysis of bZIP Transcription Factor Family and Their Expressions under Salt Stress in Chlamydomonas reinhardtii. International Journal of Molecular Sciences. 2018; 19(9):2800. https://doi.org/10.3390/ijms19092800
Chicago/Turabian StyleJi, Chunli, Xue Mao, Jingyun Hao, Xiaodan Wang, Jinai Xue, Hongli Cui, and Runzhi Li. 2018. "Analysis of bZIP Transcription Factor Family and Their Expressions under Salt Stress in Chlamydomonas reinhardtii" International Journal of Molecular Sciences 19, no. 9: 2800. https://doi.org/10.3390/ijms19092800
APA StyleJi, C., Mao, X., Hao, J., Wang, X., Xue, J., Cui, H., & Li, R. (2018). Analysis of bZIP Transcription Factor Family and Their Expressions under Salt Stress in Chlamydomonas reinhardtii. International Journal of Molecular Sciences, 19(9), 2800. https://doi.org/10.3390/ijms19092800




