Investigation of the Antioxidant Capacity, Insecticidal Ability and Oxidation Stability of Chenopodium formosanum Seed Extract
Abstract
:1. Introduction
2. Results and Discussion
2.1. Model Fitting
2.2. Analysis of the Response Surfaces
2.3. Determination of the Optimal Conditions and Model Verification
2.4. GC-MS Analysis of the Extract
2.5. Toxicity Bioassays
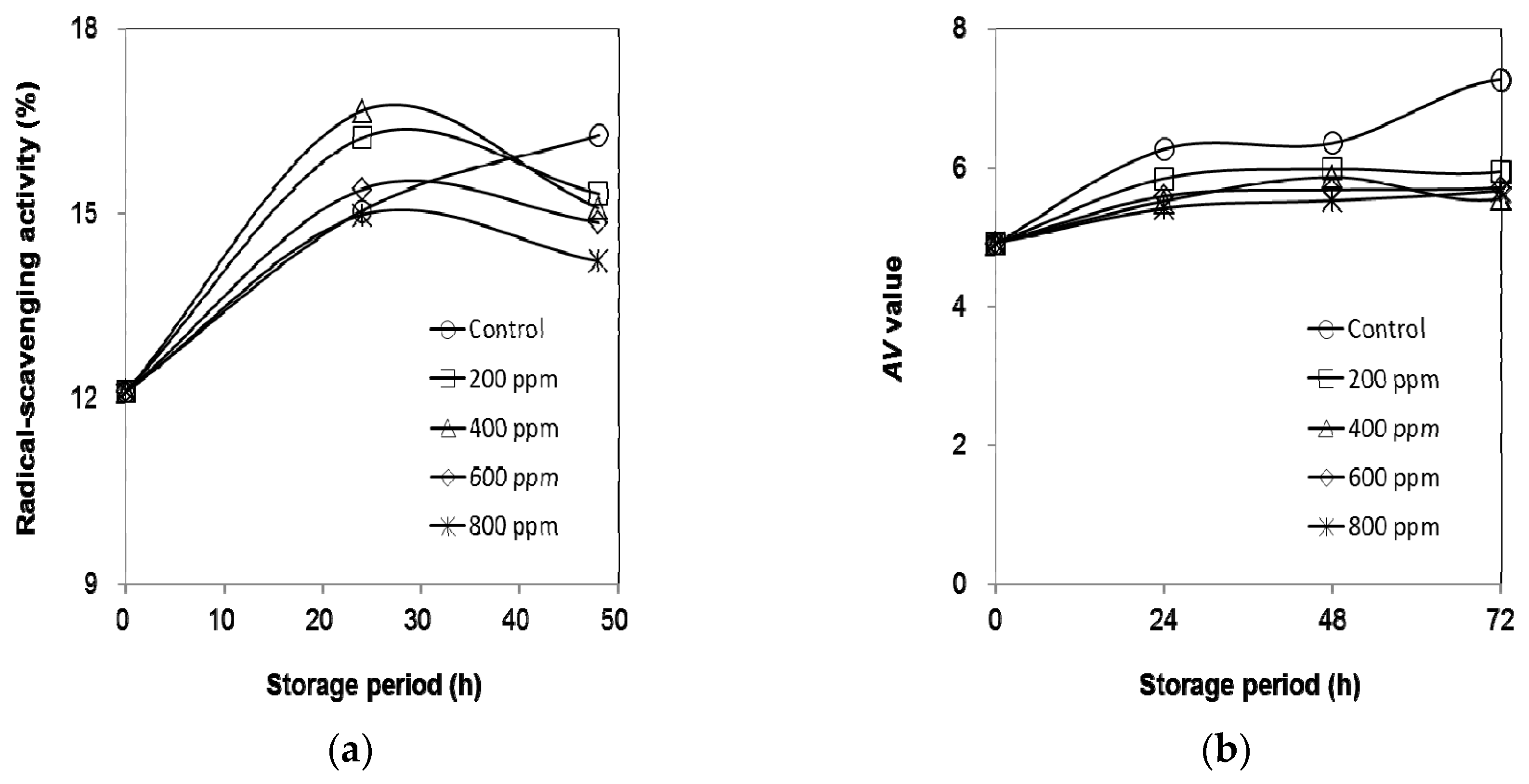
2.6. Oxidative Stability of Olive Oil
3. Materials and Methods
3.1. Materials
3.2. Extraction of Chenopodium formosanum Seeds
3.3. Phenolic Content Analysis
3.4. Antioxidant Capacity Analysis
3.5. Insect Cultures and Toxicity Bioassays
3.6. Oxidative Stability of Olive Oil
3.7. Experimental Design and Statistical Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| RSM | Response surface methodology |
| ROS | Reactive oxygen species |
| TPC | Total phenolic content |
| TFC | Total flavonoid content |
| FICA | Ferrous ion chelating activity |
| EDTA | Ethylenediaminetetraacetic acid |
References
- Chew, Y.L.; Lim, Y.Y.; Omar, M.; Khoo, K.S. Antioxidant activity of three edible seaweeds from two areas in South East Asia. LWT-Food Sci. Technol. 2008, 41, 1067–1072. [Google Scholar] [CrossRef]
- Gliszczynska-Swiglo, A. Antioxidant activity of water soluble vitamins in the TEAC (trolox equivalent antioxidant capacity) and the FRAP (ferric reducing antioxidant power) assays. Food Chem. 2006, 96, 131–136. [Google Scholar] [CrossRef]
- Chyau, C.C.; Chu, C.C.; Chen, S.Y.; Duh, P.D. Djulis (Chenopodiun formosaneum) and its bioactive compounds protect against oxidative stress in human HepG2 cells. J. Funct. Foods 2015, 18, 159–170. [Google Scholar] [CrossRef]
- Gutteridge, J.M.C.; Halliwell, B. Antioxidants: Molecules, medicines, any myths. Biochem. Biophys. Res. Commun. 2010, 393, 561–564. [Google Scholar] [CrossRef] [PubMed]
- Vattakandya, L.S.; Chaudharib, G.S. Estimation of total phenolic content and antioxidant activity of Dendrobium herbaceum Lindl. Int. J. Pharm. 2013, 104, 302–305. [Google Scholar]
- Kyarimpa, C.M.; Bohmdorfer, S.; Wasswa, J.; Kiremire, B.T.; Ndiege, I.O.; Kabasa, J.D. Essential oil and composition of Tagetes minuta from Uganda. Larvicidal activity on Anopheles gambbiae. Ind. Crops Prod. 2014, 62, 400–404. [Google Scholar] [CrossRef]
- Su, S.W.; Lee, T.M.; Mok, H.K.; Chio, E.H. Insecticidal activity and insect repellencyof four species of sea lily (comatulida: comatulidae) from Taiwan. J. Insect Sci. 2016, 16, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.M.; Cheng, C.L.; Lee, S.C.; Hong, G.B. Antioxidant capacity, insecticidal ability and heat-oxidation stability of Tagetes lemmonii leaf extract. Ecotoxicol. Environ. Saf. 2018, 151, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Hrasia, A.R.; Hadolin, M.; Kenz, Z.; Bauman, D. Comparison of antioxidative and synergistic effects of rosemary extract with a-tocopherol, ascorbyl palmitate and citric acid in sun flower oil. Food Chem. 2000, 71, 229–233. [Google Scholar] [CrossRef]
- Ilaiyaraja, N.; Likhith, K.R.; Sharath Babu, G.R.; Khanum, F. Optimisation of extraction of bioactive compounds from Feronia limonia (wood apple) fruit using response surface methodology (RSM). Food Chem. 2015, 173, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Narkprasom, K.; Su, W.L.; Cheng, M.Y.; Wang, S.P.; Hsiao, S.M.; Tsai, P.J. Relative effects of alcohol and pH on betacyanin stability in aqueous djulis extracts and their color qualities after organic acid addition. J. Food Qual. 2012, 35, 283–291. [Google Scholar] [CrossRef]
- Yang, B.Y.; Cheng, M.H.; Ko, C.H.; Wang, Y.N.; Chen, W.H.; Hwang, W.S.; Yang, Y.P.; Chen, H.T.; Chang, F.C. Potential bioethanol production from Taiwanese chenopods (Chenopodium formosanum). Energy 2014, 76, 59–65. [Google Scholar] [CrossRef]
- Chio, E.H.; Yang, E.C.; Huang, H.T.; Hsu, E.L.; Chen, C.R.; Huang, C.G.; Huang, R.N. Toxicity and repellence of Taiwanese indigenous djulis, Chenopodium formosaneum, against Aedes albopictus (Diptera: Culicidae) and Forcipomyia taiwana (Diptera: Ceratopogonidae). J. Pest Sci. 2013, 86, 705–712. [Google Scholar] [CrossRef]
- Amado, I.R.; Franco, D.; Sanchez, M.; Zapata, C.; Vazquez, J.A. Optimisation of antioxidant extraction from Solanum tuberosum potato peel waste by surface response methodology. Food Chem. 2014, 165, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, A.R.; Ghaedi, M.; Asfaram, A.; Hajati, S.; Ghaedi, A.M.; Bazrafshan, A.; Rahimi, M.R. Modeling and optimization of simultaneous removal of ternary dyes onto copper sulfide nanoparticles loaded on activated carbon using second-derivative spectrophotometry. J. Taiwan Inst. Chem. Eng. 2016, 65, 212–224. [Google Scholar] [CrossRef]
- Hemwimon, S.; Pavasant, P.; Shotipruk, A. Microwave-assisted extraction of antioxidative anthraquinones from roots of Morinda citrifolia. Sep. Purif. Technol. 2007, 54, 44–50. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Tekin, K.; Akalin, M.K.; Seker, M.G. Ultrasound bath-assisted extraction of essential oils from clove using central composite design. Ind. Crops Prod. 2015, 77, 954–960. [Google Scholar] [CrossRef]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability-a review. Int. J. Food Sci. Technol. 2009, 44, 2365–2376. [Google Scholar] [CrossRef]
- Proestos, C.; Boziaris, I.S.; Nychas, G.J.E.; Komaitis, M. Analysis of flavonoids and phenolic acids in Greek aromatic plants: Investigation of their antioxidant capacity and antimicrobial activity. Food Chem. 2006, 95, 664–671. [Google Scholar] [CrossRef]
- Dieng, H.; Zawawi, R.B.M.; Mohamed Yusof, N.I.S.B.; Ahmad, A.H.; Abang, F.; Ghani, I.A.; Satho, T.; Ahmad, H.; Zuharah, W.F.; Majid, A.H.A.; et al. Green tea and its waste attract workers of formicine ants and kill theirworkers-implications for pest management. Ind. Crops Prod. 2016, 89, 157–166. [Google Scholar] [CrossRef]
- Azadfar, M.; Gao, A.H.; Bule, M.V.; Chen, S. Structural characterization of lignin: A potential source of antioxidants guaiacol and 4-vinylguaiacol. Int. J. Biol. Macromol. 2015, 75, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Kosobutskii, V.S. Pyrocatechol and its derivatives as antioxidants and prooxidants. Russ. J. Gen. Chem. 2014, 84, 746–749. [Google Scholar] [CrossRef]
- Zhao, L.; Chen, J.; Su, J.; Li, L.; Hu, S.; Li, B.; Zhang, X.; Xu, Z.; Chen, T. In vitro antioxidant and antiproliferative activities of 5-hydroxymethylfurfural. J. Agric. Food Chem. 2013, 61, 10604–10611. [Google Scholar] [CrossRef] [PubMed]
- Tunón, H.; Thorsell, W.; Bohlin, L. Mosquito repelling activity of compounds occurring in Achillea millefolium L. (asteraceae). Econ. Bot. 1994, 48, 111–120. [Google Scholar] [CrossRef]
- Rieth, J.P.; Wilson, W.T.; Levin, M.D. Repelling honeybees from insecticide-treated flowers with 2-heptanone. J. Apicult. Res. 1986, 25, 78–84. [Google Scholar] [CrossRef]
- Miyazawa, M.; Anzai, J.; Fujioka, J.; Isikawa, Y. Insecticidal compounds against drosophila melanogaster from Cornus Officinalis Sieb. et Zucc. Nat. Prod. Res. 2003, 17, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Bird, T.G.; Hedin, P.A.; Burks, M.L. Feeding deterrent compounds to the boll weevil, Anthonomus grandis Boheman in rose-of-sharon, Hibiscus syriacus L. J. Chem. Ecol. 1987, 13, 1087–1097. [Google Scholar] [CrossRef] [PubMed]
- Chong, Y.M.; Chang, S.K.; Sia, W.C.M.; Yim, H.S. Antioxidant efficacy of mangosteen (Garcinia mangostana Linn.) peel extracts in sunflower oil during accelerated storage. Food Biosci. 2015, 12, 18–25. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Khaneghah, A.M.; Koubaa, M.; Lopez-Cervantes, J.; Yousefabad, S.H.A.; Hosseini, S.F.; Karimi, M.; Motazedian, A.; Asadifard, S. Novel edible oil sources: Microwave heating and chemical properties. Food Res. Int. 2017, 92, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic–phosphotungstic acid reagents. Am. J. Enol. Viticult. 1965, 16, 144–158. [Google Scholar]
- Chang, C.C.; Yang, M.H.; Wen, H.M.; Chern, J.C. Estimation of total flavonoid content in propolis by two complementary colorimetric methods. J. Food Drug Anal. 2002, 10, 178–182. [Google Scholar]
- Dinis, C.P.; Madeira, V.M.C.; Almeida, L.M. Action of phenolic derivatives (acetaminophen, salicylate, and 5-aminosalicylate) as inhibitors of membrane lipid peroxidation and as peroxyl radical scavengers. Arch. Biochem. Biophys. 1994, 315, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Bougherra, H.H.; Bedini, S.; Flamini, G.; Cosci, F.; Belhamel, K.; Conti, B. Pistacia lentiscus essential oil has repellent effect against three major insect pests of pasta. Ind. Crops Prod. 2015, 63, 249–255. [Google Scholar] [CrossRef]
- Peixoto, M.G.; Bacci, L.; Blank, A.F.; Araujo, A.P.A.; Alves, P.B.; Silva, J.H.S.; Santos, A.A.; Oliverira, A.P.; Costa, A.S.; Arrigoni-Blank, M.F. Toxicity and repellency of essential oils of Lippia alba chemotypes and their major monoterpenes against stored grain insects. Ind. Crops Prod. 2015, 71, 31–36. [Google Scholar] [CrossRef]
- Abbott, W.S. A method for computing the effectiveness of the insecticide. J. Am. Mosq. Control Assoc. 1987, 3, 302–303. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.S.; Chye, F.Y.; Liow, M.L.; Ho, C.W. Antioxidant potential of Pleurotus Porringens extract and application in sunflower oil during accelerated storage. Chiang Mai J. Sci. 2013, 40, 34–48. [Google Scholar]
- Martinez, M.; Maestri, D.M. Oil chemical variation in walnut (Juglans regia L.) genotypes grown in Argentina. Eur. J. Lipid Sci. Technol. 2008, 110, 1183–1189. [Google Scholar] [CrossRef]
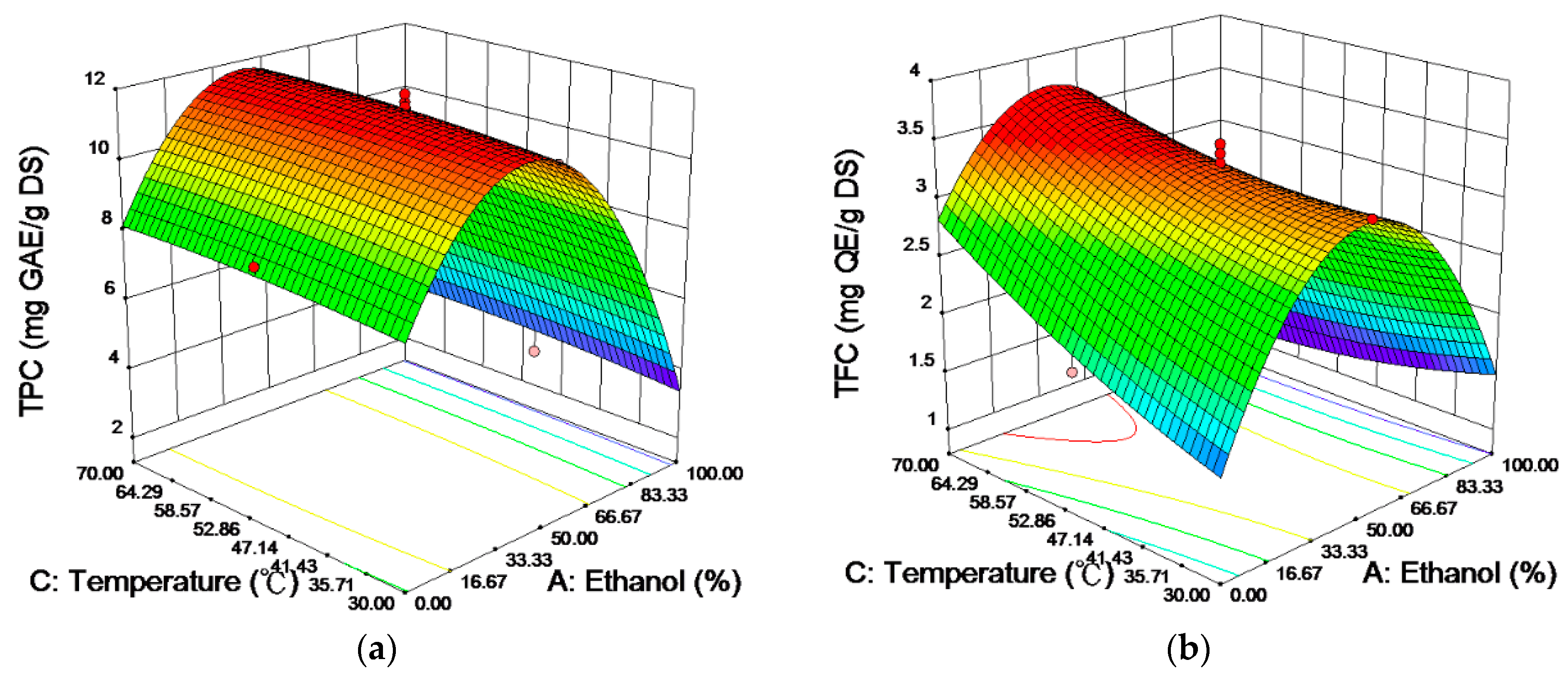
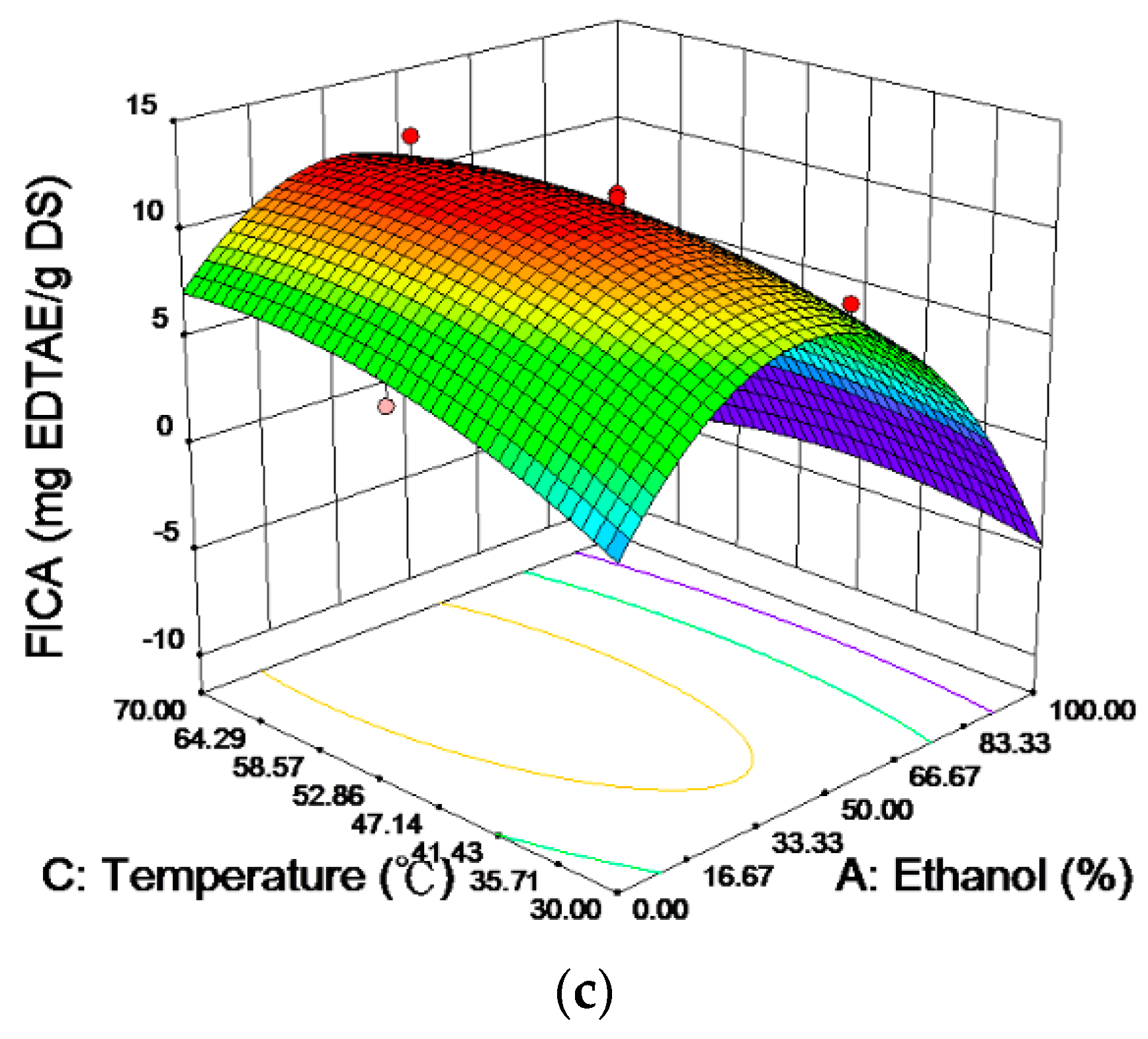
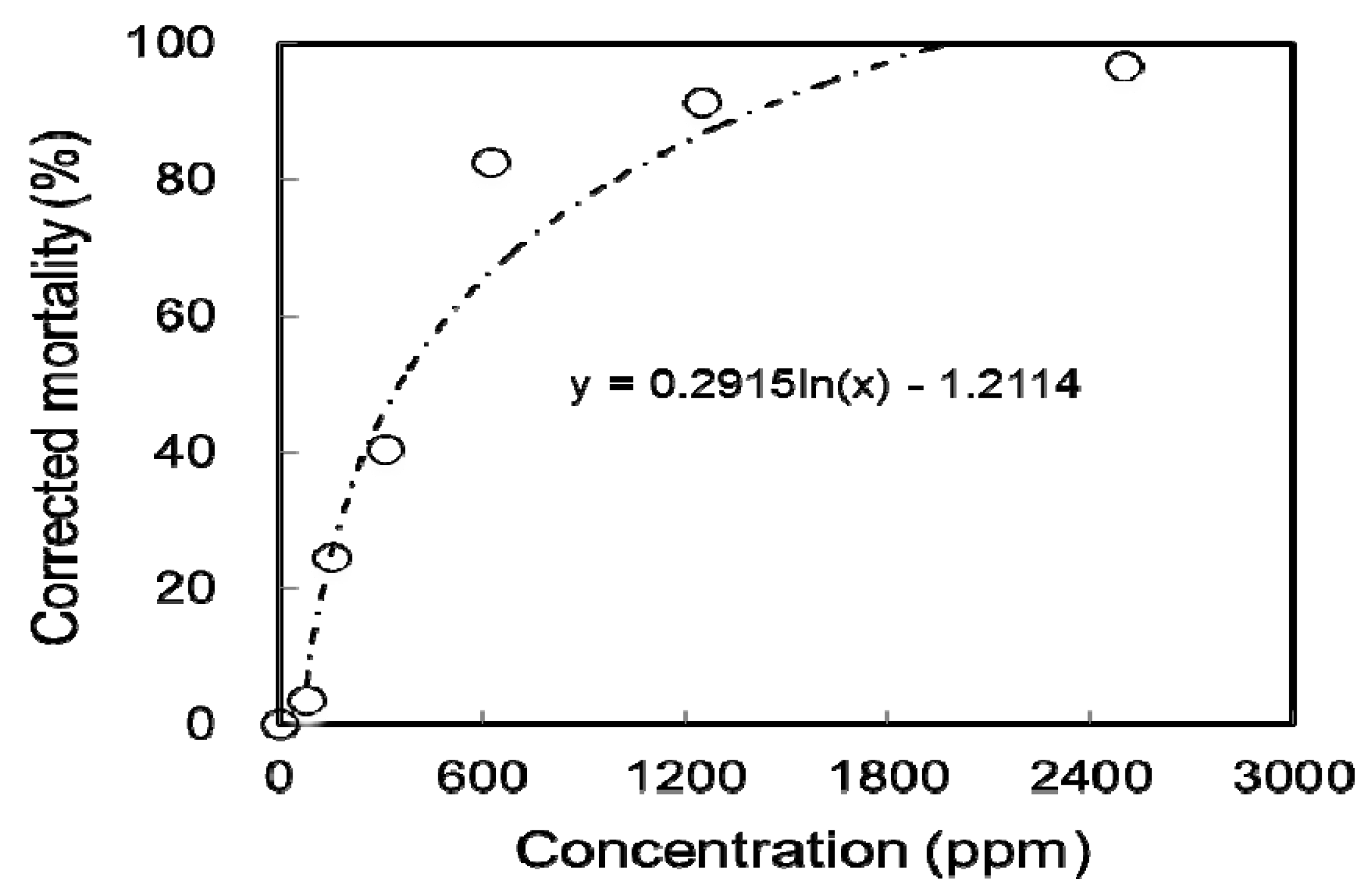
| Independent Variables | Coded | Factor Levels | ||||||
|---|---|---|---|---|---|---|---|---|
| −1.68 | −1 | 0 | 1 | 1.68 | ||||
| Ethanol concentration (%) | X1 | 0 | 20.3 | 50 | 79.7 | 100 | ||
| Extraction time (min) | X2 | 30 | 60.4 | 105 | 149.6 | 180 | ||
| Extraction Temperature (°C) | X3 | 30 | 38.1 | 50 | 61.9 | 70 | ||
| Run | Independent varialbes | Experimental values 1 | ||||||
| X1:E | X2:t | X3:T | TPC | TFC | FICA | |||
| 1 | 20.3 | 60.4 | 38.1 | 10.745 | 2.851 | 9.151 | ||
| 2 | 20.3 | 60.4 | 61.9 | 11.316 | 3.429 | 9.786 | ||
| 3 | 20.3 | 149.6 | 38.1 | 10.148 | 2.846 | 8.207 | ||
| 4 | 20.3 | 149.6 | 61.9 | 10.238 | 3.405 | 12.092 | ||
| 5 | 79.7 | 60.4 | 38.1 | 8.140 | 2.456 | 2.360 | ||
| 6 | 79.7 | 60.4 | 61.9 | 8.118 | 2.464 | 2.293 | ||
| 7 | 79.7 | 149.6 | 38.1 | 8.234 | 2.499 | 1.692 | ||
| 8 | 79.7 | 149.6 | 61.9 | 8.931 | 2.499 | 2.336 | ||
| 9 | 0 | 105 | 50 | 8.389 | 1.988 | 5.413 | ||
| 10 | 100 | 105 | 50 | 3.051 | 1.265 | 0.02 | ||
| 11 | 50 | 30 | 50 | 10.978 | 3.356 | 10.312 | ||
| 12 | 50 | 180 | 50 | 10.752 | 3.261 | 11.901 | ||
| 13 | 50 | 105 | 30 | 11.078 | 3.231 | 9.844 | ||
| 14 | 50 | 105 | 70 | 11.186 | 3.522 | 11.754 | ||
| 15 | 50 | 105 | 50 | 11.861 | 3.211 | 11.754 | ||
| 16 | 50 | 105 | 50 | 11.124 | 3.400 | 11.571 | ||
| 17 | 50 | 105 | 50 | 11.515 | 3.319 | 11.125 | ||
| 18 | 50 | 105 | 50 | 11.465 | 3.479 | 11.002 | ||
| 19 | 50 | 105 | 50 | 11.300 | 3.301 | 11.106 | ||
| 20 | 50 | 105 | 50 | 11.643 | 3.161 | 10.408 | ||
| Source | DF (Degree of Freedom) | SS (Sum of Squares) | F Value | p Value |
|---|---|---|---|---|
| TPC | ||||
| Model | 9 | 80.42 | 51.45 | <0.0001 |
| X1 | 1 | 23.73 | 136.61 | <0.0001 |
| X2 | 1 | 0.097 | 0.56 | 0.4732 |
| X3 | 1 | 0.17 | 0.97 | 0.3477 |
| X1X2 | 1 | 0.83 | 4.80 | 0.0533 |
| X1X3 | 1 | 2.45 × 10−5 | 1.41 × 10−4 | 0.9908 |
| X2X3 | 1 | 7.08 × 10−3 | 0.041 | 0.8440 |
| X12 | 1 | 55.23 | 318 | <0.0001 |
| X22 | 1 | 0.28 | 1.60 | 0.2351 |
| X32 | 1 | 0.028 | 0.16 | 0.6951 |
| Linear | 3 | 23.99 | 2.20 | 0.1278 |
| Quadratic | 3 | 55.59 | 106.68 | <0.0001 |
| Cubic | 4 | 0.94 | 1.77 | 0.2525 |
| Lack of fit | 5 | 1.40 | 4.23 | 0.0698 |
| R2 = 0.9789 | ||||
| Adj R2 = 0.9598 | ||||
| TFC | ||||
| Model | 9 | 6.49 | 51.65 | <0.0001 |
| X1 | 1 | 1.07 | 76.87 | <0.0001 |
| X2 | 1 | 8.99 × 10−4 | 0.064 | 0.8049 |
| X3 | 1 | 0.2 | 14.01 | 0.0038 |
| X1X2 | 1 | 1.43 × 10−3 | 0.1 | 0.7555 |
| X1X3 | 1 | 0.16 | 11.41 | 0.0070 |
| X2X3 | 1 | 9.11 × 10−5 | 6.53 × 10−3 | 0.9372 |
| X12 | 1 | 4.87 | 348.84 | <0.0001 |
| X22 | 1 | 2.54 × 10−3 | 0.18 | 0.6790 |
| X32 | 1 | 0.02 | 1.44 | 0.2583 |
| Linear | 3 | 1.27 | 1.26 | 0.3203 |
| Quadratic | 3 | 5.06 | 120.78 | <0.0001 |
| Cubic | 4 | 0.056 | 1.00 | 0.4750 |
| Lack of fit | 5 | 0.071 | 1.03 | 0.4876 |
| R2 = 0.9789 | ||||
| Adj R2 = 0.9600 | ||||
| FICA | ||||
| Model | 9 | 294.24 | 10.23 | 0.0006 |
| X1 | 1 | 114.97 | 35.97 | 0.0001 |
| X2 | 1 | 0.85 | 0.27 | 0.6170 |
| X3 | 1 | 5.06 | 1.58 | 0.2371 |
| X1X2 | 1 | 0.49 | 0.15 | 0.7026 |
| X1X3 | 1 | 1.94 | 0.61 | 0.4536 |
| X2X3 | 1 | 1.96 | 0.61 | 0.4516 |
| X12 | 1 | 168.15 | 52.61 | <0.0001 |
| X22 | 1 | 2.91 | 0.91 | 0.3624 |
| X32 | 1 | 4.49 | 1.40 | 0.2633 |
| Linear | 3 | 120.88 | 3.14 | 0.0545 |
| Quadratic | 3 | 168.96 | 17.62 | 0.0003 |
| Cubic | 4 | 17.57 | 1.83 | 0.2417 |
| Lack of fit | 5 | 30.84 | 27.63 | 0.0012 |
| R2 = 0.9020 | ||||
| Adj R2 = 0.8138 |
| Retention Time | Composition | Peak Area (%) |
|---|---|---|
| 4.9 | 2-Hydroxy-2-cyclopenten-1-one | 3.46 |
| 7.97 | 2-Heptanone | 4.57 |
| 9.7 | Guaiacol | 9.10 |
| 14.09 | 1,2-Benzenediol | 2.18 |
| 14.52 | 4-Methylbenzaldehyde | 3.67 |
| 15.03 | Hydroxy methyl furural | 2.04 |
| 18.45 | 2-Methoxy-4-Vinyl phenol | 3.17 |
| 26.25 | Sucrose | 25.81 |
| 31.33 | Ethyl d-glucoside | 5.50 |
| 48.94 | Methyl oleate | 8.87 |
| 49.16 | Octadecadienoic acid | 4.84 |
| Other | 26.79 | |
| Total | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuang, K.-J.; Chen, Z.-J.; Cheng, C.-L.; Hong, G.-B. Investigation of the Antioxidant Capacity, Insecticidal Ability and Oxidation Stability of Chenopodium formosanum Seed Extract. Int. J. Mol. Sci. 2018, 19, 2726. https://doi.org/10.3390/ijms19092726
Chuang K-J, Chen Z-J, Cheng C-L, Hong G-B. Investigation of the Antioxidant Capacity, Insecticidal Ability and Oxidation Stability of Chenopodium formosanum Seed Extract. International Journal of Molecular Sciences. 2018; 19(9):2726. https://doi.org/10.3390/ijms19092726
Chicago/Turabian StyleChuang, Kai-Jen, Zong-Jiang Chen, Chih-Lun Cheng, and Gui-Bing Hong. 2018. "Investigation of the Antioxidant Capacity, Insecticidal Ability and Oxidation Stability of Chenopodium formosanum Seed Extract" International Journal of Molecular Sciences 19, no. 9: 2726. https://doi.org/10.3390/ijms19092726
APA StyleChuang, K.-J., Chen, Z.-J., Cheng, C.-L., & Hong, G.-B. (2018). Investigation of the Antioxidant Capacity, Insecticidal Ability and Oxidation Stability of Chenopodium formosanum Seed Extract. International Journal of Molecular Sciences, 19(9), 2726. https://doi.org/10.3390/ijms19092726





